Abstract
Introduction
Neuropeptide inactivation is generally thought to occur via peptidase-mediated degradation. However, a recent study found increased analgesia after L-kyotorphin (L-Tyr-L-Arg; L-KTP) administration in mice lacking an oligopeptide transporter, PEPT2. The current study examines the role of PEPT2 in L-KTP uptake by astrocytes and compares it to astrocytic L-KTP degradation.
Methods
L-[3H]KTP uptake was measured in primary cultures of neonatal astrocytes from rats and from Pept2+/+ and Pept2-/- mice. Uptake was further characterized using potential inhibitors. L-[3H]KTP degradation was examined in primary astrocyte cultures from Pept2-/- mice by following the formation of L-[3H]tyrosine.
Results
The uptake of L-[3H]KTP in both rat and Pept2+/+ mouse neonatal astrocytes was inhibited by known PEPT2 inhibitors. L-[3H]KTP uptake was also reduced in Pept2-/- astrocytes as compared to those from Pept2+/+ mice. Kinetic analysis indicated the presence of a high affinity (Km ∼50 μM) transporter for L-[3H]KTP, identified as Pept2, and a low affinity transporter (Km ∼3-4 mM), inhibited by amastatin, bestatin and tyrosine. Astrocytes also degraded L-KTP through a low affinity peptidase (Km ∼2 mM).
Conclusions
Astrocytic clearance of L-KTP occurs via both peptidase activity and transport. These processes occur at similar rates and may be linked. This supports the contention that oligopeptide transport may have an impact on the extracellular clearance (and potentially activity) of certain neuropeptides.
Keywords: Kyotorphin, astrocyte, PEPT2, transport, peptidase, knockout, mice, rats
1. Introduction
A wide range of amino acids and neuropeptides function as neurotransmitters within the brain. With amino acid neurotransmitters, such as glutamate, transporters play an integral role in the clearance of neurotransmitters from the synaptic cleft, thereby limiting both the duration of exposure at the post-synaptic cell and preventing exposure of adjacent cells (Tzingounis and Wadiche, 2007). For peptide neurotransmitters, peptidases may have a similar function by degrading the neuroactive peptides into inactive peptide fragments or amino acids (McKelvy and Blumberg, 1986). Enkephalins, for example, are degraded by a variety of peptidases and differences in enzymatic degradation may affect neuropeptide activity (Roques et al., 1993). However, there are also peptide transporters in the brain. For example, members of the proton-coupled oligopeptide transporter (POT) family (PEPT2, PHT1 and PHT2) are present in the CNS (Kamal et al., 2008; Smith et al., 2004). Di- and tri-peptides as well as some peptidomimetics are substrates for those transporters (Daniel and Kottra, 2004; Smith et al., 2004). Using Pept2 null mice and PEPT2 inhibitors, we have shown that the uptake of carnosine (an endogenous dipeptide), glycylsarcosine (a synthetic dipeptide) and 5-aminolevulinic acid (a peptidomimetic) into cultured neonatal rat and mouse astrocytes are all PEPT2 dependent (Xiang et al., 2006a; Xiang et al., 2006b). However, it is unclear how important such transport is compared to peptidase activity in clearing endogenous neuropeptide substrates from the brain extracellular space.
One PEPT2 substrate is L-kyotorphin (KTP; L-Tyr-L-Arg; (Thakkar et al., 2008)). This is an endogenous analgesic that can cause receptor-mediated release of enkephalins within the brain (Takagi et al., 1979b). Compared to L-KTP, D-KTP (L-Tyr-D-Arg) has been reported to have a greater analgesic effect and it has been postulated that this is because it is resistant to hydrolysis by peptidases (Matsubayashi et al., 1984; Takagi et al., 1979a; Takagi et al., 1979b). Peptidases degrading L-KTP have been identified in brain homogenates and synaptosomes (Akasaki et al., 1991; Orawski and Simmons, 1992; Ueda et al., 1985), however, whether astrocytes can degrade L-KTP has not been examined. While these results suggest that L-KTP mediated analgesia is limited by peptidases, we recently found that such analgesia is enhanced in Pept2-/- mice (Jiang et al., 2009). This suggests that peptide transporters can modulate neuropeptide activity in the extracellular space.
Based on the above, this study aimed to determine whether PEPT2 is a transporter for L-KTP in astrocytes, to examine whether there are differences in PEPT2 affinity between D- and L-KTP (which might contribute to differences in analgesic effects) and to compare the kinetics of astrocyte transport and peptidase activity. The study examined L-[3H]KTP transport and degradation in neonatal astrocytes derived from rats and Pept2+/+ and Pept2-/- mice. While peptidase inhibitors can provide information on the role of peptidases in truncating the effects of neuropeptides, such inhibitors may also affect oligopeptide transport (Hori et al., 1993).
2. Results
L-[3H]KTP Uptake in Rat Astrocytes
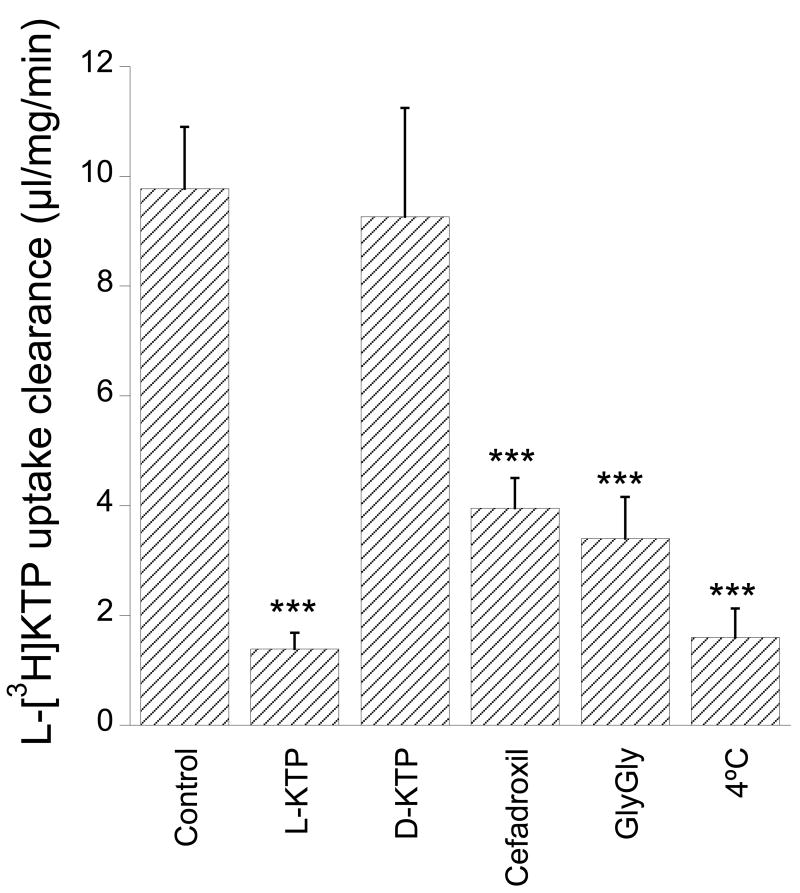
Under control conditions, in the presence of 1 μM L-KTP, the uptake clearance of L-[3H]KTP was 9.8±1.1 μl/mg/min. This was markedly reduced (∼86%) in the presence of 1 mM L-KTP but not D-KTP (Fig. 1). Uptake was temperature dependent (∼84% reduction at 4°C) and reduced by 60-65% in the presence of 1 mM cefadroxil or GlyGly, two PEPT2 substrates (Fig. 1).
Figure 1.
Uptake clearance of L-[3H]KTP (1 μM) in rat neonatal astrocytes in the presence of potential transport inhibitors (1 mM) or at 4°C. Values are means ± SE, n = 3-5 independent experiments. *** indicates a significant difference from control at the p<0.001 level.
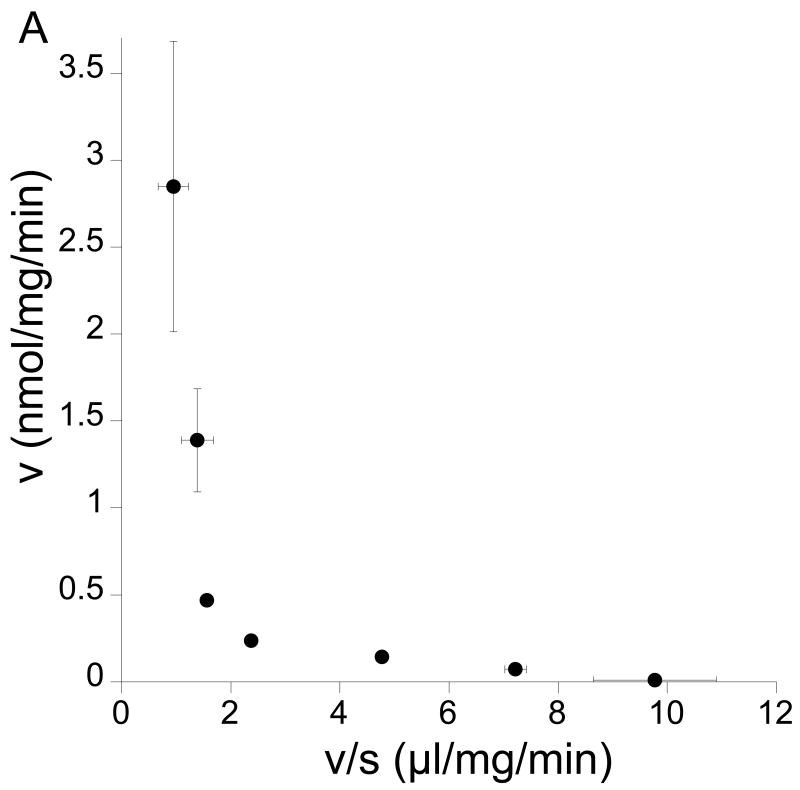
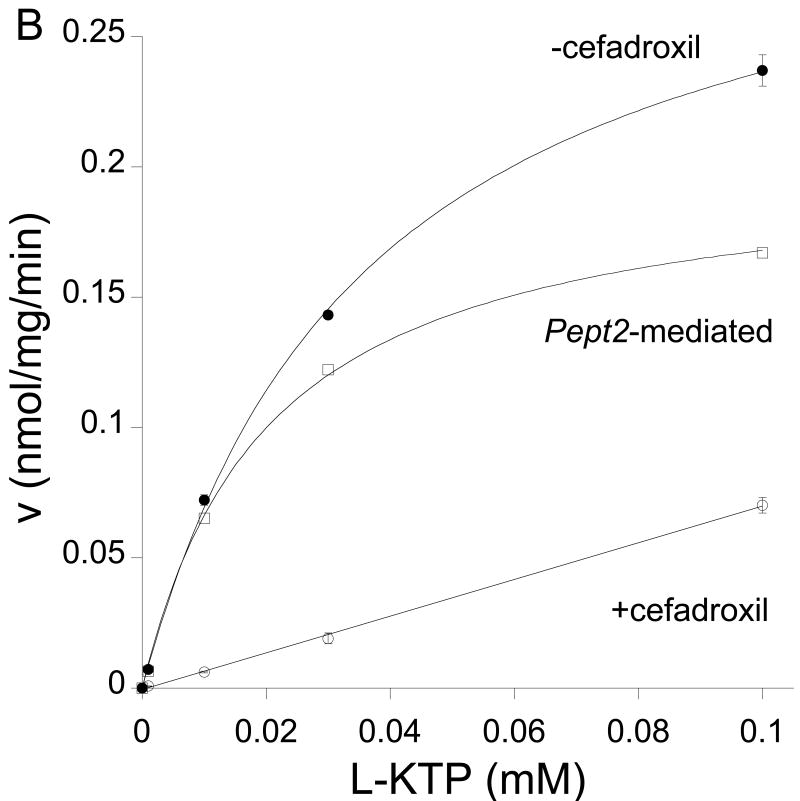
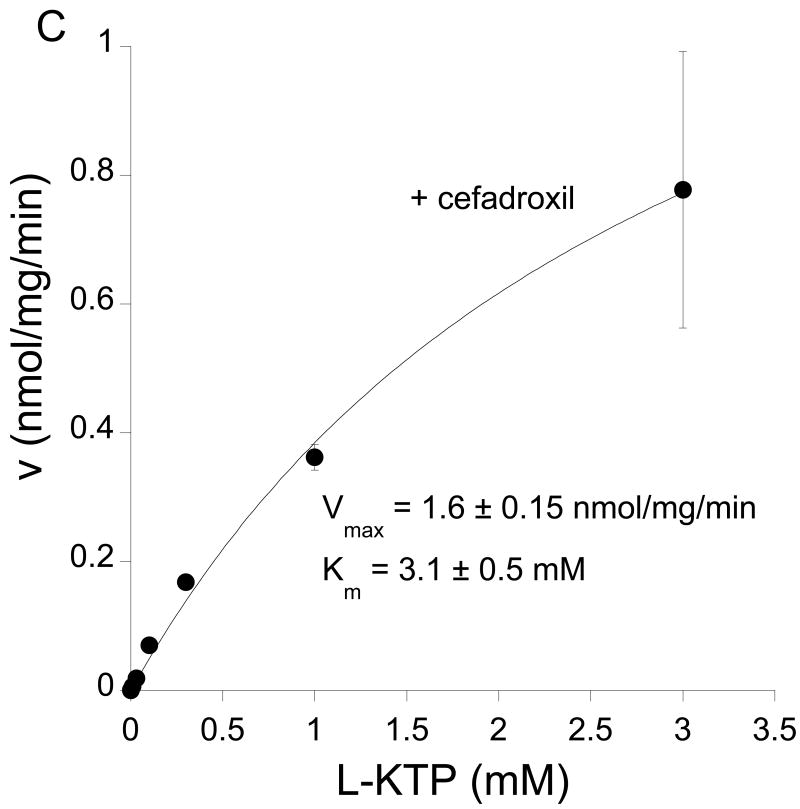
A kinetic analysis of L-KTP uptake was undertaken where concentrations were varied from 1 μM to 3 mM. A Woolf-Augustinsson-Hofstee plot indicated two transport processes, one with a high affinity and another with a low affinity (Fig 2A). The presence of two uptake mechanisms is also suggested by the incomplete inhibition of L-KTP uptake by cefadroxil and GlyGly. For example, in contrast to the 60% reduction found in L-KTP uptake, we have previously found that cefadroxil inhibits GlySar, a PEPT2 substrate, uptake by ∼96% in neonatal astrocytes (Xiang et al., 2006a). Therefore, to distinguish between the two transporters, uptake was measured in the presence and absence of excess cefadroxil. In the presence of cefadroxil, there was no evidence of saturable transport at low L-KTP concentrations (0.1 mM or less; Fig. 2B). In contrast, there was evidence of saturable transport when measurements were made in the absence of cefadroxil (Fig. 2B). The difference in L-KTP uptake in the presence and absence of cefadroxil was used to determine Pept2-mediated transport. That transport had a Km of 21±1 μM and a Vmax of 0.20±0.01 nmol/mg/min (Fig 2B). Analysis of the cefadroxil-insensitive uptake (not Pept2-mediated) over the full concentration range showed the presence of a low affinity transporter with a Km of 3.1±0.5 mM and a Vmax of 1.6 ±0.15 nmol/mg/min (Fig 2C).
Figure 2.
Effect of concentration (1-3,000 μM) on the uptake of L-[3H]KTP in rat neonatal astrocytes at 37°C. (A) Woolf-Augustinsson-Hofstee plot of the transformed data (V, nmol/mg/min versus V/S, μl/mg/min) indicated that there were two uptake mechanisms. (B) At low L-KTP concentrations, uptake was measured in the absence or presence of 1 mM cefadroxil (a Pept2 substrate). There was no evidence of saturable transport in the presence of cefadroxil (linear regression, r2 = 0.999). The difference between uptake in the absence and presence of cefadroxil (Pept2-mediated uptake) showed a Km of 21±1 μM and a Vmax of 0.20±0.01 nmol/mg/min (r2 = 0.999). (C) Uptake in the presence of 1 mM cefadroxil was measured to examine the low affinity uptake mechanism. That uptake had a Km of 3.1±0.5 mM and a Vmax of 1.6±0.15 nmol/mg/min (r2 = 0.999). Data are the averages ± SE of 6-9 measurements.
L-[3H]KTP Uptake in Pept2+/+ and Pept2-/- Mouse Astrocytes
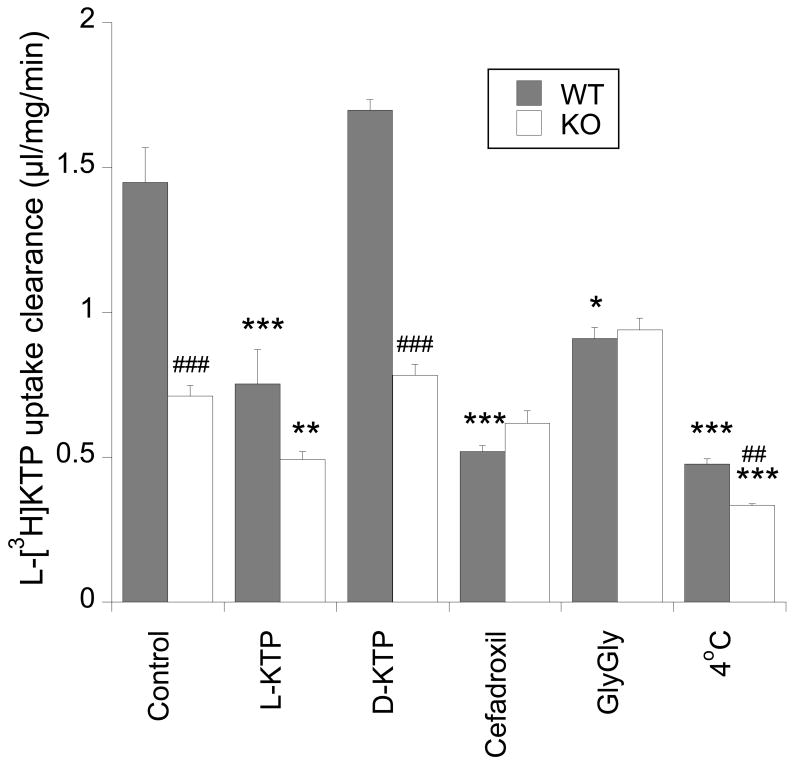
Under control conditions, in the presence of 1 μM L-KTP, the uptake clearance of L-[3H]KTP in mouse Pept2+/+ astrocytes was 1.5±0.1 μl/mg/min; i.e. only about 15% of the level in rat neonatal astrocytes. As with rat astrocytes, though, L-[3H]KTP uptake was reduced in the presence of 1 mM L-KTP, cefadroxil and GlyGly, and was temperature dependent (Fig.3). Uptake was not affected by 1 mM D-KTP (Fig.3).
Figure 3.
Uptake clearance of L-[3H]KTP in neonatal astrocytes derived from either Pept2+/+ (WT) or Pept2-/- (KO) mice. Uptake was measured under control conditions or in the presence of 1 mM L-KTP, D-KTP, cefadroxil, GlyGly, or at 4°C. Values are means±SE, n = 3-12 independent experiments, *p<0.05, **p<0.01 and ***p<0.001 levels as compared to control values for each genotype. For a given treatment, ##p<0.01 and ###p<0.001 levels between genotypes.
Compared to astrocytes derived from Pept2+/+ mice, the uptake of L-[3H]KTP was reduced by ∼50% in cells derived from Pept2-/- animals (Fig. 3; p<0.001). Also, in contrast to Pept2+/+ astrocytes, 1 mM cefadroxil and 1 mM GlyGly had no effect on L-[3H]KTP uptake in Pept2-/- astrocytes. However, there was a small reduction in uptake with 1 mM L-KTP and 4°C (Fig. 3).
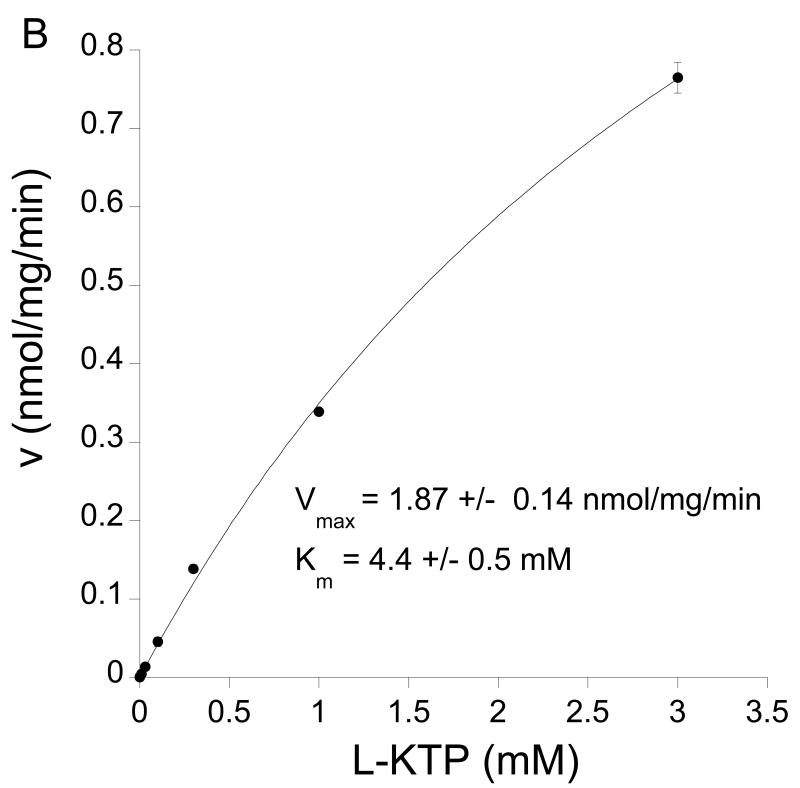
An analysis of the difference in L-[3H]KTP between Pept2+/+ and Pept2-/- astrocytes was used to examine the kinetics of Pept2-mediated transport (Fig. 4A). This again indicated that Pept2 had a high affinity for L-KTP (Km = 76±27 μM). There was no high affinity uptake in Pept2-/- astrocytes with uptake being linear with concentration (r2 = 1.000) when L-KTP was 0.3 mM or less. There was, though, evidence of a low affinity transporter in Pept2-/- astrocytes with a Km of 4.4±0.5 mM (Fig. 4B).
Figure 4.
(A) Concentration-dependent uptake of L-[3H]KTP by Pept2+/+ and Pept2-/- astrocytes. The difference between uptake in the two types of astrocytes at low L-KTP concentrations was used to calculate Pept2-mediated uptake and this was fitted to a Michaelis Menten equation. Values are the averages ± SE of 6-9 measurements. The Pept2-mediated uptake had a Km of 76±27 μM and a Vmax of 0.062±0.006 nmol/mg/min (r2 = 0.969). (B) Kinetics for uptake of L-[3H]KTP by pept2-/- astrocytes over a wider L-KTP concentration range was fit to a Michaelis Menten equation. It showed a low affinity transporter Km of 3.1±0.5 mM and a Vmax of 1.6±0.15 nmol/mg/min.
Peptide Degradation in Pept2-/- Mouse Astrocytes
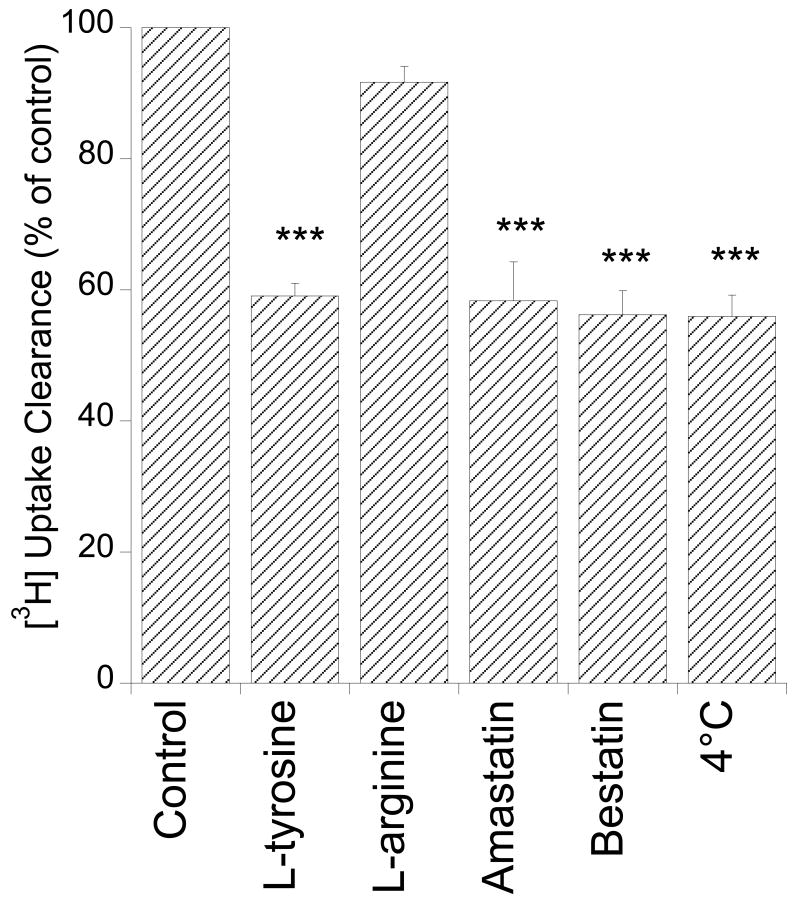
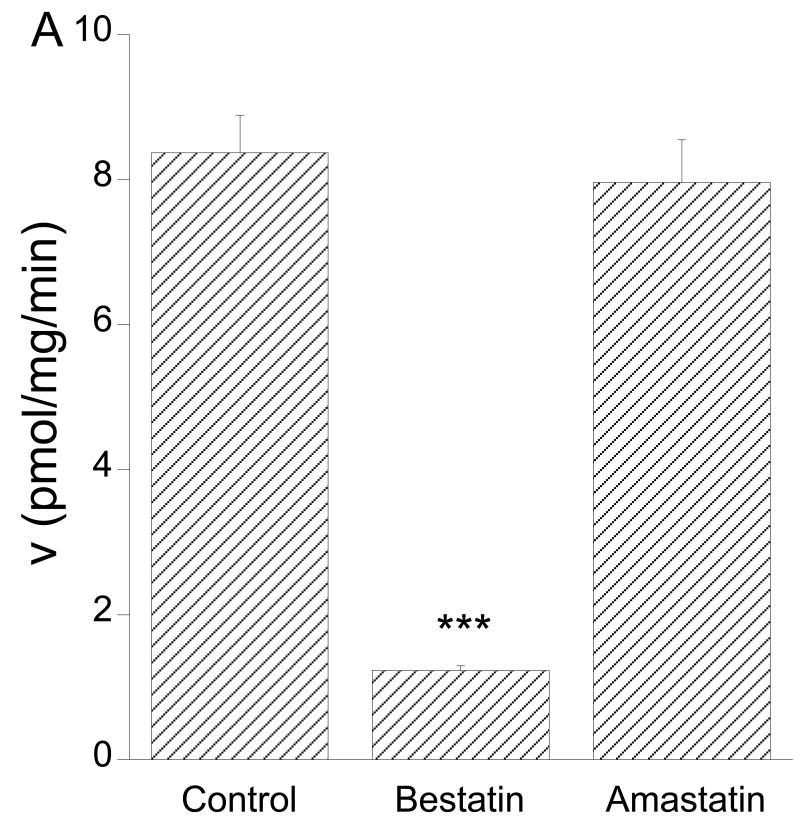
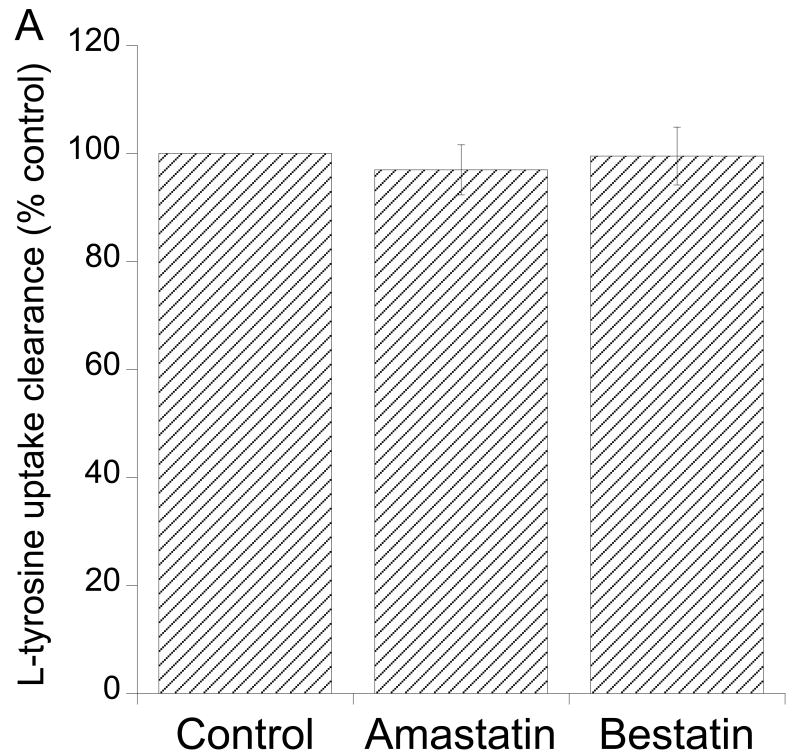
L-KTP is composed of L-tyrosine and L-arginine. To examine whether L-KTP degradation and the uptake of [3H]arginine or [3H]tyrosine might contribute to [3H] uptake after incubation of Pept2-/- astrocytes with L-[3H]KTP, experiments were performed in the presence of 1 mM arginine or 1 mM tyrosine. Arginine had little or no effect on [3H] uptake (almost all [3H] in L-[3H]KTP is expected to be on tyrosine; Moraveck personal communication), but incubation with 1 mM tyrosine reduced [3H] uptake by ∼40%, to a level similar to that found at 4°C (Fig. 5). Two peptidase inhibitors, amastatin and bestatin (10 μM), also inhibited [3H] uptake in Pept2-/- astrocytes which might support the concept of degradation followed by [3H]tyrosine uptake. However, a direct examination of the degradation of L-[3H]KTP to [3H]tyrosine in the media surrounding the Pept2-/- astrocytes indicated that this was slow (∼1% over 3 minutes with 1 μM L-KTP present). In addition, while bestatin inhibited degradation by 85%, amastatin had no effect on degradation (Fig. 6A) and neither bestatin nor amastatin inhibited the uptake of [3H]tyrosine into Pept2-/- astrocytes (Fig. 7A). It appears, therefore, that there is a non-Pept2 mediated L-KTP uptake mechanism that is temperature dependent and inhibited by bestatin, amastatin and tyrosine.
Figure 5.
Effect of L-tyrosine and L-arginine (both 1 mM) and amastatin and bestatin (both 10 μM) on the [3H] uptake clearance (expressed as % of control) in neonatal astrocytes from Pept2-/- mice exposed to L-[3H]KTP. Values are means±SE, n = 3-9 independent experiments, *p<0.05, **p<0.01 and ***p<0.001 levels as compared to control values.
Figure 6.
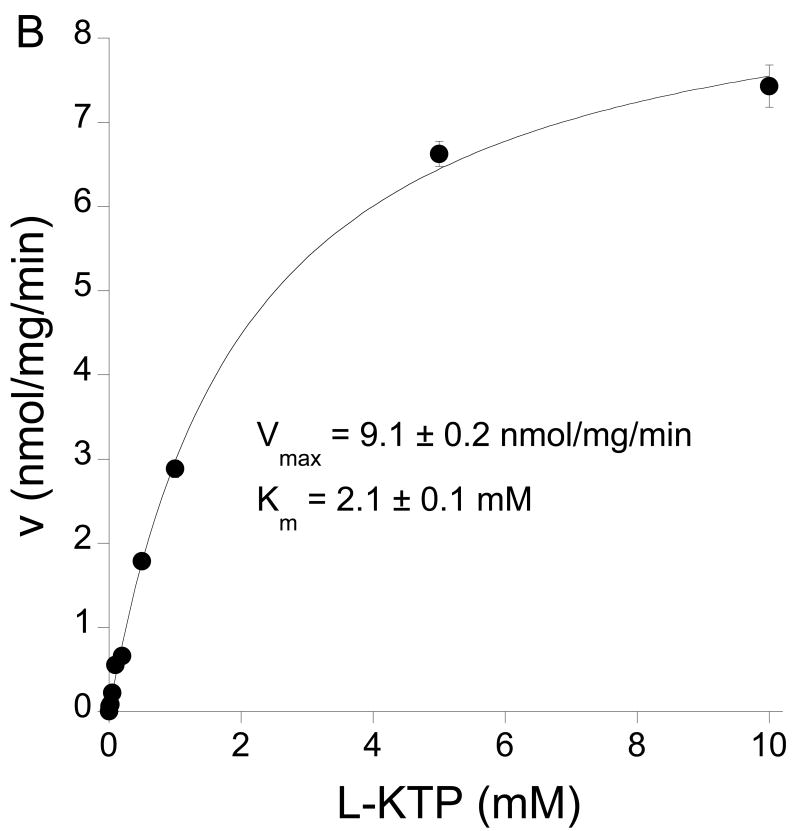
(A) Effect of two peptidase inhibitors, bestatin and amastatin (10 μM) on the degradation rate for L-[3H]KTP (1 μM) by Pept2-/- astrocytes. Values are means±SE, n = 3-4, ***p<0.001 level as compared to control. (B) Kinetics for the degradation of L-[3H]KTP by Pept2-/- astrocytes. Values are the means of 3-4 experiments. The Michaelis-Menten fit indicated a Km of 2.07±0.12 mM and a Vmax of 9.1±0.2 nmol/mg/min.
Figure 7.
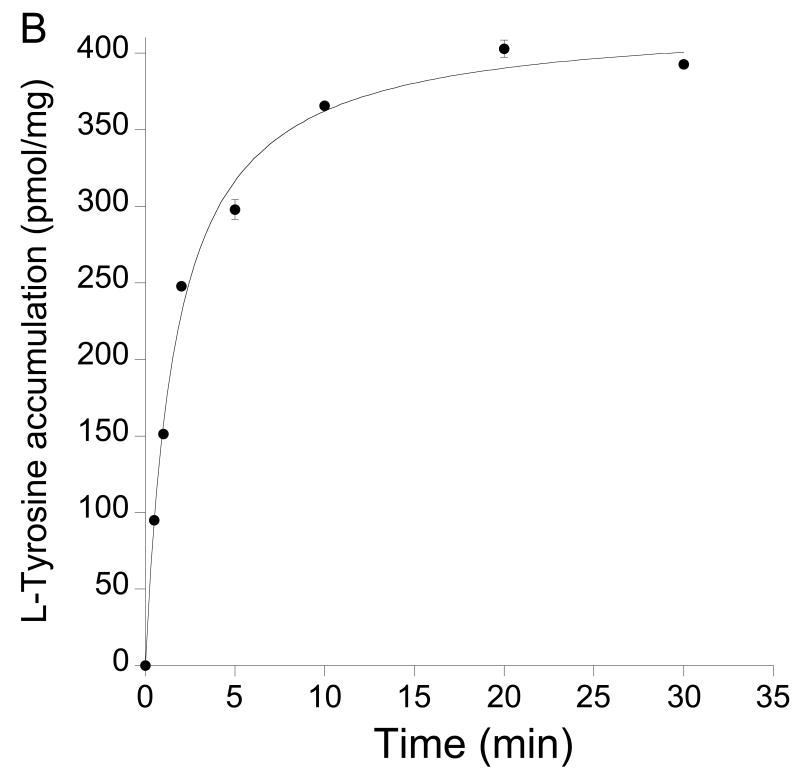
(A) Lack of effect of amastatin and bestatin (both 10 μM) on L-[3H]tyrosine uptake clearance (expressed as % of control) in neonatal astrocytes from Pept2-/- mice. Values are means±SE, n = 3. (B) Time course of L-[3H]tyrosine accumulation (in the presence of 1 μM tyrosine) in neonatal astrocytes from Pept2-/- mice. The initial uptake rate (0.5 min) was 190±2 pmol/mg/min and uptake plateaued at 423±10 pmol/mg. At equilibrium, efflux = influx and, therefore, the efflux rate can be estimated as 45%/min. Values are means±SE, n = 3.
Degradation is another mechanism by which astrocytes might clear L-KTP from the extracellular space. The kinetics of such degradation (appearance of extracellular L-[3H]tyrosine) was examined in Pept2-/- astrocytes. The Km for degradation was 2.07±0.12 mM and the Vmax 9.1±0.2 nmol/mg/min (Fig. 6B). The degradation appears, therefore, to be a low affinity system compared to Pept2. The degradation of L-KTP may occur extracellularly or intracellularly, with the subsequent movement of L-[3H]tyrosine to the extracellular space. It was notable that the rate of L-[3H]tyrosine appearance in the extracellular space was of the same order of magnitude as the rate of L-[3H]KTP uptake into Pept2-/- astrocytes (see Discussion). To examine whether intracellular degradation might significantly impact the appearance of L-[3H]tyrosine in the extracellular space, the rate of L-[3H]tyrosine efflux was determined in Pept2-/- astrocytes (Fig. 7B). The efflux rate was rapid at 45%/min.
3. Discussion
Determining how neuropeptides are cleared from the brain extracellular space is important for understanding the kinetics of neuropeptide-mediated neurotransmission and for designing agents to affect neurotransmission. While there has been a focus on peptidases in clearing extracellular neuropeptides, we recently found that the actions of the analgesic dipeptide, L-KTP, were enhanced in Pept2-/- mice indicating a role for transporter-mediated clearance (Jiang et al., 2009). The current study shows that neonatal astrocytes have the ability to take up L-KTP by two mechanisms, a high affinity system corresponding to Pept2 and an unknown low affinity system that is inhibited by bestatin, amastatin and tyrosine. In addition, astrocytes were capable of metabolizing L-KTP. That degradation was via a low affinity peptidase (Km ∼2 mM). The rate of metabolism of L-KTP was similar in magnitude to its uptake into astrocytes and may be subsequent to uptake. D-KTP has a lack of affinity for PEPT2 and this may contribute to the greater analgesic effect of this enantiomer compared to L-KTP.
L-KTP uptake in rat and mouse neonatal astrocytes occurs via two systems, one with high affinity (Km 21-76 μM) and one with low affinity (Km ∼3-4 mM) for L-KTP. Several pieces of evidence indicate that the high affinity system is mediated by Pept2: 1) Pept2 KO astrocytes only had the low affinity system; 2) cefadroxil, a PEPT2 substrate (Shen et al., 2005; Shen et al., 2007), blocked the high affinity system; 3) L-KTP is a substrate for PEPT2 (Thakkar et al., 2008) which is a high affinity oligopeptide transporter; and 4) L-KTP blocks PEPT2-mediated GlySar uptake with IC50 values of 5-30 μM in choroid plexus epithelial cells, synaptosomes and kidney cells, suggesting a high affinity for PEPT2 (Bravo et al., 2005; Fujita et al., 2004; Teuscher et al., 2001). In rat, the concentration of L-KTP in different brain regions varies between 0.14 – 2.1 μmol/kg tissue (Ueda et al., 1980). While this may suggest that L-KTP concentrations are below the Km for Pept2, the concentration of L-KTP in synaptosomes is several-fold higher than in brain homogenate (Ueda et al., 1982) and the concentration at or adjacent to the synaptic cleft is unknown.
The nature of the low affinity uptake system that is present in Pept2 null astrocytes is as yet uncertain. We initially hypothesized that it might represent the uptake of [3H]tyrosine after [3H]KTP degradation. However, while two peptidase inhibitors, amastatin and bestatin, as well as tyrosine could inhibit the apparent uptake of [3H], one peptidase inhibitor, amastatin, did not affect the degradation of L-KTP and neither peptidase inhibitor affected L-[3H]tyrosine uptake directly. Thus, it appears likely that amastatin and bestatin are affecting uptake via a non-peptidase mediated mechanism. This is not unprecedented. For example, bestatin is also a PEPT1 and PEPT2 substrate (Hori et al., 1993; Inui et al., 1992). Hussain et al. (Hussain et al., 2001) found an L-KTP transporter in PC12 cells that had a Km of ∼1mM which did not appear to be PEPT2 or PHT. That transporter might be the same one as described in this study. In contrast to PEPT2, which is electrogenic, Hussain et al. (Hussain et al., 2001) found the new transporter to be electroneutral.
In this study, astrocyte degradation of L-KTP was via a low affinity (Km ∼ 2 mM) system. This is the first demonstration of such activity in astrocytes. There have, though, been a number of studies on L-KTP peptidase activity in brain homogenates and synaptosomes (Akasaki et al., 1991; Orawski and Simmons, 1992; Ueda et al., 1985). Initial studies on brain homogenates showed a high affinity peptidase for L-KTP (Km ∼20 μM; (Akasaki et al., 1991; Ueda et al., 1985)) and a high affinity peptidase is present in synaptosomes (Km ∼8 μM; (Orawski and Simmons, 1992)), suggesting that the peptidase is neuronal. Akasaki et al. (Akasaki et al., 1991) also found evidence of another peptidase in the soluble fraction from brain homogenates with a Km of ∼100 μM which accounted for about 5% of L-KTP degradation. Based on affinity, it appears that the astrocyte L-KTP peptidase may be different from either of these previously identified peptidases, although the synaptosomal peptidase activity was also blocked by bestatin and not amastatin (Orawski and Simmons, 1992).
A possible function of the astrocyte peptidase is to degrade neuropeptides that diffuse away from the synaptic cleft. In relation to this point, it is important to state that our experiments were performed on neonatal astrocytes and that the need for astrocytic degradation of neuropeptides may vary during development. Struckhoff (Struckhoff, 1993) also found that astrocytic expression of dipeptidyl peptidase II decreased during postnatal development in the rat. Interestingly, the amount of astrocytic Pept2, which may also act to clear spillover from the synaptic cleft, also decreases postnatally in the rat (Shen et al., 2004). The effect of brain development on astrocyte peptidase activity merits further investigation although, even early in development, the fact that the synaptosomal peptidase has a Km for L-KTP of ∼8 μM (Orawski and Simmons, 1992) compared to 2 mM in astrocytes suggests that degradation at the synapse may predominate over astrocytic degradation.
The current study raises a fundamental question of how important are peptidases compared to transporters in clearing oligopeptide neurotransmitters from the extracellular space? For KTP, the evidence supporting the greater role of peptidases has come from the enhanced analgesia with D-KTP, which is peptidase resistant, compared to L-KTP (Matsubayashi et al., 1984) and the fact that bestatin, a peptidase inhibitor, can increase the L-KTP-induced analgesia (Matsubayashi et al., 1984; Ueda et al., 1985). As noted above, though, evidence in this study and others (Teuscher et al., 2001) indicates that, as well as being hydrolysis resistant, D-KTP is not a substrate for PEPT2 and that bestatin can affect peptide transport as well as peptidase activity (Hori et al., 1993; Inui et al., 1992). Indeed, it is interesting that we found that while D-KTP was more potent than L-KTP at inducing analgesia in Pept2+/+ mice, this was not the case in Pept2-/- animals (Jiang et al., 2009). To fully elucidate the role of peptidases in truncating L-KTP-induced analgesia probably requires peptidase KO mice as have been used to examine enkephalin-induced analgesia (Fischer et al., 2002; Saria et al., 1997).
For cultured neonatal astrocytes it is possible to estimate the relative rates of L-KTP transport and L-KTP degradation. Under linear conditions, the clearance via transport was 9.8 and 1.5 μl/mg/min in rat and mouse, respectively, while the peptidase-mediated degradation in mouse astrocytes was 4.4 μl/mg/min. This suggests transport and degradation, at low L-KTP concentrations, are of the same order of magnitude. Indeed, it is possible that the degradation of L-[3H]KTP observed in our experiments occurred intracellularly with the subsequent efflux of L-[3H]tyrosine to the extracellular space; i.e. that transport and degradation are linked. We found that L-[3H]tyrosine can rapidly efflux from astrocytes (45%/min) indicating that any L-[3H]tyrosine generated by an intracellular peptidase can rapidly migrate to the extracellular space.
In conclusion, these results support the concept that oligopeptide transport as well as peptidase activity can significantly impact the clearance of select neuropeptides, such at L-KTP, from the extracellular space. Thus, modulating transporter activity or affinity may be one method of modulating neuropeptide action.
4. Methods and Materials
Materials
Sprague Dawley rats were purchased from Charles River (Portage, MI). Pept2 knockout mice were generated on a C57BL/6 mouse background and genotyped by PCR, as described by Shen et al. (Shen et al., 2003). L-[3H]KTP (560mCi/mmol; labeled predominantly on tyrosine) was purchased from Moravek Biochemicals (Brea, CA,) and [14C]mannitol (53 mCi/mmol) and L-[3H]tyrosine (50 Ci/mmol) were from American Radiolabeled Chemicals (St Louis, MO). L- and D-KTP, glycylglycine (GlyGly), cefadroxil, amastatin, bestatin, L-tyrosine and L-arginine were purchased from Sigma-Aldrich (St Louis, MO). All other reagents for cell culture were purchased from Invitrogen Corporation (Grand Island, NY).
Neonatal astrocyte cultures were prepared from 1-2 day old rats or mice according to the method of Hertz et al. (Hertz et al., 1982) with slight modifications as described previously (Stamatovic et al., 2005; Xiang et al., 2006a). Mice were either Pept2-/- (null) or Pept2+/+ (wild type). Brain tissue was mechanically dissociated in Ca2+/Mg2+-free Hanks balanced salt solution (HBSS) and then digested with 0.25% trypsin-EDTA and 10 U/μl DNAse I at 37°C for 20 min. Trypsin digestion was stopped by adding DMEM containing 10% fetal bovine serum (FBS), followed by low speed centrifugation to remove debris. To obtain single cell suspensions, the brain tissue was triturated with a solution of Ca2+/Mg2+-free HBSS supplemented with 10 U/μl DNAse I and 3.8% (w/w) MgSO4. Cells were then washed twice in HBSS, resuspended in complete astrocyte media (DMEM, 10% inactivated fetal bovine serum, 1× glutamine, 1× antibiotic/antimycotic) and seeded on 12-well plates. Cells were grown under an atmosphere of 5% CO2/95% air at 37°C. Two weeks after initial plating, the cells were shaken at 220 rpm for 2 h at 4°C. After this time, the supernatant, containing mainly microglia, was removed. The remaining cells were cultured for 2 days and then used for uptake experiments.
Transport Studies
For L-[3H]KTP uptake measurements, experiments were performed in triplicate on each individual preparation. Preliminary experiments showed that L-[3H]KTP uptake was linear over 3 min and all subsequent experiments employed this uptake time. At the start of the experiment, cells were transferred to artificial CSF containing (in mM) 127 NaCl, 20 NaHCO3, 2.4 KCl, 0.5 KH2PO4, 1.1 CaCl2, 0.85 MgCl2, 0.5 Na2SO4 and 5.0 glucose (pH 7.4), bubbled with 5% CO2 and 95% O2. After 30 sec at 37°C, the buffer was removed and fresh uptake buffer containing L-[3H]KTP and [14C]mannitol (0.2 and 0.1 μCi/ml, respectively) were added to initiate uptake. Transport was measured at 37°C. At the end of the experiment, the medium was aspirated and the cells rapidly washed four times with ice-cold uptake buffer. The cells were solubilized in methylbenzethonium hydroxide and counted in a liquid scintillation counter. The protein content of the solublized cell monolayers was determined using a protein assay kit (Bio-Rad Laboratories, Hercules, CA). The volume of distribution (VD) for L-[3H]KTP (corrected for extracellular contamination) was determined as:
where KTPtiss and Manntiss are the dpm per mg protein for cellular L-[3H]KTP and [14C]mannitol, R is the ratio of L-[3H]KTP to [14C]mannitol radioactivity in the media, and KTPmedia is the dpm per μl of media. Dividing VD (μl/mg) by the duration of uptake (min) equals the influx clearance (μl/mg/min).
For L-[3H]tyrosine uptake studies, the same methodology was used. Uptakes were measured up to 30 minutes to determine the time course of uptake and to calculate the rate of efflux (see text). The rate of L-[3H]tyrosine uptake was linear for 0.5 minutes and this time was used for inhibitor studies.
For kinetic studies, the concentration-dependent uptake of L-KTP was fit to a Michaelis-Menten model:
Where Vmax is the maximal rate of saturable uptake, Km is the Michaelis constant and C is the substrate concentration.
Enzymatic Kinetic Degradation Studies
Neonatal Pept2-/- mouse astrocytes were grown on 12-well plates at 37°C. The growth media was replaced by 1 ml artificial CSF with L-[3H]KTP and [14C]mannitol (0.6 and 0.1 μCi/ml, respectively), with varying amounts of unlabeled L-KTP added at the start of the experiment. Initial experiments showed that the rate of degradation of L-[3H]KTP to L-[3H]tyrosine was linear over two hours (in presence of 10 μM L-KTP) and this time point was used for all subsequent experiments. After 2 hours, 50 μl of the incubating CSF was sampled, 5 μl of 1% trichloroacetic acid added to precipitate proteins and the sample centrifuged at 15,000 g for 10 min. The resultant supernatant was subjected to HPLC (Jiang et al., 2009). A Hypersil C18 column (250 mm × 4.6 mm, 5 μm) and a radiochemical detector (500TR Flow Scintillation Analyzer) were used. The mobile phase (20 mM K2PO4 containing 0.1% TFA [v/v], 3% CAN) was isocratically pumped at 1 ml/min (Waters, Model 515) under ambient conditions. The retention times for L-KTP, tyrosine and mannitol were 8.3, 4.7 and 3.1 min, respectively. The kinetics of degradation (conversion of L-[3H]KTP to L-[3H]tyrosine) was fit to a single component Michaelis-Menten model.
Statistics
All data are reported as the mean ± standard error of the mean (S.E.). Statistical differences were evaluated by analysis of variance (ANOVA) with a Newman-Keuls post-hoc test for multiple groups. Michaelis-Menten curve fits were performed with KaleidaGraph (Version 4.02; Synergy Software, Reading, PA).
Acknowledgments
This study was partially funded by NIH Grants R01 GM035498 (DES) and R01 NS034709 (RFK). H Jiang was supported by Scholarship of Zhejiang University and China Scholarship Council. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- KTP
kyotorphin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akasaki K, Nakamura A, Shiomi H, Tsuji H. Identification and characterization of two distinct kyotorphin-hydrolyzing enzymes in rat brain. Neuropeptides. 1991;20:103–7. doi: 10.1016/0143-4179(91)90059-r. [DOI] [PubMed] [Google Scholar]
- Bravo SA, Nielsen CU, Frokjaer S, Brodin B. Characterization of rPEPT2-mediated Gly-Sar transport parameters in the rat kidney proximal tubule cell line SKPT-0193 cl.2 cultured in basic growth media. Molecular Pharmaceutics. 2005;2:98–108. doi: 10.1021/mp049892q. [DOI] [PubMed] [Google Scholar]
- Daniel H, Kottra G. The proton oligopeptide cotransporter family SLC15 in physiology and pharmacology. Pflugers Arch. 2004;447:610–8. doi: 10.1007/s00424-003-1101-4. [DOI] [PubMed] [Google Scholar]
- Fischer HS, Zernig G, Hauser KF, Gerard C, Hersh LB, Saria A. Neutral endopeptidase knockout induces hyperalgesia in a model of visceral pain, an effect related to bradykinin and nitric oxide. J Mol Neurosci. 2002;18:129–34. doi: 10.1385/JMN:18:1-2:129. [DOI] [PubMed] [Google Scholar]
- Fujita T, Kishida T, Wada M, Okada N, Yamamoto A, Leibach FH, Ganapathy V. Functional characterization of brain peptide transporter in rat cerebral cortex: identification of the high-affinity type H+/peptide transporter PEPT2. Brain Research. 2004;997:52–61. doi: 10.1016/j.brainres.2003.10.049. [DOI] [PubMed] [Google Scholar]
- Hertz L, Juurilink BHL, Fosmark H, Schouboe A. Astrocytes in the primary culture. In: Pfeiffer SE, editor. Neuroscience Approached Through Cell Culture. Vol. 1. CRL Press; Boca Raton: 1982. pp. 175–186. [Google Scholar]
- Hori R, Tomita Y, Katsura T, Yasuhara M, Inui K, Takano M. Transport of bestatin in rat renal brush-border membrane vesicles. Biochem Pharmacol. 1993;45:1763–8. doi: 10.1016/0006-2952(93)90431-u. [DOI] [PubMed] [Google Scholar]
- Hussain I, Zanic-Grubisic T, Kudo Y, Boyd CA. Functional and molecular characterization of a peptide transporter in the rat PC12 neuroendocrine cell line. FEBS Letters. 2001;508:350–4. doi: 10.1016/s0014-5793(01)03081-2. [DOI] [PubMed] [Google Scholar]
- Inui K, Tomita Y, Katsura T, Okano T, Takano M, Hori R. H+ coupled active transport of bestatin via the dipeptide transport system in rabbit intestinal brush-border membranes. Journal of Pharmacology & Experimental Therapeutics. 1992;260:482–6. [PubMed] [Google Scholar]
- Jiang H, Hu Y, Keep RF, Smith DE. Enhanced antinociceptive response to intracerebroventricular kyotorphin in Pept2 null mice. Journal of Neurochemistry. 2009;109:1536–43. doi: 10.1111/j.1471-4159.2009.06090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal MA, Keep RF, Smith DE. Role and relevance of PEPT2 in drug disposition, dynamics, and toxicity. Drug Metabolism & Pharmacokinetics. 2008;23:236–42. doi: 10.2133/dmpk.23.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubayashi K, Kojima C, Kawajiri S, Ono K, Takegoshi T, Ueda H, Takagi H. Hydrolytic deactivation of kyotorphin by the rodent brain homogenates and sera. J Pharmacobiodyn. 1984;7:479–84. doi: 10.1248/bpb1978.7.479. [DOI] [PubMed] [Google Scholar]
- McKelvy JF, Blumberg S. Inactivation and metabolism of neuropeptides. Annu Rev Neurosci. 1986;9:415–34. doi: 10.1146/annurev.ne.09.030186.002215. [DOI] [PubMed] [Google Scholar]
- Orawski AT, Simmons WH. Dipeptidase activities in rat brain synaptosomes can be distinguished on the basis of inhibition by bestatin and amastatin: identification of a kyotorphin (Tyr-Arg)-degrading enzyme. Neurochemical Research. 1992;17:817–20. doi: 10.1007/BF00969018. [DOI] [PubMed] [Google Scholar]
- Roques BP, Noble F, Dauge V, Fournie-Zaluski MC, Beaumont A. Neutral endopeptidase 24.11: structure, inhibition, and experimental and clinical pharmacology. Pharmacol Rev. 1993;45:87–146. [PubMed] [Google Scholar]
- Saria A, Hauser KF, Traurig HH, Turbek CS, Hersh L, Gerard C. Opioid-related changes in nociceptive threshold and in tissue levels of enkephalins after target disruption of the gene for neutral endopeptidase (EC 3.4.24.11) in mice. Neuroscience Letters. 1997;234:27–30. doi: 10.1016/s0304-3940(97)00660-5. [DOI] [PubMed] [Google Scholar]
- Shen H, Smith DE, Keep RF, Xiang J, Brosius FC., 3rd Targeted disruption of the PEPT2 gene markedly reduces dipeptide uptake in choroid plexus. J Biol Chem. 2003;278:4786–91. doi: 10.1074/jbc.M207397200. [DOI] [PubMed] [Google Scholar]
- Shen H, Smith DE, Keep RF, Brosius FC., 3rd Immunolocalization of the proton-coupled oligopeptide transporter PEPT2 in developing rat brain. Molecular Pharmaceutics. 2004;1:248–56. doi: 10.1021/mp049944b. [DOI] [PubMed] [Google Scholar]
- Shen H, Keep RF, Hu Y, Smith DE. PEPT2 (Slc15a2)-mediated unidirectional transport of cefadroxil from cerebrospinal fluid into choroid plexus. Journal of Pharmacology & Experimental Therapeutics. 2005;315:1101–8. doi: 10.1124/jpet.105.090654. [DOI] [PubMed] [Google Scholar]
- Shen H, Ocheltree SM, Hu Y, Keep RF, Smith DE. Impact of genetic knockout of PEPT2 on cefadroxil pharmacokinetics, renal tubular reabsorption, and brain penetration in mice. Drug Metabolism & Disposition. 2007;35:1209–16. doi: 10.1124/dmd.107.015263. [DOI] [PubMed] [Google Scholar]
- Smith DE, Johanson CE, Keep RF. Peptide and peptide analog transport systems at the blood-CSF barrier. Adv Drug Deliv Rev. 2004;56:1765–91. doi: 10.1016/j.addr.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Stamatovic SM, Shakui P, Keep RF, Moore BB, Kunkel SL, Van Rooijen N, Andjelkovic AV. Monocyte chemoattractant protein-1 regulation of blood-brain barrier permeability. J Cereb Blood Flow Metab. 2005;25:593–606. doi: 10.1038/sj.jcbfm.9600055. [DOI] [PubMed] [Google Scholar]
- Struckhoff G. Dipeptidyl peptidase II in astrocytes of the rat brain. Meningeal cells increase enzymic activity in cultivated astrocytes. Brain Research. 1993;620:49–57. doi: 10.1016/0006-8993(93)90269-s. [DOI] [PubMed] [Google Scholar]
- Takagi H, Shiomi H, Ueda H, Amano H. Morphine-like analgesia by a new dipeptide, L-tyrosyl-L-arginine (Kyotorphin) and its analogue. Eur J Pharmacol. 1979a;55:109–11. doi: 10.1016/0014-2999(79)90154-7. [DOI] [PubMed] [Google Scholar]
- Takagi H, Shiomi H, Ueda H, Amano H. A novel analgesic dipeptide from bovine brain is a possible Met-enkephalin releaser. Nature. 1979b;282:410–2. doi: 10.1038/282410a0. [DOI] [PubMed] [Google Scholar]
- Teuscher NS, Keep RF, Smith DE. PEPT2-mediated uptake of neuropeptides in rat choroid plexus. Pharmaceutical Research. 2001;18:807–13. doi: 10.1023/a:1011088413043. [DOI] [PubMed] [Google Scholar]
- Thakkar SV, Miyauchi S, Prasad PD, Ganapathy V. Stimulation of Na+/Cl--coupled opioid peptide transport system in SK-N-SH cells by L-kyotorphin, an endogenous substrate for H+-coupled peptide transporter PEPT2. Drug Metab Pharmacokinet. 2008;23:254–62. doi: 10.2133/dmpk.23.254. [DOI] [PubMed] [Google Scholar]
- Tzingounis AV, Wadiche JI. Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat Rev Neurosci. 2007;8:935–47. doi: 10.1038/nrn2274. [DOI] [PubMed] [Google Scholar]
- Ueda H, Shiomi H, Takagi H. Regional distribution of a novel analgesic dipeptide kyotorphin (Tyr-Arg) in the rat brain and spinal cord. Brain Research. 1980;198:460–4. doi: 10.1016/0006-8993(80)90761-1. [DOI] [PubMed] [Google Scholar]
- Ueda H, Tatsumi K, Shiomi H, Takagi H. Analgesic dipeptide, kyotorphin (Tyr-Arg), is highly concentrated in the synaptosomal fraction of the rat brain. Brain Research. 1982;231:222–4. doi: 10.1016/0006-8993(82)90023-3. [DOI] [PubMed] [Google Scholar]
- Ueda H, Ming G, Hazato T, Katayama T, Takagi H. Degradation of kyotorphin by a purified membrane-bound-aminopeptidase from monkey brain: potentiation of kyotorphin-induced analgesia by a highly effective inhibitor, bestatin. Life Sciences. 1985;36:1865–71. doi: 10.1016/0024-3205(85)90160-2. [DOI] [PubMed] [Google Scholar]
- Xiang J, Chiang PP, Hu Y, Smith DE, Keep RF. Role of PEPT2 in glycylsarcosine transport in astrocyte and glioma cultures. Neuroscience Letters. 2006a;396:225–9. doi: 10.1016/j.neulet.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Xiang J, Hu Y, Smith DE, Keep RF. PEPT2-mediated transport of 5-aminolevulinic acid and carnosine in astrocytes. Brain Research. 2006b;1122:18–23. doi: 10.1016/j.brainres.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]