Abstract
One of the pathological hallmarks of Alzheimer’s disease (AD) is neurofibrillary tangles (NFTs), which are composed of abnormally hyperphosphorylated tau, but the mechanism of tau hyperphosphorylation in AD is still unclear. To investigate the effects of estrogens on tau phosphorylation, SH-SY5Y cells were treated with okadaic acid (OA), a serine/threonine phosphatase inhibitor, to induce tau phosphorylation and the effects of estrogen were observed by co-treatment with 17β-estradiol (E2). We found that OA induced in vitro tau hyperphosphorylation, which was prevented by E2 in a dose-dependent manner. This effect of E2 was partially blocked by an estrogen receptor (ER) antagonist, ICI 182,780. In addition to tau hyperphosphorylation, inhibition of serine/threonine phosphorylation induced upregulation of cdk5 levels, which was attenuated by E2 in a manner that was counteracted by ICI 182,780. Our results show that cdk5 is involved in OA induced tau hyperphosphorylation, and estrogens ameliorate the tau hyperphosphorylation, which may be mediated in part by ER.
Keywords: Alzheimer’s disease, estrogen, tau, cdk5, phosphatase
1. Introduction
Tau proteins are microtubule associated proteins that are abundant in neurons (Weingarten et al., 1975). Physiologically, tau plays a key role in microtubules stabilization, axonal transportation and neurite outgrowth (Avila et al., 2004; Devred et al., 2004; Johnson and Stoothoff 2004; Weingarten et al., 1975). Pathologically, tau is abnormally heyperphosphorylated and its aggregation and deposition is found in many neurodegenerative disorders including AD (Devred et al., 2004; Grundke-Iqbal et al., 1986a; Grundke-Iqbal et al., 1986b; Johnson and Stoothoff 2004; Lace et al., 2009). Tau is a phosphoprotein whose expression and phosphorylation is well regulated (Baudier and Cole 1987; Grundke-Iqbal et al., 1986a; Ihara et al., 1986). The longest human tau contains 441 residues, including 79 putative serine and threonine residues and 5 tyrosine residues located in two proline-rich regions (Johnson and Stoothoff 2004). The phosphorylation of these residues affects the binding of tau to microtubules, leads to tau dysfunction and ultimately results in cell death (Goedert et al., 1989; Hernandez and Avila 2007; Johnson and Stoothoff 2004). Although many protein kinases are able to phosphorylate tau in vitro (Correas et al., 1992; Drewes et al., 1992; Hanger et al., 1992; Liu et al., 2008), only a few are thought to be good candidates in vivo. GSK3β and cdk5 were first isolated from bovine brain microtubules and named tau protein kinase I (TPK I) and TPK II (Ishiguro et al., 1992; Ishiguro et al., 1993; Uchida et al., 1994). The dephosphorylation of phospho-tau is mainly mediated by protein phosphatases (PPs), among which PP2A is considered the major phosphatase in vivo (Gong et al., 2000; Liu et al., 2005). In selected areas of AD brain, both the expression and activity of PP2A have been reported to be reduced (Gong et al., 1993; Gong et al., 1995; Sontag et al., 2004; Vogelsberg-Ragaglia et al., 2001). It has been proposed that the imbalance between tau phosphorylation and dephosphorylation is critical to AD (Arendt et al., 1998; Gong et al., 2006). This disturbance could be due to the increase of tau kinases activity, decrease of tau PPs activity, or both.
Estrogens, which have been established as potent neuroprotectant, have been considered as a potential treatment for AD (for review see Singh et al., 2006). The prevalence of AD is higher in woman, and epidemiological studies have indicated that estrogen protects against AD (Filley 1997). Clinical studies have shown that postmenopausal women with estrogen deficiency are at risk for neurodegenerative diseases (Paganini-Hill and Henderson 1994) and postmenopausal estrogen therapy reduces the risk or delay the onset of AD (Henderson et al., 1994). This evidence suggests that estrogen deficiency may be a contributing factor in AD and estrogen could be a treatment for AD. Based on this, we hypothesize that estrogen can prevent tau phosphorylation. To test the hypothesis, we treated female human neuroblastoma SH-SY5Y cells with a PP1/2A inhibitor, okadaic acid (OA), to induce tau phosphorylation and the neuroprotective effect of 17β-estradiol (E2) was observed via co-treatment with E2. We also examined the role of certain tau kinases, cdk5 and GSK3 β, in the estrogen neuroprotective pathway.
2. Results
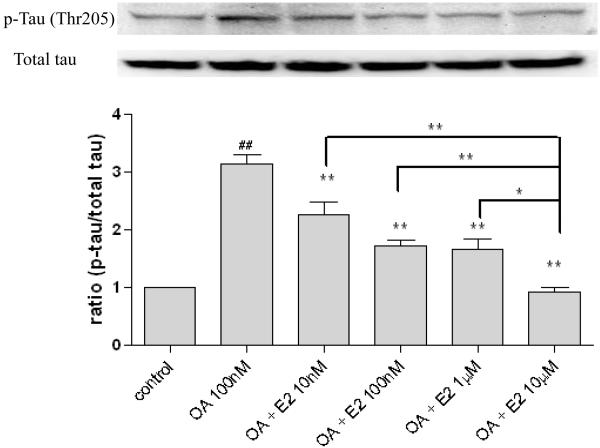
2.1 Estrogen prevent OA-induced tau phosphorylation in a dose-dependent manner
To examine the effects of OA and/or estrogen on tau phosphorylation, human neuroblastoma SH-SY5Y cells were treated with OA (100 nM) in the absence or presence of various concentrations of 17β-estradiol (0, 10 nM, 100 nM, 1 μM, 10 μM). After three hours co-treatment, protein samples were collected to assess the phosphorylation state of tau by Western blot analysis. As Figure 1 shows, OA (100 nM) induced a 3-fold increase in tau phosphorylation at a proline-directed site (Thr 205) (p < 0.05) in SH-SY5Y cells, which was attenuated by 17β-estradiol in a dose-dependent manner. 10 μM of estradiol reduced phosphorylated tau to control level (p < 0.01) and was significantly difference from the other three estrogen groups.
Figure 1. The dose-dependent effects of 17β-estradiol on tau phosphorylation in OA treated SH-SY5Y cells.
SH-SY5Y cells were co-treated with OA (100 nM) and 17β-estradiol (10 nM, 100 nM, 1 μM, 10 μM) for three hours. Phospho-tau (Thr 205) was assessed via western blot analysis. OA and E2 were dissolved in 0.1% DMSO. Vehicle control was treated with 0.1% DMSO. All data are normalized to total tau and are expressed as a percentage of control. Data are presented as mean ± SEM for n=4. ## means p<0.01 compared to control and ** <0.01 compared to OA only. Groups connected by the line were different at *p<0.05 and **p<0.01.
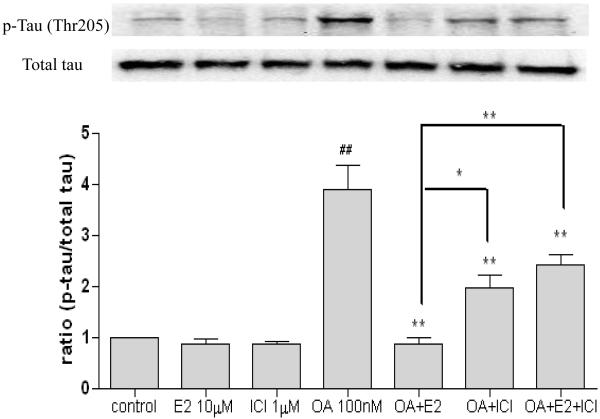
2.2 Estrogen prevent OA induced tau phosphorylation mediated by ER
To determine the role of ER, we treated SH-SY5Y cells with 17 β-estradiol (10 μM) of in the presence or absence of OA (100 nM) and ICI 182,780 (1μM) for three hours. As shown in Figure 2A, OA induced tau phosphorylation by 4-folds. Neither 17β-estradiol nor ICI 182,780 alone had any effect on tau phosphorylation (Figure 2), but both prevented the OA-induced tau phosphorylation (p < 0.05). In the presence of ICI 182,780, the E2-mediated effect on tau phosphroylation was partially blocked (p < 0.05) (Figure 2).
Figure 2. The effect of 17β-estradiol and ICI 182,780 on tau phosphorylation in OA treated SH-SY5Y cells.
SH-SY5Y cells were treated with OA (100 nM) in the presence of absence of 17β-estradiol (E2, 10 μM) and/or ICI 182,780 (1 μM) for three hours. Phospho-tau (Thr 205) was assessed via western blot analysis. OA, E2 and ICI 182,780 were dissolved in 0.1% DMSO. Vehicle control was treated with 0.1% DMSO as vehicle. All data are normalized to total tau and are expressed as a percentage of control. Data are presented as mean ± SEM for n=4. ## means p<0.01 compared to control and ** <0.01 compared to OA only. Groups connected by the line were different at *p<0.05 and **p<0.01.
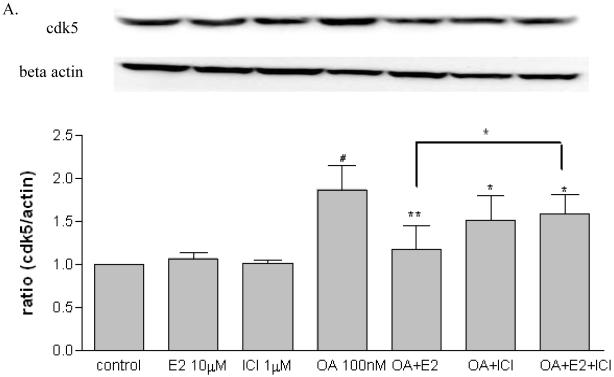
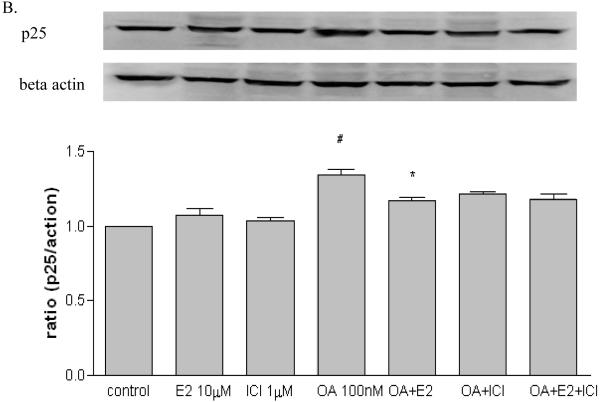
2.3 The effects of estrogen on tau kinases
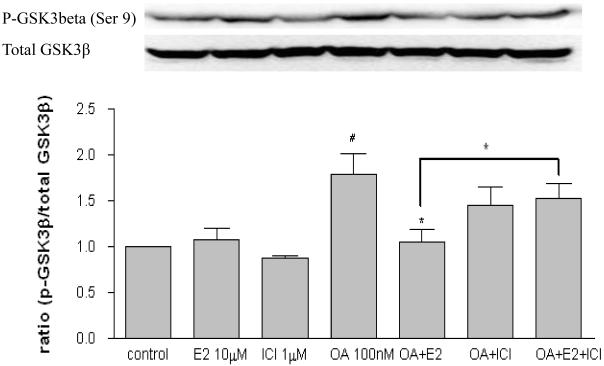
In order to investigate the possible mechanisms of estrogen-mediated prevention of OA-induced tau phosphorylation, SH-SY5Y cells were treated with OA (100 nM) in the presence or absence of 17 β-estradiol (10 μM) and ICI 182,780 (1μM). After three hours exposure, protein samples were collected for assessing certain tau kinases levels including cdk5, GSK3β and ERK1/2. As shown in Figure 3A, OA alone increased cdk5 (p < 0.05) while 17β-estradiol or ICI 182,780 had no effect. The OA-induced cdk5 increase was attenuated in the presence of 17β-estradiol (p < 0.01) but not ICI 182,780 (p > 0.05). Further, the effects of 17β-estradiol were partially blocked by ICI 182,780 (p < 0.05). OA increased p25 expression (p < 0.05) which was attenuated by 17β-estradiol (p < 0.05), while 17β-estradiol or ICI 182,780 alone had no effect (Figure 3B). As shown in Figure 4, OA alone increased GSK3β (p-Ser 9) expression, which is the inactive state of the enzyme (p < 0.05); 17β-estradiol or ICI 182,780 alone did not have any effect. The OA-induced GSK3β increase was attenuated by co-treatment with 17β-estradiol (p < 0.05) but not ICI 182,780 (p > 0.05); and this effect of 17β-estradiol was partially blocked by ICI 182,780 (p < 0.01). There were no changes observed on p-ERK/total ERK after treatment (data not shown).
Figure 3. Effects of 17β-estradiol and ICI 182,780 on cdk5 and p25 in OA treated SH-SY5Y cells.
SH-SY5Y cells were treated with OA (100 nM) in the presence of absence of 17β-estradiol (E2, 10 μM) and ICI 182,780 (1 μM) for three hours. cdk5 (A) and p25 (B) were assessed via western blot analysis. OA, E2 and/or ICI 182,780 were dissolved in 0.1% DMSO. Vehicle control was treated with 0.1% DMSO as vehicle. All data are normalized to β-actin and are expressed as a percentage of control. Data are presented as mean ± SEM for n=5. # means p<0.05 compared to control and * <0.05, ** <0.01 compared to OA only. Groups connected by the line were different at p<0.05.
Figure 4. Effects of 17β-estradiol and ICI 182,780 on GSK3β in OA treated SH-SY5Y cells.
Human neuroblastoma SH-SY5Y cells were treated with OA (100 nM) in the presence of absence of 17β-estradiol (E2, 10 μM) and ICI 182,780 (1 μM) for three hours. Total GSK3β and p-GSK3β (Ser 9) were assessed via western blot analysis. OA, E2 and/or ICI 182,780 were dissolved in 0.1% DMSO. Vehicle control was treated with 0.1% DMSO as vehicle. All data are normalized to total GSK3β and are expressed as a percentage of control. Data are presented in mean ± SEM for n=4. # <0.05 compared to control and * <0.05 compared to OA only. Groups connected by the line were different at p<0.05.
3. Discussion
In the present study, we showed that OA induces tau phosphorylation in SH-SY5Y neuroblastoma cells. This OA effect was dose-dependently reduced by E2 in a manner that was partially antagonized by the ER antagonist, ICI182,780. OA also causes an increase in cdk5 and p25 levels, an effect that may exacerbate tau hyerphosphorylation, but OA stimulated the inactive form of GSK3β. Collectively, these data indicate that OA exposure induces an imbalance between phosphatases and kinases leading to the hyperphospharylation of tau and E2 largely prevents these effects.
The tau phosphorylation at proline-directed site (Thr 205) seen in SH-SY5Y neuroblastoma cells induced by inhibition of PP1/2A is consistent with our previous in vivo study showing that OA dorsal hippocampal infusion of OA induces tau phosphorylation (Thr 205) in the hippocampus and cortex (Zhang and Simpkins, 2008). Studies from Alvarez-de-la-Rosa et al. also showed that OA induced hyperphosphorylation of tau in non-proline-directed site (Ser 262) (Alvarez-de-la-Rosa et al., 2005). Studies from other groups have been shown that OA induce phosphorylation of tau at different sites, including Ser 396/404 (Ekinci and Shea 1999), Ser 202/205 (Ekinci et al., 2003) and Ser 262 (Alvarez-de-la-Rosa et al., 2005). Abnormal tau hyperphosphorylation has been associated with the decreased stability of microtubules and accumulation as tangles of PHFs in neurons undergoing degeneration (Alonso et al., 1996; Ballatore et al., 2007). The microtubules binding ability of tau, which is considered to be the main mechanism that regulates the affinity of tau to the microtubules (Ballatore et al., 2007; Mazanetz and Fischer 2007), is post-translationally regulated by serine/threonine-directed phosphorylation. Responding to phosphorylation and dephosphorylation, the cycles of binding and detachment of tau from microtubules affect axonal transportation (Avila et al., 2004; Devred et al., 2004; Johnson and Stoothoff 2004; Weingarten et al., 1975). This equilibrium is determined by the phosphorylation state of tau, which is further controlled by the actions of kinases and phosphatases (Sergeant et al., 2005). We previously found that dorsal hippocampal infusion of OA can induce tau phosphorylation and cognitive deficits similar to those seen in AD, which are also seen in other in vivo studies (Alonso et al., 1996; Arias et al., 1993).
We found that the OA induced tau phosphorylation at proline-directed site (Thr 205) in SH-SY5Y cells can be prevented by 17β-estradiol in a dose-dependent manner. A 50% reduction in the effects of OA were observed by the 100 nM concentration of E2, and 10 M E2 completely prevented tau hyperphosphorylation induced by OA. This dosimetry argues for an ER-mediated effect at low E2 concentrations, and a non-ER-mediated effect at higher concentrations. This hypothesis is consistent with our observation that OA cause a profound pro-oxidant stress (Yi et al., 2009) and that E2 has antioxidant properties (Green and Simpkins 2000) and our observation that ICI 182,780 only partially blocked the E2 effect. Other study also showed that ER antagonist can block the effect of estrogen on tau phosphorylation induced by OA at non-proline-directed site (Ser 262) (Alvarez-de-la-Rosa et al., 2005). It has been reported that both ERα and ERβ are expressed in SH-SY5Y cell line (Bang et al., 2004). Although ICI 182,780 has been shown to bind to ER receptor as a pure ER antagonist, the actual mechanisms of ICI 182,780 remains poorly understood. It has been reported that ICI 182,780, at very high concentration, is able to induce ER dimerization and ER-dependent transcription (Dudley et al., 2000), which may explain why only partial blockade of estrogen effect on tau phosphorylation by ICI 182,780 was seen in our study; and our observation that ICI 182,780 also reduced tau phosphorylation by itself. Another possibility is that the high concentration of estrogen used exerts its protective effect via a non-receptor pathway masking the receptor-mediated effect.
We found that the OA-induced increase in GSK3β (p-Ser 9) and cdk5 levels were prevented by estrogen and this effect of estrogen could be partially blocked by ICI 182,780. Our data indicates that the regulation of tau kinases could be one of the post-receptor events by which estrogen preventing tau phosphorylation. The imbalance between tau phosphorylation mediated by tau kinases and dephosphorylation mediated by PPs is critical to AD (Arendt et al., 1998; Gong et al., 2006). Besides inhibiting PP1/2A, OA is also reported to activate calpain (Yoon et al., 2006), which cleaves p35 to release p25 (Lee et al., 2000). Conversion of p35 to p25 causes prolonged activation and mislocalization of cdk5, which leads to aberrant tau hyperphosphorylation (Lee et al., 2000). It is consistent with our data showed that OA induced increase of cdk5 and p25 levels and further increase of phospho-tau (Thr205). The activation of GSK3β is mediated by dephosphorylation at Ser 9 which is regulated by Akt (Grimes and Jope 2001). PP2A can activate GSK3β directly by dephosphorylation at Ser 9, or indirectly by dephosphorylating Akt (Lin et al., 2007). Inhibition of PP2A by OA led to inactivation of GSK3β by increasing GSK3β (p-Ser 9). Although our data do not indicate GSK3β is a factor in pathological tau phosphorylation, it may still play key role in tau phosphorylation because the model of tau phosphorylation we produced is to inhibit PP2A, which leads to the deactivation of GSK3β. Further, no significant changes of p-ERK/ERK ratio were found in SH-SY5Y cells with the insult of OA. Moreover, these changes in proline-directed kinases induced by OA were found to be prevented by co-treatment with estrogen, an effect that was attenuated by ICI 182,780. Our data indicates that inhibition of PP2A/B by OA leads to tau phosphorylation, which can be prevented by estrogen in a partially receptor-mediated manner.
Collectively, our data indicate that the balance between tau kinases and phosphatases is important for tau phosphorylation. Our data also suggest that cdk5 may be involved in OA induced tau phosphorylation via inhibition of PP1/2A; estrogen can prevent tau phosphorylation which may be an ER-mediated effect and cdk5 may be a downstream target of estrogen.
4. Experimental procedures
4.1 Materials
Okadaic acid (Cat #: 495604) was purchased from Calbiochem (Gibbstown, NJ) and dissolved in dimethyl sulfoxide (DMSO) at a concentration of 1 μM and diluted to appropriate concentration in artificial cerebrospinal fluid. Anti-cdk5 (C-8), anti-p25, anti-tau (T1) and anti-p-ERK (E4) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-ERK1/2 and anti-phospho-tau (Thr 205) antibody was purchased from Invitrogen (Carlsbad, CA). Anti-GSK3β and GSK3β (p-Ser 9) antibody was purchased from Cell Signaling Technology (Danvers, MA). SH-SY5Y cell line was purchased from ATCC (Manassas, VA) and DME/F-12 media was obtained from Hyclone Laboratories, Inc. (Logan, Utah). Fulvestrant (ICI 182,780) and other reagents were purchased from Sigma-Aldrich (St Louis, MO).
4.2 Cell culture and treatment
SH-SY5Y cells were grown in DME/F-12 medium supplemented with 10% charcoal-stripped FBS and penicillin/streptomycin (50 μg/ml) at 37°C in an atmosphere containing 5% CO2 and 95% air. SH-SY5Y cells cultures were maintained at 50 and 100% confluency, respectively, in monolayers in plastic 75 cm2 flasks.
OA was added into the media to produce a final concentration of 100 nM for three hours in the presence or absence of various concentrations of 17β-estradiol (10 nM, 100 nM, 1μM, 10 μM) to determine the dose-dependent effects of 17β-estradiol on the phospho-tau levels. For mechanism study, OA was added into the media to produce a final concentration of 100 nM for three hours in the presence or absence of 17β-estradiol (10 μM) and ICI 182,780 (1 μM), an ER antagonist.
4.3 Western blotting
For immunoblotting analysis, cells were harvested by scraping, washed in PBS, resuspended, then homogenized and sonicated in RIPA buffer (1X PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 1mM sodium orthovanadate, 10mg/ml Aprotinin, 100 mg/ml Phenylmethyl Sulphonyl Fluoride (PMSF)). Lysates were then centrifuged at 12,000g for 10 min at 4 °C, and supernatants were collected for analysis. The protein contents in the supernatants were determined by Bradford reagent assay. Protein from the treated cells was mixed with loading buffer, boiled for 5 minutes, separated by SDS-PAGE and then transferred to Immunobilon-P polyvinylidene difluoride (PVDF) (Millipore, Bedford, MA) membrane. Membranes were blocked with 5% try milk in PBS. Proteins were probed with specific antibodies at proper dilutions according to the manufacturer’s instruction and incubated overnight at 4 °C. The blots were rinsed and applied with the appropriate secondary antibodies. After washing, the blots were developed with an enhanced chemiluminescent kit (Pierce, Rockford, IL). ECL results were digitized and quantified by using UVP (Upland, CA) Bioimaging System. Blots were normalized to beta actin, which was probed and detected on the same blots after stripping and re-blocking the membranes. For phosphor-GSK3β, GSK3β was used for normalization of blots.
4.4 Statistics
The results were analyzed with one-way ANOVA with prism software (Graphpad Inc., San Diego, CA). The significance of differences among groups was determined by Tukey’s multiple comparison tests. p<0.05 was considered significant for all the experiments. All results were expressed as mean ± SEM.
Acknowledgements
This study was supported by NIH Grants P01 AG10485, P01 AG22550 and P01 AG27956.
Abbreviations
- OA
okadaic acid
- E2
17β-estradiol
- ER
estrogen receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Section: Regulatory Systems
References
- Alonso AC, Grundke-Iqbal I, Iqbal K. Alzheimer’s disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat Med. 1996;2:783–787. doi: 10.1038/nm0796-783. [DOI] [PubMed] [Google Scholar]
- Alvarez-de-la-Rosa M, Silva I, Nilsen J, Perez MM, Garcia-Segura LM, Avila J, Naftolin F. Estradiol prevents neural tau hyperphosphorylation characteristic of alzheimer’s disease. Ann N Y Acad Sci. 2005;1052:210–224. doi: 10.1196/annals.1347.016. [DOI] [PubMed] [Google Scholar]
- Arendt T, Holzer M, Bruckner MK, Janke C, Gartner U. The use of okadaic acid in vivo and the induction of molecular changes typical for alzheimer’s disease. Neuroscience. 1998;85:1337–1340. doi: 10.1016/s0306-4522(97)00697-0. [DOI] [PubMed] [Google Scholar]
- Arias C, Sharma N, Davies P, Shafit-Zagardo B. Okadaic acid induces early changes in microtubule-associated protein 2 and tau phosphorylation prior to neurodegeneration in cultured cortical neurons. J Neurochem. 1993;61:673–682. doi: 10.1111/j.1471-4159.1993.tb02172.x. [DOI] [PubMed] [Google Scholar]
- Avila J, Lucas JJ, Perez M, Hernandez F. Role of tau protein in both physiological and pathological conditions. Physiol Rev. 2004;84:361–384. doi: 10.1152/physrev.00024.2003. [DOI] [PubMed] [Google Scholar]
- Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in alzheimer’s disease and related disorders. Nat Rev Neurosci. 2007;8:663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- Bang OY, Hong HS, Kim DH, Kim H, Boo JH, Huh K, Mook-Jung I. Neuroprotective effect of genistein against beta amyloid-induced neurotoxicity. Neurobiol Dis. 2004;16:21–28. doi: 10.1016/j.nbd.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Baudier J, Cole RD. Phosphorylation of tau proteins to a state like that in alzheimer’s brain is catalyzed by a calcium/calmodulin-dependent kinase and modulated by phospholipids. J Biol Chem. 1987;262:17577–17583. [PubMed] [Google Scholar]
- Brookmeyer R, Gray S, Kawas C. Projections of alzheimer’s disease in the united states and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correas I, Diaz-Nido J, Avila J. Microtubule-associated protein tau is phosphorylated by protein kinase C on its tubulin binding domain. J Biol Chem. 1992;267:15721–15728. [PubMed] [Google Scholar]
- Devred F, Barbier P, Douillard S, Monasterio O, Andreu JM, Peyrot V. Tau induces ring and microtubule formation from alphabeta-tubulin dimers under nonassembly conditions. Biochemistry. 2004;43:10520–10531. doi: 10.1021/bi0493160. [DOI] [PubMed] [Google Scholar]
- Drewes G, Lichtenberg-Kraag B, Doring F, Mandelkow EM, Biernat J, Goris J, Doree M, Mandelkow E. Mitogen activated protein (MAP) kinase transforms tau protein into an alzheimer-like state. EMBO J. 1992;11:2131–2138. doi: 10.1002/j.1460-2075.1992.tb05272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley MW, Sheeler CQ, Wang H, Khan S. Activation of the human estrogen receptor by the antiestrogens ICI 182,780 and tamoxifen in yeast genetic systems: Implications for their mechanism of action. Proc Natl Acad Sci U S A. 2000;97:3696–3701. doi: 10.1073/pnas.040558197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekinci FJ, Ortiz D, Shea TB. Okadaic acid mediates tau phosphorylation via sustained activation of the L-voltage-sensitive calcium channel. Brain Res Mol Brain Res. 2003;117:145–151. doi: 10.1016/s0169-328x(03)00294-8. [DOI] [PubMed] [Google Scholar]
- Ekinci FJ, Shea TB. Hyperactivation of mitogen-activated protein kinase increases phospho-tau immunoreactivity within human neuroblastoma: Additive and synergistic influence of alteration of additional kinase activities. Cell Mol Neurobiol. 1999;19:249–260. doi: 10.1023/a:1006981228331. [DOI] [PubMed] [Google Scholar]
- Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M, Alzheimer’s Disease International Global prevalence of dementia: A delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filley CM. Alzheimer’s disease in women. Am J Obstet Gynecol. 1997;176:1–7. doi: 10.1016/s0002-9378(97)80003-8. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: Sequences and localization in neurofibrillary tangles of alzheimer’s disease. Neuron. 1989;3:519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- Gong CX, Liu F, Grundke-Iqbal I, Iqbal K. Dysregulation of protein phosphorylation/dephosphorylation in alzheimer’s disease: A therapeutic target. J Biomed Biotechnol. 2006:31825. doi: 10.1155/JBB/2006/31825. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong CX, Lidsky T, Wegiel J, Zuck L, Grundke-Iqbal I, Iqbal K. Phosphorylation of microtubule-associated protein tau is regulated by protein phosphatase 2A in mammalian brain. implications for neurofibrillary degeneration in alzheimer’s disease. J Biol Chem. 2000;275:5535–5544. doi: 10.1074/jbc.275.8.5535. [DOI] [PubMed] [Google Scholar]
- Gong CX, Shaikh S, Wang JZ, Zaidi T, Grundke-Iqbal I, Iqbal K. Phosphatase activity toward abnormally phosphorylated tau: Decrease in alzheimer disease brain. J Neurochem. 1995;65:732–738. doi: 10.1046/j.1471-4159.1995.65020732.x. [DOI] [PubMed] [Google Scholar]
- Gong CX, Singh TJ, Grundke-Iqbal I, Iqbal K. Phosphoprotein phosphatase activities in alzheimer disease brain. J Neurochem. 1993;61:921–927. doi: 10.1111/j.1471-4159.1993.tb03603.x. [DOI] [PubMed] [Google Scholar]
- Green PS, Simpkins JW. Neuroprotective effects of estrogens: Potential mechanisms of action. Int J Dev Neurosci. 2000;18:347–358. doi: 10.1016/s0736-5748(00)00017-4. [DOI] [PubMed] [Google Scholar]
- Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau. A component of alzheimer paired helical filaments. J Biol Chem. 1986a;261:6084–6089. [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986b;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanger DP, Hughes K, Woodgett JR, Brion JP, Anderton BH. Glycogen synthase kinase-3 induces alzheimer’s disease-like phosphorylation of tau: Generation of paired helical filament epitopes and neuronal localisation of the kinase. Neurosci Lett. 1992;147:58–62. doi: 10.1016/0304-3940(92)90774-2. [DOI] [PubMed] [Google Scholar]
- Henderson VW, Paganini-Hill A, Emanuel CK, Dunn ME, Buckwalter JG. Estrogen replacement therapy in older women. comparisons between alzheimer’s disease cases and nondemented control subjects. Arch Neurol. 1994;51:896–900. doi: 10.1001/archneur.1994.00540210068014. [DOI] [PubMed] [Google Scholar]
- Hernandez F, Avila J. Tauopathies. Cell Mol Life Sci. 2007;64:2219–2233. doi: 10.1007/s00018-007-7220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara Y, Nukina N, Miura R, Ogawara M. Phosphorylated tau protein is integrated into paired helical filaments in alzheimer’s disease. J Biochem. 1986;99:1807–1810. doi: 10.1093/oxfordjournals.jbchem.a135662. [DOI] [PubMed] [Google Scholar]
- Ishiguro K, Shiratsuchi A, Sato S, Omori A, Arioka M, Kobayashi S, Uchida T, Imahori K. Glycogen synthase kinase 3 beta is identical to tau protein kinase I generating several epitopes of paired helical filaments. FEBS Lett. 1993;325:167–172. doi: 10.1016/0014-5793(93)81066-9. [DOI] [PubMed] [Google Scholar]
- Ishiguro K, Takamatsu M, Tomizawa K, Omori A, Takahashi M, Arioka M, Uchida T, Imahori K. Tau protein kinase I converts normal tau protein into A68-like component of paired helical filaments. J Biol Chem. 1992;267:10897–10901. [PubMed] [Google Scholar]
- Johnson GV, Stoothoff WH. Tau phosphorylation in neuronal cell function and dysfunction. J Cell Sci. 2004;117:5721–5729. doi: 10.1242/jcs.01558. [DOI] [PubMed] [Google Scholar]
- Kopke E, Tung YC, Shaikh S, Alonso AC, Iqbal K, Grundke-Iqbal I. Microtubule-associated protein tau. abnormal phosphorylation of a non-paired helical filament pool in alzheimer disease. J Biol Chem. 1993;268:24374–24384. [PubMed] [Google Scholar]
- Lace G, Savva GM, Forster G, de Silva R, Brayne C, Matthews FE, Barclay JJ, Dakin L, Ince PG, Wharton SB, MRC-CFAS Hippocampal tau pathology is related to neuroanatomical connections: An ageing population-based study. Brain. 2009;132:1324–1334. doi: 10.1093/brain/awp059. [DOI] [PubMed] [Google Scholar]
- Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai LH. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405:360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- Lin CF, Chen CL, Chiang CW, Jan MS, Huang WC, Lin YS. GSK-3beta acts downstream of PP2A and the PI 3-kinase-akt pathway, and upstream of caspase-2 in ceramide-induced mitochondrial apoptosis. J Cell Sci. 2007;120:2935–2943. doi: 10.1242/jcs.03473. [DOI] [PubMed] [Google Scholar]
- Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur J Neurosci. 2005;22:1942–1950. doi: 10.1111/j.1460-9568.2005.04391.x. [DOI] [PubMed] [Google Scholar]
- Liu M, Choi S, Cuny GD, Ding K, Dobson BC, Glicksman MA, Auerbach K, Stein RL. Kinetic studies of Cdk5/p25 kinase: Phosphorylation of tau and complex inhibition by two prototype inhibitors. Biochemistry. 2008;47:8367–8377. doi: 10.1021/bi800732v. [DOI] [PubMed] [Google Scholar]
- Mazanetz MP, Fischer PM. Untangling tau hyperphosphorylation in drug design for neurodegenerative diseases. Nat Rev Drug Discov. 2007;6:464–479. doi: 10.1038/nrd2111. [DOI] [PubMed] [Google Scholar]
- Mudher A, Lovestone S. Alzheimer’s disease-do tauists and baptists finally shake hands? Trends Neurosci. 2002;25:22–26. doi: 10.1016/s0166-2236(00)02031-2. [DOI] [PubMed] [Google Scholar]
- Paganini-Hill A, Henderson VW. Estrogen deficiency and risk of alzheimer’s disease in women. Am J Epidemiol. 1994;140:256–261. doi: 10.1093/oxfordjournals.aje.a117244. [DOI] [PubMed] [Google Scholar]
- Sergeant N, Delacourte A, Buee L. Tau protein as a differential biomarker of tauopathies. Biochim Biophys Acta. 2005;1739:179–197. doi: 10.1016/j.bbadis.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Yi KD, Yang S. Role of protein phosphatases and mitochondria in the neuroprotective effects of estrogens. Front Neuroendocrinol. 2009 doi: 10.1016/j.yfrne.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Dykens JA, Simpkins JW. Novel mechanisms for estrogen-induced neuroprotection. Exp Biol Med (Maywood) 2006;231:514–521. doi: 10.1177/153537020623100505. [DOI] [PubMed] [Google Scholar]
- Sontag E, Luangpirom A, Hladik C, Mudrak I, Ogris E, Speciale S, White CL., 3rd Altered expression levels of the protein phosphatase 2A ABalphaC enzyme are associated with alzheimer disease pathology. J Neuropathol Exp Neurol. 2004;63:287–301. doi: 10.1093/jnen/63.4.287. [DOI] [PubMed] [Google Scholar]
- Uchida T, Ishiguro K, Ohnuma J, Takamatsu M, Yonekura S, Imahori K. Precursor of cdk5 activator, the 23 kDa subunit of tau protein kinase II: Its sequence and developmental change in brain. FEBS Lett. 1994;355:35–40. doi: 10.1016/0014-5793(94)01163-x. [DOI] [PubMed] [Google Scholar]
- Vogelsberg-Ragaglia V, Schuck T, Trojanowski JQ, Lee VM. PP2A mRNA expression is quantitatively decreased in alzheimer’s disease hippocampus. Exp Neurol. 2001;168:402–412. doi: 10.1006/exnr.2001.7630. [DOI] [PubMed] [Google Scholar]
- Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975;72:1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi KD, Covey DF, Simpkins JW. Mechanism of okadaic acid-induced neuronal death and the effect of estrogens. J Neurochem. 2009;108:732–740. doi: 10.1111/j.1471-4159.2008.05805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi KD, Simpkins JW. Protein phosphatase 1, protein phosphatase 2A, and calcineurin play a role in estrogen-mediated neuroprotection. Endocrinology. 2008;149:5235–5243. doi: 10.1210/en.2008-0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi KD, Chung J, Pang P, Simpkins JW. Role of protein phosphatases in estrogen-mediated neuroprotection. J Neurosci. 2005;25:7191–7198. doi: 10.1523/JNEUROSCI.1328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S, Choi J, Huh JW, Hwang O, Kim D. Calpain activation in okadaic-acid-induced neurodegeneration. Neuroreport. 2006;17:689–692. doi: 10.1097/01.wnr.0000214398.04093.7f. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Simpkins JW. Okadaic acid induces cognitive deficiency in rats. 2008 doi: 10.1016/j.brainres.2010.08.077. Society for Neuroscience Abstract No. 556.4/BB25. [DOI] [PMC free article] [PubMed] [Google Scholar]