Abstract
We herein report a dramatically improved total synthesis of the high-affinity translocator protein (TSPO) ligand DPA-714, featuring microwave-assisted organic synthesis (MAOS). Compared with previously described approaches, our novel MAOS method dramatically reduces overall reaction time without adversely effecting reaction yields. We envision that the described MAOS protocol may be suitably applied to high-throughput, diversity-oriented synthesis of novel compounds based on the pyrazolo-pyrimidinyl scaffold. Such an approach could accelerate the development of focused libraries of novel TSPO ligands with potential for future development as molecular imaging and therapeutic agents.
Translocator protein (TSPO), formerly known as the peripheral benzodiazepine receptor (PBR), is an 18kDa outer-mitochondrial membrane protein that participates in the regulation of numerous cellular processes, including cholesterol metabolism, steroid biosynthesis, proliferation and apoptosis.1a–c Under normal circumstances, TSPO expression tends to be highest in steroid producing tissues and mitochondrial enriched tissues such as skeletal muscle, kidney and heart. TSPO expression is also elevated in disease states such as neuroinflammation and cancer.2a–c For this reason, TSPO is regarded as a potentially important target for drug and molecular imaging probe development. Our previous research involving TSPO resulted in the development of several labeled TSPO ligands for in vitro and in vivo fluorescence imaging and high-throughput screening applications.3a–e Presently, a diverse array of TSPO-targeted probes has been developed and radiolabeled with positron emitting isotopes such as carbon-11 and fluorine-18 for positron emission tomography (PET) imaging.4 One such compound, [18F]DPA-714 (N,N-diethyl-2-(2-(4-(2-[18F])fluoroethoxy)phenyl)-5,7-dimethylpyrazolo[1,5-a]pyrimidin-3-yl)acetamide, is a high-affinity TSPO ligand that has shown promise for PET imaging of neuroinflammation.5a,5b Previous publications report total syntheses of this compound and its tosylate precursor, suitable for radiolabeling with fluorine-18, that incorporate lengthy, multi-step schema.6a–d Our interest in evaluating pyrazolo-pyrimidinyl compounds as TSPO imaging ligands, such as [18F]DPA-714, led us to explore methodologies applicable to rapid, high-throughput synthesis of this compound and potentially novel analogues thereof. To this end, we have evaluated microwave assisted organic synthesis (MAOS) as an approach to accelerate the synthesis of DPA-714 and similar compounds. Compared with previously reported approaches, this study illustrates that MAOS applied to each reaction step dramatically reduces the overall time required to prepare the compound while achieving comparable, or in most cases, superior synthetic yields. According to the authors, this is the first MAOS-facilitated synthesis of DPA-714 and accordingly, this methodology can be extended to the synthesis of similar pyrazolo-pyrimidinyl-based compounds. As evidence of this, we illustrate application of MAOS to several additional pyrazolo-pyrimidinyl synthetic intermediates with good utility.
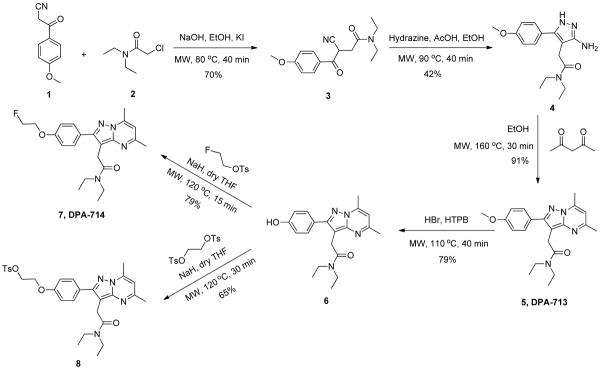
The overall synthetic methodology utilized in this study is shown in Scheme 1. Though the synthesis reported here utilized starting materials similar to previous methods, those syntheses were carried out either at room temperature or with conventional thermal heating. By applying MAOS to each step as further described below, the overall reaction time could be reduced from over 50 hours to approximately three, while maintaining consistently high yields. Full synthetic methodology and characterization data can be found in Supplementary Data.
Scheme 1.
Microwave-assisted organic synthesis of DPA-714 (7) and the tosylate precursor (8).
1. MAOS of 3-cyano-N,N-diethyl-4-(4-methoxyphenoyl)-4-oxobutanamide (3)
The consecutive five-step synthesis begins with formation of 3 by reaction of 3-(4-methoxyphenyl)-3-oxopropanenitrile (1) and 2-chloro-N,N-diethylacetamide (2) in 80% EtOH, NaI/KI and NaOH. Previous studies carried out this reaction at either room temperature or reflux over 8 h with reported yields ranging from 10 – 70%.6a,6b To reduce the reaction time required, microwave irradiation provided a reaction temperature of 80 °C. Brief irradiation for 40 min and flash chromatography on silica gel gave 3 with a slightly higher yield when compared with the best previously reported method.6b (Table 1)
Table 1.
Synthesis of 3-cyano-N,N-diethyl-4-(4-methoxyphenoyl)-4-oxobutanamide (3).
| Entry | MAOS | Reaction Conditions | Temperature | Time | Yielda |
|---|---|---|---|---|---|
| 16a | No | 80% EtOH, NaI, NaOH | RT | 8 h | 10% |
| 26b | No | 80% EtOH, NaI, NaOH | RT | 8 h | 64% |
| 3 | Yes | 80% EtOH, KI, NaOH | 80 °C | 40 min | 70% |
Isolated yield. See reference.6a,6b
2. Synthesis of 2(3-amino-5-(4-methoxyphenyl)-1H-pyrazol-4-yl)-N,N-diethylacetamide (4)
Synthesis of 4 features pyrazolo ring formation from the reaction of 3 with hydrazine. According to previous reports, this reaction can be carried out at room temperature or with traditional thermal heating for 4 – 6 h in ethanol/acetic acid to achieve yields ranging from 68 – 72%.6a,6b In this study, this reaction could be adapted to MAOS via microwave irradiation at 90 °C for 40 min, albeit with a somewhat lower yield (42%) than previous studies (Table 2). Efforts to increase the reaction yield above 42% while maintaining the rapid 40 min reaction time included elevated temperature (> 90 °C) and hydrazine concentration. However, these conditions resulted in increased byproduct formation and further diminished synthetic yields (data not shown). Despite the slightly reduced yield, the significantly increased speed with which this reaction could be performed using MAOS is a considerable advantage over traditional methods.
Table 2.
Synthesis of 2-(3-amino-5-(4-methoxyphenyl)-lH-pyrazol-4-yl)-N,N-diethylacetamide(4).
| Entry | MAOS | Reaction Conditions | Temperature | Time | Yielda |
|---|---|---|---|---|---|
| 16a | No | EtOH, acetic acid | RT | 6 h | 72% |
| 26b | No | EtOH, acetic acid | Reflux | 4 h | 68% |
| 3 | Yes | EtOH, acetic acid | 90 °C | 40 min | 42% |
Isolated yield. See reference.6a,6b
3. MAOS of N,N-diethyl-2-(2-(4-methoxyphenyl)-5,7-dimethylpyrazolo[1,5-a] pyrimidin-3-yl) acetamide (5, DPA-713)
In the third step, 5 (DPA-713) was synthesized from the reaction of 4 and 2,4-pentanedione in ethanol. Previous studies have reported carrying out this reaction at room temperature or with conventional thermal heating.6a,6b Optimization with microwave irradiation resulted in final reaction conditions of 160 °C in ethanol for 30 min. Purification by flash chromatography on silica gel gave 5 in near quantitative yield (91%), comparable to the best previously reported synthesis while requiring only a fraction of the reaction time.6b (Table 3)
Table 3.
Synthesis of N,N-diethyl-2-(2-(4-methoxyphenyl)-5,7-dimethylpyrazolo[l,5-a] pynmidin-3-yl) acetamide (5, DPA-713).
| Entry | MAOS | Reaction Conditions | Temperature | Time | Yielda |
|---|---|---|---|---|---|
| 16a | No | EtOH | RT | 4 h | 44% |
| 26b | No | EtOH | Reflux | 12 h | 93% |
| 3 | Yes | EtOH | 160 °C | 30 min | 91% |
Isolated yield. See reference.6a,6b
4. MAOS of N,N-diethyl-2-(2-(4-hydroxyphenyl)-5,7-dimethylpyrazolo[1,5-a]pyrimidin-3-yl) acetamide (6)
The fourth reaction in the synthesis of DPA-714 features deprotection of 5, performed using either BBr3 in CH2Cl2 at −60 °C or with aqueous HBr under conventional thermal conditions.6b,6d To optimize this reaction with MAOS, examination of various reaction temperatures ranging from 100 to 140 °C demonstrated that temperatures > 120 °C resulted in byproduct formation. However, irradiation at 110 °C for 40 min in aqueous HBr with addition of hexadecyl tributyl phosphonium bromide (HTPB) proved successful. Compared to previously reported methods,6b,6d this approach yielded 6 in higher yield (79%), with a significantly reduced reaction time (40 min versus 2 – 7 h) (Table 4).
Table 4.
Synthesis of N,N-diethyl-2-(2-(4-hydroxyphenyl)-5,7-dimethylpyrazolo[1,5-a]pyTimidin-3-yl) acetamide (6).
| Entry | MAOS | Reaction Conditions | Temperature | Time | Yielda |
|---|---|---|---|---|---|
| 16b | No | HTPB, HBr, H2O | 100 °C | 7 h | 54% |
| 26d | No | BBr3, CH2Cl2 | -60 °C | 2 h | 55% |
| 3 | Yes | HTPB, HBr, H2O | 110 °C | 40 min | 79% |
Isolated yield. See reference.6b,6d
5. MAOS of N,N-diethyl-2-(2-(4-(2-fluoroethoxy)phenyl)-5,7-dimethylpyrazolo[1,5-a]pyrimidin-3-yl) acetamide (7, DPA-714)
From compound 6, reported syntheses of 7 describe reactions carried out at room temperature with NaH in dry THF for 16 h with yields ranging from 60 – 80%.6c,6d Employing microwave irradiation, an optimized reaction temperature of 120 °C was achieved with a subsequent reduction in reaction time from 16 h to 15 min, a factor of 64, while still achieving a comparable yield (79%) (Table 5)
Table 5.
Synthesis of N,Ndiethyl-2-(2-(4-(2-fluoroethoxy)phenyl)-5,7-dimethylpyrazolo[l,5-a]pyrimidin-3-yl)acetamide (7, DPA-714).
| Entry | MAOS | Reaction Conditions | Temperature | Time | Yielda |
|---|---|---|---|---|---|
| 16c | No | NaH, THF | RT | 16 h | 80% |
| 26d | No | NaH, THF | RT | 16 h | 58% |
| 3 | Yes | NaH, THF | 120 °C | 15 min | 79% |
Isolated yield. See reference.6c,6d
6. MAOS of 2-(4-(3-(2-(diethylamino)-2-oxoethyl)-5,7-dimethylpyrazolo[1,5-a]pyrimidin-2-yl)phenoxy)ethyl-4-methylbenzenesulfonate (8), a precursor for radiosynthesis of [18F]DPA-714
In order to produce 18F-labeled DPA-714 as a PET tracer, 8 is commonly prepared as the precursor. Previous syntheses of this compound were carried out at room temperature in high yield.6c,6d However, the prolonged reaction time reported (16 h) is a considerable disadvantage. We found that this reaction could be optimized using microwave irradiation for 30 min at 120 °C. Following purification, we obtained 8 in a yield of 65%, comparable to previous reports (Table 6).
Table 6.
Synthesis of 2-(4-(3-(2-(diethylamino)-2-oxoethyl)-5,7-dimethylpyrazolo[1,5-a]pyrimidin-2-yl)phenoxy)ethyl 4-methylbenzenesulfonate (8).
| Entry | MAOS | Reaction Conditions | Temperature | Time | Yielda |
|---|---|---|---|---|---|
| 16c | No | NaH, THF | RT | 16 h | 59% |
| 26d | No | NaH, THF | RT | 16 h | 77% |
| 3 | Yes | NaH, THF | 120 °C | 30 min | 65% |
Isolated yield. See reference.6c,6d
Extending the utility of MAOS to the synthesis of additional pyrazolo-pyrimidinyl-based compounds, we performed the first and third reactions shown in Scheme 1 using a variety of different reagents, mimicking a library-based synthetic approach. For example, substitution of 1 with the corresponding p-chloro or p-methyl reagent results in rapid synthesis of 3a and 3b in acceptable yields (Table 7). Similarly, application of MAOS to reactions featuring unique diones also appears feasible, enabling synthesis of potential TSPO ligands following the third reaction (Table 8). Interestingly, in evaluating somewhat bulkier diones, we observed significantly reduced reaction yield, presumably due to the geometric constraints afforded by groups in R1, R2 and R3. We anticipate that synthetic optimization of the bulkier pyrazolo-pyrimidines is possible, though beyond the scope of this focused work.
Table 7.
Synthesis of pyrazolo-pyrimidine intermediates.
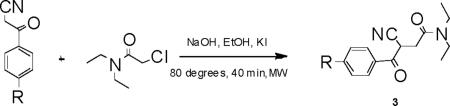
| Entry | Product | Yield (%)a |
|---|---|---|
| 3a |
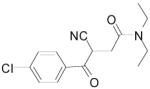
|
42 |
| 3b |
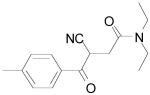
|
40 |
Isolated yield.
Table 8.
Synthesis of pyrazolo-pyrimidines.
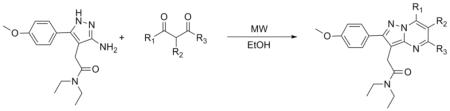
| Entry | R1 | R2 | R3 | Temperature (°C) | Time (min) | Yield (%)a |
|---|---|---|---|---|---|---|
| 5a | -CH3 | -CH3 | -CH3 | 160 | 30 | 77 |
| 5b | -CH3 | -CH2CH3 | -CH3 | 160 | 30 | 24 |
| 5c | -CH(CH3)2 | -CH(CH3)2 | 180 | 45 | 6.0 | |
| 5d | -Ph | -Ph | 180 | 45 | 5.0 |
Isolated Yield.
In summary, a detailed optimization of the total synthesis of a high-affinity TSPO ligand, DPA-714, utilizing MAOS, is described. The protocol reported here significantly improves overall reaction times while maintaining or even improving synthetic yields. We envision that this protocol can be extended to library synthesis of novel TSPO ligands with potential use for noninvasive visualization of TSPO expression in vivo as well as treatment of TSPO-expressing disease.
Supplementary Material
Acknowledgements
The authors wish to gratefully acknowledge funding from the National Cancer Institute (NCI): 1R01 CA140628, K25 CA127349, and 1P50CA128323 (Vanderbilt ICMIC Program). We thank Dr(s). Ronald Baldwin, Ph. D. and R. Adam Smith, Ph.D. for editorial input and insightful discussion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.(a) Papadopoulos V, Amri H, Boujrad N, Cascio C, Culty M, Garnier M, Hardwick M, Li H, Vidic B, Brown AS, Reversa JL, Bernassau JM, Drieu K. Steroids. 1997;62:21–28. doi: 10.1016/s0039-128x(96)00154-7. [DOI] [PubMed] [Google Scholar]; (b) Veenman L, Shandalov Y, Gavish M. J. Bioenerg. Biomembr. 2008;40:199–205. doi: 10.1007/s10863-008-9142-1. [DOI] [PubMed] [Google Scholar]; (c) Galiegue S, Tinel N, Casellas P. Curr. Med. Chem. 2003;10:1563–1572. doi: 10.2174/0929867033457223. [DOI] [PubMed] [Google Scholar]
- 2.(a) Chen MK, Guilarte TR. Pharmacol. Therapeut. 2008;118:1–17. doi: 10.1016/j.pharmthera.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Maaser K, Grabowski P, Ozdem Y, Krahn A, Heine B, Stein H, Buhr HJ, Zeitz M, Scherubl H. Clin. Cancer Res. 2005;11:1751–1756. doi: 10.1158/1078-0432.CCR-04-1955. [DOI] [PubMed] [Google Scholar]; (c) Maaser K, Grabowski P, Sutter AP, Hopfner M, Foss HD, Stein H, Berger G, Gavish M, Zeitz M, Scherubl H. Clin. Cancer Res. 2002;8:3205–3209. [PubMed] [Google Scholar]
- 3.(a) Manning HC, Goebel T, Marx JN, Bornhop DJ. Org. Lett. 2002;4:1075–1078. doi: 10.1021/ol017155b. [DOI] [PubMed] [Google Scholar]; (b) Manning HC, Goebel T, Thompson RC, Price RR, Lee H, Bornhop DJ. Bioconjugate Chem. 2004;15:1488–1495. doi: 10.1021/bc049904q. [DOI] [PubMed] [Google Scholar]; (c) Manning HC, Bai MF, Anderson BM, Lisiak R, Samuelson LE, Bornhop DJ. Tetrahedron Lett. 2005;46:4707–4710. [Google Scholar]; (d) Manning HC, Smith SM, Sexton M, Haviland S, Bai MF, Cederquist K, Stella N, Bornhop DJ. ioconjugate Chem. 2006;17:735–740. doi: 10.1021/bc060020b. [DOI] [PubMed] [Google Scholar]; (e) Deane NG, Manning HC, Foutch AC, Washington MK, Aronow BA, Bornhop DJ, Coffey RJ. Mol. Cancer Res. 2007;5:341–349. doi: 10.1158/1541-7786.MCR-06-0225. [DOI] [PubMed] [Google Scholar]
- 4.Dolle F, Luus C, Reynolds A, Kassiou M. Curr. Med. Chem. 2009;16:2899–2923. doi: 10.2174/092986709788803150. [DOI] [PubMed] [Google Scholar]
- 5.(a) James ML, Fulton RR, Vercoullie J, Henderson DJ, Garreau L, Chalon S, Dolle F, Selleri S, Guilloteau D, Kassiou M. J. Nucl. Med. 2008;49:814–822. doi: 10.2967/jnumed.107.046151. [DOI] [PubMed] [Google Scholar]; (b) Martin A, Boisgard R, Theze B, Van Camp N, Kuhnast B, Damont A, Kassiou M, Dolle F, Tavitian B. J Cerebr. Blood F Met. 2010;30:230–241. doi: 10.1038/jcbfm.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Selleri S, Bruni F, Costagli C, Costanzo A, Guerrini G, Ciciani G, Costa B, Martini C. Bioorg. Med. Chem. 2001;9:2661–71. doi: 10.1016/s0968-0896(01)00192-4. [DOI] [PubMed] [Google Scholar]; (b) James ML, Fulton RR, Henderson DJ, Eberl S, Meikle SR, Thomson S, Allan RD, Dolle F, Fulham MJ, Kassiou M. Bioorg. Med. Chem. 2005;13:6188–6194. doi: 10.1016/j.bmc.2005.06.030. [DOI] [PubMed] [Google Scholar]; (c) Doorduin J, Klein HC, Dierckx RA, James M, Kassiou M, de Vries EF. Mol. Imaging Biol. 2009;11:386–398. doi: 10.1007/s11307-009-0211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Damont A, Hinnen F, Kuhnast B, Schollhorn-Peyronneau MA, James M, Luus C, Tavitian B, Kassiou M, Dolle F. J Labelled Compd. Rad. 2008;51:286–292. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.