Synopsis
Network analysis of 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) is an innovative approach for the study of in movement disorders, such as Parkinson’s disease (PD). Spatial covariance analysis of imaging data acquired from PD patients has revealed characteristic regional patterns associated with the motor and cognitive features of disease. Quantification of pattern expression in individual patients can be used for diagnosis, assessment of disease severity, and evaluation of novel medical and surgical therapies. Identification of disease-specific patterns in other parkinsonian syndromes, such as multiple system atrophy and progressive supranuclear palsy, has improved diagnostic accuracy in patients with difficult to diagnose parkinsonism. Further developments of these techniques are likely to enhance the role of functional imaging in investigating underlying abnormalities and potential new therapies in these neurodegenerative diseases.
Keywords: Positron emission topography (PET), parkinsonism, movement disorders, differential diagnosis, brain metabolism, biomarkers, treatment response
Introduction
Parkinson’s disease (PD) is the second most common age-related neurodegenerative disorder, affecting 1–2 million individuals in North America. Given that the incidence of PD increases with age, the number of PD patients is estimated to triple in the next 50 years as the average age of the population increases [1]. Classically, PD is defined by motor features of bradykinesia (slow movement), rest tremor, muscle rigidity, and gait abnormalities. However, non-motor features, including cognitive decline, dementia, and depression can significantly contribute to patient disability and even increase mortality [2,3]. Diagnosis is typically made solely on clinical findings and can be quite challenging, particularly early in the disease course. Even among specialists, diagnosis at disease onset can be elusive, as the clinical symptoms in early PD overlap with several ‘look-alike’ disorders [4]. Conventional anatomical imaging techniques, such as MRI and CT, are generally not helpful in diagnosing PD, particularly in early patients, nor are they helpful in monitoring changes in disease over time. More accurate clinical diagnosis requires at least two years of clinical follow-up by a movement disorders specialist with expertise in rarer causes of parkinsonism, such as progressive supranuclear palsy (PSP) and multiple system atrophy (MSA) [4]. These more aggressive and less treatable syndromes are the most common atypical forms of parkinsonism misdiagnosed as PD [5]; therefore accurate diagnosis is critical for patient counseling and treatment decisions.
Current research in neurodegenerative diseases has centered on the development of biomarkers to aid clinicians and researchers in making earlier, more accurate diagnoses, specifically in PD and other atypical parkinsonian disorders [6]. In addition, biomarkers for the objective assessment of disease progression and response to treatment are critical for researching new therapies for these progressive, incurable diseases. Indeed, such biomarkers are vital to the development of potentially neuromodulatory medical and surgical treatments for PD, including cell-based approaches [7] and gene transfer therapy [8,9].
Functional imaging with PET has the potential to fulfill the need for such biomarkers by providing objective, quantifiable, and stable markers for the diagnosis of parkinsonian syndromes, and for the assessment of the progression and response to treatment of these disorders. Over a decade ago it was first recognized that regional differences in glucose metabolism measured with 18F-fluorodeoxyglucose (FDG) PET could be used to distinguish between different forms of parkinsonism [10]. However, broad application of metabolic imaging in these neurodegenerative diseases has been limited due to the substantial variability in brain activity between subjects as well as difficulty analyzing large datasets. A relatively novel approach aimed to overcome these limitations is a spatial covariance method based on principal components analysis (PCA) [11]. This method, termed the scaled subprofile model (SSM), has been applied to FDG PET scans to identify disease-specific metabolic patterns in patients with various neurodegenerative disorders [12,13]. Indeed, spatial covariance mapping has been used with FDG PET to identify abnormal metabolic patterns (i.e., large-scale brain networks) associated with a variety of neurodegenerative diseases, including PD, MSA, PSP, Alzheimer’s disease (AD) [14], and Huntington’s disease (HD) [15].
In this review, we will describe current applications of FDG PET with pattern analysis to the characterization of parkinsonian disorders. We will focus on the use of this approach in aiding clinical diagnosis, monitoring disease progression, and in studying novel treatments for PD and related disorders.
Metabolic networks in parkinsonism
The PD-related motor pattern
The cardinal motor abnormalities of PD have been attributed not only to dysfunction within the basal ganglia, but also to broader functional abnormalities involving the cortico-striato-pallido-thalamocortical (CSPTC) and related pathways [16]. Therefore, it is not surprising that the abnormal spatial covariance pattern consistently identified in PD patients involves metabolic changes at key nodes of these circuits [17–19]. This Parkinson’s disease-related metabolic pattern (PDRP) is characterized by increased pallido-thalamic and pontine metabolic activity associated with relative reductions in premotor cortex, supplementary motor area (SMA), and in parietal association areas (Fig. 1, left). Indeed, similar PDRP topographies have been detected in seven independent patient populations using a variety of resting state imaging techniques [12].
Figure 1. Parkinson’s Disease-Related Spatial Covariance Patterns.
Left: Parkinson’s disease motor-related spatial covariance pattern (PDRP; [20]) characterized by pallidothalamic, pontine, and motor cortical hypermetabolism, associated with relative metabolic reductions in the lateral premotor and posterior parietal areas.
Right: Parkinson’s disease cognition-related spatial covariance pattern (PDCP; [28]) characterized by hypometabolism of prefrontal cortex, rostral supplementary motor area, and superior parietal regions.
[Relative metabolic increases are displayed in red; relative metabolic decreases are displayed in blue. Both patterns were overlaid on a standard MRI brain template. The left hemisphere was cut in the transverse plane at z=−5 mm. The right hemisphere was displayed as a surface projection on the same brain template.] [Hirano S, Asanuma K, Ma Y, Tang C, Feigin A, Dhawan V, Carbon M, Eidelberg D. Dissociation of metabolic and neurovascular responses to levodopa in the treatment of Parkinson’s disease. J Neurosci 2008;28:4203, Reprinted with permission from The Society for Neuroscience, Copyright © 2008]
By forward application of networks into individual cases, disease-related spatial patterns can be quantified prospectively in single scans [13]. Indeed, PDRP subject scores, measuring pattern expression in individual subjects, have been found to be highly reproducible with stable network activity recorded over hours to weeks [20]. In PD patients, pattern expression has been found to correlate with validated clinical markers of disease severity, such as standardized motor rating scales, as well as symptom duration [21,22]. However, unlike clinical ratings, PDRP scores are fully objective and unbiased by inter-rater variability. In addition, current PD rating scales, such as the Unified Parkinson’s Disease Rating Scale (UPDRS) [23], are relatively more sensitive for detecting changes in certain motor aspects of disease, such as worsening tremor or bradykinesia. However, these clinical scales are less sensitive to changes in other PD symptoms, such as freezing of gait and falls, and may therefore fail to capture total motor progression in many patients. The PDRP, however, is generated without a priori assumptions regarding patient symptomatology and is present regardless of patient phenotype [24,25]. Although the correlations between the motor UPDRS and PDRP activity are significant and consistent across populations, the overall effect is modest, with 40–50% variability in common for the two measures [13]. Rather than a biomarker for specific clinical manifestations of PD, PDRP expression likely reflects overall abnormalities in motor circuitry associated with this neurodegenerative disease.
The PD-related cognitive pattern
Cognitive disturbances, including mild cognitive impairment (MCI) and PD dementia (PDD), are among the most concerning non-motor symptoms in PD and are present in over 80% of patients with 15 years of disease duration [2]. Indeed, dementia is associated with a doubling of mortality risk in older patients with PD [26]. Earlier in the course of disease, a wide variety of more subtle cognitive changes occur, the most prominent being deficits in executive function, followed by visuospatial and working memory deficits [27]. Clinically, these cognitive changes are non-responsive to therapies aimed at treating PD motor symptoms and develop later in the disease course. Therefore, PD associated cognitive symptoms are generally attributed to a separate set of regional changes than the motor symptoms [28,29].
FDG PET with spatial covariance analysis has also been used to study the cognitive changes associated with PD. A voxel-based spatial covariance approach has been used to identify a specific PD-related metabolic pattern associated with cognitive dysfunction in non-demented patients [28]. This PD-related cognitive pattern (PDCP) is characterized by metabolic reductions in the medial frontal and parietal association regions with relative increases in the cerebellar vermis and dentate nuclei (Fig. 1, right). This pattern is distinct from the PDRP and its expression has been shown to correlate with performance on neuropsychological tests of executive functioning and memory. Indeed, PDCP expression was found to increase stepwise in PD patients categorized clinically by the degree of cognitive impairment on a psychometric battery [29,30]. Similar to the PDRP, PDCP expression is highly reproducible in individual patients [20]. Although both PDRP and PDCP expression increase with disease duration, PDCP increases at a slower rate, reflecting the relatively later development of neurodegenerative changes in the cerebral cortex [30]. Indeed, the findings suggest that the PDCP metabolic network can be used as a specific biomarker for the evaluation of cognitive dysfunction in PD patients.
Metabolic patterns in atypical parkinsonian syndromes
Atypical parkinsonian syndromes (APS) share many clinical symptoms seen in classical PD, but are associated with different underlying pathological processes and carry a significantly worse prognosis [5]. MSA and PSP are the two most common APS and account for more than 80% of the APS patients initially misdiagnosed as PD [4]. In addition to parkinsonism, patients with MSA develop autonomic symptoms, such as severe orthostatic hypotension, and cerebellar symptoms, such as ataxia. By contrast, PSP patients develop parkinsonism with early postural instability, falls, and occulomotor abnormalities. Nonetheless, the diagnostic features of atypical parkinsonian syndromes may not be clinically evident at the early stages of disease. Death is common in MSA and PSP after 7–9 years of symptoms and the pathologic etiologies for both syndromes differ from PD and from one another [5].
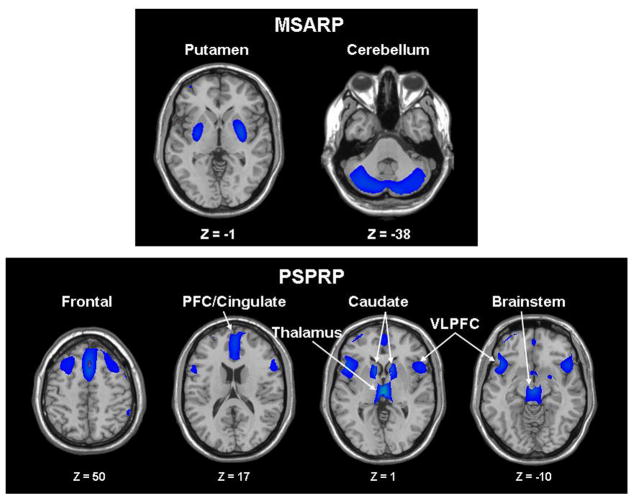
Using FDG PET and spatial covariance analysis, we have recently identified abnormal metabolic patterns for MSA and PSP [31]. By applying strictly defined statistical criteria to the imaging data to define disease-related patterns [13], we found that these atypical syndromes are each associated with a specific and highly stable metabolic brain network [32]. The MSA-related pattern (MSARP) is characterized by metabolic decreases in the putamen and the cerebellum (Fig. 2, top). By contrast, the PSP-related pattern (PSPRP) is characterized by metabolic decreases predominately in the upper brainstem and medial prefrontal cortex as well as in the medial thalamus, the caudate nuclei, the anterior cingulate area, and the superior frontal cortex (Fig. 2, bottom). In both diseases, pattern expression was found to be stable and significantly elevated (p<0.001) in patients relative to age-matched healthy control subjects [32]. In addition, pattern expression was also elevated (p>0.001) in two independent patient groups when compared to a prospectively scanned healthy age-matched control group that was different from those originally used to identify the patterns. In MSA patients, the activity of the disease-related pattern in individual subjects increases with disease progression and correlates well with findings at postmortem [33]. Further prospective studies are needed to understand how changes in pattern expression relate to the emergence of symptoms over time. Nonetheless, the preliminary studies suggest the potential utility of the MSARP and the PSPRP as functional imaging biomarkers for these atypical parkinsonian disorders.
Figure 2. Spatial Covariance Patterns Associated with Atypical Parkinsonism.
A. Metabolic pattern associated with multiple system atrophy (MSARP) characterized by covarying metabolic decreases in the putamen and the cerebellum.
B. Metabolic pattern associated with progressive supranuclear palsy (PSPRP) characterized by covarying metabolic decreases in the medial prefrontal cortex (PFC), the frontal eye fields, the ventrolateral prefrontal cortex (VLPFC), the caudate nuclei, the medial thalamus, and the upper brainstem.
[The covariance patterns were overlaid on T1-weighted MR-template images. The displays represent regions that contributed significantly to the network and that were demonstrated to be reliable by bootstrap resampling. Voxels with negative region weights (metabolic decreases) are color-coded blue.] [Eckert T, Tang C, Ma Y, Brown N, Lin T, Frucht S, Feigin A, Eidelberg D. Abnormal metabolic networks in atypical parkinsonism. Movement Disorders, 2008; 23(5):730,731, Reprinted with permission from John Wiley & Sons, Inc., Copyright © 2008 Movement Disorders Society]
Differential diagnosis using PET
Patients with early parkinsonism often present with overlapping signs and symptoms and only later develop the specific findings needed to make a definitive diagnosis of PD, MSA, or PSP. Accurate clinical diagnosis can often be made only after two years of clinical follow-up. Indeed almost a quarter of patients initially diagnosed as PD are ultimately found to have APS on pathology [4,34]. Under ideal circumstances, such patients should be excluded from trials of disease modifying interventions for PD. Moreover, up to 15% of patients recruited for early, pre-treatment clinical trials were found to have a different syndrome on reassessment [23,35]. Diagnostic inclusion criteria for these studies are typically based on clinical evaluation alone, potentially exposing misdiagnosed early APS patients to experimental treatments intended for PD patients. Clinically, patients with APS do not have substantial improvement with dopaminergic medications [5], and have been found to develop serious complications from standard PD deep brain stimulation (DBS) surgery [36]. For these reasons, accurate diagnosis is critical for patient counseling and treatment decisions. In addition, as new disease-specific treatments emerge targeting the underlying pathophysiology for each of these disorders [37] there will be increased need for early, accurate diagnosis.
Metabolic imaging is a valuable tool to differentiate among these parkinsonian syndromes, particularly in patients with early symptoms. A number of FDG PET studies have described characteristic regional patterns of glucose metabolism in PD, MSA, PSP, and corticobasal degeneration (CBD) [38–41]. In one study, FDG PET scans from patients with PD, MSA, PSP, and CBD as well as healthy subjects were compared using a voxel-based statistical mapping technique [39]. Maps of regional metabolic differences between patients and controls were used to create characteristic templates for each of these parkinsonian “look-alike” conditions. Disease defining features of individual scans can be visually matched to the templates allowing for the best “match” to be made for image classification. A reader with no expertise in PET diagnosis of movement disorders could use this computer-aided visual approach to determine a specific diagnostic category. Indeed, we found that using this qualitative technique, the blinded non-expert’s determination agreed with the clinical diagnosis in 92.4% of cases. The accuracy of this template-based approach suggests that it may prove useful clinically, especially when experienced readers of brain FDG PET scans are not readily available.
Highly accurate scan classification can also be achieved using a fully automated pattern quantification approach [42]. In one study, parkinsonian patients with an unclear diagnosis who exhibited normal nigrostriatal dopamine function with 18F-fluorodopa PET were found to have normal PDRP expression, suggesting a diagnosis other than PD [43]. These findings were confirmed when a non-PD diagnosis was established in each patient years later on clinical follow-up. Similarly, prospective PDRP quantification in cerebral perfusion images acquired with 99mTc ECD SPECT revealed excellent separation of PD and MSA patients [44]. Furthermore, the application of multiple disease-specific spatial covariance patterns to interrogate individual FDG PET images from early stage patients can provide an objective and completely user-independent means of classification [42,45]. These findings suggest that disease-related metabolic networks can constitute a powerful adjunct in the differential diagnosis of parkinsonism.
Changes in network activity with PD disease progression
FDG PET has also been found to be useful for monitoring different aspects of disease progression in PD patients. Despite having a unifying underlying pathology, patients with PD can vary significantly with respect to the rate of progression of motor and non-motor symptoms. For example, motor subtypes of PD, such as tremor-dominant and postural instability with gait difficulty, have been found to have different rates of progression [46]. Moreover, within these subtypes, other factors, such as age of onset, can influence the progression of motor impairment [47]. Indeed, these differences can confound clinically-based measures of progression rate in the clinical setting and in research trials.
It has recently been shown that FDG PET can provide an objective and accurate means for monitoring disease progression in PD. Longitudinal metabolic imaging studies with PDRP and PDCP quantification have provided unique information concerning functional changes in network activity in patients with early PD [21]. In one study, patients with less than two years of clinical symptoms were studied with FDG PET at baseline, 24 months, and 48 months; caudate and putamen dopamine transporter (DAT) binding and clinical motor rating scales (UPDRS) were also measured at each time point. PDRP expression was abnormally elevated in patients at baseline, compared to age-matched controls, and continued to increase at each successive time point (Fig. 3). These studies also revealed significant correlations between increases in PDRP expression over time and concurrent deterioration in UPDRS motor scores and DAT binding. However, these progression measures were not interchangeable, since less than a third of the variability in each is explained by the others. Rather, these three biomarkers are likely best used in concert, as each captures unique features of disease progression [12]. Larger cross-sectional studies of patients with varying symptom duration corroborate the results of the longitudinal studies. In aggregate, these investigations verify PDRP expression as a sensitive biomarker of PD motor severity throughout the disease course [13].
Figure 3. Longitudinal Progression of PD-Related Pattern Expression.
Mean network activity at baseline, 24, and 48 months. Values for the PD-motor and cognitive spatial covariance patterns (PDRP and PDCP; see Fig. 1) were computed at each time point and displayed relative to the mean for 15 age-matched healthy subjects. Network activity increased significantly over time for both patterns (p<0.001; RMANOVA), with the PDRP progressing faster than the PDCP (p<0.04). Relative to controls, PDRP activity in the patient group was elevated at all three time points, while PDCP activity reached abnormal levels only at the final time point. [Huang C, Tang C, Feigin A, Lesser M, Ma Y, Pourfar M, Dhawan V, Eidelberg D. Changes in network activity with the progression of Parkinson’s disease. Brain, 2007; 130(Pt 7):1842, Reprinted with permission from Oxford University Press]
Prior to the onset of clinically diagnostic symptoms there is suggestion that the PDRP motor network is already abnormal. Neuronal death begins in PD years prior to symptom onset, and less than 50% of the neurons in the substantia nigra (SN) are functional at the time patients present in clinic [48]. It is likely that SN neuronal decline is accompanied by compensatory metabolic changes downstream in the CSPTC loops. Since most early patients present with only unilateral appendicular symptoms, the brain hemisphere contralateral to the still asymptomatic side is ideal for studying the ‘pre-clinical’ condition. Indeed, analysis of ‘hemispheric’ network activity reveals changes in PDRP expression in the asymptomatic hemisphere of early unilateral patients [49]. Longitudinal studies in carriers of susceptibility genes for PD will provide further data concerning changes in metabolic networks prior to the emergence of symptoms.
Longitudinal studies also demonstrate that PDCP expression increases with time in non-demented PD patients, albeit at a different rate of progression than the motor pattern [21]. While the PDRP is elevated in patients at baseline, the PDCP is not elevated until approximately 6 years after symptom onset. Further in the disease course, both the motor and cognitive pattern continue to increase linearly with disease duration, but with different tragectories [30]. The difference in the rate of development of motor and cognitive pattern expression parallels the observed time courses over which motor and cognitive symptoms develop [2]. Increases in PDCP expression are not only associated with symptom duration, but also with the degree of cognitive dysfunction that is present in patients. When compared to controls, PD patients exhibit significant increases in PDCP expression prior to the development of cognitive changes on neuropsychological assessment (Fig. 3). As cognitive symptoms emerge, PDCP expression continues to increase in patients exhibiting mild cognitive impairment (MCI) in single and multiple domains [29], with further increases as patients develop dementia [30]. These studies demonstrate the specificity of PDCP expression for cognitive dysfunction in PD and highlight this metabolic network as an objective biomarker for this devastating and progressive disease manifestation.
Network modulation with PD therapy
In the last decade, research efforts have focused on the development of novel surgical and pharmacologic treatment strategies for the motor and non-motor symptoms of PD. Objective biomarkers that accurately quantify the clinical response to a therapeutic intervention can be extremely useful in determining the efficacy of such new approaches [6]. In this regard, using FDG PET to assess network activity before and during treatment can provide a quantitative index of treatment-mediated changes in brain function at the systems level. Indeed the quantification of network activity in small therapeutic trials (Phases I and II) may be used to objectively screen anti-parkinsonian treatments before proceeding with costly and time consuming efficacy studies [22,50].
Dopaminergic therapy
Regional and network changes in metabolic activity have been described in PD patients undergoing treatment with dopaminergic medications [22,51]. Specifically, changes in pallidal metabolism and PDRP expression have been noted with levodopa administration. Indeed, individual differences in the network-level treatment response correlated closely with concurrent changes in UPDRS motor ratings. In the non-medicated state, regional glucose metabolism is tightly linked to cerebral blood flow [52]. However, a recent study has shown dissociation between cerebral blood flow and glucose metabolism in PD patients during levodopa therapy [53]. This effect was most pronounced in patients with levodopa-induced dyskinesias (LID) and was not observed in patients undergoing deep brain stimulation (DBS). The basis of flow-metabolism dissociation with levodopa is thought to reflect vasodilation induced by exogenous dopamine. A better understanding of this mechanism may reveal new targets for treatments aimed at medication-induced dyskinesias.
While many of the motor symptoms of PD improve with dopaminergic therapy, cognitive dysfunction is largely non-dopamine responsive. Similarly, PDCP expression is not significantly changed in patients receiving levodopa [28]. Thus, PDRP expression is an index of the progression of PD motor symptoms and their response to treatment. By contrast, PDCP expression constitutes a marker of the severity of PD cognitive dysfunction in its early stages.
Stereotaxic-based surgical therapy
Modulation of subthalamic nucleus (STN) activity with deep brain stimulation (DBS) has been used to produce clinical improvement in PD patients comparable to that achieved with dopaminergic drugs. Indeed, STN ablation and STN DBS are thought to ameliorate PD motor symptoms by similar mechanisms as dopamine therapy [54]. Intraoperative microelectrode recordings during STN DBS electrode placement in PD patients have revealed an increase in the mean firing rate of STN neurons [55], which is correlated significantly with PDRP expression [56]. This suggests that network activity might be modulated by surgical interventions in this region. Indeed, STN DBS is associated with significant reductions in PDRP expression, correlating with clinical changes in motor function [22].
Although both dopamine therapy and STN DBS similarly suppress PDRP expression, the effects on network activity are not additive [22]. Data from patients with both therapies suggest that it is unlikely for combination therapy to lower network activity beyond a naturally defined “floor”. Similarly, a recent study using FDG PET addressed changes in PDRP expression after STN “microlesion” [57], defined as the change in network activity that occurs after STN mirco-electrode recording and electrode insertion but prior to STN stimulation. Regional changes in glucose metabolism were evident in regions receiving either direct or indirect input from the STN, such as the putamen, the globus pallidus, and the ventral thalamus [56]. However, the magnitude of these changes was smaller compared to changes seen with therapeutic STN lesioning (subthalamotomy) [58] or DBS [22],. These findings imply that upper and lower thresholds exist for therapeutic PDRP modulation, defining the minimum and maximum functional changes achievable at the STN target site.
Gene transfer therapy
Gene transfer therapy is a novel form of treatment for neurodegenerative disease, such as PD [50]. Small, well designed Phase I trials are critical for evaluating the safety and tolerability of this technique. Moreover, neuroimaging techniques such as FDG PET can be invaluable as objective in vivo biomarkers for the assessment of treatment response. In a recent Phase I clinical trial, we used this approach to measure the safety, tolerability, and potential efficacy of an adeno-associated virus (AAV) borne glutamic acid decarboxylase (GAD) gene transferred into the STN of PD patients [8,9,50]. FDG PET was performed on patients at baseline, 6 months, and 12 months after unilateral gene transfer therapy of the STN. There were significant reductions in thalamic metabolism on the operated side as well as concurrent metabolic increases in ipsilateral motor and premotor cortical regions (Fig. 4A). PDRP activity on the operated side was significantly reduced at 6 months with continued reduction at 12 months (Fig. 4B). This change correlated with improvement in clinical motor UPDRS ratings contralateral to the operated side [8]. By contrast, there was no change in PDCP activity or neuropsychological rates at any time point (Fig. 4C). These studies indicate the use of FDG PET and network analysis as an objective, blinded biomarker for use in open label Phase I clinical trials for the development of novel therapies in PD.
Figure 4. Changes in Regional Metabolism Following Gene Therapy.
A. Voxel based analysis of changes in regional metabolic activity following unilateral STN AAV-GAD gene therapy for advanced PD. Following unilateral gene therapy, a significant reduction in metabolism (top) was found in the operated thalamus, involving the ventrolateral and mediodorsal nuclei. The analysis also revealed a significant metabolic increase (bottom) following surgery in the ipsilateral primary motor region (BA 4), which extended into the adjacent lateral premotor cortex (PMC; BA 6). [Representative axial T1-weighted MRI with merged FDG PET slices; the operated (OP) side is signified on the left. Metabolic increases following surgery are displayed using a red-yellow scale. Metabolic declines are displayed using a blue-purple scale. The displays were thresholded at p<0.05, corrected for multiple comparisons].
B. Postoperative changes in PDRP activity controlling for the effect of disease progression. These progression-corrected values (PDRPc scores) reflect the net effect of STN AAV-GAD on network expression for each subject and timepoint (see text). Relative to baseline, PDRPc scores declined following gene therapy (p<0.001, RMANOVA), with significant reductions relative to baseline at both 6 (gray) and 12 (black) months. These changes correlated (p<0.03) with clinical outcome over the course of the study. [**p<0.005, Bonferroni tests; Bars represent standard error].
C. Changes in mean PDCP network activity over time for the operated (filled circles) and the unoperated (open circles) hemispheres. Following gene therapy, there was no change in PDCP activity over time in either of the two hemispheres (p=0.72). [The dashed line represents one standard error above the normal mean value of zero].
[Feigin A, Kaplitt MG, Tang C, Lin T, Mattis P, Dhawan V, During MJ, Eidelberg D. Modulation of metabolic brain networks following subthalamic gene therapy for Parkinson’s disease. Proceedings of the National Academy of Sciences USA, 2007; 104(49):19560, 19561, Reprinted with permission © 2007 by The National Academy of Sciences of the USA]
Summary
Spatial covariance analysis of metabolic imaging data from patients with neurodegenerative disorders has provided valuable insights into the pathophysiology of these diseases. Recently, this approach has been used with FDG PET to characterize and measure disease-related metabolic patterns as specific network biomarkers for differential diagnosis and the assessment of responses to therapy. For instance, distinct metabolic patterns associated with the motor and cognitive manifestations of PD have been used to assess network modulation following gene therapy. Ongoing FDG PET investigations are being conducted to assess the accuracy of early differential diagnosis of parkinsonism based solely on pattern analysis. Other studies are being planned to validate disease-related patterns for atypical parkinsonian syndromes such as MSA and PSP. Validated metabolic networks for these atypical syndromes will aid in the development of novel therapeutic targets for these refractory conditions. Overall, metabolic imaging with FDG PET in conjunction with pattern analysis is likely to enhance diagnostic accuracy and facilitate the development of new treatments for PD and related disorders.
Acknowledgments
This work was supported by NIH NINDS R01 NS 035069, R01 NS 37564, P50 NS 38370, and the General Clinical Research Center of The Feinstein Institute for Medical Research (M01 RR018535). The authors wish to thank Ms. Toni Flanagan for her valuable editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Rijk MC, Breteler MM, Graveland GA, et al. Prevalence of Parkinson’s disease in the elderly: the Rotterdam Study. Neurology. 1995;45(12):2143–6. doi: 10.1212/wnl.45.12.2143. [DOI] [PubMed] [Google Scholar]
- 2.Hely MA, Morris JG, Reid WG, et al. Sydney Multicenter Study of Parkinson’s disease: non-L-dopa-responsive problems dominate at 15 years. Mov Disord. 2005;20(2):190–9. doi: 10.1002/mds.20324. [DOI] [PubMed] [Google Scholar]
- 3.Hely MA, Reid WG, Adena MA, et al. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23(6):837–44. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- 4.Hughes AJ, Daniel SE, Ben-Shlomo Y, et al. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain. 2002;125(Pt 4):861–70. doi: 10.1093/brain/awf080. [DOI] [PubMed] [Google Scholar]
- 5.O’Sullivan SS, Massey LA, Williams DR, et al. Clinical outcomes of progressive supranuclear palsy and multiple system atrophy. Brain. 2008;131(Pt 5):1362–72. doi: 10.1093/brain/awn065. [DOI] [PubMed] [Google Scholar]
- 6.Poston KL, Eidelberg D. Network biomarkers for the diagnosis and treatment of movement disorders. Neurobiol Dis. 2009;35(2):141–7. doi: 10.1016/j.nbd.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 7.Freed CR, Greene PE, Breeze RE, et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med. 2001;344(10):710–9. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 8.Feigin A, Kaplitt MG, Tang C, et al. Modulation of metabolic brain networks after subthalamic gene therapy for Parkinson’s disease. Proc Natl Acad Sci U S A. 2007;104(49):19559–64. doi: 10.1073/pnas.0706006104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplitt MG, Feigin A, Tang C, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet. 2007;369(9579):2097–105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- 10.Eidelberg D, Takikawa S, Moeller JR, et al. Striatal hypometabolism distinguishes striatonigral degeneration from Parkinson’s disease. Ann Neurol. 1993;33(5):518–27. doi: 10.1002/ana.410330517. [DOI] [PubMed] [Google Scholar]
- 11.Alexander GE, Moeller JR. Application of the scaled subprofile model to functional imaging in neuropsychiatric disorders: A principal component approach to modeling brain function in disease. Hum Brain Mapp. 1994;2:1–16. [Google Scholar]
- 12.Eckert T, Tang C, Eidelberg D. Assessment of the progression of Parkinson’s disease: a metabolic network approach. Lancet Neurol. 2007;6(10):926–32. doi: 10.1016/S1474-4422(07)70245-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eidelberg D. Metabolic brain networks in neurodegenerative disorders: A functional imaging approach. Trends Neurosci. 2009 doi: 10.1016/j.tins.2009.06.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Habeck C, Foster NL, Perneczky R, et al. Multivariate and univariate neuroimaging biomarkers of Alzheimer’s disease. Neuroimage. 2008;40(4):1503–15. doi: 10.1016/j.neuroimage.2008.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feigin A, Tang C, Ma Y, et al. Thalamic metabolism and symptom onset in preclinical Huntington’s disease. Brain. 2007;130(Pt 11):2858–67. doi: 10.1093/brain/awm217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64(1):20–4. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- 17.Ma Y, Tang C, Moeller JR, et al. Abnormal regional brain function in Parkinson’s disease: truth or fiction? Neuroimage. 2009;45(2):260–6. doi: 10.1016/j.neuroimage.2008.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eidelberg D, Moeller JR, Dhawan V, et al. The metabolic topography of parkinsonism. J Cereb Blood Flow Metab. 1994;14(5):783–801. doi: 10.1038/jcbfm.1994.99. [DOI] [PubMed] [Google Scholar]
- 19.Eidelberg D, Moeller JR, Kazumata K, et al. Metabolic correlates of pallidal neuronal activity in Parkinson’s disease. Brain. 1997;120 (Pt 8):1315–24. doi: 10.1093/brain/120.8.1315. [DOI] [PubMed] [Google Scholar]
- 20.Ma Y, Tang C, Spetsieris PG, et al. Abnormal metabolic network activity in Parkinson’s disease: test-retest reproducibility. J Cereb Blood Flow Metab. 2007;27(3):597–605. doi: 10.1038/sj.jcbfm.9600358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C, Tang C, Feigin A, et al. Changes in network activity with the progression of Parkinson’s disease. Brain. 2007;130(Pt 7):1834–46. doi: 10.1093/brain/awm086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asanuma K, Tang C, Ma Y, et al. Network modulation in the treatment of Parkinson’s disease. Brain. 2006;129(Pt 10):2667–78. doi: 10.1093/brain/awl162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fahn S, Oakes D, Shoulson I, et al. Levodopa and the progression of Parkinson’s disease. N Engl J Med. 2004;351(24):2498–508. doi: 10.1056/NEJMoa033447. [DOI] [PubMed] [Google Scholar]
- 24.Antonini A, Moeller JR, Nakamura T, et al. The metabolic anatomy of tremor in Parkinson’s disease. Neurology. 1998;51(3):803–10. doi: 10.1212/wnl.51.3.803. [DOI] [PubMed] [Google Scholar]
- 25.Isaias IU, Marotta G, Hirano S, et al. Imaging essential tremor. Mov Disord. 2009 doi: 10.1002/mds.22870. in press. [DOI] [PubMed] [Google Scholar]
- 26.Levy G, Tang MX, Louis ED, et al. The association of incident dementia with mortality in PD. Neurology. 2002;59(11):1708–13. doi: 10.1212/01.wnl.0000036610.36834.e0. [DOI] [PubMed] [Google Scholar]
- 27.Bosboom JL, Stoffers D, Wolters E. Cognitive dysfunction and dementia in Parkinson’s disease. J Neural Transm. 2004;111(10–11):1303–15. doi: 10.1007/s00702-004-0168-1. [DOI] [PubMed] [Google Scholar]
- 28.Huang C, Mattis P, Tang C, et al. Metabolic brain networks associated with cognitive function in Parkinson’s disease. Neuroimage. 2007;34(2):714–23. doi: 10.1016/j.neuroimage.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang C, Mattis P, Perrine K, et al. Metabolic abnormalities associated with mild cognitive impairment in Parkinson disease. Neurology. 2008;70(16 Pt 2):1470–7. doi: 10.1212/01.wnl.0000304050.05332.9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poston KL, Mattis P, Tang C, et al. Metabolic abnormalities associated with progressive cognitive dysfunction in Parkinson’s disease. Neurology. 2009;72(11):A114. [Google Scholar]
- 31.Eckert T, Edwards C. The application of network mapping in differential diagnosis of parkinsonian disorders. Clin Neurosci Res. 2007;6:359–66. [Google Scholar]
- 32.Eckert T, Tang C, Ma Y, et al. Abnormal metabolic networks in atypical parkinsonism. Mov Disord. 2008;23(5):727–33. doi: 10.1002/mds.21933. [DOI] [PubMed] [Google Scholar]
- 33.Poston KL, Tang C, Eckert T, et al. Longitudinal Changes in Regional Metabolism and Network Activity in Multiple System Atrophy. Neurology. 2009;72(Suppl 3):A67. [Google Scholar]
- 34.Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jankovic J, Rajput AH, McDermott MP, et al. The evolution of diagnosis in early Parkinson disease. Parkinson Study Group. Arch Neurol. 2000;57(3):369–72. doi: 10.1001/archneur.57.3.369. [DOI] [PubMed] [Google Scholar]
- 36.Shih LC, Tarsy D. Deep brain stimulation for the treatment of atypical parkinsonism. Mov Disord. 2007;22(15):2149–55. doi: 10.1002/mds.21648. [DOI] [PubMed] [Google Scholar]
- 37.Zhou J, Yu Q, Zou T. Alternative splicing of exon 10 in the tau gene as a target for treatment of tauopathies. BMC Neurosci. 2008;9 (Suppl 2):S10. doi: 10.1186/1471-2202-9-S2-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng T, Wang Y, Ouyang Q, et al. Comparison of cerebral glucose metabolism between multiple system atrophy Parkinsonian type and Parkinson’s disease. Neurol Res. 2008;30(4):377–82. doi: 10.1179/174313208X300396. [DOI] [PubMed] [Google Scholar]
- 39.Eckert T, Barnes A, Dhawan V, et al. FDG PET in the differential diagnosis of parkinsonian disorders. Neuroimage. 2005;26(3):912–21. doi: 10.1016/j.neuroimage.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 40.Juh R, Pae CU, Lee CU, et al. Voxel based comparison of glucose metabolism in the differential diagnosis of the multiple system atrophy using statistical parametric mapping. Neurosci Res. 2005;52(3):211–9. doi: 10.1016/j.neures.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 41.Otsuka M, Kuwabara Y, Ichiya Y, et al. Differentiating between multiple system atrophy and Parkinson’s disease by positron emission tomography with 18F-dopa and 18F-FDG. Ann Nucl Med. 1997;11(3):251–7. doi: 10.1007/BF03164771. [DOI] [PubMed] [Google Scholar]
- 42.Spetsieris PG, Ma Y, Dhawan V, et al. Differential diagnosis of parkinsonian syndromes using PCA-based functional imaging features. Neuroimage. 2009;45(4):1241–52. doi: 10.1016/j.neuroimage.2008.12.063. [DOI] [PubMed] [Google Scholar]
- 43.Eckert T, Feigin A, Lewis DE, et al. Regional metabolic changes in parkinsonian patients with normal dopaminergic imaging. Mov Disord. 2007;22(2):167–73. doi: 10.1002/mds.21185. [DOI] [PubMed] [Google Scholar]
- 44.Eckert T, Van Laere K, Tang C, et al. Quantification of Parkinson’s disease-related network expression with ECD SPECT. Eur J Nucl Med Mol Imaging. 2007;34(4):496–501. doi: 10.1007/s00259-006-0261-9. [DOI] [PubMed] [Google Scholar]
- 45.Tang C, Eckert T, Ma Y, et al. Application of disease-related metabolic networks for automated differential diagnosis of parkinsonian disorders. Neurology. 2008;70(11):A384. [Google Scholar]
- 46.Alves G, Larsen JP, Emre M, et al. Changes in motor subtype and risk for incident dementia in Parkinson’s disease. Mov Disord. 2006;21(8):1123–30. doi: 10.1002/mds.20897. [DOI] [PubMed] [Google Scholar]
- 47.Alves G, Wentzel-Larsen T, Aarsland D, et al. Progression of motor impairment and disability in Parkinson disease: a population-based study. Neurology. 2005;65(9):1436–41. doi: 10.1212/01.wnl.0000183359.50822.f2. [DOI] [PubMed] [Google Scholar]
- 48.Bernheimer H, Birkmayer W, Hornykiewicz O, et al. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20:415–55. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- 49.Tang C, Eckert T, Dhawan V, et al. Parkinson’s disease: Evidence for a Short Metabolic Preclinical Period. Neurology. 2008;70(11):A436. [Google Scholar]
- 50.Feigin A, Eidelberg D. Gene transfer therapy for neurodegenerative disorders. Mov Disord. 2007;22(9):1223–8. doi: 10.1002/mds.21423. [DOI] [PubMed] [Google Scholar]
- 51.Feigin A, Fukuda M, Dhawan V, et al. Metabolic correlates of levodopa response in Parkinson’s disease. Neurology. 2001;57(11):2083–8. doi: 10.1212/wnl.57.11.2083. [DOI] [PubMed] [Google Scholar]
- 52.Raichle ME. Behind the scenes of functional brain imaging: a historical and physiological perspective. Proc Natl Acad Sci U S A. 1998;95(3):765–72. doi: 10.1073/pnas.95.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hirano S, Asanuma K, Ma Y, et al. Dissociation of metabolic and neurovascular responses to levodopa in the treatment of Parkinson’s disease. J Neurosci. 2008;28(16):4201–9. doi: 10.1523/JNEUROSCI.0582-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vingerhoets FJ, Villemure JG, Temperli P, et al. Subthalamic DBS replaces levodopa in Parkinson’s disease: two-year follow-up. Neurology. 2002;58(3):396–401. doi: 10.1212/wnl.58.3.396. [DOI] [PubMed] [Google Scholar]
- 55.Steigerwald F, Potter M, Herzog J, et al. Neuronal activity of the human subthalamic nucleus in the parkinsonian and nonparkinsonian state. J Neurophysiol. 2008;100(5):2515–24. doi: 10.1152/jn.90574.2008. [DOI] [PubMed] [Google Scholar]
- 56.Lin TP, Carbon M, Tang C, et al. Metabolic correlates of subthalamic nucleus activity in Parkinson’s disease. Brain. 2008;131(5):1373–80. doi: 10.1093/brain/awn031. [DOI] [PubMed] [Google Scholar]
- 57.Pourfar M, Tang C, Lin T, et al. Assessing the microlesion effect of subthalamic deep brain stimulation surgery with FDG PET. J Neurosurg. 2009;110(6):1278–82. doi: 10.3171/2008.12.JNS08991. [DOI] [PubMed] [Google Scholar]
- 58.Trost M, Su S, Su P, et al. Network modulation by the subthalamic nucleus in the treatment of Parkinson’s disease. Neuroimage. 2006;31(1):301–7. doi: 10.1016/j.neuroimage.2005.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]