Abstract
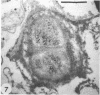
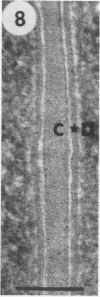
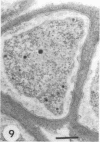
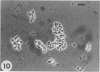
Azospirillum brasilense was reisolated from associations with callus tissue cultures of sugarcane and compared with stock cultures of the inoculated bacterium and related strains. Although the reisolate had a growth rate similar to stock cultures, it exhibited a severalfold increase in maximum specific activity of nitrogenase. The reisolate and the parent culture had similar ultrastructure. The general ultrastructure of Azospirillum is described. The bacterium was capsulated when grown on nitrogen-free nutrient agar plates and on callus, but was not capsulated when growing in a subsurface zone in N-free semisolid nutrient agar, except rarely in aging cultures. Capsulation may be a protective mechanism against unfavorable pO2 under dinitrogen-fixing conditions. Pleomorphism occurred in capsulated forms, and the ultrastructure of these forms is described.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber L. E., Tjepkema J. D., Russell S. A., Evans H. J. Acetylene reduction (nitrogen fixation) associated with corn inoculated with Spirillum. Appl Environ Microbiol. 1976 Jul;32(1):108–113. doi: 10.1128/aem.32.1.108-113.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barea J. M., Brown M. E. Effects on plant growth produced by Azotobacter paspali related to synthesis of plant growth regulating substances. J Appl Bacteriol. 1974 Dec;37(4):583–593. doi: 10.1111/j.1365-2672.1974.tb00483.x. [DOI] [PubMed] [Google Scholar]
- Bednarski M. A., Reporter M. Expression of rhizobial nitrogenase: influence of plant cell-conditioned medium. Appl Environ Microbiol. 1978 Jul;36(1):115–120. doi: 10.1128/aem.36.1.115-120.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D., Kaufman P., McNeil M., Albersheim P. The Structure of Plant Cell Walls: VI. A Survey of the Walls of Suspension-cultured Monocots. Plant Physiol. 1974 Jul;54(1):109–115. doi: 10.1104/pp.54.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhlen P., Stein S., Dairman W., Udenfriend S. Fluorometric assay of proteins in the nanogram range. Arch Biochem Biophys. 1973 Mar;155(1):213–220. doi: 10.1016/s0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- Child J. J., Kurz W. G. Inducing effect of plant cells on nitrogenase activity by Spirillum and Rhizobium in vitro. Can J Microbiol. 1978 Feb;24(2):143–148. doi: 10.1139/m78-026. [DOI] [PubMed] [Google Scholar]
- De Martino C., Zamboni L. Silver methenamine stain for electron microscopy. J Ultrastruct Res. 1967 Aug;19(3):273–282. doi: 10.1016/s0022-5320(67)80221-1. [DOI] [PubMed] [Google Scholar]
- Dobereiner J., Marriel I. E., Nery M. Ecological distribution of Spirillum lipoferum Beijerinck. Can J Microbiol. 1976 Oct;22(10):1464–1473. doi: 10.1139/m76-217. [DOI] [PubMed] [Google Scholar]
- Eskew D. L., Focht D. D., Ting I. P. Nitrogen fixation, denitrification, and pleomorphic growth in a highly pigmented Spirillum lipoferum. Appl Environ Microbiol. 1977 Nov;34(5):582–585. doi: 10.1128/aem.34.5.582-585.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson L. M., Knowles R. Effect of oxygen and nitrate on nitrogen fixation and denitrification by Azospirillum brasilense grown in continuous culture. Can J Microbiol. 1978 Nov;24(11):1395–1403. doi: 10.1139/m78-223. [DOI] [PubMed] [Google Scholar]
- Okon Y., Albrecht S. L., Burris R. H. Factors affecting growth and nitrogen fixation of Spirillum lipoferum. J Bacteriol. 1976 Sep;127(3):1248–1254. doi: 10.1128/jb.127.3.1248-1254.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okon Y., Albrecht S. L., Burris R. H. Methods for Growing Spirillum lipoferum and for Counting It in Pure Culture and in Association with Plants. Appl Environ Microbiol. 1977 Jan;33(1):85–88. doi: 10.1128/aem.33.1.85-88.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okon Y., Houchins J. P., Albrecht S. L., Burris R. H. Growth of Spirillum lipoferum at constant partial pressures of oxygen, and the properties of its nitrogenase in cell-free extracts. J Gen Microbiol. 1977 Jan;98(1):87–93. doi: 10.1099/00221287-98-1-87. [DOI] [PubMed] [Google Scholar]
- Pickett-Heaps J. D. Preliminary attempts at ultrastructural polysaccharide localization in root tip cells. J Histochem Cytochem. 1967 Aug;15(8):442–455. doi: 10.1177/15.8.442. [DOI] [PubMed] [Google Scholar]
- Pope L. M., Wyss O. Outer layers of the Azotobacter vinelandii cyst. J Bacteriol. 1970 Apr;102(1):234–239. doi: 10.1128/jb.102.1.234-239.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. L., Bouton J. H., Schank S. C., Quesenberry K. H., Tyler M. E., Milam J. R., Gaskins M. H., Littell R. C. Nitrogen Fixation in Grasses Inoculated with Spirillum lipoferum. Science. 1976 Sep 10;193(4257):1003–1005. doi: 10.1126/science.193.4257.1003. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Tarrand J. J., Krieg N. R., Döbereiner J. A taxonomic study of the Spirillum lipoferum group, with descriptions of a new genus, Azospirillum gen. nov. and two species, Azospirillum lipoferum (Beijerinck) comb. nov. and Azospirillum brasilense sp. nov. Can J Microbiol. 1978 Aug;24(8):967–980. doi: 10.1139/m78-160. [DOI] [PubMed] [Google Scholar]
- Tien T. M., Gaskins M. H., Hubbell D. H. Plant Growth Substances Produced by Azospirillum brasilense and Their Effect on the Growth of Pearl Millet (Pennisetum americanum L.). Appl Environ Microbiol. 1979 May;37(5):1016–1024. doi: 10.1128/aem.37.5.1016-1024.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler M. E., Milam J. R., Smith R. L., Schank S. C., Zuberer D. A. Isolation of Azospirullum from diverse geographic regions. Can J Microbiol. 1979 Jun;25(6):693–697. doi: 10.1139/m79-100. [DOI] [PubMed] [Google Scholar]
- Vasil I. K., Vasil V., Hubbell D. H. Engineered plant cell or fungal association with bacteria that fix nitrogen. Basic Life Sci. 1977;9:197–211. doi: 10.1007/978-1-4684-0880-5_14. [DOI] [PubMed] [Google Scholar]