Abstract
The purpose of this study was to determine what effect aerobic and resistance exercise training has on gain of visceral fat during the year following weight loss. After being randomly assigned to aerobic training, resistance training, or no exercise training, 45 European-American and 52 African-American women lost 12.3±2.5 kg on a 800 kcal/day diet. Computed tomography was used to measure abdominal subcutaneous and visceral adipose tissue while total fat and regional fat (leg, arm, and trunk) were measured by Dual Energy X-Ray Absorptiometry after weight loss and one year following the weight loss. Since not all the subjects adhered to the 2 time/week 40 minutes/day exercise training during the one year follow-up, subjects were divided into five groups for analysis; aerobic adherers, aerobic non-adherers, resistance adherers, resistance non-adherers and no exercise. No significant differences were observed between the aerobic training and resistance training adherers for any variable. However, the aerobic (3.1 kg) and resistance (3.9 kg) exercise adherers gained less weight than any of the other 3 groups (all more than 6.2 kg). In addition, the two exercise adherence groups did not significantly increase visceral fat (< 0.8%) as compared with the 38% increase for the two non-adhering exercise groups and the 25% for the non-exercise group. In conclusion, as little as 80 minutes/week aerobic or resistance training had modest positive effects on preventing weight regain following a diet induced weight loss. More importantly, both aerobic and resistance training prevented regain of potentially harmful visceral fat.
Keywords: resistance training, aerobic training, fat distribution
Introduction
It is well accepted that obesity, especially visceral obesity, is independently related to an atherogenic blood lipid profile, increased blood pressure and reduced insulin sensitivity (1, 2, 3, 4). In addition, in a large epidemiological study, Tricia et al (5) have recently shown that obesity is independently related to incidence of cardiovascular disease during 20 years of follow-up in the Nurse’s Health Study. Studies have reported that weight gain is associated with increased risk of colon cancer (6) breast cancer (7) clinical diabetes (8, 9), and cardiovascular disease even if the gain occurred in relatively lean women, under BMI of 25 kg/m2 (10). Therefore, factors that may influence weight gain have important implications.
High physical activity is strongly associated with little or no weight regain over one year (11, 12). This is particularly the case when physical activity levels are equivalent to 60 or more minutes/day of moderate intensity exercise, e.g. walking at 3 mph or equivalent energy expenditures from higher intensity exercise (13, 14, 15, 16), (17). Exercise training studies have not shown a consistent training effect on long-term weight maintenance, presumably because of poor adherence or insufficient training induced energy expenditure in some of the studies (16).
Few, if any, studies have compared the effects of resistance and aerobic training on long-term weight regain. After an 11 week diet induced weight loss of 9 kg, Ballor et al (18) reported that both resistance training and aerobic training conserved both weight loss and fat mass loss over 12 weeks in older presumably European American (EA) adults (average age 61 years). In another study, also with presumably EA subjects, Weinstock et al (reference) evaluated the effects of dietary restriction with either aerobic or resistance training with dietary resistance alone. No difference was found between the three groups for weight loss during the first 16 weeks of the study, and for a small subset of 22 subjects for weight regain 96 weeks after beginning the study (19). Despite obesity being a major problem for African Americans (AA), especially AA women, we know of no studies that have evaluated the effects of aerobic and resistance training on long term weight loss maintenance in AAs.
Since visceral adipose tissue is more strongly related to cardiovascular disease and diabetes risk than overall obesity (1, 4, 20, 21, 22, 23) it is particularly important to know what impact exercise training has on distribution of fat. Exercise training typically results in a loss of adipose tissue, especially from the viscera (24, 25, 26, 27). However, studies comparing weight loss from diet restriction with weight loss from exercise have not shown a consistent pattern, with some showing a proportionally larger loss from the viscera with exercise training compared to diet (28) and others not (29). To our knowledge no one has attempted to determine whether exercise is associated with a smaller proportional regain in visceral fat one year following weight loss. Therefore, the purpose of this paper is to compare the effects of aerobic, resistance, and no training on weight and fat distribution one-year following a diet induced weight loss of approximately 12 kg in AA and EA premenopausal women.
Research Methods and Procedures
Subjects
The study group was comprised of 208 healthy premenopausal women, with BMI between 27 and 30 kg/m2. Age ranged from 21 to 46 years. All subjects were non-smokers, of overall good health, and had normal menstrual cycles. Normal glucose tolerance was documented by 2-hour post-prandial blood glucose levels after an oral glucose load. None of the subjects used oral contraceptives at the time of enrollment into the study or medications known to affect body composition. The Institutional Review Board for Human Subjects-approved informed consent was obtained prior to participation in the study in compliance with the Department of Health and Human Services Regulations for Protection of Human Research Subjects.
Study Design
Subjects were randomly assigned to 3 different weight loss groups: diet and aerobic exercise, diet and resistance exercise, and diet only. All food was furnished during the weight loss and consisted of 800 kcal/day that was 20–22% fat, 18–22% protein, and 58–62% carbohydrate. Subjects picked up food at the General Clinical Research Center (GCRC) twice weekly and were instructed to remain on the 800 kcal/day diet until a BMI of less than 25 kg/m2 was reached. Time needed to reach the goal of a 25 kg/m2 BMI was variable with a mean of 154±61 days. However, no differences between the exercise groups was found (p = 0.2). Subjects were evaluated two times, once after losing an average of 12.3±2.5 kg (121 subjects reached the target BMI, defined as baseline) and once one year following the weight loss (97 subjects returned for the 1 year post weight loss evaluation). During the one year following weight loss subjects were given instructions on a balanced diet that focused on low density food intake according to EatRight Weight Management Program principles (30). Data presented in this paper is for the 97 subjects (52 AA and 45 EA) that we have data one-year following weight loss. Prior to evaluations subjects were maintained in weight-stable state for 4 weeks. During those 4 weeks, body weight measurements were made at visits to the General Clinical Research Center (GCRC) 3 days/wk for the first 2 weeks, and 5 days/wk for the last 2 weeks. During the final 2 weeks, all meals were provided through the GCRC Research Kitchen to ensure weight stability of less than 1% variation from initial weight and to maintain daily macronutrient intake within the range of 20–22% of energy as fat, 18–22% as protein, and 58–62% as carbohydrate. Subjects were then admitted to the GCRC for 4 days, during the follicular phase of the menstrual cycle, and underwent assessment of body composition/fat distribution. After all assessments were completed subjects were discharged from the GCRC.
Those subjects assigned to exercise groups were scheduled to train three times each week during weight loss and two times each week during the one year following weight loss. All exercise training was done in an exercise training facility devoted entirely to research and was supervised by exercise physiology study personnel. Subjects that were assigned to exercise groups were required to exercise train during weight loss to remain in the study. However, after weight loss adherence to exercise training was only strongly encouraged but not required for study participation. There was considerable variation in exercise adherence for the year that followed weight loss. Subjects assigned to exercise were thus divided into adherers (adherence to 60% or more of the scheduled exercise sessions) and non-adherers (adherence to less than 60% of the scheduled exercise sessions). Sixty % adherence was selected as a cut-point for adherence for two reasons. First it is accepted that one-day per week training maintains aerobic (31) and strength fitness (32) and some studies even show that it is sufficient to increase fitness (33, 34). Adherence of 60% would mean that the subjects would train a minimum of at least 1.2 days/wk. Second 15 of the 39 individuals in the two exercise groups had adherence levels between 60 and 69%, making it necessary to select 60% as a minimum to have sufficient sample size in the adherence groups. Comparisons between five groups (aerobic adherers, aerobic non-adherers, resistance adherers, resistance non-adherers, and no exercise) were done in all statistical evaluations.
Body Composition
Regional and whole body lean and fat tissue were determined with the use of dual-energy X-ray absorptiometry (GE Medical Systems Lunar., Madison Wisconsin). The scans were analyzed with the use of ADULT software, LUNAR DPX-L Version 1.35.
Abdominal adipose tissue
Cross-sectional area of intra-abdominal adipose tissue (IAAT), deep subcutaneous adipose tissue (DSAT), and superficial subcutaneous adipose tissue (SSAT) was determined by CT with the use of a HiLight/HTD Advantage scanner (General Electric Co, Milwaukee) set at 120 kVp (peak kilovoltlage) and 40 mA. Subjects were examined in the supine position with their arms stretched above their heads, taking a 5-mm scan for 2 s at approximately the level of the fourth and fifth lumbar vertebrae. With the use of procedures established by Kvist et al (35), the attenuation range for adipose tissue was − 30 to − 190 Hounsfield units. Cross sections of adipose tissue were determined by using a computerized fat tissue-highlighting technique. Tissue cross-sections between − 30 and − 190 Hounsfield units in the respective areas were considered to be IAAT. The fascia superficialis was used to delineate the SSAT from the DSAT as described by Smith et al (36). Both intra- and inter-observer test-retest reliability for IAAT, DSAT, and SSAT had an r value of 0.99 with a CV of < 2% for re-evaluation of 20 scans.
Aerobic training
Aerobic training entailed continuous walking/jogging on a treadmill, commencing with a warm-up of 3 minutes and 3–5 minutes of stretching. During the first week of training, the subjects performed 20 minutes of continuous exercise at 67% maximum heart rate. Each week after the 1st week, duration and intensity increased so that by the beginning of the 8th week subjects exercised continuously at 80% of maximum HR for 40 minutes. Subjects were encouraged to increase intensity (either speed or grade) when average exercise heart rate was consistently below 80% of maximum heart rate during both the weight loss and 1-yr weight maintenance phases. After the exercise session, subjects cooled down for 3–5 minutes with gradually decreasing exercise intensity.
Resistance training
After a warm-up on the treadmill or bike ergometer for 5 minutes and 3–5 minutes of stretching, subjects performed the following exercises: squats, leg extension, leg curl, elbow flexion, triceps extension, lateral pull-down, bench press, military press, lower back extension, and bent leg sit-ups. One set of 10 repetitions was performed during the first 4 weeks, after which two sets of 10 repetitions were performed for each exercise with 2 minutes rest between sets. The training was progressive with intensity based on 80% of the maximum weight that an individual lifted one time (1RM). Strength was evaluated every 3 weeks and adjustments in training resistance were made based on the most current 1RM in both the weight loss and 1-yr weight maintenance phases.
In both the aerobic and resistance exercise groups subjects were expected to train 3 days/wk during the weight loss and 2 days/wk during the 1-yr weight maintenance phase.
Statistics
A two (time) by two (race) by five (group) analysis of variance (ANOVA) was run on the primary variables of interest, weight, percent fat, IAAT, SAAT, and leg fat. No significant time by race or time by group by race interactions were found for any of the variables, indicating that the AA and EA women were responding similarly to the exercise interventions. Therefore, the analysis was collapsed to a two (time) by five (group) ANOVA with repeated measures on time for all variables except age and exercise adherence in which a one-way ANOVA was run for the five group categories. Changes in the ratio between IAAT and leg fat (IAAT/leg fat) across the one-year follow-up were calculated. The dependent variable IAAT/leg fat was evaluated in a one-way ANOVA, with independent variable adherence consisting of: 1) combined aerobic and resistance exercise adherers; 2) combined aerobic and resistance exercise non-adherers; and 3) no exercisers. T-tests with bonferroni corrections were run to compare post hoc analyses of interest.
Results
No significant time by race or time by group by race interactions were found for any of the variables indicating that the AA and EA women were responding similarly to the exercise interventions. Therefore, the analyses are collapsed into a two (time) by group analyses. No group effects were found for any variable of interest in the study with the exception of arm fat and adherence with the aerobic and resistance adherence exercise groups adhering similarly (82 and 79% respectively) and the aerobic and resistance non-adherence exercise groups adhering similarly (18 and 22 % respectively, Table 1). The significant time effect indicated that weight, BMI, and % fat and waist increased significantly during the one-year of follow-up (Table 1). There was also a significant time × group interaction for each of these variables with post hoc tests showing the exercise adherence groups (both aerobic and resistance) increased less than the combined non-adherence and no exercise groups. No difference was found between the aerobic and resistance adherence groups.
Table 1.
Descriptives.
| Age (yrs) | Adherence to training (%) | Body Weight (kg) | BMI (kg/m2) | % fat | Waist Circumference | ||
|---|---|---|---|---|---|---|---|
| Aerobic Adherers N=18 |
Baseline Follow-up Change |
34.7±8.4 | 82±15 | 62.8±5.2 65.9±7.1 3.1a |
23.5±1.0 24.7±2.0 1.2 a |
31.8±3.6 35.2±6.0 3.4 a |
74.3±5.2 76.2±6.6 1.9 |
| Aerobic Non-adherers N=14 |
Baseline Follow-up Change |
36.2±6.6 | 18±13 | 65.0±6.0 72.1±8.9 7.1 |
24.1±1.2 26.3±2.5 2.2 |
35.1±3.5 41.5±4.8 6.4 |
76.5±4.4 84.4±6.3 7.9 |
| Resistance Adherers N=21 |
Baseline Follow-up Change |
34.1±7.2 | 79±13 | 66.0±8.3 69.9±8.0 3.9 a |
23.9±1.0 25.3±1.5 1.2 a |
32.7±5.5 37.1±5.9 4.4 a |
75.6±5.5 78.5±5.7 2.9 |
| Resistance Non-adherers N=14 |
Baseline Follow-up Change |
34.1±5.2 | 22±13 | 65.4±6.2 71.6±7.9 6.2 |
24.0±1.2 26.3±2.4 2.3 |
33.4±4.2 39.2±5.0 5.8 |
77.0±6.1 82.3±6.5 5.3 |
| No Exercise N=30 |
Baseline Follow-up Change |
34.8±5.6 | 65.0±5.7 71.4±7.5 6.4 |
23.9±1.1 26.5±2.0 2.6 |
33.7±4.8 39.0±5.0 5.3 |
77.0±5.7 82.5±7.8 5.5 |
|
| P | G = 0.92 | G < 0.01 | T < 0.03 G = 0.17 TxG = 0.01 |
T = 0.03 G = 0.14 TxG< 0.02 |
T < 0.01 G = 0.11 TxG = 0.03 |
T < 0.01 G = 0.06 TxG < 0.01 |
|
Significant different post hoc test (p < 0.05) from cells not containing a
A significant time effect and time by group interaction were found for IAAT and DSAT (Table 2). Post hoc analyses indicated that the diet only control group significantly increased IAAT, DSAT, SSAT, while the aerobic nonadherers significantly increased IAAT and DSAT. The aerobic adherers significantly increased DSAT. No other significant changes in computed tomography adipose tissue measures were found.
Table 2.
CT derived abdominal adipose tissue.
| IAAT (cm2) | DSAT (cm2) | SSAT (cm2) | ||
|---|---|---|---|---|
| Aerobic Adherers N=15 |
Baseline Follow-up Change |
48.0±17.7 48.8±20.1 0.8 cm2 1.6 % |
65.7±29.4 a 101.0±46.4 35.3 cm2 53.7 % |
129.4±64.5 142.1±32.9 22.7 cm2 17.5 % |
| Aerobic Non-adherers N=13 |
Baseline Follow-up Change |
46.9±19.5 a 72.4±26.8 25.5 cm2 54.4 % |
84.7±27.4 a 140.4±51.4 55.7 cm2 a 65.8 % |
142.5±46.0 188.4±71.1 45.9 cm2 32.2 % |
| Resistance Adherers N=18 |
Baseline Follow-up Change |
43.7±14.4 43.3±15.5 − 0.4 cm2 0.0% |
82.3±35.0 89.4±35.0 7.1 cm2 8.6 % |
132.0±41.7 146.1±47.1 14.1 cm2 9.9 % |
| Resistance Non-adherers N=11 |
Baseline Follow-up Change |
47.4±27.6 57.8±28.0 10.4 cm2 21.9 % |
74.5±30.2 100.3±39.0 25.8 cm2 34.6 % |
127.0±42.5 150.8±41.2 23.8 cm2 18.7 % |
| No Exercise N=26 |
Baseline Follow-up Change |
50.0±20.8 a 62.4±28.2 12.4 cm2 24.8 % |
79.4±34.4 a 114.2±51.5 34.8 cm2 43.8 % |
127.8±43.6 a 158.9±47.7 31.1 cm2 24.3 % |
| P | T < 0.01 G = 0.23 TxG <0.01 |
T < 0.01 G = 0.14 TxG < 0.02 |
T = 0.12 G = 0.27 TxG = 0.23 |
|
Significant difference between baseline and follow-up
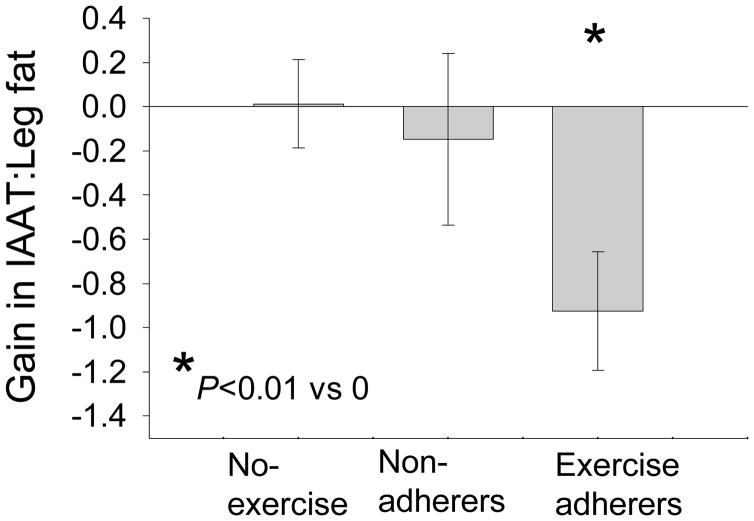
Arm fat, leg fat and trunk fat all had a significant time and time by group interaction (Table 3). Post hoc analysis showed that all groups increased fat mass in all three fat depots. A significant difference in change in fat mass was found between the aerobic non adherers and aerobic adherers for both arm fat and leg fat. No other significant differences in fat mass change were found. Figure 1 contains the changes in the ratio between IAAT and leg fat (IAAT/leg fat) across the one-year follow-up. The two adherence groups (aerobic and resistance) and the two non-adherence groups are combined in this analysis. The combined adherence groups decreased the IAAT/leg fat ratio significantly (P less than 0.01) whereas the combined non-adherers and no exercise groups did not significantly change.
Table 3.
DXA derived regional body fat.
| Arm Fat (kg) | Leg Fat (kg) | Trunk Fat (kg) | ||
|---|---|---|---|---|
| Aerobic Adherers |
Baseline Follow-up Change |
1.9±0.4 2.1±0.6 0.2 kg 10.5 % |
8.7±1.8 10.3±2.4 1.6 kg 18.4 % |
9.9±1.5 11.6±2.7 1.7 kg 17.2 % |
| Aerobic Non-adherers |
Baseline Follow-up Change |
2.2±0.4 a 2.9±0.7 0.7 kg 31.8 % |
9.6±2.5 a 12.6±3.7 3.0 kg 31.3 % |
9.4±1.8 12.8±2.8 3.4 kg 36.3 % |
| Resistance Adherers |
Baseline Follow-up Change |
2.1±0.4 2.4±0.7 0.3 kg 14.3 % |
9.6±2.4 11.4±2.4 1.8 kg 19.0 % |
10.1±2.4 12.1±2.7 2.0 kg 19.8 % |
| Resistance Non-adherers |
Baseline Follow-up Change |
2.1±0.5 2.7±0.7 0.6 kg 28.6 % |
9.1±2.2 11.7±2.9 2.6 kg 29.9 % |
9.3±2.3 12.3±3.1 3.0 kg 32.0 % |
| No Exercise | Baseline Follow-up Change |
2.0±0.5 2.5±0.7 0.5kg 25.0 % |
8.7±1.9 10.9±2.3 2.2 kg 25.3 % |
10.0±2.5 13.2±3.6 3.2 kg 32.0 % |
| P | T < 0.01 G < 0.05 TxG< 0.01 |
T < 0.01 G = 0.14 TxG < 0.05 |
T < 0.02 G = 0.64 TxG < 0.03 |
|
Baseline to follow-up changes in fat mass all significantly different from zero.
Significantly different from aerobic adherers
Figure 1.
Change in IAAT (visceral fat) to leg fat ratio. Results show that the ratio decreased for the exercise adherers (aerobic and resistance combined) favoring distribution away from the potentially harmful viscera (p < 0.01) but did not change significantly for the no exercise and non adherence groups. Change in IAAT to leg fat ratio for the exercise adherers was significantly different from the changes in IAAT to leg fat ratio for the no exercise and non adherence groups (both p<0.01). No significant difference in IAAT to leg fat ratio was found between the no exercise and non adherence groups (p > 0.50).
Discussion
Following a 12 kg weight loss, adherence to either moderate volume (about 80 minutes/week) aerobic or resistance training resulted in reduced one-year weight regain (3.1 kg aerobic and 3.9 kg resistance versus 6.4 kg for the no exercise group). Perhaps more important, the aerobic and resistance training prevented visceral fat (IAAT) regain, despite modest weight regains, while the group that did not participate in exercise training increased visceral fat 25%. This was the case for both AA and EA women, suggesting that the exercise intervention was equally affective for both races. The adhering exercisers did regain body fat in other regions, however, such as an increase of about 18% in leg fat. These results not only are supportive of the concept that moderate volumes of exercise training are beneficial for decreasing weight regain following weight loss, but supportive of the concept that exercise training might direct energy storage during fat regain to depots other than the viscera.
It is important to point out that the smaller weight regain and prevention of visceral fat gain was achieved with a small volume of exercise training, only 80 minutes/week. Since other studies have reported that much longer training durations of more than 60 minutes/day are necessary to prevent weight regain (11, 12, 13, 14, 15, 16, 17) it is not too surprising that weight regain was not totally prevented in this study. It is very encouraging, however, that this relatively small volume of exercise was sufficient to prevent visceral fat gain.
No consensus has been reached concerning the effects of exercise training on fat distribution. Both aerobic and resistance exercise training are associated with loss of body fat, particularly visceral fat suggesting either type of exercise training may result in proportionately small amounts of fat stored in the viscera (24, 25, 26, 26, 27). Since diet induced weight loss is also associated with a disproportionately large visceral fat loss, it is possible that caloric deficit will create similar visceral fat losses whether the restriction is induced by diet or exercise. For example, a 10% weight loss is typically associated with a 30 to 35% visceral fat loss following diet induced weight loss (37). In one of the few studies comparing exercise and diet induced weight loss, Ross et al (29) showed similar visceral fat losses between diet and exercise interventions. However, others have found over two times more visceral fat loss for exercise induced weight loss when weight loss is matched between exercise and diet induction (28). In addition, detrained athletes increase waist circumference despite decreasing body weight (38) suggesting an increase in visceral fat as the athletes detrained. Although not conclusive, these studies, as well as the present study, suggest that exercise training may be beneficial in prevention of visceral obesity.
Although there was no increase in visceral adipose tissue in those who adhered to exercise, fat in other depots did increase. Prevention of visceral adipose tissue gain is important since visceral adipose tissue is more strongly related to cardiovascular disease and diabetes risk than fat in other depots (1, 4, 20, 21, 22, 23). In fact, multiple regression modeling shows that independent of visceral fat, leg fat is typically associated with improved blood lipid profile and insulin sensitivity (4, 1). Although this could be interpreted to mean that leg fat is protective, other interpretations are possible. For example, it is possible that the same factors that may cause an individual to preferentially partition fat in the legs may be responsible for improving risk. No definitive interpretation of these correlational findings are available at this time, however, it is clear that leg fat does little to increase cardiovascular and diabetes risk. Therefore, from a health risk standpoint repartitioning of fat away from the viscera by exercise training would be considered beneficial.
No significant differences were observed between those subjects who were assigned to exercise training but adhered poorly to the training and those subjects who were assigned to the no exercise group. This was not too surprising since adherence for the nonadhering exercisers was about 20 % of the twice weekly scheduled exercise sessions.
Little is known concerning potential mechanisms for exercise training success in preventing visceral fat regain, despite weight regains. One possibility would be that the exercise training prevented body fat regains despite modest weight regain. However, percent body fat increased even among the exercise adherers in our study (3.4 % and 4.4 % for the aerobic and resistance adherence groups). Despite this increase in percent body fat the two adherence exercise groups did not significantly increase visceral fat (mean of 1.6 % for the aerobic group and 0 % for the resistance group). Another plausible explanation, considering our results that suggest a repartitioning of fat gain, would be that the hormonal milieu changed so that it favored storage of fat in periphery rather than the viscera. Cortisol (39), exogenous androgens (40), and dopamine (41) may increase storage of fat in the viscera while growth hormone (39) may have a negative effect on storage of fat in the viscera in women. Exercise training has well established effects on a number of hormones including decreasing cortisol (42) and increasing growth hormone (43), (44). More research is needed to confirm any potential positive effects exercise training may have on fat distribution and to explore potential mechanisms by which exercise might achieve in repartitioning fat distribution.
In conclusion, as little as 80 minutes/week aerobic or resistance training had modest positive effects on preventing weight regain following a diet induced weight loss. More importantly, though, both aerobic and resistance training prevented regain of potentially harmful visceral fat.
Acknowledgments
We wish to thank our sources of support: NIH grants R01 DK 49779 and R01 DK51684, General Clinical Research Center grant M01-RR00032, Clinical Nutrition Research Unit grant P30-DK56336, and UAB University-Wide Clinical Nutrition Research Center grant. Stouffer’s Le NIH grants R01 DK 49779 and R01 DK51684. The Nestlé Food Co., Solon, OH., provided the Stouffer’s Lean Cuisine® entrées.
Reference List
- 1.Williams MJ, Hunter GR, Kekes-Szabo T, Snyder S, Treuth MS. Regional fat distribution in women and risk of cardiovascular disease. Am J Clin Nutr. 1997;65:855–60. doi: 10.1093/ajcn/65.3.855. [DOI] [PubMed] [Google Scholar]
- 2.Gower BA, Ard JD, Hunter GR, Fernandez J, Ovalle F. Elements of the metabolic syndrome: association with insulin sensitivity and effects of ethnicity. Metab Syndr Rel Dis. 2007;5:77–86. doi: 10.1089/met.2006.0027. [DOI] [PubMed] [Google Scholar]
- 3.Hunter GR, Newman Giger J, Weaver M, Strickland OL, Zuckerman P, Taylor H. Fat distribution and cardiovascular disease risk in African-American women. J Nat Black Nurses Assoc. 2000;11:7–11. [PubMed] [Google Scholar]
- 4.Hunter GR, Kekes-Szabo T, Snyder S, Nicholson C, Nyikos I, Berland L. Fat distribution, physical activity, and cardiovascular risk factors. Med Sci Sports Exerc. 1997;29:362–9. doi: 10.1097/00005768-199703000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Tricia YL, Rana JS, Manson JE, et al. Obesity as compared with physical activity in predicting risk of coronary heart disease in women. Circulation. 2006;113:499–506. doi: 10.1161/CIRCULATIONAHA.105.574087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thygesen LC, Gronbaek M, Johansen C, Fuchs CS, Willett WC, Giovanncci E. Int J Cancer. 2008;123:1160–5. doi: 10.1002/ijc.23612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eliassen EH, Colditz GA, Rosner B, Willett WC, Hankinson SE. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006;296:193–201. doi: 10.1001/jama.296.2.193. [DOI] [PubMed] [Google Scholar]
- 8.Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. An Intern Med. 1995;122:481–6. doi: 10.7326/0003-4819-122-7-199504010-00001. [DOI] [PubMed] [Google Scholar]
- 9.Koh-Banerjee P, Wang Y, Hu FB, Spiegelman D, Willettt WC, Rimm EB. Changes in body weight and body fat distribution as risk factors for clinical diabetes in US men. Am J Epidemiol. 2004;159:1150–9. doi: 10.1093/aje/kwh167. [DOI] [PubMed] [Google Scholar]
- 10.Willett WC, Manson JE, Stampfer MJ, et al. Weight, weight change, and coronary heart disease in women. Risk within the ‘normal’ weight range. JAm Med Assoc. 1995;273:461–5. doi: 10.1001/jama.1995.03520300035033. [DOI] [PubMed] [Google Scholar]
- 11.Weinsier RL, Hunter GR, Desmond RA, Byrne NM, Zuckerman PA, Darnell BE. Free-living activity energy expenditure in women successful and unsuccessful in maintaining a normal body weight. Am J Clin Nutr. 2002;75:499–504. doi: 10.1093/ajcn/75.3.499. [DOI] [PubMed] [Google Scholar]
- 12.Schoeller DA, Shay K, Kushner RF. How much physical activity is neded to minimize weight gain in previously obese women? Am J Clin Nutr. 1997;66:551–6. doi: 10.1093/ajcn/66.3.551. [DOI] [PubMed] [Google Scholar]
- 13.Williams PT. Maintaining vigorous activity attenuates 7-yr weight gain in 8340 runners. Med Sci Sports Exerc. 2007;39:801–9. doi: 10.1249/mss.0b013e31803349b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Votruba SB, Blac S, Schoeller DA. Pattern and cost of weight gain in previously obese women. Am J Physiol. 2001;282:E923–E930. doi: 10.1152/ajpendo.00265.2001. [DOI] [PubMed] [Google Scholar]
- 15.Erlichman J, Kerbey AL, James WPT. Physical activity and its impact on health outcomes. Paper 2: prevention of unhealthy weight gain and obesity by physical activity: an analysis of the evidence. Obes Rev. 2002;3:273–87. doi: 10.1046/j.1467-789x.2002.00078.x. [DOI] [PubMed] [Google Scholar]
- 16.Fogelholm M, Kukkonen-Harjula K. Does physical activity prevent weight gain - a systematic review. Obes Rev. 2000;1:95–111. doi: 10.1046/j.1467-789x.2000.00016.x. [DOI] [PubMed] [Google Scholar]
- 17.DiPietro L. Physical activity in the prevention of obesity: current evidence and research issues. Med Sci Sports Exerc. 1999 doi: 10.1097/00005768-199911001-00009. [DOI] [PubMed] [Google Scholar]
- 18.Ballor DL, Harvey-Brino JR, Ades PA, Cryan J, Calles-Escandon J. Contrasting effects of resistance and aerobic training on body composition and metabolism after diet-induced weight loss. Metabolism. 1996;45:179–83. doi: 10.1016/s0026-0495(96)90050-5. [DOI] [PubMed] [Google Scholar]
- 19.Weinstock RS, Dai H, Wadden TA. Diet and exercise in the treatment of obesity: effects of 3 interventions on insulin resistance. Arch Intern Med. 1998;158:2477–83. doi: 10.1001/archinte.158.22.2477. [DOI] [PubMed] [Google Scholar]
- 20.Pouliot MC, Despres JP, Nadeau A, et al. Associations with glucose tolerance, plasma insulin, and lipoprotein levels. Diabetes. 1992;41:826–34. doi: 10.2337/diab.41.7.826. [DOI] [PubMed] [Google Scholar]
- 21.Despres JP. Abdominal obesity as important component of insulin-resistance syndrome. Nutrition. 1993;9:452–9. [PubMed] [Google Scholar]
- 22.Peiris AN, Sothmann MS, Hoffmann RG, et al. Adiposity, fat distribution, and cardiovascular risk. Ann Intern Med. 1989;110:867–72. doi: 10.7326/0003-4819-110-11-867. [DOI] [PubMed] [Google Scholar]
- 23.DeNino WF, Tchernof A, Dionne IJ, et al. Contribution of abdominal adiposity to age-related differences in insulin sensitivity and plasma lipids in healthy nonobese women. Diabetes Care. 2001;24:925–32. doi: 10.2337/diacare.24.5.925. [DOI] [PubMed] [Google Scholar]
- 24.Treuth MS, Hunter GR, Kekes-Szabo T, Weinsier RL, Goran MI, Berland L. Reduction in intra-abdominal adipose tissue after strength training in older women. J Appl Physiol. 1995;78:1425–31. doi: 10.1152/jappl.1995.78.4.1425. [DOI] [PubMed] [Google Scholar]
- 25.Hunter GR, Bryan DR, Wetzstein CJ, Zuckerman PA, Bamman MM. Resistance training and intra-abdominal adipose tissue in older men and women. Med Sci Sports Exerc. 2002;34:1023–8. doi: 10.1097/00005768-200206000-00019. [DOI] [PubMed] [Google Scholar]
- 26.Kohrt WM, Obert KA, Holloszy JO. Exercise training improves fat distribution patterns in 60- to 70-year old men and women. J Gerontol. 1992;47:M99–M105. doi: 10.1093/geronj/47.4.m99. [DOI] [PubMed] [Google Scholar]
- 27.Oppert J-M, Nadeau ATA, Despres J-P, Theriault G, Bouchard C. Negative energy balance with exercise in identical twins: plasma glucose and insulin responses. Am J Physiol. 1997;272:E248–E254. doi: 10.1152/ajpendo.1997.272.2.E248. [DOI] [PubMed] [Google Scholar]
- 28.Coker RH, Yeo SE, Williams RH, Kortebein PM, Evans WJ. Exercise training - versus caloric restriction - induced weight loss: effects on hepatic and peripheral insulin sensitivity. ACSM Integrative Physiology Meeting 2007. Poster Session. 2007;3:50. abstr. [Google Scholar]
- 29.Ross R, Rissanen J, Pedwell H, Clifford J, Shragge P. Influence of diet and exercise on skeletal muscle and visceral adipose tissue in men. J Appl Physiol. 1996;81:2445–55. doi: 10.1152/jappl.1996.81.6.2445. [DOI] [PubMed] [Google Scholar]
- 30.Weinsier RL, Wilson NP, Morgan SL, Cornwell AR, Craig CB. EatRight Lose Weight: Seven Simple Steps. Birmingham: Oxmoor House; 1997. [Google Scholar]
- 31.Fox E, Bowers R, Foss M. The Physiological Basis for Exercise and Sport. WCB Brown & Benchmark; Madison, WI: 1993. pp. 355–357. [Google Scholar]
- 32.Graves JE, Pollock ML, Leggett SH, Braith RW, Carpenter DM, Bishop LE. Effect of reduced frequency on muscular strength. Int J Sports Med. 1988;9:316–9. doi: 10.1055/s-2007-1025031. [DOI] [PubMed] [Google Scholar]
- 33.Gettman LR, Pollock ML, Durstine JL, Ward A, Ayres J, Linnerud AC. Physiological responses of men to 1, 3, and 5 day per week training programs. Res Quart. 1976;47:638–46. [PubMed] [Google Scholar]
- 34.Pollock ML. How Much Exercise Is Enough? Physic Sports Med. 1978;6(6) [Google Scholar]
- 35.Kvist H, Chowdhury B, Grangard U, Tylen U, Sjostrom L. Total and visceral adipose-tissue volumes derived from measurements with computed tomography in adult men and women: predictive equations. Am Clinical Nutrition. 1988;48:1351–61. doi: 10.1093/ajcn/48.6.1351. [DOI] [PubMed] [Google Scholar]
- 36.Smith SR, Lovejoy JC, Greenway F, et al. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism. 2001;50:425–35. doi: 10.1053/meta.2001.21693. [DOI] [PubMed] [Google Scholar]
- 37.Ross R, Freeman JA, Jansen I. Exercise alone is an effective strategy for reducing obesity and related comorbidities. Exerc Sports Scie Rev. 2001;28:165–70. [PubMed] [Google Scholar]
- 38.Liu TC, Liu YY, Lee SD, et al. Effects of short-term detraining on measures of obesity and glucose tolerance in elite athletes. Int J Sports Sports Sci. 2008;26:912–25. doi: 10.1080/02640410801885925. [DOI] [PubMed] [Google Scholar]
- 39.Misra M, Brdella MA, Tsai P, Mendes N, Miller KK, Klibanski A. Lower growth hormone and higher cortisol are associated with greater viscral adiposity, intramyyocellular lipids, and insulin resistance in overweight girls. Am J Physiol. 2008;295:E385–E392. doi: 10.1152/ajpendo.00052.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lovejoy JC, Bray GA, Bourgeois MO, et al. Exogenous androgens influence body composition and regional body fat distribution in obese postmenopausal women--a clinical research center study. Journal of Clinical Endocrinology Metabolism. 1996;81:2198–203. doi: 10.1210/jcem.81.6.8964851. [DOI] [PubMed] [Google Scholar]
- 41.Blaudeau TE, Hunter GR, St Onge M-P, et al. IAAT, catecholemines, and parity in African-American and European-American women. Obesity. 2008;16:797–803. doi: 10.1038/oby.2007.137. [DOI] [PubMed] [Google Scholar]
- 42.Viru M, Hackney AC, Janson T, Karlson K, Viru A. Characterization of the cortisol response to incremental exercise in physically active young men. Acta Phisiologica Hungary. 2008;95:219–27. doi: 10.1556/APhysiol.95.2008.2.6. [DOI] [PubMed] [Google Scholar]
- 43.Weltman A, Weltman JY, Roy CP, et al. Growth hormone response to graded exercise intensities is attenuated and the gender difference abolished in older adults. J Appl Physiol. 2006;100:1623–9. doi: 10.1152/japplphysiol.01312.2005. [DOI] [PubMed] [Google Scholar]
- 44.Wideman L, Consitt L, Parrie J, et al. The impact of sex and exercise duration on growth hormone secretion. J Appl Physiol. 2006;101:1641–7. doi: 10.1152/japplphysiol.00518.2006. [DOI] [PubMed] [Google Scholar]