Abstract
Phthalates are known reproductive and developmental toxicants in experimental animals. However, in humans, there are few data on the exposure of pregnant women that can be used to assess the potential developmental exposure experienced by the fetus. We measured several phthalate metabolites in maternal urine, maternal serum, and cord serum samples collected at the time of delivery from 150 pregnant women from central New Jersey. The urinary concentrations of most metabolites were comparable to or less than among the U.S. general population, except for mono(2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), and mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), three metabolites of di(2-ethylhexyl) phthalate (DEHP). The median urinary concentrations of MEHHP (109 μg/l) and MEOHP (95.1 μg/l) were more than 5 times their population-based concentrations, whereas the median urinary concentration of MEHP was more than 20 times higher. High concentration of MEHP may indicate a recent exposure to the parent chemical DEHP in the hospital shortly before the collection of the samples. Calculation of daily intakes using the urinary biomarker data reveals that none of the pregnant women tested had integrated exposures to DEHP greater than the Agency for Toxic Substances and Disease Registry’s minimal risk levels (MRLs chronic 60, intermediate 100 μg/kg/day). No abnormal birth outcomes (e .g., birth weight, Apgar Score, and gestational age) were noted in those newborns whose mothers had relatively greater exposure to DEHP during the perinatal period than others in this study. Significantly greater concentrations and detection frequencies in maternal urine than in maternal serum and cord serum suggest that the urinary concentrations of the phthalate metabolites may be more reliable biomarkers of exposure than their concentrations in other biological specimens.
Keywords: phthalates, metabolites, daily intake, risk assessment, women, children
INTRODUCTION
Chemical contamination of the environment is a pervasive, insidious side effect of human population growth and technological development. A wide range of species, ranging from crustaceans, fish, and birds to mammals, have been reported as being affected by chemicals that interfere with the normal functioning of the endocrine systems, which consequently results in adverse effects on reproduction, growth, and development (Crisp et al. 1998). Defined as endocrine disruptors (EDs) by the U.S. Environmental Protection Agency (USEPA), EDs can be naturally occurring (e .g., soy isoflavones, whole grain lignans, microbial products, wood components) or anthropogenic chemicals, such as pesticides and phthalates.
Phthalates, one class of potential EDs, are diesters of 1, 2-benzenedicarboxylic acid (phthalic acid) produced by reaction of phthalic acid with a specific alcohol to form the desired ester with the side chain(s) of interest. They are high production volume chemicals (>1 million tons produced or imported into the United States per year) and are used in many consumer products, including personal care products (e .g., perfumes, lotions, cosmetics), paints, industrial plastics, and pharmaceuticals. Their primary functions in these products are to hold color or fragrance, to provide a film or gloss, to make certain plastics more flexible, or provide timed release for some pharmaceuticals (ATSDR 1995, 1997, 2001, 2002). A large market for several phthalates is as plasticizers for polyvinyl chloride (PVC), and certain types of elastomers. Given their use in a vast range of consumable products and because they are not covalently bound to the other chemicals in the formulations, the potential for human exposure to phthalates is high.
Phthalates can enter the human body by several routes, including ingestion through diet, absorption through skin, and inhalation of indoor and outdoor air. For several phthalates, the principal route of exposure is assumed to be the ingestion of contaminated food products (CERHR 2000, 2005). Phthalates can be absorbed from the intestinal tract, the intraperitoneal cavity, and the lung. Dermal absorption is lower with the high molecular weight phthalates (e .g., di(2-ethylhexyl) phthalate (DEHP)) than with the low molecular weight phthalates, such as diethyl phthalate (DEP) and di(n-butyl) phthalate (DBP) (ATSDR 1995, 2001, 2002). Metabolism of most phthalates in humans occurs by an initial phase I biotransformation in which the diesters primarily metabolize into their hydrolytic monoesters. The monoesters of high molecular weight phthalates (e .g., DEHP) may be further metabolized to produce more hydrophilic oxidative products (Albro and Moore 1974; Albro et al. 1973; ATSDR 1997; Barr et al. 2003). Monoesters and the oxidative metabolites of phthalates can be excreted in urine and feces in their free form or as glucuronide conjugates with increased water solubility and increased urinary excretion (ATSDR 1995, 2002).
Phthalates have been reported as developmental and reproductive toxicants in experimental animals. Developmental anomalies were seen in rodents dosed during gestation and/or lactation with DBP (Mylchreest et al. 1999, 2000), DEHP (Gray et al. 2000; Koch et al. 2006), butylbenzyl phthalate (BBzP) (Ema et al. 1998, 2003; Ema and Miyawaki 2002), and diisononyl phthalate (Gray et al. 2000). The commonly observed anomalies at experimentally high doses included reductions in androgen-dependent tissue weights (e .g., seminal vesicles, epididymis, and prostate), increased incidence of hypospadias, cryptorchidism, decreased anogenital distance (AGD), delayed preputial separation (pubertal milestone), retention of thoracic nipples, and testicular lesions (e .g., seminiferous tubule atrophy and Leydig cell hyperplasia) (Agarwal et al. 1985; Ema et al. 2003; Foster et al. 2002; Gray et al. 2000; Mylchreest et al. 1998, 2000). Phthalates and their metabolites act functionally as antiandrogens during the prenatal period by interference of normal androgenic signaling, rather than interaction with the androgen receptors (Hotchkiss et al. 2004; Mylchreest et al. 1998; Parks et al. 2000).
There is currently limited or inadequate human data on the relationships between exposure to phthalates and human health effects. In a recent multicenter epidemiologic study, prenatal exposure to monoethyl phthalate (MEP), monobutyl phthalate (MBP), monobenzyl phthalate (MBzP), and monoisobutyl phthalate (MiBP) was associated with shortened AGD in human male infants (Swan et al. 2005). There was some evidence of associations between environmental exposure to MBP and MBzP and altered semen quality (Duty et al. 2003a,b, 2004), MEP and sperm DNA damage (Duty et al. 2003a,b), and MBP and MBzP and reproductive hormones (Duty et al. 2005a,b). An Italian study revealed that newborns who had positive MEHP in the cord blood (n = 65) had a younger gestational age than MEHP negative newborns (n = 19) (p = 0.033) (Latini et al. 2003).
Just because people have an environmental chemical in their blood or urine does not mean the chemical causes disease. The toxicity of a chemical is related to its dose, time of exposure, dose frequency and duration, and an individual’s susceptibility. The common limitations of the published studies include small human populations, low epidemiologic sensitivity and selectivity, potential preanalytic and analytic contamination, and lack of adequate exposure assessment measurements (either environmental or biological measures). Our study was undertaken to characterize the distribution of pesticides and other EDs in various matrices collected from pregnant women, to identify the predictive biomarkers of exposure, and to assess the exposure and risk levels in this vulnerable population.
METHODS
Our study was a prospective observational study of EDs in the maternal and fetal compartments. We recruited 150 mothers (Table 1) and their children born at a major central New Jersey teaching hospital, St. Peter’s University Hospital in New Brunswick, which averages from 12 to 30 births per day. It is centrally located and serves both urban and suburban populations in a large portion of the state. All subjects provided informed consent prior to participation in the study, which was approved by the Institutional Review Board (IRB) at Rutgers University and the Centers for Disease Control and Prevention (CDC). A detailed medical history, information on household product use, occupation, hobbies, diet, demographic variables, ethnicity, and medical examination were collected as part of the study.
Table 1.
Characteristics of study subjects (n = 150)
| Characteristic | Value | |
|---|---|---|
| Age | [Median (25%, 75%)] | 33 [30, 35] |
| Smoking | Never [n(%)] | 144 (96%) |
| Sometimes [n(%)] | 6 (4%) | |
| Use of plastic products | Microwave food in plastic container | 141 (94%) |
| Use plastic tableware | 111 (74%) | |
| Save items in plastic container | 145 (97%) | |
| Gestational age | Mean (SD) (wks) | 39 (±0.79) |
| Birth outcome | Birth weight (g) | 3575 (±689) |
| Apgar score (5 min) | 9 (±0.092) |
Eligible healthy subjects included women with singleton pregnancies scheduled for an elective cesarean birth at term (≥37 weeks) who had blood hemoglobin concentration ≥8 mg/dl. Women were excluded if they took medications that could interfere with the metabolism and/or measurement of environmental chemicals, if they had pregnancy-related complications, or if there was evidence of labor or rupture of membranes at the time of operative delivery. Standard sample collection protocols were followed to collect all biological samples. All women were put on intravenous (IV) injection for glucose, water, and electrolytes balance support after arrival to the hospital. Maternal blood samples (10 ml) were obtained on the day of surgery. The maternal urine specimens were collected before delivery, but after the Foley tube and the IV injection was placed. After the fetus was delivered, the umbilical cord was clamped, and 30–60 ml of cord blood was aspirated directly from the umbilical vein. All umbilical vein samples were obtained within 15 minutes of delivery. Standard procedures were followed to isolate serum from the whole blood samples in the hospital. Finally, maternal pregnancy characteristics and neonatal outcome data were also recorded.
Prior to initiation of the study, representative samples of all equipment used for either collection or storage of samples were examined by the CDC laboratory to verify the absence of the phthalate metabolites measured for this study. Ten percent of all samples collected were quality assurance samples. No preservatives were added prior to sampling of the urine, but phosphoric acid was added to maternal and cord serum samples to quench the esterase activity (Kato et al. 2004). Then all samples were stored at −70°C until transferred to CDC for analysis. Specimens were sent overnight express packed in dry ice to avoid the potential freeze thaw effects. They were maintained at −70°C until analyzed.
Phthalate metabolites were measured in maternal urine, maternal serum, and cord serum at CDC. The analytical methods involved the enzymatic deconjugation of the phthalate metabolites from their glucuronidated form, automated solid-phase extraction, separation with high performance liquid chromatography, and detection by isotope-dilution tandem mass spectrometry (Kato et al. 2004; Silva et al. 2004). The limits of detection (LODs) were in the low ng/ml range; 1 ml of sample was used for the analysis. Each analytical run also included analytical standards, quality control [QC] materials of high concentration, QC materials of low concentration, and reagent blanks. The QC samples were analyzed along with the study samples to monitor for accuracy and precision. QC results were evaluated according to modified Westgard statistical probability rules.
The statistical analysis consisted of summary statistics of each metabolite, such as means, medians, percentiles, ranges, and comparisons of the concentrations of the metabolites from each biological matrix (i.e., maternal urine, maternal serum, and cord serum). In addition, the relation of urinary metabolite data to household products use was evaluated. In all statistical analyses, concentration values below the LOD were imputed a concentration equal to LOD/ for the calculations (Hornung and Reed 1990). A p < .05 was considered to indicate statistically significant findings, and all tests were two-tailed.
The daily exposure of the phthalate parent compounds was estimated, assuming steady-state intake of the phthalate diester and metabolic clearance of the metabolite, from the concentrations of the urinary concentrations of the phthalate metabolites using the method proposed by David (2000) as expressed by Koch et al. (2003a): DI = [(ME × CE)/(FUE×1,000)] ×MWd/MWm) where DI is the daily intake in micrograms per kilogram per day; ME is the creatinine corrected urinary metabolite concentration in micrograms per gram; CE is the creatinine clearance rate, normalized for body weight, in milligrams per kilogram per day; FUE is the molar conversion factor that relates urinary excretion of metabolite to diester ingested; and MWd and MWm are the molecular weights of diester and metabolite, respectively. For these calculations, we set CE at 20 mg/kg/day for adults (Jacobs et al. 2001; Tietz 1990), and used the following FUE values: 0.69 for MEP/DEP and MBP/DBP; 0.059 for MEHP/DEHP, 0.23 for MEHHP/DEHP, 0.15 for MEOHP/DEHP, and 0.73 for MBzP/BBzP (Koch et al. 2004a; Anderson et al. 2001).
RESULTS AND DISCUSSION
In Table 1 are presented the characteristics of the 150 women from the central New Jersey area, the number of women who reported using plastic household products in their homes during pregnancy, and the clinical birth outcomes (e .g., birth weight, Apgar score, and gestational age) of the 150 newborns. The median age of participants was 33 years. Only 4% of the women smoked sometimes during pregnancy, and none of them reported drinking during pregnancy. The average gestation of the women was 39 weeks (±0.79); the average birth weight was 3575 grams (±689 SD); and the average Apgar score at 5 minutes was 9 (±0.09).
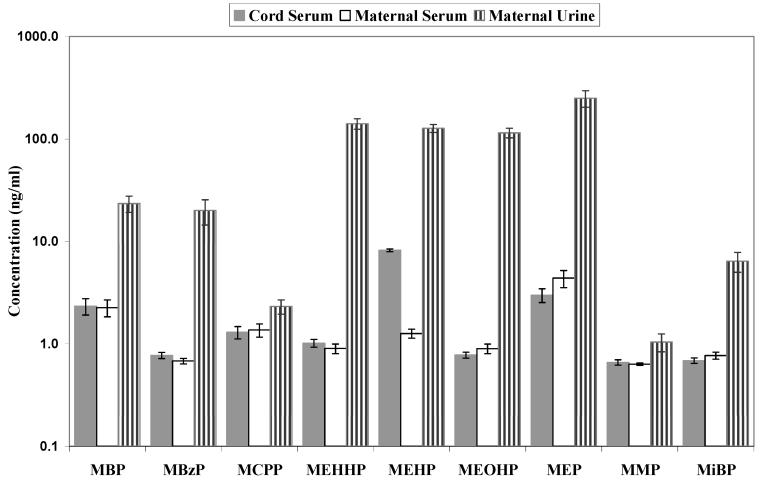
Nine phthalate metabolites were detected in the three biological matrices analyzed. Except for MMP, which was not detectable in most of the subjects (LOD 1 ng/ml), the other phthalate metabolites were found in maternal urine at significantly greater concentrations than in cord serum (CS) and maternal serum (MS) (p < .05, Table 2, Figure 1). The significantly greater concentration of phthalate metabolites in urine supports the use of urinary metabolites as exposure biomarkers rather than using those in other matrices (e.g., MS, CS), because the former often contribute to exposure increments that differ by several orders of magnitude. Further, the concentrations of hydrolytic monoesters in MS and CS, even though they can be determined accurately, may include an unknown contribution from hydrolysis of contaminant (exogenous) phthalates by endogenous serum esterases even when phosphoric acid is used to quench the enzymatic activity, and their use as biomarkers of exposure should generally be avoided (Calafat and Needham 2008). In addition, for the high molecular weight phthalates, such as DEHP, the secondary oxidative metabolites (e.g., MEHHP, MEOHP), which cannot be formed as a result of contamination during sampling or analysis (Koch et al. 2003b), offer more value as biomarkers of exposure than the hydrolytic metabolite MEHP due to their longer T1/2(10 h), and recent discovery that these oxidative metabolites might be the ultimate developmental DEHP toxicants (Koch et al. 2003b, 2004b).
Table 2.
Phthalate metabolite concentrations (ng/ml) in biological matrices
| Analytes | Matrices | Arithmetic Mean |
Standard Error |
Median | 95th Percentile |
Range | LOD (ng/ml) |
Sample Size |
DF (%) |
|---|---|---|---|---|---|---|---|---|---|
| MBP | CS | 2.3 | 0.4 | <LOD | 10.9 | <LOD-49.4 | 1.07 | 150 | 28.0 |
| MS | 2.3 | 0.4 | <LOD | 12.2 | <LOD-45.7 | 1.07 | 148 | 22.3 | |
| MU | 23.3 | 4.2 | 14.8 | 68.9 | 3.4–107.9 | 1.07 | 34 | 100.0 | |
| MBzP | CS | <LOD | 0.1 | <LOD | 2.1 | <LOD-5.2 | 1.00 | 150 | 8.0 |
| MS | <LOD | 0.0 | <LOD | 1.3 | <LOD-3.7 | 1.00 | 148 | 5.4 | |
| MU | 20.0 | 5.5 | 9.2 | 52.8 | 1.0–181.3 | 1.00 | 34 | 97.1 | |
| MCPP | CS | 1.3 | 0.2 | <LOD | 4.6 | <LOD-16.9 | 1.00 | 150 | 16.0 |
| MS | 1.4 | 0.2 | <LOD | 4.3 | <LOD-19.9 | 1.00 | 148 | 16.2 | |
| MU | 2.3 | 0.4 | 1.6 | 7.1 | <LOD-9.3 | 1.00 | 34 | 70.6 | |
| MEHHP | CS | 1.0 | 0.1 | <LOD | 2.8 | <LOD-10.6 | 0.95 | 150 | 26.0 |
| MS | <LOD | 0.1 | <LOD | 2.9 | <LOD-10.9 | 0.95 | 148 | 14.2 | |
| MU | 140.7 | 16.5 | 108.9 | 308.9 | 21.0–444.6 | 0.95 | 34 | 100.0 | |
| MEHP | CS | 8.2 | 0.3 | 7.7 | 14.1 | 1.0–21.8 | 0.98 | 150 | 99.3 |
| MS | 1.3 | 0.1 | <LOD | 3.9 | <LOD-10.3 | 0.98 | 148 | 26.4 | |
| MU | 126.9 | 11.1 | 114.7 | 246.2 | 38.4–274.6 | 0.98 | 34 | 100.0 | |
| MEOHP | CS | <LOD | 0.1 | <LOD | 2.3 | <LOD-3.7 | 1.07 | 150 | 13.3 |
| MS | <LOD | 0.1 | <LOD | 2.7 | <LOD-10.1 | 1.07 | 148 | 12.8 | |
| MU | 114.8 | 12.3 | 95.1 | 233.0 | 17.7–321.6 | 1.07 | 34 | 100.0 | |
| MEP | CS | 3.0 | 0.5 | <LOD | 12.9 | <LOD-45.3 | 1.00 | 150 | 40.7 |
| MS | 4.4 | 0.8 | <LOD | 20.4 | <LOD-89.3 | 1.00 | 148 | 42.6 | |
| MU | 249.8 | 45.7 | 168.5 | 749.1 | 7.7–830.2 | 1.00 | 29 | 100.0 | |
| MMP | CS | <LOD | 0.0 | <LOD | <LOD | <LOD-5.3 | 1.00 | 150 | 2.0 |
| MS | <LOD | 0.0 | <LOD | <LOD | <LOD-1.6 | 1.00 | 148 | 0.7 | |
| MU | 1.0 | 0.2 | <LOD | 2.5 | <LOD-7.3 | 1.00 | 34 | 11.8 | |
| MiBP | CS | <LOD | 0.0 | <LOD | <LOD | <LOD-5.6 | 1.04 | 150 | 3.3 |
| MS | <LOD | 0.1 | <LOD | 1.3 | <LOD-7.6 | 1.04 | 148 | 4.7 | |
| MU | 6.4 | 1.4 | 4.6 | 13.5 | <LOD-47.5 | 1.04 | 34 | 94.1 |
Abbreviations: CS: cord serun; MS: maternal serum; MU: maternal urine.
DF: detection frequency; LOD: limit of detection.
Figure 1.
Mean concentrations* of phthalate metabolites in cord serum, maternal serum, and maternal urine. Except for MMP, the phthalate metabolite concentrations in maternal urine are significantly greater than in maternal and cord serum concentrations (p < .05). *LOD/SQRT(2) was used for the mean calculations in cord and maternal serum when they were < LOD.
Prior to the study, a questionnaire was distributed to collect information on commonly used household products to investigate if some products might contribute to phthalate exposure. Particularly, the usage information on plastic products that may contain phthalate plasticizers were gathered by questions such as “Did the mother microwave food in PVC plastic containers while pregnant (Never/Sometimes/Often)”? “Did the mother use plastic tableware while pregnant (Never/Sometimes/Often)”? “Did the mother save items in plastic containers while pregnant (Never/Sometimes/Often)”? Because all mothers received an IV injection in the hospital and DEHP is the most commonly used plasticizer in medical bags and tubes, it is most likely that the dose of DEHP the mothers received through the IV was much greater than the dose they could have received from use of plastic household products. For this reason, the association between use of products and DEHP exposure in this particular population was not examined. Instead, the association between use of the plastic household products and exposure to other phthalates using urinary biomarkers was tested. The data presented in Table 3 suggested that the urinary concentrations of some phthalate metabolites, such as MBP, MBzP, and mono-3-carboxypropyl phthalate (MCPP), were significantly different between plastic product users and non-users, suggesting that the use of household products that contain phthalates may contribute to the body burden. As phthalates are not chemically bound to the plastic polymer in these products, under high temperature or as the plastic wears out, phthalates may leach out of the polymer and migrate into the foodstuff, and so add to the total exposure for small molecular phthalates (e .g., DBP, BBzP).
Table 3.
Association of self-reported use of plastic products and phthalate levels (ng/ml) in maternal urine
| Parameters | Metabolites | Non User a | Usera | P valueb |
|---|---|---|---|---|
| “Microwave food using plastic container” | MBP | 11.2 /4.0/5 | 25.4/25.8/29 | 0.0091 |
| MBzP | 4.9/3.2/5 | 22.6/34.3/29 | 0.0112 | |
| MCPP | 1.1/0.7/5 | 2.5/2.3/29 | 0.0124 | |
| “Using plastic tableware” | MBzP | 8.0/5.1/6 | 22.5/35.0/28 | 0.0452 |
| “Save items using plastic container” | MBP | 10.6/2.9/2 | 24.1/24.9/32 | 0.0118 |
| MBzP | 4.2/1.8/2 | 20.9/33/32 | 0.0083 | |
| MCPP | 0.5/0.3/2 | 2.4/2.2/32 | 0.0004 |
All values expressed as: mean concentration/standard deviation/subject number.
Significant differences in urinary concentrations between plastic product users and non-users (p < 0.05).
In this group of pregnant women, the urinary monoester concentrations (except for the DEHP metabolites) were less or similar to the concentrations of the U.S. general population based on the National Health and Nutrition Examination Survey (NHANES) 2001–2002 data (CDC 2005), but DEHP metabolite concentrations were much greater (Table 4). The major source of DEHP exposure for these women was likely related to their medical interventions in the hospital. DEHP/MEHP could leach out from the IV bags and tubes directly into the women’s system circulation, and contribute significantly to their DEHP exposure and body burden. Previous studies showed much lower concentrations in urine of MEHP than of the oxidative metabolites MEHHP and MEOHP (Albro et al. 1973; Barr et al. 2003; Kato et al. 2004; Kohn et al. 2000; Silva et al. 2006a,b). However, in this study, we observed similar urinary concentrations for all three DEHP metabolites measured (Tables 2, 4). This may confirm the recent exposure to DEHP from IV injection during the mothers’ stay in the hospital, as the hydrolytic monoester MEHP was not completely metabolized yet at the time point of urine sample collection.
Table 4.
Comparison of the urinary levels (ng/ml) of phthalate metabolites in the NJ study population to US general and female population (NHANES 2001–2002 data)
| Metabolites | Subjects | GM | 50th | 75th | 90th | 95th |
|---|---|---|---|---|---|---|
| MMP | NJ women | * | <LODa | <LODa | 1.47 | 2.48 |
| US total | 1.15 | 1.50 | 3.30 | 6.00 | 9.80 | |
| US female | 1.13 | 1.30 | 3.30 | 6.40 | 10.3 | |
| MEP | NJ women | 135 | 169 | 400 | 721 | 749 |
| US total | 178 | 169 | 465 | 1230 | 2500 | |
| US female | 174 | 167 | 427 | 1050 | 1840 | |
| MBP | NJ women | 15.7 | 14.8 | 29.6 | 48.1 | 68.9 |
| US total | 18.9 | 20.4 | 40.4 | 73.6 | 108 | |
| US female | 20.2 | 21.6 | 46.7 | 85.0 | 120 | |
| MiBP | NJ women | 4.05 | 4.60 | 7.48 | 12.6 | 13.5 |
| US total | 2.71 | 2.60 | 5.70 | 11.9 | 17.9 | |
| US female | 2.68 | 2.50 | 5.70 | 12.6 | 18.7 | |
| MBzP | NJ women | 10.0 | 9.15 | 21.4 | 44.3 | 52.8 |
| US total | 15.1 | 15.7 | 38.0 | 80.8 | 122 | |
| US female | 14.6 | 15.4 | 38.1 | 81.4 | 122 | |
| MCPP | NJ women | 1.63 | 1.55 | 3.20 | 4.17 | 7.09 |
| US total | 2.75 | 3.00 | 5.70 | 10.0 | 14.6 | |
| US female | 2.62 | 3.00 | 5.60 | 10.0 | 14.7 | |
| MEHP | NJ women | 109 | 115 | 155 | 227 | 246 |
| US total | 4.27 | 4.10 | 9.80 | 22.8 | 38.9 | |
| US female | 4.23 | 4.10 | 9.70 | 23.0 | 42.5 | |
| MEHHP | NJ women | 112 | 109 | 203 | 245 | 309 |
| US total | 20.0 | 20.1 | 43.6 | 91.3 | 192 | |
| US female | 18.3 | 18.2 | 39.8 | 86.0 | 170 | |
| MEOHP | NJ women | 93.4 | 95.1 | 166 | 213 | 233 |
| US total | 13.5 | 14.0 | 29.6 | 59.9 | 120 | |
| US female | 12.5 | 13.0 | 28.1 | 57.5 | 115 |
Geometric mean not calculated because detection of frequency < 60%.
The urinary concentrations of phthalate monoesters were converted to intake levels using the mathematical model described by David (2000) and were related to doses (below which we do not expect to see effects) developed from animal toxicology studies such as the USEPA oral reference doses (RfDs), European Union tolerable daily intakes (TDIs), Agency for Toxic Substances and Disease Registry (ATSDR) minimal risk levels for chronic effects (MRLs), and National Toxicology Program/Center for the Evaluation of Risks to Human Reproduction (NTP/CERHR) no observable adverse effect levels (NOAELs) (CERHR 2000, 2005). The estimated daily exposures to BBzP, DBP, DEP (Table 5) were well below the RfDs, TDIs, or MRLs. Exposures for the general population, estimated by the NTP/CERHR Expert Panels are in good agreement with human daily intake estimates for most of the phthalates from this study.
Table 5.
Estimated daily intake (μg/kg/day) of phthalate for the NJ pregnant women based on the David (2000) method
| Metabolites | Diesters (Parent) | 25th | Median | 75th | 95th | Max |
|---|---|---|---|---|---|---|
| MMP | DMP | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| MEP | DEP | 1.98 | 4.72 | 16.70 | 50.08 | 152.83 |
| MBP + MiBP | DBP + DiBP | 0.37 | 0.49 | 1.08 | 2.38 | 2.95 |
| MBzP | BBzP | 0.11 | 0.23 | 0.55 | 1.35 | 4.62 |
| MEHP | DEHP | 30.94 | 49.87 | 67.38 | 107.09 | 119.45 |
| MEHHP | DEHP | 6.39 | 10.57 | 19.69 | 30.00 | 43.18 |
| MEOHP | DEHP | 8.65 | 14.26 | 24.92 | 34.93 | 48.22 |
EPA RfDs (μg/kg/day): DEP = 800, DBP = 100, BBzP = 200.
EU TDIs (μg/kg/day): DEP = 8,000; DBP = 10; BBzP = 370; DEHP = 40.
ATSDR reproductive MRL (chronic) DEHP = 60 μg/kg/day.
ATSDR reproductive MRL (intermediate) DEHP = 100 μg/kg/day.
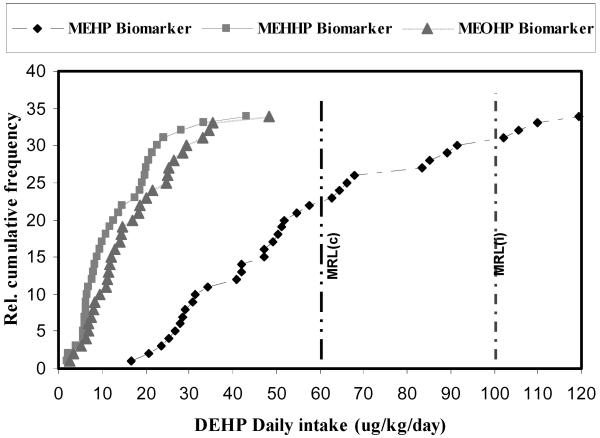
Using MEHHP and MEOHP as biomarkers, the median DEHP daily intakes were 10.57 and 14.26 μg/kg/day, and the 95th-percentile values 30.00 and 34.93 μg/kg/day, respectively (Table 5). As discussed earlier, the greater concentrations of MEHP in maternal urine in this study compared to the urinary concentrations reported in other studies could result from the early transient phase of MEHP metabolism at the time point of urine sample collection as a result of the recent exposure to DEHP from IV injection during the mothers’ stay in the hospital. Thus, using urinary MEHP as the biomarker might not be appropriate to calculate the chronic daily exposure to DEHP, and the dose estimate could easily be overly exaggerated (Figure 2).
Figure 2.
Relative cumulative frequency of DEHP daily intakes. MRL(c): ATSDR reproductive minimal risk level for chronic duration 60 μg/kg/day. MRL(i): ATSDR reproductive minimal risk level for intermediate duration 100 μg/kg/day.
The USEPA derived a chronic RfD of 20 μg/kg/day for DEHP based on a low observable adverse effect level of 19 mg/kg/day for hepatic effects in guinea pigs (Carpenter et al. 1953). However, this RfD is outdated, and a reevaluation of the DEHP toxicity assessment is underway at the USEPA. In addition, the hepatic effect is not a known critical endpoint of DEHP toxicity from long duration studies. Considering the male reproductive effects, a known critical endpoint of DEHP based on long duration studies, ATSDR has established a MRL of 60 μg/kg/day for chronic-duration oral exposure to DEHP based on a NOAEL of 5.8 mg/kg/day for testicular pathology in male rats, and a MRL of 100 μg/kg/day intermediate-duration oral exposure based on a NOAEL of 14 mg/kg/day (uncertainty factor 100) for decreased fertility in mice (ATSDR 2002). In our study, none of the pregnant woman had greater DEHP daily dose than the ATSDR exposure guidance limits (Figure 2), and only one woman had a DEHP intake estimate (43.18 or 48.22 μg/kg/day) that was above the TDI of 40 μg/kg/day (Table 5, Figure 2). These data suggest that although these pregnant women had much greater urinary concentrations of MEHP, MEHHP, and MEOHP than the U.S. general and female population, and accordingly a fraction of these pregnant women had their daily intake estimates of DEHP greater than those reported in previous studies, their dose estimates were well below the regulatory exposure limits (e .g., MRLs). In addition, no abnormal birth outcomes (e .g., birth weight, Apgar score, and gestational age) or other clinical reproductive endpoints were noted in those newborns whose mothers had relatively greater exposure estimate to DEHP during the perinatal period than others in this study. Our data suggest that enough margin of safety may have been built in the various regulatory exposure limits for DEHP. Because we have asserted that this high exposure to DEHP could be explained by exposure through the medical devices in the hospital, which was a transient acute exposure, we speculate that we would not see adverse health effects in this pregnant population and their newborns during follow up.
Because of the modest sample size (n = 150) from the local central New Jersey region, it is not possible to generalize our findings to other populations. Future studies should involve a larger cohort that includes women from diverse geographic areas. More importantly, a major limitation of this study was the potential contamination of the biological samples collected in the hospital from these pregnant women with phthalate plasticizers, most notably DEHP, as a result of the medical interventions required for cesarean deliveries. This fact highlights the critical importance of selecting collection protocols and timing for sampling that would avoid such contamination in future studies.
CONCLUSION
In this New Jersey pregnant population, nine phthalate metabolites were detected in their maternal serum, cord serum, and maternal urine samples, indicating widespread exposure to five phthalate parent compounds. Significantly greater concentrations in maternal urine than in serum suggest that for the non-persistent phthalates, urinary concentrations of phthalate metabolites are better biomarkers for exposure assessment than their respective concentrations in serum. In our study, the urinary concentrations of phthalate metabolites were similar or less than the concentrations in the U.S. general and female population based on NHANES 2001–2002 data, except for the three metabolites of DEHP: MEHP, MEHHP, and MEOHP. The transient exposure to plastic medical devices in the hospital by these women before delivery could explain the high urinary concentrations of DEHP metabolites. Calculation of the daily intakes using the urinary concentrations of the oxidative metabolites (MEHHP and MEOHP) as the biomarkers indicated that the estimated daily dose of DEHP was well below regulatory guidance limits, such as the ATSDR MRLs. No abnormal birth outcomes or other clinical endpoints were noted in those newborns whose mothers had relatively greater exposure estimate to DEHP during the perinatal period than others in this study.
ACKNOWLEDGMENTS
We thank Dr. Jack Reidy, Jim Preau, and Arnetra Herbert for their assistance in the phthalate analytical measurements. We also thank Dr. Kenneth R. Reuhl in the Department of Pharmacology and Toxicology, Rutgers University/UMDNJ, Piscataway, NJ, for his assistance. This work was supported by grants from New Jersey Department of Environmental Protection (DEP SR02-038, SR04-058, SR05-042) and the NIEHS P30ES005022 and NIEHS 5T32ES07148.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention or the New Jersey Department of Environmental Protection.
REFERENCES
- Agarwal DK, Maronpot RR, Lamb JC, et al. Adverse-effects of butyl benzyl phthalate on the reproductive and hematopoietic systems of male-rats. Toxicolog. 1985;35:189–206. doi: 10.1016/0300-483x(85)90015-0. [DOI] [PubMed] [Google Scholar]
- Albro PW, Moore B. Identification of the metabolites of simple phthalate diesters in rat urine. J Chromatogr. 1974;94:209–18. doi: 10.1016/s0021-9673(01)92368-4. [DOI] [PubMed] [Google Scholar]
- Albro PW, Thomas R, Fishbein L. Metabolism of diethylhexyl phthalate by rats. Isolation and characterization of the urinary metabolites. J Chromatogr. 1973;76:321–30. doi: 10.1016/s0021-9673(01)96915-8. [DOI] [PubMed] [Google Scholar]
- Anderson WAC, Castle L, Scotter MJ, et al. A biomarker approach to measuring human dietary exposure to certain phthalate diesters. Food Addit Contam. 2001;18:1068–74. doi: 10.1080/02652030110050113. [DOI] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry) Toxicological Profile for Diethyl Phthalate (DEP) Department of Health and Human Services; Atlanta, GA, USA: 1995. [PubMed] [Google Scholar]
- ATSDR . Toxicological Profile for Di-N-Octyl Phthalate (DnOP) Department of Health and Human Services; Atlanta, GA, USA: 1997. [Google Scholar]
- ATSDR . Toxicological Profile for Di-N-Butyl Phthalate (DBP) Department of Health and Human Services; Atlanta, GA, USA: 2001. [Google Scholar]
- ATSDR . Toxicological Profile for Di(2-Ethylhexyl) Phthalate (DEHP) Department of Health and Human Services; Atlanta, GA, USA: 2002. [Google Scholar]
- Barr DB, Silva MJ, Kato K, et al. Assessing human exposure to phthalates using monoesters and their oxidized metabolites as biomarkers. Environ Health Perspect. 2003;111:1148–51. doi: 10.1289/ehp.6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Needham LL. Factors affecting the evaluation of biomonitoring data for human exposure assessment. Int J Androl. 2008;31:139–43. doi: 10.1111/j.1365-2605.2007.00826.x. [DOI] [PubMed] [Google Scholar]
- Carpenter CP, Weil CS, Smyth HF., Jr Chronic oral toxicity of di(2-ethylhexyl) phthalate for rats, guinea pigs, and dogs. Arch Indust Hyg. 1953;8:219–26. [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) Third National Report on Human Exposure to Environmental Chemicals. National Center for Environmental Health, Division of Laboratory Sciences; Atlanta, GA, USA: 2005. [Google Scholar]
- CERHR (Center for the Evaluation of Risks to Human Reproduction) NTP-CERHR Expert Panel Report on Di(2-ethylhexyl) Phthalate. Science International, Inc; Alexandria, VA, USA: 2000. [Google Scholar]
- CERHR . NTP-CERHR Expert Panel Update Report on the Reproductive and Developmental Toxicity of Di (2-Ethylhexyl) Phthalate. Science International, Inc; Alexandria, VA, USA: 2005. [Google Scholar]
- Crisp TM, Clegg ED, Cooper RL, et al. Environmental endocrine disruption: An effects assessment and analysis. Environ Health Perspect. 1998;106:11–56. doi: 10.1289/ehp.98106s111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David RM. Exposure to phthalate esters. Environ. Health Perspect. 2000;108:A440. doi: 10.1289/ehp.108-a440a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duty SM, Silva MJ, Barr DB, et al. Phthalate exposure and human semen parameters. Epidemiology. 2003a;14:269–77. [PubMed] [Google Scholar]
- Duty SM, Singh NP, Silva MJ, et al. The relationship between environmental exposures to phthalates and DNA damage in human sperm using the neutral comet assay. Environ Health Perspect. 2003b;111:1164–9. doi: 10.1289/ehp.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duty SM, Calafat AM, Silva MJ, et al. The relationship between environmental exposure to phthalates and computer-aided sperm analysis motion parameters. J Androl. 2004;25:293–302. doi: 10.1002/j.1939-4640.2004.tb02790.x. [DOI] [PubMed] [Google Scholar]
- Duty SM, Ackerman RM, Calafat AM, et al. Personal care product use predicts urinary concentrations of some phthalate monoesters. Environ Health Perspect. 2005a;113:1530–5. doi: 10.1289/ehp.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duty SM, Calafat AM, Silva MJ, et al. Phthalate exposure and reproductive hormones in adult men. Human Reprod. 2005b;20:604–10. doi: 10.1093/humrep/deh656. [DOI] [PubMed] [Google Scholar]
- Ema M, Miyawaki E. Effects on development of the reproductive system in male offspring of rats given butyl benzyl phthalate during late pregnancy. Reprod Toxicol. 2002;16:71–6. doi: 10.1016/s0890-6238(01)00200-3. [DOI] [PubMed] [Google Scholar]
- Ema M, Miyawaki E, Kawashima K. Reproductive effects of butyl benzyl phthalate in pregnant and pseudopregnant rats. Reprod Toxicol. 1998;12:127–32. doi: 10.1016/s0890-6238(97)00127-5. [DOI] [PubMed] [Google Scholar]
- Ema M, Miyawaki E, Hirose A, et al. Decreased anogenital distance and increased incidence of undescended testes in fetuses of rats given monobenzyl phthalate, a major metabolite of butyl benzyl phthalate. Reprod Toxicol. 2003;17:407–12. doi: 10.1016/s0890-6238(03)00037-6. [DOI] [PubMed] [Google Scholar]
- Foster PMD, Mylchreest E, Gaido KW, et al. Effects of phthalate esters on the developing reproductive tract of male rats. Human Reprod Update. 2002;7:231–5. doi: 10.1093/humupd/7.3.231. [DOI] [PubMed] [Google Scholar]
- Gray LE, Ostby J, Furr J, et al. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol Sci. 2000;58:350–65. doi: 10.1093/toxsci/58.2.350. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5:46–51. [Google Scholar]
- Hotchkiss AK, Parks-Saldutti LG, Ostby JS, et al. A mixture of the “antiandrogen” linuron and butyl benzyl phthalate alters sexual differentiation of male rat in a cumulative fashion. Biology of Reproduction. 2004;71:1852–61. doi: 10.1095/biolreprod.104.031674. [DOI] [PubMed] [Google Scholar]
- Jacobs DS, Oxley DK, DeMott WR, editors. Laboratory Test Handbook. Lexi-Comp, Hudson; OH, USA: 2001. [Google Scholar]
- Kato K, Silva MJ, Reidy JA, et al. Mono(2-ethyl-5-hydroxyhexyl) phthalate and mono-(2-ethyl-5-oxohexyl) phthalate as biomarkers for human exposure assessment to di-(2-ethylhexyl) phthalate. Environ Health Perspect. 2004;112:327–30. doi: 10.1289/ehp.6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch HM, Drexler H, Angerer J. An estimation of the daily intake of di(2-ethylhexyl)phthalate (DEHP) and other phthalates in the general population. Int J Hyg Environ Health. 2003a;206:1–7. doi: 10.1078/1438-4639-00205. [DOI] [PubMed] [Google Scholar]
- Koch HM, Gonzalez-Reche LM, Angerer J. On-line clean-up by multidimensional liquid chromatography-electrospray ionization tandem mass spectrometry for high throughput quantification of primary and secondary phthalate metabolites in human urine. J Chromatogr B. 2003b;784:169–82. doi: 10.1016/s1570-0232(02)00785-7. [DOI] [PubMed] [Google Scholar]
- Koch HM, Bolt HM, Angerer J. Di(2-ethylhexyl)phthalate (DEHP) metabolites in human urine and serum after a single oral dose of deuterium-labelled DEHP. Arch Toxicol. 2004a;78:123–30. doi: 10.1007/s00204-003-0522-3. [DOI] [PubMed] [Google Scholar]
- Koch HM, Drexler H, Angerer J. Internal exposure of nursery-school children and their parents and teachers to di(2-ethylhexyl)phthalate (DEHP) Int J Hyg Environ Health. 2004b;207:15–22. doi: 10.1078/1438-4639-00270. [DOI] [PubMed] [Google Scholar]
- Koch HM, Preuss R, Angerer J. Di(2-ethylhexyl) phthalate (DEHP): Human metabolism and internal exposure—An update and latest results. Internat J Andrology. 2006;29:155–65. doi: 10.1111/j.1365-2605.2005.00607.x. [DOI] [PubMed] [Google Scholar]
- Kohn MC, Parham F, Masten SA, et al. Human exposure estimates for phthalates. Environ Health Perspect. 2000;108:A440–A442. doi: 10.1289/ehp.108-a440b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latini G, De Felice C, Presta G, et al. In utero exposure to di-(2-ethylhexyl)phthalate and duration of human pregnancy. Environ Health Perspect. 2003;111:1783–5. doi: 10.1289/ehp.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylchreest E, Cattley RC, Foster PM. Male reproductive tract malformations in rats following gestational and lactational exposure to di(n-butyl) phthalate: An antiandrogenic mechanism. Toxicol Sci. 1998;43:47–60. doi: 10.1006/toxs.1998.2436. [DOI] [PubMed] [Google Scholar]
- Mylchreest E, Sar M, Cattley RC, et al. Disruption of androgen-regulated male reproductive development by di(n-butyl) phthalate during late gestation in rats is different from flutamide. Toxicol Appl Pharmacol. 1999;156:81–95. doi: 10.1006/taap.1999.8643. [DOI] [PubMed] [Google Scholar]
- Mylchreest E, Wallace DG, Cattley RC, et al. Dose-dependent alterations in androgen-regulated male reproductive development in rats exposed to di(n-butyl) phthalate during late gestation. Toxicol Sci. 2000;55:143–51. doi: 10.1093/toxsci/55.1.143. [DOI] [PubMed] [Google Scholar]
- Parks LG, Ostby JS, Lambright CR, et al. The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol Sci. 2000;58:339–49. doi: 10.1093/toxsci/58.2.339. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Slakman AR, Reidy JA, et al. Analysis of human urine for 15 phthalate metabolites using automated solid phase extraction. J Chromatogr B. 2004;805:161–7. doi: 10.1016/j.jchromb.2004.02.038. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Reidy JA, Preau JL, Jr., et al. Measurement of eight urinary metabolites of di(2-ethylhexyl) phthalate as biomarkers for human exposure assessment. Biomarkers. 2006a;11:1–13. doi: 10.1080/13547500500382868. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Samandar E, Preau JL, Jr., et al. Urinary oxidative metabolites of di(2-ethylhexyl) phthalate in humans. Toxicology. 2006b;219:22–32. doi: 10.1016/j.tox.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Swan SH, Main KM, Liu F, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005;113:1056–61. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietz NW, editor. Clinical Guide to Laboratory Tests. W.B. Saunders; Philadelphia, PA, USA: 1990. [Google Scholar]