Abstract
Hepatic stellate cells (HSCs) store retinoids and triacylglycerols in cytoplasmic lipid droplets. Two prominent features of HSC activation in liver fibrosis are loss of lipid droplets along with increase of α-smooth muscle actin (α-SMA), but the link between these responses and HSC activation remains elusive. In non-adipose cells, adipose differentiation-related protein (ADRP) coats lipid droplets and regulates their formation and lipolysis; however its function in HSCs is unknown. Here, we observed, in human liver sections or primary HSC culture, ADRP localization to lipid droplets of HSCs, and reduced staining coincident with loss of lipid droplets in liver fibrosis and in culture-activated HSCs, consistent with HSC activation. In the LX-2 human immortalized HSCs, with scant lipid droplets and features of activated HSCs, we found that the upregulation of ADRP mRNA by palmitate is potentiated by retinol, accompanied by increased ADRP protein, generation of retinyl palmitate and lipid droplet formation. ADRP induction also led to decreased expression of α-SMA mRNA and its protein, while ADRP knockdown with small interfering RNA (siRNA) normalized α-SMA expression. Furthermore, ADRP induction by retinol and palmitate resulted in decreased expression of collagen I and matrix metalloproteinase-2 mRNA, fibrogenic genes associated with activated HSCs, while increasing matrix metalloproteinase-1 mRNA; ADRP knockdown with siRNA reversed these changes. Tissue inhibitor of metalloproteinase-1 was not affected. Thus, ADRP upregulation mediated by retinol and palmitate promotes downregulation of HSC activation and is functionally linked to the expression of fibrogenic genes.
Keywords: retinol, palmitate, retinyl palmitate, lipid droplets, hepatic stellate cells, adipose differentiation-related protein, ADRP siRNAs, stellate cell activation, fibrogenic genes
Introduction
Hepatic stellate cells (HSCs) are the primary cellular site of vitamin A (retinoids) storage in the body. In normal liver, HSCs are quiescent and are filled with cytoplasmic lipid droplets containing retinyl esters (especially retinyl palmitate) and triacylglycerols, accounting for more than 70% of their lipid content (Moriwaki et al., 1998). Upon injury, HSCs are activated to myofibroblast-like cells with expanded rough endoplasmic reticulum that produce fibrotic scar tissue (Friedman, 2008; Mak et al., 1993). HSC activation, in which the cell changes from a quiescent to a more fibrogenic phenotype, is characterized by the progressive loss of lipid droplets and retinoid content, along with an increase in α-smooth muscle acitn (α-SMA) expression. Despite this striking decrease in retinoid content, little is known about intracellular events underlying this response, and whether loss of lipid droplets is functionally linked to fibrogenic gene expression that is associated with cellular activation. A previous study from our laboratory demonstrated that Kupffer cell-conditioned medium and platelet-derived growth factor provoke retinol release from primary activated rat HSCs, but the intracellular mediators responsible for this effect were not uncovered (Friedman et al., 1993). Moreover, treatment with retinol restores lipid content, suppresses collagen production and inhibits HSC proliferation (Davis and Vucic, 1988; Pinzani et al., 1992; Sato et al., 1995), suggesting a role for retinol in the process of HSC activation.
Cytoplasmic lipid droplets are coated by lipid droplet-associated proteins (Londos et al., 1999; Wolins et al., 2006), mostly members of the PAT domain family, whose founding members are perilipin, adipose differentiation related-protein (ADRP or adipophilin), and tail-interacting protein of 47 kDa (TIP47). ADRP is ubiquitous in non-adipose lipid droplet-containing cells (Brasaemle et al., 1997; Heid et al., 1998), and plays important roles in lipid droplet formation and stabilization, as well as lipolysis (Londos et al., 2005). In the liver, ADRP is upregulated in association with drug- and diet-induced hepatic steatogenesis (Steiner et al., 1996; Motomura et al., 2006). ADRP expression is a reliable and sensitive marker for lipid droplets in alcohol-induced fatty liver (Mak et al., 2008). Reduced ADRP expression either in ADRP-deficient mice (Chang et al., 2006), or in mice administered ADRP antisense oligonucleotides while on a high fat diet (Imai et al., 2007), attenuates hepatic steatosis.
Despite these findings, little is known about the expression and function of ADRP in HSCs in liver injury and fibrosis. Recently, ADRP was identified in human HSCs by immunohistochemistry (Straub et al., 2008). In preliminary studies (unpublished observation), we observed that in histologically normal liver, lipid droplet-rich HSCs stained strongly with an ADRP antibody, while in fibrotic liver, lipid droplet-poor HSCs showed reduced ADRP staining. These observations led us to question whether ADRP expression is related to lipid droplet formation, and whether this molecule is functionally linked to HSC activation in liver fibrogenesis. Therefore, our aims were to determine: 1) ADRP expression by HSCs in vivo and in culture; 2) ADRP behavior following lipid droplet formation induced by retinol and palmitic acid in cultured HSCs; and 3) the impact of ADRP on HSC activation and fibrogenic gene expression.
Material and Methods
Immunohistochemical staining for ADRP in human liver
De-identified formalin-fixed, paraffin-embedded liver sections (histologically normal or fibrotic liver) were obtained from the Pathology Department of Mount Sinai School of Medicine in accordance with IRB guidelines. Deparaffinized sections were immersed in antigen retrieval citra buffer, pH 6.0 (BioGenex, San Ramon, CA), and repeatedly microwaved 3 times, 5 min each. Endogenous peroxidases were inactivated with hydrogen peroxide. The slides were then incubated with mouse monoclonal ADRP antibody (Fitzgerald Industries Intl, Concord, MA) at a dilution of 1:500 overnight at 4°C. The immunoreaction was detected using peroxidase-labeled biotin-streptavidin and visualized by diaminobenzidine tetrahydrochloride using the Super SensitiveTM Link-Label ICH Detection System (BioGenex). Nuclei were counterstained with hematoxylin.
Cell cultures
The human LX-2 stellate cell line, which retains key features of activated HSCs (Xu et al., 2005), was cultured in Dulbecco’s modified essential medium containing 1% fetal bovine serum and 1% penicillin and streptomycin. Human primary HSCs were isolated as previously described (Friedman et al., 1992), and grown in primary culture or passaged using trypsin.
Retinol and palmitic acid treatment of HSC cultures
To evaluate the effects of retinol and palmitate on lipid droplet formation and ADRP expression, LX-2 cells and human HSC culture (passage 3) were incubated with either 5 μM retinol (Acros Organics, Leicestershire, UK), 100 μM palmitic acid (MP Biomedicals, Irvine, CA), or their combination. Retinol and palmitic acid were dissolved in ethanol, added to the media and then vigorously mixed (Xu et al., 2005). Analyses were performed 16, 24 and 48 hr after the start of incubation. In some experiments with the LX-2 line, cells were treated with the combination of retinol and palmitate for 24 hr, followed by replacement with fresh retinol and palmitate, and then incubated for an additional 24 hr.
Detection of lipid droplets and ADRP in HSC by light and fluorescence microscopy
LX-2 cells and primary or passaged HSCs on glass coverslips were fixed with 4% paraformaldehyde in PBS for 15 min. Cells were stained with Oil red O (0.5% in propylene glycol) revealing lipid droplets. Nuclei were counterstained with hematoxylin for light microscopic examination. Dual fluorescence was performed to demonstrate co-localization of lipid droplets and ADRP in HSC. Oil red O-stained cells were treated with 5% normal goat serum for 30 min and then incubated with mouse monoclonal ADRP antibody (Fitzgerald Industries Intl) at 1:50 dilution in PBS (containing 1% BSA) overnight at 4°C. This was followed by incubation with goat anti-mouse AlexaFluor 488 (Molecular Probes, Eugene, OR) at a dilution of 1:1000 for 30 min at room temperature. The coverslips were mounted using anti-fade DAPI mounting media (Molecular Probes). Cells were viewed with a Nikon Eclipse E600 fluorescence microscope (Nikon Instruments, Melville, NY) and photographed using a RT slider camera (Diagnostics, Sterling, MI) coupled to a SPOT Software v. 3.1 (Diagnostics).
RNA extraction and real-time polymerase chain reaction (PCR)
The abundance of mRNA for ADRP and fibrogenic genes was determined by real-time PCR. We tested collagen I, matrix metalloproteinase (MMP)-1, MMP-2, tissue inhibitor of metalloproteinase (TIMP)-1 and α-smooth muscle actin (α-SMA), which all are well known for their respective roles in liver fibrogenesis (vide infra). Cellular RNA was extracted using Qiagen mini-columns (Qiagen, Germantown, MD) with an on-column DNAase treatment. One μg RNA was reverse transcribed using RT complete-double pre-primed kit (Clontech, Mountain View, CA). FastStart SYBER Green Master (Roche, Indianapolis, IN) was used for PCR. Samples were analyzed in triplicate in an ABI PRISM 7900HT Sequence Detection System (Applied Biosystems, Austin, TX) and normalized to GAPDH expression. The sequences of the primers used are listed in Table 1.
Table 1.
Primers used for real-time polymerase chain reaction
| Forward primer | Reverse primer | |
|---|---|---|
| GAPDH | 5′-CAATGACCCCTTCATTGACC | 5′-GATCTCGCTCCTGGAAGATG |
| ADRP | 5′-AGCAGGCTCTCAGCAGGG | 5′-GTACACCTTGGATGTTGG |
| α-SMA | 5′-AGGCACCCCTGAACCCCAA | 5′-CAGCACCGCCTGGATAGCC |
| α2(I) collagen | 5′-GTCCCTGAAGTCAGCTGCATA | 5′-TGGGACAGTCCAGTTCTTCAT |
| MMP-1 | 5′-GATCATCGGGACAACTCTCCT | 5′-TCCGGGTAGAAGGGATTTGTG |
| MMP-2 | 5′-CCCCAAGCTCATCGCAGAT | 5′-GGTCCACGACGGCATCC |
| TIMP-1 | 5′-TGACATCCGGTTCGTCTACA | 5′-TGATGTGCAAGAGTCCATCC |
| TIP47 | 5′-GGACTCGACCTGCTCTGG | 5′-AGGAACAGAGCTACTTCG |
| Pan-perilipin | 5′-GCAGCATTGAGAAGGTGG | 5′-CCATCAGCGACAGCCTGG |
ADRP small interfering RNA (siRNA) transfection
ADRP knockdown was performed by transfecting ADRP siRNA into LX-2 cells. Ninety percent confluent LX-2 cells were seeded onto 6-well plates. We used TransIT-LTI (Mirus, Madison, WI) as transfection reagent. GAPDH siRNA (Ambion Silencer Select, Ambion, Austin, TX) was used at a concentration of 5 nM as a positive control for transfection efficiency, according to the manufacturer’s instruction, and 60% to 90% GAPDH knockdown was detected by real-time PCR after 6 to 48 hr of transfection. For ADRP knockdown, LX-2 cells were transfected with two ADRP siRNAs or negative control siRNA. These were obtained from Ambion: Silencer® Pre-designed siRNA-1 (ID# s1057) and siRNA-2 (ID# s1055). The concentration of siRNA was 5 nM. After 8 hr, retinol (5 μM) + palmitate (100 μM) were added to the culture medium and another dose of the combination was added 24 hr later. Cells were collected 48 hr after the transfection. A negative siRNA (Silencer Negative Control #1; Ambion) that does not target any endogenous transcript was also included to control for nonspecific effects on gene expression caused by siRNA transfection.
Western blot
Protein levels of ADRP and α-SMA in LX-2 cells were determined by Western blotting. Fifty μg of whole cell lysate was subjected to SDS-PAGE. Primary antibodies and their dilutions were mouse monoclonal ADRP (1:100; Fitzgerald Industries Intl) and mouse monoclonal α-SMA (1:2000 dilution; Sigma-Aldrich). The reactions were detected with HRP-conjugated secondary antibodies. Blots were developed using ECL detection system (Amersham Pharmacia Biotech, Buckinghamshire, UK). GAPDH or α-tubulin was used as equal protein loading control.
Quantification of retinyl palmitate in LX-2 cells by high pressure liquid chromatography (HPLC)
LX-2 cells were incubated with two different concentrations of retinol and palmitic acid (5 μM retinol + 100 μM palmitate, and 10 μM retinol + 300 μM palmitate) based on our previous study with LX-2 cells (Xu et al., 2005). Cells were treated with the combination of retinol and palmitate for 24 hr, followed by replacement with fresh retinol and palmitate, and then incubated for an additional 24 hr. Control cells were incubated with ethanol vehicle.
Following incubation, cells from triplicate cultures were scraped into 2 ml ice cold PBS and homogenized (Blaner et al., 1994). The retinoids in the homogenate were extracted into hexane. The hexane was evaporated under N2. The residue was resuspended in benzene and analyzed on a 250 × 4.6-mm Intersil ODS-3 (5 μm) column (GL Sciences, Inc., Tokyo, Japan), using acetonitrile:methanol:dichloromethane (70:15:15 v/v) as the mobile phase at a flow rate of 1.8 ml/min. Retinyl palmitate was identified on the basis of the retention time, and the spectrum of the peak was monitored by UV absorbance at 325 nm with a Hitachi diode-array detector (Hitachi Corp., Tokyo, Japan). The level of retinyl palmitate was quantified from the integrated area under its peak using a standard curve constructed with known amounts of the authentic standard. In addition, cellular RNA was extracted from LX-2 incubated with 2 different concentrations of retinol and palmitate for PCR analysis to monitor the concurrent induction of ADRP mRNA by retinol and palmitate.
Cell viability/toxicity assay
LX-2 cells were assayed for their viability/toxicity after treatment with palmitic acid alone or in combination with retinol by 3-(4, 5-dimethylthiazole-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay according as described (Hansen et al., 1989)
Statistics
Data are reported as means ± SEM. Statistical analyses include Student’s t-test and one-way analysis of variance (ANOVA) followed by Newman-Keuls post hoc tests. A value of p < 0.05 was considered to be significant.
Results
ADRP immunostaining of HSCs in human liver and in culture
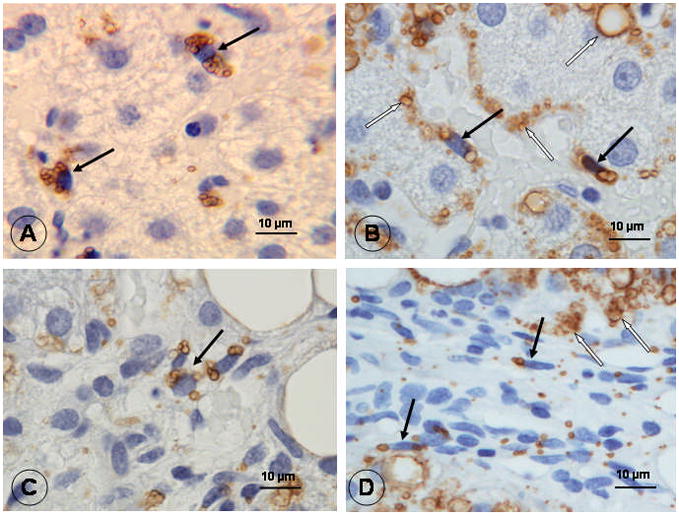
In histologically normal liver, HSCs in the lobular area expressed ADRP. ADRP staining was localized to the surface of lipid droplets which appear as unstained microvesicles due to lipid extraction during paraffin embedding (Fig. 1A). In fibrotic liver, lipid droplet-poor HSCs in the perisinusoidal space, fibrotic scars and fibrotic septa were clearly labeled with ADRP (Fig. 1B, C, and D). No ADRP staining was observed in sinusoidal endothelial cells, Kupffer cells or any cells in the portal tracts of normal and fibrotic livers. Lipid droplets of steatotic hepatocytes were positive for ADRP, as reported previously (Mak et al., 2008).
Figure 1.
Next, we examined ADRP expression in cultured human HSCs. Primary HSCs (day 2 in culture), which contained abundant lipid droplets, stained positively for ADRP and Oil red O (Fig. 2A). ADRP and lipid droplets were hardly visible in passage 3 HSCs (Fig. 2B), reflecting loss of lipids in association with progressive HSC activation in culture.
Figure 2.

Effects of retinol, palmitate or their combination on ADRP mRNA expression by cultured HSCs
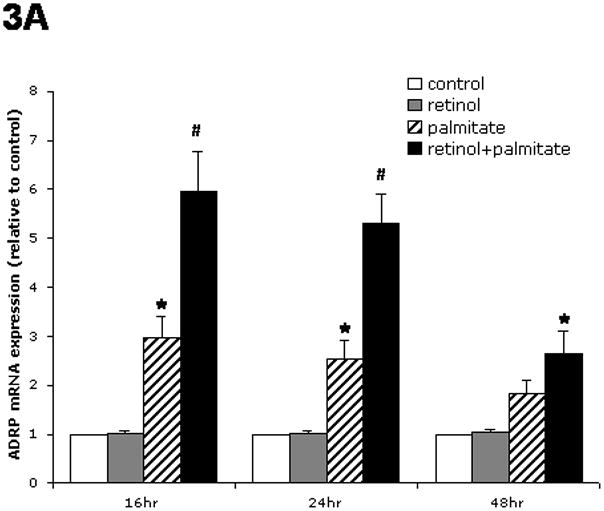
We examined the impact of two main forms of lipid molecules associated with quiescent HSCs, namely retinoids and palmitate. Retinol alone had no effect on ADRP mRNA expression in LX-2 cells (Fig. 3A). Palmitate alone significantly stimulated ADRP mRNA about three-fold after 16 hr and 24 hr of incubation. The combination of retinol and palmitate nearly doubled the increase produced by palmitate alone (p < 0.05 by ANOVA), demonstrating that retinol potentiates ADRP upregulation induced by palmitate. A similar trend of ADRP induction was observed at 48 hr, but the effect was smaller. The addition of palmitic acid to LX-2 cells, alone or together with retinol, for 24 hr or 48 hr had no cytotoxic effects, as determined by MTT assay. Thus, in subsequent experiments, we combined retinol and palmitate to maximally affect ADRP expression. The combination of retinol and palmitate also stimulated ADRP mRNA induction in primary human HSCs (Fig. 3B), confirming that the effects in LX-2 were relevant to non-immortalized cells directly isolated from human liver. In contrast to ADRP, TIP47 levels did not change in LX-2 cells, and there was no detectable perilipin mRNA with or without retinol and palmitate addition (data not shown).
Figure 3.

The combination of retinol and palmitate increases lipid droplet formation, ADRP protein and retinyl palmitate in LX-2 cells
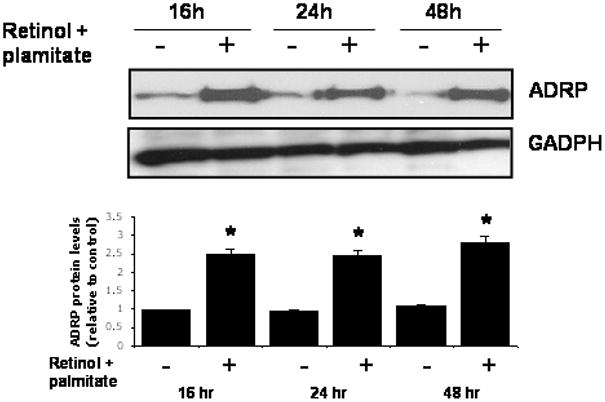
LX-2 cells displayed an increased number of Oil red O-stained lipid droplets after incubation with retinol combined with palmitate (Fig. 4A and B). Using immunofluorescence microscopy, we observed enhanced ADRP staining in cells treated with retinol + palmitate (Fig. 4C and D). Dual fluorescence staining demonstrated ADRP and lipid droplet co-localization (Fig. 4E, F and G). The enhancement of ADRP staining was corroborated by Western blotting, which revealed a 2.5-fold rise of ADRP protein, determined at 16, 24, and 48 hr after treatment (Fig. 5). These data demonstrate that ADRP upregulation after retinol and palmitate stimulates lipid droplet formation, leading to HSC quiescence.
Figure 4.
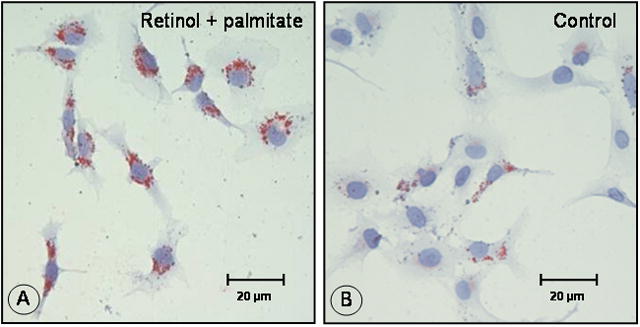
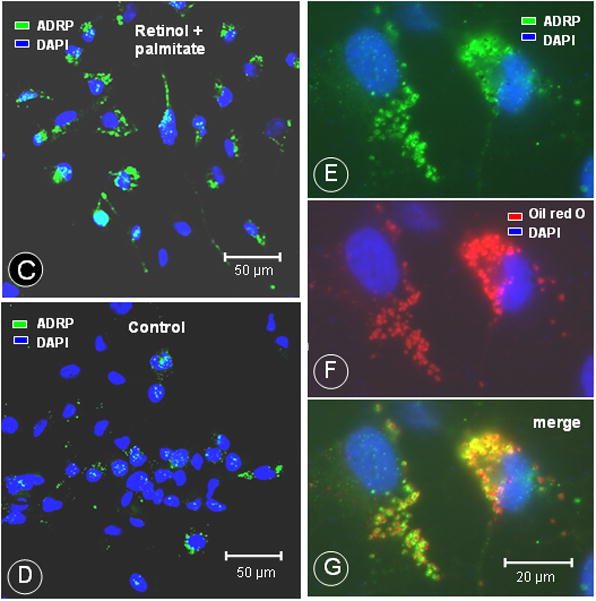
Figure 5.
Because lipid droplets of HSCs contain retinyl palmitate as the major storage form of retinol (Moriwaki et al., 1998), we explored the question of whether LX-2 cells esterified retinol and palmitic acid to retinyl palmitate. HPLC analyses revealed formation of retinyl palmitate in LX-2 cells. The amount of retinyl palmitate formed increased nearly 4 times at higher concentrations of added retinol and palmitic acid (Table 2). No retinyl palmitate was detected in untreated control LX-2 cells. We also found that the level of ADRP mRNA in cells incubated with 10 μM retinol + 300 μM palmitate was two-fold greater than in cells incubated with 5 μM retinol + 100 μM palmitate. Thus, retinol, together with palmitate, stimulates the production of retinyl palmitate coincident with the induction of ADRP gene in a concentration-dependent manner; addition of these compounds leads to the formation of lipid droplets in LX-2 cells (vide supra).
Table 2.
Combination of retinol and palmitate stimulates retinyl palmitate production and ADRP mRNA induction
| Concentrations added to culture | Retinyl palmitate (pmoles/mg cell protein) | ADRP mRNA level (Fold, relative to control) |
|---|---|---|
| 5 μM retinol + 100 μM palmitate | 8.1 ± 1.1 | 4.6 ± 0.4 |
| 10 μM retinol + 300 μM palmitate | 30.7 ± 2.8** | 9.6 ± 1.3# |
Values are means = SE of three cultures. LX-2 cells were incubated with retinol + palmitic acid as described in the Materials and Methods. The levels of retinyl palmitate and ADRP mRNA were determined by HPLC and PCR, respectively, at 48 hr after the start of incubation. No retinyl palmitate was detected in untreated control cells. ADRP mRNA level in control cells was assigned a value of 1.
p < 0.01 and
p < 0.01 vs. the respective 5 μM retinol + 100 μM palmitate
ADRP knockdown by siRNAs in LX-2 cells
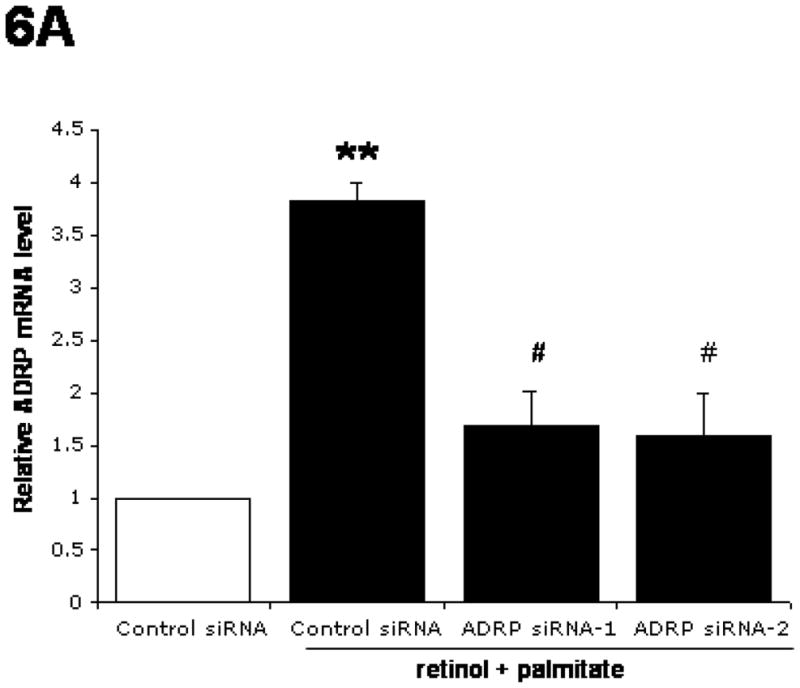
ADRP expression was knocked down by siRNA-1 and -2 transfection in LX-2 cells incubated with retinol together with palmitate (Fig. 6A and B). Both siRNAs were equally effective in reducing the retinol + palmitate-mediated stimulation of ADRP mRNA and protein expression levels by 57% and 43%, respectively.
Figure 6.

ADRP modulates α-SMA protein content in LX-2 cells
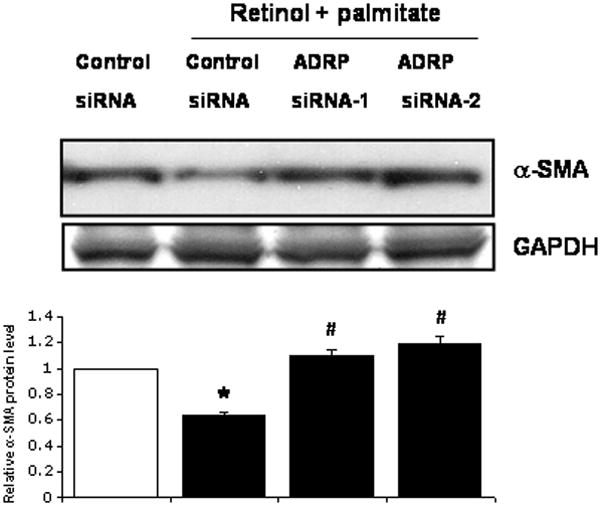
To substantiate that the ADRP induction mediated by combined retinol and palmitate promotes downregulation of LX-2 cell activation, we determined α-SMA protein content, a marker of HSC activation (Ding et al., 2005). Figure 7 shows that retinol + palmitate diminished α-SMA by 40% in negative control siRNA-transfected cells. Knockdown of ADRP using ADRP siRNA-1 or -2 in cells incubated with retinol + palmitate normalized the α-SMA protein content. These data demonstrate that ADRP modulates α-SMA expression and that its inhibition promotes HSC activation.
Figure 7.
Effects of retinol and palmitate on fibrogenic gene expression by LX-2 cells
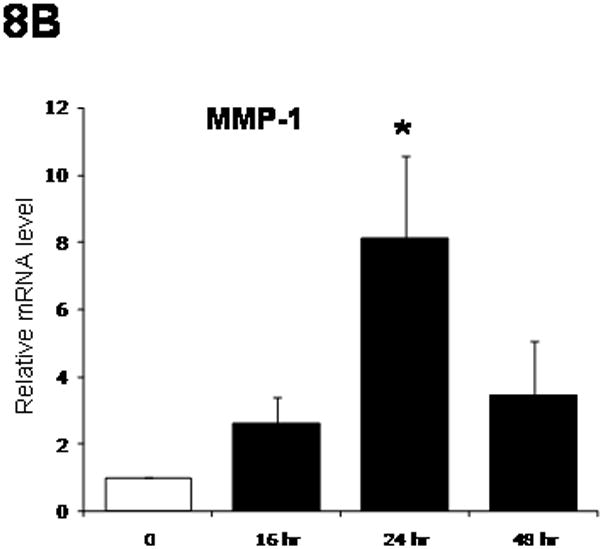
Having shown that alterations of ADRP expression levels affect LX-2 cell activation, we assessed the effects of ADRP on fibrogenic genes. As summarized in Figure 8A, there was a progressive decrease of mRNA expression for α-SMA, collagen I, and MMP-2 from16 hr to 48 hr after the start of treatment. TIMP-1 mRNA was not affected, but MMP-1 mRNA did increase after retinol + palmitate treatment, peaking at 24 hr (Fig. 8B).
Figure 8.

ADRP downregulation opposes fibrogenic gene expression induced by retinol and palmitate
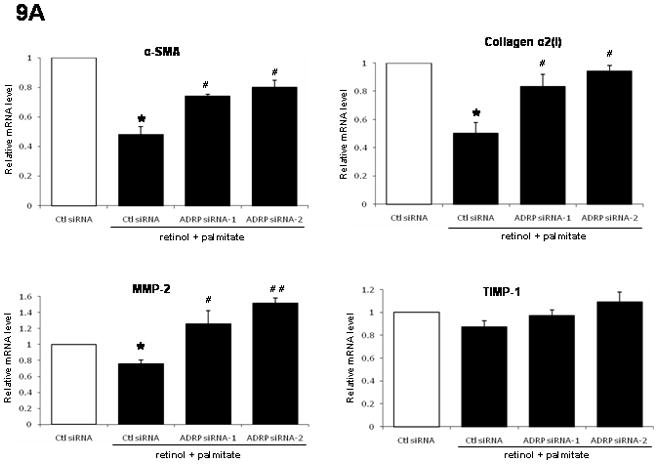
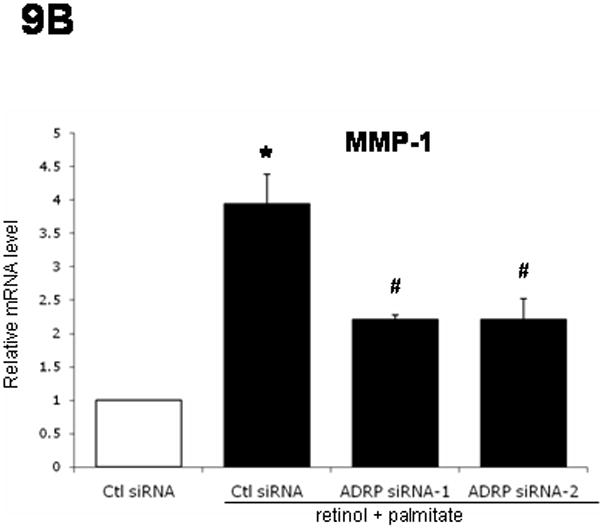
As shown in Figure 9A, while retinol combined with palmitate decreased mRNA expression of α-SMA (50%), collagen I (50%), and MMP-2 (25%) in control siRNA-transfected LX-2 cells, ADRP knockdown induced by siRNA transfection reversed these effects. Interestingly, TIMP-1 gene expression was not affected. On the other hand, ADRP siRNA-1 or -2 transfection nearly halved the increased MMP-1 mRNA after retinol + palmitate (Fig. 9B). Together with the data in Figure 8, the results support the notion that ADRP is functionally linked to fibrogenic gene expression.
Figure 9.
Discussion
The morphological hallmark of HSCs is the appearance of microscopic cytoplasmic lipid droplets. These droplets are easily discernible using electron microscopy or 1 μm thick plastic sections by light microscopy, but the preparations are not routinely available in diagnostic histopathology. Hematoxylin and eosin stained paraffin sections generally used in histopathology are not suitable for the detection of HSCs, because the fats are extracted during tissue processing for paraffin embedment, appearing as unstained vesicles. Because ADRP immuno-reactivity is retained after formalin fixation and paraffin embedment, it can be detected with the ADRP antibody (Heid et al., 1998; Mak et al., 2008). As demonstrated in the present study, using immunoperoxidase staining, ADRP clearly labels the surface of micro-vesicles (representing remnants of extracted lipid droplets) in lipid-rich HSCs, as well as lipid-poor HSCs in liver fibrosis. Furthermore, loss of lipid droplets by HSCs in liver fibrosis and during culture activation is accompanied by diminished ADRP immunostaining of HSCs. In HSC culture, ADRP co-localizes to lipid droplets by dual immunofluorescence, and enhanced ADRP immunostaining parallels increased ADRP mRNA and protein expression. Therefore, ADRP expression offers a sensitive histological marker for the detection of HSCs in the liver.
In the present study, we evaluated HSC activation in LX-2 cells using two key markers, namely lipid storage droplets and α-SMA: loss of lipid droplets along with increase of α-SMA protein content are associated with activation, while the converse connotes quiescence (Ding et al., 2005; Friedman 2008). The LX-2 human immortalized HSC line retains key features of activated HSCs with diminished lipid droplets and increased α-SMA, among others (Cao et al., 2004; Xu et al., 2005). Here, we demonstrate that ADRP upregulation induced by the combination of retinol and palmitate, two main forms of lipid molecules associated with HSC quiescence, stimulates lipid droplet formation and concomitantly diminishes α-SMA protein content, thereby promoting LX-2 cell quiescence. We next assess whether ADRP expression is linked functionally to fibrogenic gene expression in association with HSC phenotype (vide infra).
LX-2 cells express collagen I, MMP-1, MMP-2, TIMP-1 and α-SMA (Cao et al., 2007; Xu et al., 2005), which are responsive to a number of soluble signals, including leptin (Cao et al., 2004; 2006; 2007). Additionally, collagen deposition in liver fibrosis is the outcome of an imbalance between the production of MMP-1 and its physiological inhibitor TIMP-1 (Arthur, 2000). Among these genes, collagen I, MMP-2, TIMP-1 and α-SMA are upregulated during progressive liver fibrosis and in culture-induced activation, which mimics HSC activation in vivo (Friedman, 2008). Therefore, expression of these transcripts is related to stellate cell activation. MMP-1 mRNA expression, which is reduced in liver fibrosis (Milani et al., 1994), is enhanced in early primary HSC culture but then decreases in late culture (Iredale et al.,1996; Knittle et al., 1999); its expression reflects quiescent stellate cells. By either inducing ADRP with retinol and palmitate, or reducing it using siRNA, we evaluated ADRP’s impact on expression levels of fibrogenic genes by LX-2 cells. We found that ADRP upregulation decreases expression levels of α-SMA, collagen I, and MMP-2 mRNA, while increasing that of MMP-1, in accordance with quiescent HSCs. Conversely, ADRP knockdown reverses these changes and thereby enhances activation of HSCs. As noted, TIMP-1 expression was not altered under these experimental conditions, suggesting its regulation may be independent of retinoid and palmitate contents and ADRP levels. Nonetheless, the present findings implicate a regulatory role for ADRP in expression of fibrogenic genes associated with HSC activation.
Retinyl palmitate is the major storage form of retinol in HSC and account for 37% of the lipid droplet composition (Moriwaki et al., 1998). Prior studies showed that incubation with retinol plus palmitic acid led to the esterification of retinyl esters in LX-2 (Xu et al., 2005) as well as in rat HSC-T6 lines (Vogel et al., 2000), but ADRP expression was not studied. We now show that treatment of LX-2 cells with retinol together with palmitate stimulates lipid droplet formation through a process likely involving enhanced ADRP expression concurrent with increased retinyl palmitate generation. The ability of fatty acids to induce ADRP is well-documented (Bell et al., 2008; Gao et al., 2000), but the involvement of retinol is not previously described. Unexpectedly, retinol alone does not affect ADRP expression, but its presence potentiates the induction of ADRP mediated by palmitate. The observed effects are not related to cytotoxicity of palmitic acid, because the amount added has no toxic effect toward LX-2 cells.
The lipid storage droplet is now recognized as a complex organelle that functions in lipid homeostasis. In non-adipose cells, ADRP is implicated in the regulation of lipolysis of lipid storage droplets. ADRP over-expression in human embryonic kidney 293 cells decreases the association of adipose triglyceride lipase (ATGL) at the lipid droplet surface (Listenberger et al., 2007) and, consequently, reduces triacylglycerol hydrolysis. Conversely, in murine hepatic AML12 cells, knocking down ADRP using siRNA resulted in a higher lipolytic rate accompanied by an increased association of ATGL at the lipid droplet surface (Bell et al., 2008). ADRP has been proposed to regulate the access of lipases from the cytosol to the droplet surface. In HSCs, retinyl esters are catalyzed by lecithin:retinol acyltransferase (LRAT) (Fortuna et al., 2001; Matsuura et al., 1997) and retinol release by HSC is mediated by retinyl ester hydrolases (Friedman et al., 1993). These enzymes are primarily localized to microsomes and cytosol (Matsuura et al., 1997). It will be informative to assess whether ADRP modulates the activities of these enzymes and promotes their association with the lipid droplet surface in a manner analogous to that of ATGL and thereby plays a regulatory role of retinol and fatty acid contents of lipid storage droplets of HSCs.
In conclusion, ADRP is localized to lipid droplets of HSCs in vivo and in culture. Retinol potentiates ADRP upregulation induced by palmitate in LX-2 cells. The combination of retinol and palmitic acid promotes lipid droplet formation and retinyl palmitate production coincident with ADRP upregulation in HSCs. ADRP expression correlates with the state of HSC activation and is functionally linked to fibrogenic gene expression through an as-yet unknown mechanism. Nonetheless, these data provide a critical new link between retinoid and palmitate homeostasis and HSC activation, which could ultimately yield new approaches to manipulating HSC phenotype in vivo in hepatic fibrosis.
Acknowledgments
This study was supported by NIH Grant DK56621 (SLF), and the Mount Sinai School of Medicine Dean’s Student Research Program (OR and AJK). Ting Fang Lee was supported in part by a scholarship from the Graduate Students Study Abroad Program sponsored by National Science Council, Taiwan. We also thank Dr. William S. Blaner, Department of Medicine at Columbia University, New York, for sharing methods of retinoid analysis by HPLC analysis.
Grant sponsor: NIH; Grant number: DK56621 (SLF)
Literature cited
- Arthur MJP. Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2000;279:G245–G249. doi: 10.1152/ajpgi.2000.279.2.G245. [DOI] [PubMed] [Google Scholar]
- Bell M, Wang H, Chen H, McLenithan JC, Gong D, Yang R, Yu D, Fried SR, Quon MJ, Londos C, Sztalryd C. Consequences of lipid droplet coat proteins down-regulation in liver cells: Abnormal lipid droplet metabolism and induction of insulin resistance. Diabetes. 2008;57:2037–2045. doi: 10.2337/db07-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaner WS, Obunike JC, Kurlandsky SB, Al-Haideri M, Piantedosi R, Deckelbaum RM, Goldberg IJ. Lipoprotein lipase hydrolysis of retinyl ester: possible implications for retinoid uptake by cells. J Biol Chem. 1994;269:16559–16565. [PubMed] [Google Scholar]
- Brasaemle DL, Barber T, Wolins NE, Serrero G, Blanchette-Mackie EJ, Londos C. Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J Lipid Res. 1997;38:2249–2263. [PubMed] [Google Scholar]
- Cao Q, Mak KM, Lieber CS. Leptin stimulates tissue inhibitor of metalloproteinase-1 in human hepatic stellate cells: respective roles of the JAK/STAT and JAK-mediated H2O2-dependant MAPK pathways. J Biol Chem. 2004;279:4292–4304. doi: 10.1074/jbc.M308351200. [DOI] [PubMed] [Google Scholar]
- Cao Q, Mak KM, Lieber CS. Leptin enhances α1(I) collagen gene expression in LX-2 human hepatic stellate cells through JAK-mediated H2O2-dependent MAPK pathways. J Cell Biochem. 2006;97:188–197. doi: 10.1002/jcb.20622. [DOI] [PubMed] [Google Scholar]
- Cao Q, Mak KM, Lieber CS. Leptin represses matrix metalloproteinase-1 gene expression in LX2 human hepatic stellate cells. J Hepatol. 2007;46:124–133. doi: 10.1016/j.jhep.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Chang BH, Li L, Paul A, Taniguchi S, Nannegari V, Heird WC, Chan L. Protection against fatty liver but normal adipogenesis in mice lacking adipose differentiation-related protein. Mol Cell Biol. 2006;26:1063–1076. doi: 10.1128/MCB.26.3.1063-1076.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BH, Vucic A. The effect of retinol on Ito cell proliferation in vitro. Hepatology. 1988;8:788–793. doi: 10.1002/hep.1840080416. [DOI] [PubMed] [Google Scholar]
- Ding X, Saxena NK, Lin S, Xu A, Srinvasan S, Anania FA. The roles of leptin and adiponectin. A novel paradigm in adipocytokine regulation of liver fibrosis and stellate cell biology. Am J Pathol. 2005;166:1655–1669. doi: 10.1016/S0002-9440(10)62476-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortuna VA, Trugo LC, Borojevic R. Acyl-CoA:retinol acyltransferase (ARAT) and lecithin:retinol acyltransferase (LRAT) activation during the lipocyte phenotype induction in hepatic stellate cells. J Nutr Biochem. 12:610–621. doi: 10.1016/s0955-2863(01)00179-6. [DOI] [PubMed] [Google Scholar]
- Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SL, Rockey DC, McGuire RF, Maher JJ, Boyles JK, Yamasaki G. Isolated hepatic lipocytes and Kupffer cells from normal human liver: morphological and functional characteristics in primary culture. Hepatology. 1992;15:234–243. doi: 10.1002/hep.1840150211. [DOI] [PubMed] [Google Scholar]
- Friedman SL, Wei S, Blaner WS. Retinol release by activated hepatic lipocytes: regulation by Kupffer cell-conditioned medium and PDGF. Am J Physiol Gastrointest Liver Physiol. 1993;264:G947–G952. doi: 10.1152/ajpgi.1993.264.5.G947. [DOI] [PubMed] [Google Scholar]
- Gao J, Ye H, Serrero G. Stimulation of adipose differentiation-related protein (ADRP) expression in adipose precursors by long-chain fatty acids. J Cell Physiol. 2000;182:297–302. doi: 10.1002/(SICI)1097-4652(200002)182:2<297::AID-JCP19>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Hanson MB, Nielsen SE, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989;119:203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- Heid HW, Moll R, Schwetlick I, Rackwitz HR, Keenan TW. Adipophilin is a specific marker of lipid accumulation in diverse cell types and diseases. Cell Tissue Res. 1998;294:309–321. doi: 10.1007/s004410051181. [DOI] [PubMed] [Google Scholar]
- Imai Y, Varela GM, Jackson MB, Graham MJ, Crooke RM, Ahima RS. Reduction of hepatosteatosis and lipid levels by an adipose differentiation-related protein antisense oligonucleotide. Gastroenterology. 2007;132:1947–1954. doi: 10.1053/j.gastro.2007.02.046. [DOI] [PubMed] [Google Scholar]
- Iredale JP, Benyon RC, Arthur MJP, Ferris WF, Alcolado R, Winwood PJ, Clark N, Murphy G. Tissue inhibitor of metalloproteinase-1 messenger RNA expression is enhanced relative to interstitial collagenase messenger RNA in experimental liver injury and fibrosis. Hepatology. 1996;24:176–184. doi: 10.1002/hep.510240129. [DOI] [PubMed] [Google Scholar]
- Knittel T, Mehde M, Kobold D, Saile B, Dinter C, Ramadori G. Expression patterns of matrix metalloproteinases and their inhibitors in parenchymal and non-parenchymal cells of rat liver: regulation by TNF-alpha and TGF-beta 1. J Hepatol. 1999;30:48–60. doi: 10.1016/s0168-8278(99)80007-5. [DOI] [PubMed] [Google Scholar]
- Listenberger LL, Ostermeyer-Fay AG, Goldberg EB, Brown WJ, Brown D. Adipocyte differentiation-related protein reduces the lipid droplet association of adipose triglyceride lipase and slows triacylglycerol turnover. J Lipid Res. 2007;48:2751–2761. doi: 10.1194/jlr.M700359-JLR200. [DOI] [PubMed] [Google Scholar]
- Londos C, Brasaemle DL, Schultz CJ, Segrest JP, Kimmel AR. Perilipins, ADRP, and other proteins that associate with intracellular neutral lipid droplets in animal cells. Semin Cell Develop Biol. 1999;10:51–58. doi: 10.1006/scdb.1998.0275. [DOI] [PubMed] [Google Scholar]
- Londos C, Sztalryd C, Tansey JT, Kimmel AR. Role of PAT proteins in lipid metabolism. Biochemie. 2005;87:45–49. doi: 10.1016/j.biochi.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Mak KM, Leo MA, Lieber CS. Transformation of fat-storing cells into transitional cells in alcoholic liver fibrosis. In: Surrenti C, Casini A, Milani S, Pinzani M, editors. Fat-Storing Cells and Liver Fibrosis; International Falk Symposium 71; Dordrecht/Boston, London: Kluwer Academic Publishers; 1993. pp. 167–179. [Google Scholar]
- Mak KM, Ren C, Ponomarenko A, Cao Q, Lieber CS. Adipose differentiation-related protein is a reliable lipid droplet marker in alcoholic fatty liver of rats. Alcohol: Clin Exp Res. 2008;32:683–689. doi: 10.1111/j.1530-0277.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- Matsuura T, Gad MZ, Harrison EH, Ross AC. Lecithin:retinol acyltransferase and retinyl ester hydrolase activities are differentially regulated and have distinct distributions between hepatocytes and nonparenchymal cell fractions of rat liver. J Nutr. 1997;127:218–224. doi: 10.1093/jn/127.2.218. [DOI] [PubMed] [Google Scholar]
- Milani S, Herbst H, Schuppan D, Grappone C, Pellegrini G, Pinzani M, Casini A, Calabro A, Ciancio G, Stefanini F, Burroughs AK, Surrenti C. Differential expression of matrix-metalloproteinase-1 and -2 genes in normal and fibrotic human liver. Am J Pathol. 1994;144:528–537. [PMC free article] [PubMed] [Google Scholar]
- Moriwaki H, Blaner WS, Piantedosi R, Goodman DS. Effects of dietary retinoid and triglyceride on the liver composition of rat liver stellate cells and stellate cell lipid droplets. J Lipid Res. 1998;29:1523–1534. [PubMed] [Google Scholar]
- Motomura W, Inoue M, Ohtake T, Takahashi N, Nagamine M, Tanno S, Kohgo Y, Okumura T. Up-regulation of ADRP in fatty liver in human and liver steatosis in mice fed with high fat diet. Biochem Biophys Res Commun. 2006;340:1111–1118. doi: 10.1016/j.bbrc.2005.12.121. [DOI] [PubMed] [Google Scholar]
- Pinzani M, Gentilini P, Abboud HE. Phenotypical modulation of liver fat-storing cells by retinoids. Influence on unstimulated and growth factor-induced cell proliferation. J Hepatol. 1992;14:211–220. doi: 10.1016/0168-8278(92)90160-q. [DOI] [PubMed] [Google Scholar]
- Sato T, Kato R, Tyson CA. Regulation of differentiated phenotype of rat hepatic lipocytes by retinoids in primary culture. Exp Cell Res. 1995;217:72–83. doi: 10.1006/excr.1995.1065. [DOI] [PubMed] [Google Scholar]
- Steiner S, Wahl D, Mangold BLK, Robison R, Raymackers J, Meheus L, Anderson L, Cordier A. Induction of the adipose differentiation-related protein in liver of etomoxir-treated rats. Biochem Biophys Res Commun. 1996;218:777–782. doi: 10.1006/bbrc.1996.0138. [DOI] [PubMed] [Google Scholar]
- Straub BK, Stoeffel P, Heid H, Zimbelmann R, Schirmacher P. Differential pattern of lipid droplet-associated proteins and de novo perilipin expression in hepatocyte steatogenesis. Hepatology. 2008;47:1936–1946. doi: 10.1002/hep.22268. [DOI] [PubMed] [Google Scholar]
- Vogel S, Piantedosi R, Frank J, Lalazar A, Rockey DC, Friedman SL, Blaner WS. An immortalized rat liver stellate cell line (HSC-T6): a new cell model for the study of retinoid metabolism in vitro. J Lipid Res. 2000;41:882–893. [PubMed] [Google Scholar]
- Wolins NE, Brasaemle DL, Bickel PE. A proposed model of fat packaging by exchangeable lipid droplet proteins. FEBS Lett. 2006;580:5484–5491. doi: 10.1016/j.febslet.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Xu L, Hui AY, Albanis E, Arthur MJ, O’Byrne SM, Blaner WS, Mukherjee P, Friedman SL, Eng FJ. Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut. 2005;54:142–151. doi: 10.1136/gut.2004.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]


