Abstract
Easy and effective vaccination methods could reduce mortality and morbidity due to vaccine-preventable influenza infections. In this study, we examined the use of microneedle patches to increase patient coverage through possible self administration and enhance vaccine immunogenicity by targeted delivery to skin. We carried out a detailed study of protective immune responses after a single influenza vaccination to the skin of mice with a novel microneedle patch designed to facilitate simple and reliable vaccine delivery. Skin vaccination with inactivated virus-coated microneedles provided superior protection against lethal challenge compared to intramuscular injection as evidenced by effective virus clearance in lungs. Detailed immunologic analysis suggests that induction of virus neutralizing antibodies as well as enhanced anamnestic humoral and cellular responses contributed to improved protection by microneedle vaccination to the skin. These findings suggest that vaccination in the skin using a microneedle patch can improve protective immunity, and simplify delivery of influenza and possibly other vaccines.
Keywords: microneedle, influenza vaccine, inactivated virus, single immunization, intradermal vaccination
1. Introduction
Influenza virus causes 17,000–51,000 deaths in the United States annually [1]; a global pandemic could kill millions [2]. Improving the delivery of influenza vaccine antigens such as transdermal vaccination would be an important advance, which may be enabled by targeting skin’s specialized antigen-presenting Langerhans and dermal dendritic cells across the skin layer [3, 4]. However, topical antigen delivery is blocked by skin’s outermost barrier layer of stratum corneum [4].
Intradermal (ID) administration has been proposed to improve immunogenicity of influenza vaccines and limited data suggest this approach offers promise. For example, a pair of clinical studies showed that a reduced dose of influenza vaccine delivered to the skin generated similar hemagglutination inhibition (HAI) responses compared to the full intramuscular (IM) dose, which suggested a dose-sparing strategy [5-8]. Intraepidermal delivery by jet injection showed both increased protection and dose sparing compared to subcutaneous (SC) injection in mouse [9]. Human trials using other vaccines, such as inactivated polio, rabies and hepatitis B, have more definitively shown enhanced immune responses and protection after low-dose ID delivery compared to IM immunization [10]. Overall, these findings suggest that influenza vaccination in the skin would benefit from detailed immunologic study to determine the possible benefits of this route of administration.
Such detailed immunologic studies have been difficult to carry out in humans due to their invasive nature as well as the noted unreliability of ID delivery using conventional Mantoux injection [11]. In animals, it is even harder to inject into the thin skin of rodents, which is often thinner than the bevel on the tip of a hypodermic needle and thus attempts to inject ID often go subcutaneous (SC) or IM. In this study, we have used microneedles to reliably target vaccine delivery to the skin of mice using a device designed for simple administration with minimal training.
We and others have fabricated microneedles by adapting tools of the microelectronics industry to create micron-scale needles that pierce skin’s outer barrier layer of stratum corneum and administer compounds into skin [12, 13]. Microneedles can be assembled into patches suitable for self-administration using low-cost manufacturing [14] and have been reported as painless and well-tolerated by human subjects [15, 16]. Some work has addressed vaccine delivery via the ID route using hollow microneedles requiring delivery of a liquid vaccine formulation by clinical personnel [8, 17, 18]. Microneedles have also been also developed as solid microneedle patches that are coated with inactivated influenza virus vaccine [19, 20] for subsequent dissolution of coated vaccines from the microneedles in the skin and may be suitable for self administration.
Only 113 million vaccinations were given in the 2007-2008 influenza season, although influenza vaccine is currently recommended in United States for 220 million people [1]. Barriers to wider coverage include the need for injection by trained medical personnel and anxiety associated with hypodermic needles [21]. These limitations would be amplified during rapid mass vaccination during a possible pandemic, because hypodermic injection has risks of cross-contamination and spread of a pathogen [22]. Vaccination using a self-administered microneedle patch could address these limitations.
This study sought to develop solid microneedles for influenza vaccine not only to enable detailed immunologic analysis of influenza vaccination in the skin, but also as a delivery technology to enable simple and reliable vaccination for wider patient coverage in clinical practice. The results from the present study provides evidence that microneedle skin immunization can be superior to IM immunization in inducing protective immunity as demonstrated by improved lung viral clearance as well as recall humoral and cellular immune responses to influenza virus.
2. Materials and methods
2.1. Preparation of inactivated influenza virus
Formalin-inactivated influenza A/PR/8/34 virus (A/PR8) was prepared as described previously [23] and used as a vaccine antigen through this study. Briefly, influenza virus A/PR8 was grown in 10 day old embryonated hen’s eggs and purified from allantonic fluid by using a discontinuous sucrose gradient (15, 30, and 60%) layers. The purified virus was inactivated by mixing the virus with formalin at a final concentration of 1:4000 (v/v).
2.2. Fabrication, coating and imaging of microneedles
Stainless steel microneedles were fabricated using laser cutting and electropolishing, as described previously [24]. To apply a vaccine coating, microneedles were dipped six times at 25°C into coating solution using a dip-coating device described previously [24] and air dried. The coating solution was composed of 1% (w/v) carboxymethylcellulose (CMC) sodium salt (Carbo-Mer, San Diego, CA), 0.5% (w/v) Lutrol F-68 NF (BASF, Mt. Olive, NJ), 15% (w/v) trehalose (Sigma Aldrich, St. Louis, MO) and 1 mg/ml inactivated virus in phosphate buffered saline (PBS).
Microneedles were imaged by, bright-field stereo microscope (Olympus SZX12, Center Valley, PA) with a CCD camera (Leica DC 300, Leica Microsystems, Bannockburn, IL). To image the microneedles after delivery of viral antigen into skin, microneedles coated with inactivated virus were inserted into mouse cadaver skin for 10 min.
2.3. Immunization and viral challenge infection
BALB/c mice (Charles River, Wilmington, MA) were anesthetized intramuscularly with 110 mg/kg ketamine (Abbott Laboratories, Abbott Park, IL) mixed with 11 mg/kg xylaxine (Phoenix Scientific, St. Joseph, MO). The skin on the back of the mouse was exposed by removing hair with depilatory cream (Nair, Princeton, NJ), washed with 70% ethanol, and dried with a hair dryer. An in-plane five-needle array of microneedles coated with 0.4 μg of inactivated influenza virus was manually inserted into the skin and left for 10 min to dissolve the vaccine coating in the skin. To determine the amount of inactivated virus vaccine coated on microneedle, vaccine coated microneedles were soaked in PBS solution for 12 h at 4°C, and the amount of released protein measured by a BCA protein assay kit (Pierce Biotechnology, Rockford, IL).
As a positive control, mice were similarly treated, but no depilatory cream was applied, and 0.4 μg of inactivated influenza virus in 100 μl PBS solution was injected intramuscularly into the mice. IM immunization was performed using the same inactivated influenza virus dose and in the absence of any adjuvant. Naïve (i.e., negative control) mice received no treatment. Mock control (i.e., negative control) mice received microneedle vaccination in the skin with coating buffer but not containing vaccine.
For virus challenge, isoflurane-anesthetized mice were intranasally infected with the mouse-adapted A/PR8 virus (20 LD50) five weeks after vaccination. Mice were observed daily to monitor changes in body weight and to record mortality (mice were compassionately sacrificed at 25% body weight loss). In some cases, mice (4 out of 10 mice per group) were sacrificed four days after challenge to harvest lung and spleen for additional analysis described below. All animal studies were approved by the Emory University Institutional Animal Care and Use Committee.
2.4. Antibody responses
Influenza virus-specific antibodies of different isotypes (IgG, IgG1, IgG2a and IgG2b) were determined by enzyme-linked immunosorbent assay (ELISA) following standard protocols described previously [23]. Data are presented as optical densities read at 450 nm or end-point titers. Titers are expressed as the highest dilution having a mean optical density at 450nm greater than the mean value plus 3 standard deviations of naïve serum samples.
2.5. Neutralization, hemagglutination inhibition titer (HAI), lung viral titer and lung inflammatory cytokine assays
Virus neutralization assay of serum samples collected at week 4 post immunization was performed using MDCK cells (American Type Culture Collection, Manassas, VA) following a previously described procedure [23]. Neutralization activity was expressed as the percentage of plaque reduction as a function of serum dilution.
Hemagglutination-inhibition (HAI) titers were determined as previously [23]. Briefly, serum samples were first treated with receptor-destroying enzyme in 1:3 ratio (Denka Seiken, Tokyo, Japan) overnight at 37°C and then incubated for 30 min at 56°C. Sera were then serially diluted in separate wells, mixed with 4 HA units (HAU) of influenza A/PR8 virus, and incubated for 30 min at room temperature prior to adding 0.5% chicken red blood cells.
Lung viral titers at day 4 post challenge were determined by counting the number of plaques formed on the MDCK cells as described previously [23] . Inflammatory cytokines (IL-6, IFN-γ) in lungs collected at day 4 post challenge were analyzed by Ready-Set-Go cytokine kits (eBioscience, San Diego, CA) following the manufacturer’s procedure.
2.6. Virus-specific immune recall responses upon virus challenge
Spleen cells harvested from immunized and naïve mice at day 4 post-challenge were also used to determine cytokine producing T cell responses by ELISPOT (BD/PharMingen, San Diego, CA)_upon the in vitro stimulation with a mixture of two major A/PR8 hemagglutinin-specific histocompatibility complex class I (MHC-I) peptides (IYSTVASSL and LYEKVKSQL) or a pool of five MHC-II peptides (SFERFEIFPKE, HNTNGVTAACSH, CPKYVRSAKLRM, KLKNSYVNKKGK, and NAYVSVVTSNYNRRF), as described previously [23]
2.7. Statistical Analysis
Every assay was measured using at least three samples, from which the arithmetic mean and standard error of the mean were calculated (unless otherwise noted). A two-tailed Student’s t-test (α=0.05) was performed when comparing two different conditions. When comparing three or more conditions, a one-way analysis of variance (ANOVA; α=0.05) was performed. In all cases, a value p<0.05 was considered statistically significant.
3. Results
3.1. Fabrication of microneedle and stabilization of influenza vaccines by trehalose
To carry out this study, microneedles fabricated by laser-cutting stainless steel sheets were designed to be long enough to penetrate through stratum corneum and viable epidermis and into superficial dermis by gentle manual insertion, but short enough to avoid pain compare to hypodermic needle. Figure 1A illustrates a size comparison between an array of 5 microneedles and a conventional hypodermic needle [16]. The microneedle length is approximately 700 μm. For comparison, the thickness of mouse epidermis and full-thickness skin are approximately 30 μm and 700 μm respectively [25], which suggests that microneedles penetrated through the full thickness of mouse skin.
Fig.1.
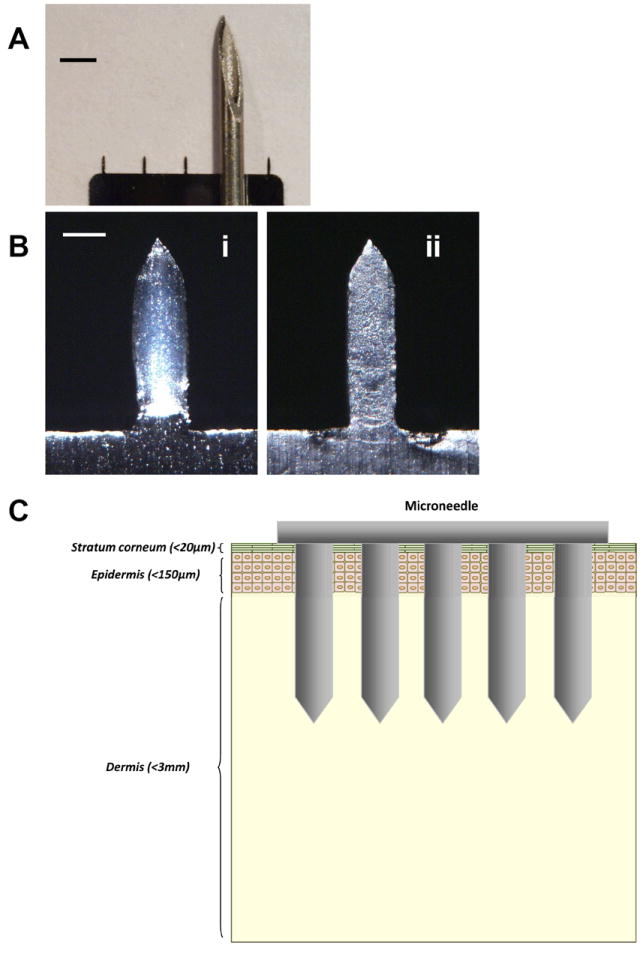
(A) Size comparison between a microneedle array and the tip of a 20 gauge hypodermic needle (scale bar = 1500 μm). (B) Bright-field micrographs of a microneedle (scale bar = 150μm). (i) A microneedle coated with inactivated influenza virus as seen in white, bulky parts covering the microneedle. (ii) The same microneedle after insertion and removal from mouse skin after 10 min, indicating the disappearance of the vaccine coating as a result of dissolution of the vaccine coating in the skin. (C) Insertion of microneedles across the skin layer.
Our delivery strategy involved coating solid microneedles with formulations of influenza vaccine that dissolve in skin. We developed aqueous coating formulazztions including surfactant to facilitate uniform coatings by reducing surface tension, viscosity enhancer to enable thicker coatings by increasing coating solution residence time during a drying process, and inactivated whole influenza virus (A/PR/8/34) as a vaccine antigen. To prevent vaccine activity loss, we used trehalose, which is known to stabilize biomolecules during drying [26]. Dip coating influenza vaccine onto microneedles in the coating buffer produced thick, uniform coatings localized to microneedle shafts as seen in white (Fig.1B-i). Insertion of microneedles into skin led to dissolution of coated vaccines from the microneedles within minutes (Fig. 1B-ii). As shown in Fig. 1B-ii, the white, bulky vaccine coating was dissolved in the skin and disappeared from microneedle surface.
3.2. Antibody responses after delivery of solid microneedle influenza vaccine
We next evaluated vaccine efficacy via microneedle delivery to the skin compared with IM injection in mice. Groups of mice (n=10 BALB/c mice per group) were IM immunized with unprocessed inactivated virus vaccine (0.4 μg total protein contents) or administered via skin with an array of microneedle coated with 0.4 μg of inactivated virus in a solid formulation. A mock control group of mice was immunized with microneedles coated with coating buffer only in the absence of vaccine. Visual examination of the skin of animals in all three groups showed that the sites of microneedle insertion (either with vaccine or mock control) show barely perceptible erythema that resolved within hours. IM vaccinated mice also showed very mild erythema, although somewhat more pronounced that the microneedle-treated sites, which also resolved within hours.
Both IM and microneedle groups showed gradual increases in virus specific antibody levels with time up to week 4 after a single immunization (Fig. 2). For total IgG, IgG2a, and IgG2b (Fig 2A, 2C, 2D), antibody levels after skin vaccination were statistically the same as IM vaccination (p>0.1). Interestingly, microneedle delivery induced higher levels of IgG1 antibodies compared to IM immunization at weeks 2 and 4 (p<0.05, Fig 2B). For both delivery methods, IgG2a was the dominant isotype (ratios of virus specific IgG2a and IgG1 antibodies based on titers for microneedle and IM is 8 and 16, respectively), indicating T helper type 1 (Th1) biased immune responses. When antibody levels were compared in terms of titers as end-point dilutions, a similar pattern of antibody profiles was observed in both total IgG and isotype antibodies (data not shown). Thus, by systemic humoral measures, microneedle delivery to the skin and IM immunization provided similar antibody responses, but microneedles increased IgG1 while maintaining IgG2 levels. These results suggest that microneedle skin immunization can induce more balanced IgG1 and IgG2a antibody responses compared to IM immunization although inactivated whole virus vaccine delivered by either IM immunization or microneedle skin vaccination induced a dominant IgG2a isotype antibody response.
Fig.2.
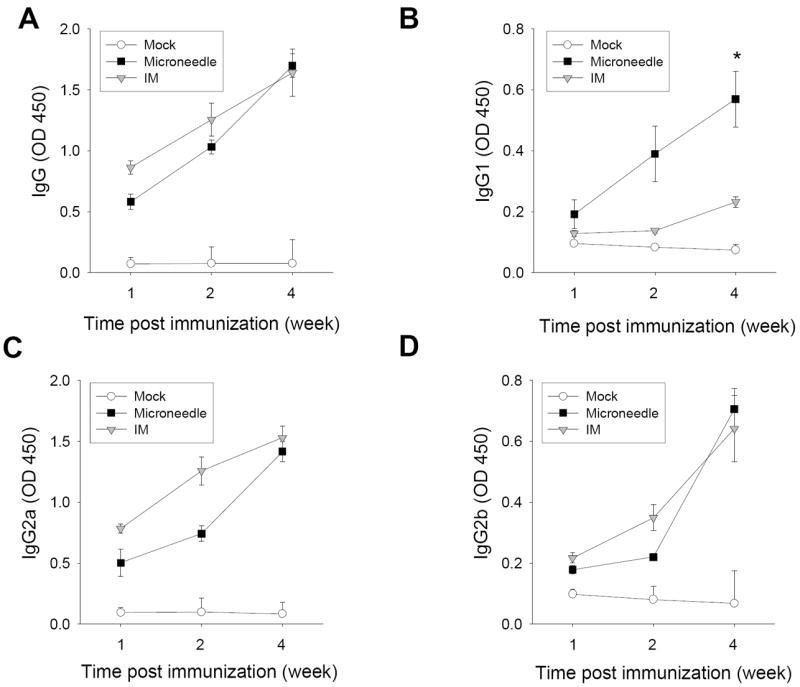
Kinetics of virus-specific total IgG and isotype antibodies after a single dose of vaccination. Kinetics of influenza virus specific serum antibody responses were determined at week 1, 2, and 4 post-immunization with microneedle (MN) vaccine in the skin, IM immunization, or mock immunization (n=10 mice). Antibody levels in the diluted serum samples (100x) were determined by ELISA using inactivated virus as a coating antigen and presented as optical densities (OD at 450 nm). (A) Total serum IgG. (B) IgG1. (C) IgG2a. (D) IgG2b (*p<0.05).
3.3. Induction of functional antibodies by microneedle vaccination
Viral neutralizing activity is a direct and sensitive measure for functional antibodies [27]. Even the high level of 540 fold-diluted serum samples from the microneedle group showed 100% inhibition of virus plaque formation whereas a smaller dilution factor of 270 was needed for 100% inhibition in the IM immunization group although (Fig. 3). Hemagglutination inhibition titers (HAI) are another important serological response when assessing vaccine efficacy. HAI titers of 40 are generally expected to provide 50% protective immunity [28]. Both microneedle and IM immunization groups showed HAI titers of approximately 300, whereas the mock control showed an HAI titer of less than 15. Therefore, microneedle skin immunization can induce comparable (or higher) levels of virus neutralizing antibody and HAI responses compared to IM immunization.
Fig. 3.
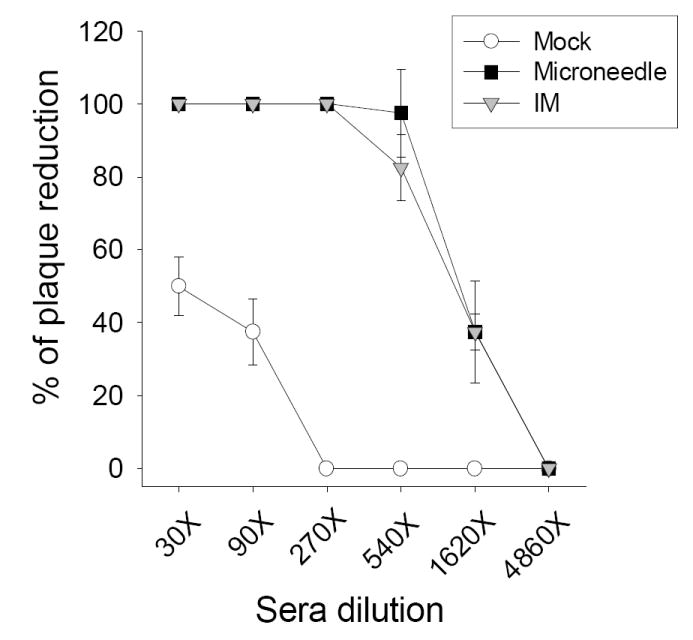
Induction of functional antibodies after a single vaccination. Neutralizing activity expressed as percentages of plaque reduction on MDCK cells 4 weeks after immunization (n=10 mice).
3.4. Protection against lethal challenge infection
To determine protection, microneedle, IM immunized, and mock control mice were challenged with a lethal dose of mouse-adapted A/PR8 virus (20 × LD50) at 5 weeks after a single vaccination. Mock control mice showed pathologic signs including ruffled fur, lethargy, and significant weight loss, and then died or had to be euthanized by day 6. Microneedle and IM immunized mice were protected without body weight loss (p>0.4) (Fig.4). Thus, microneedle delivery to the skin and IM immunization both provided apparently similar protection against challenge.
Fig. 4.
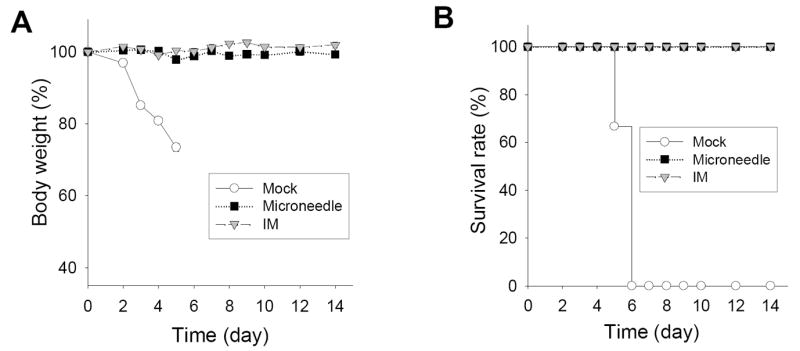
Protection against lethal challenge with A/PR8 virus (20 LD50) five weeks after immunization. Change of (A) body weight and (B) survival rate (n=6 mice).
3.5. Protective efficacy of microneedle vaccination
We next assessed local immunity in the lung as a more direct measure of immune protection at the major site for virus replication. At four days post-challenge, IM-immunized mice had 2000-fold lower lung viral titers than mock control mice, indicating significant suppression of viral replication (Table 1). Interestingly, the group of mice that received microneedle vaccination in the skin had no detectable lung viral titers indicating effective control of challenge virus replication by day 4. Moreover, mock challenged mice exhibited high levels of inflammatory cytokines known to cause tissue damage and increased mortality [29], whereas no IFN-γ and an order of magnitude less IL-6 were detected in lungs of both groups of immunized mice (Table 1). Importantly, microneedle-immunized mice showed even lower levels of IL-6 compared to IM immunization, indicating less pathological symptoms due to viral replication. Therefore, microneedle delivery to the skin provided superior protection in the lung compared to IM immunization.
Table 1.
Lung virus titer, lung inflammatory cytokines, and total virus-specific IgG antibody from lung extracts A.
| Group | Lung virus titer (log10) B | Lung IFN-γ (pg/ml) C | Lung IL-6 (pg/ml) C | Lung IgG (OD450) D |
|---|---|---|---|---|
| MockE | 6.64 ± 0.7 | 650 ±50 | 1500 ± 200 | 0.28 ± 0.02 |
| MN+TreF | 1.69 ± 0.2* | 0 | 60 ± 20* | 1.5 ± 0.23* |
| IMG | 3.25 ± 0.4 | 0 | 200 ± 60 | 0.8 ± 0.1 |
Lung extracts harvested four days post-challenge. Averages of 4 individual mice pre group are shown from two independent experiments (*p<0.05 compared with IM).
Lung virus titer expressed as plaque-forming units/ml. The detection limit for lung viral titers was 50 pfu/ml.
Lung inflammatory cytokines presented as geometric mean.
Total virus-specific IgG antibody from lung extracts. (100x diluted samples, n=4 mice)
The mock control mice.
Microneedle immunization with trehalose formulation.
Intramuscularly immunization with intact influenza vaccine.
3.6 .Rapid recall humoral and cellular immune responses induced by microneedle vaccination after challenge infection
To better understand the underlying host immune mechanism for improved lung viral clearance after microneedle immunization in the skin, we performed detailed immunologic studies. Improved responses to skin immunization using microneedles could be caused by more effective induction of rapid recall immune responses from immunologic memory upon pathogen exposure. We first assessed this by measuring antibody responses four days after virus challenge. Microneedle vaccination in the skin was found to induce higher levels of virus-specific IgG than IM immunization both in sera (Fig. 5) and, more significantly, in lungs (Table 1). The microneedle vaccine group showed significantly higher levels of virus specific total IgG antibody than IM immunization after challenge (Fig. 5), indicating rapid host anamnestic immune responses probably due to the exposure and replication of challenge virus. Whereas, the IM immunization group showed decreases in levels of virus-specific antibodies at this early time post challenge infection, which might be related with delayed virus clearance.
Fig. 5.
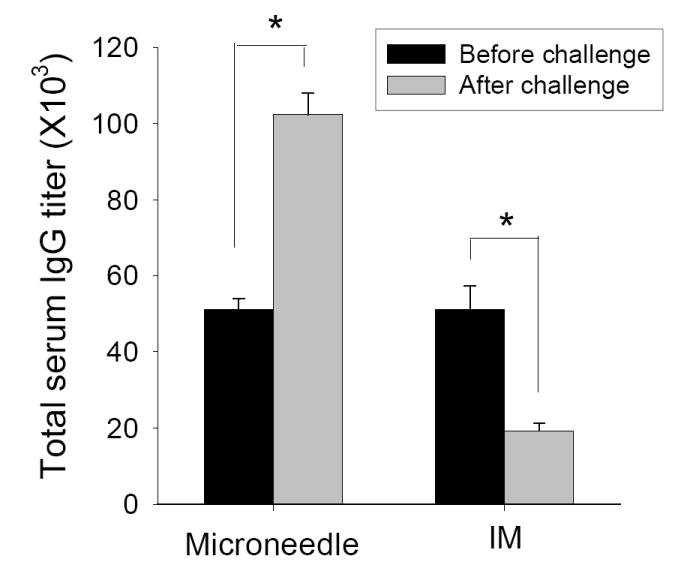
Virus specific serum antibody levels before and after challenge. Averages (n=4 mice) are shown from three independent experiments (*p<0.05). Titers are expressed as the highest dilution fold having a mean optical density at 450nm greater than the mean value plus 3 standard deviations of naïve serum samples.
We next assessed cellular recall responses by measuring cytokine-secreting spleen cells harvested four days post-challenge following stimulation with immunodominant major histocompatibility complex (MHC) class I and II hemagluttanin peptides (Fig.6). Microneedle-vaccinated mice induced approximately three-fold more IFN-γ and IL-4 cytokine-secreting splenocytes compared to IM immunization upon MHC II peptide stimulation. Altogether, these results indicate that microneedle delivery to the skin induced rapidly responsive anamnestic recalls upon pathogen exposure particularly through lung and serum antibodies as well as MHC II-associated CD4+ T helper cells, which might be especially important to protection in the elderly [30]. Enhanced immune responses at the time of day 4 post challenge observed particularly in the group of microneedle vaccination in the skin reflect the recall responses of memory, since it is too early for naïve mice to induce protective virus-specific antibodies or cellular immunity [31]. In summary, our results indicate that influenza vaccine delivery to the skin by solid microneedles were superior to IM immunization in inducing effective recall immune responses contributing to improved protective immunity.
Fig. 6.
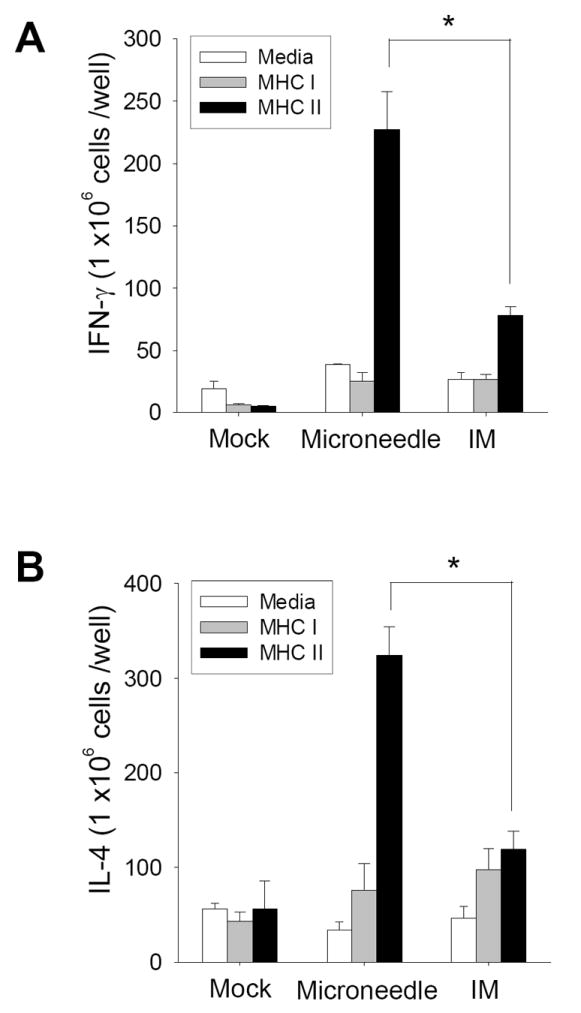
Cellular responses to influenza vaccination. (A) IFN-γ and (B) IL-4 secreting splenocytes in response to A/PR8 hemagglutinin-specific MHC I and II peptide stimulation determined by ELISPOT (n=4, *p<0.05). Averages (n=4 mice) are shown from duplicate wells.
4. Discussion
In the present study, we demonstrated that microneedle vaccination in the skin was superior to IM immunization in inducing rapid increases in virus-specific antibodies in lung and sera, and recall responses of T cells. One of the striking findings was that microneedle vaccination was more effective in controlling challenge virus replication than IM immunization. At the early time point of day 4 post-challenge, virus specific serum total IgG was significantly higher in the microneedle vaccine group than those observed by IM immunization. These rapidly enhanced recall immune responses indicate that the vaccine-induced pre-existing memory B cells in the microneedle group might be rapidly responsive to the exposure of challenge virus and its replication. These rapid host recall responses after microneedle vaccination were reflected by the significant increases in sera and lungs at an early time point of day 4 after challenge. In contrast, in the IM immunization group, the serum antibodies were transiently decreased by day 4 post challenge probably due to recruitment of circulating antibodies for binding to rapidly replicating viruses. Thus, it is speculated that the IM immunized group might be less effective in rapidly responding to the exposure of challenge virus than the microneedle vaccine group, which results in a delay in clearing virus compared to the microneedle delivery.
In the spleen, cytokine-secreting splenocytes are generated by a MHC II-dependent pathway to produce both Th1 and Th2 CD4+ T helper cells [32]. Skin-derived dendritic cells are known to express high levels of MHC II molecules upon activation [33]. The combination of these elevated humoral and cellular anamnestic responses can explain the lower lung viral titers resulting in lower levels of lung inflammatory cytokines and the associated excellent protection against viral challenge. More detailed kinetic studies after challenge are needed to better understand the control of viral replication and the generation of memory immune responses with microneedle vaccination in comparison with IM immunization.
The humoral, cellular and protective immune responses to influenza vaccination in the skin are not well understood. Only limited studies on intradermal immunizations have been reported using animals probably due to difficulties in intradermally delivering vaccines in solution (10 to 100 μl) using a relatively large hypodermic needle into relatively thin skin [18, 34]. There have also been intradermal immunization studies in humans by delivering liquid formulations of vaccines (100 to 500 μl) using hypodermic needles [5-8, 17, 35, 36]. Based on HAI antibody responses after vaccination with low doses delivered by ID injection, some clinical studies demonstrated dose sparing effects although equivalent dose comparing groups are missing [5, 7, 8]. A well-controlled and direct comparison of low-dose influenza vaccination ID versus IM showed similar HAI for both delivery routes among adult subjects, which indicated that ID delivery was not dose sparing [35]. In contrast, a separate study did show elevated HAI responses after low-dose ID delivery among the elderly [17]. However, none of these studies reported detailed immune responses or protective immunity due to intrinsic technical limitations. Clearly, such experiments would be difficult to be carried out on humans. Thus, microneedle immunizations can provide an enabling tool to gain insight into the immunologic basis for improved protection. More detailed dosage studies will be needed to better define the optimization for microneedle vaccination.
In addition to apparent immunologic advantages, microneedles also offer potential logistic opportunities. The small size of solid microneedle patches should facilitate storage, stockpiling and transportation of influenza vaccines, possibly via mail [37]. Vaccination should be faster and simpler because microneedles are painless [16], inherently avoid need for reconstitution, and may be administered by minimally trained personnel or possibly patients themselves. Used microneedles have small disposal volume and are difficult to reuse, either intentionally or accidentally. Finally, mass-produced microneedles are expected to be cost-competitive with hypodermic needle and syringe.
5. Conclusion
Skin vaccination using microneedles induced robust humoral and cellular immune responses that were, by some measures, significantly stronger than IM injection and provided full protection against viral challenge. Although this research used microneedles to study skin vaccination, we hypothesize that the advantages reported here may be more broadly representative of vaccination in the skin, which may be harnessed to develop better influenza vaccines. These immunologic advantages, combined with logistic benefits, indicate that microneedle delivery to the skin may offer a strategy for improved influenza vaccination.
Acknowledgments
This work was carried out at the Emory Vaccine Center and the Georgia Tech Center for Drug Design, Development and Delivery and Institute for Bioengineering and Biosciences. It was partially supported by NIH grants EB006369 (M.R.P.) and AI0680003 (R.W.C.), and by the Korea Ginseng Society (S.M.K). We thank Dr. Vladimir Zarnitsyn for microneedle fabrication and Dr. Mark Allen for use of his laser microfabrication facilities.
References
- 1.Centers for Disease Control and Prevention. ACIP. (2008) Prevention and control of influenza, recommendations of the Advisory Committee on Immunization Practices. Morbidity and Mortality Weekly Report (MMWR) 2008;57:1–60. [PubMed] [Google Scholar]
- 2.Tumpey TM, Basler CF, Aguilar PV, Zeng H, Solorzano A, Swayne DE, et al. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science. 2005;310(5745):77–80. doi: 10.1126/science.1119392. [DOI] [PubMed] [Google Scholar]
- 3.Glenn GM, Kenney RT, Hammond SA, Ellingsworth LR. Transcutaneous immunization and immunostimulant strategies. Immunol Allergy Clin North Am. 2003;23(4):787–813. doi: 10.1016/s0889-8561(03)00094-8. [DOI] [PubMed] [Google Scholar]
- 4.Glenn GM, Taylor DN, Li XR, Frankel S, Montemarano A, Alving CR. Transcutaneous immunization: A human vaccine delivery strategy using a patch. Nat Med. 2000;6(12):1403–6. doi: 10.1038/82225. [DOI] [PubMed] [Google Scholar]
- 5.Belshe RB, Newman FK, Cannon J, Duane C, Treanor J, Van Hoecke C, et al. Serum antibody responses after intradermal vaccination against influenza. N Engl J Med. 2004;351(22):2286–94. doi: 10.1056/NEJMoa043555. [DOI] [PubMed] [Google Scholar]
- 6.Beran J, Ambrozaitis A, Laiskonis A, Mickuviene N, Bacart P, Calozet Y, et al. Intradermal influenza vaccination of healthy adults using a new microinjection system: a 3-year randomised controlled safety and immunogenicity trial. BMC Med. 2009;7(1):13. doi: 10.1186/1741-7015-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. N Engl J Med. 2004;351(22):2295–301. doi: 10.1056/NEJMoa043540. [DOI] [PubMed] [Google Scholar]
- 8.Van Damme P, Oosterhuis-Kafeja F, Van der Wielen M, Almagor Y, Sharon O, Levin Y. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine. 2009;27(3):454–9. doi: 10.1016/j.vaccine.2008.10.077. [DOI] [PubMed] [Google Scholar]
- 9.Chen DX, Endres RL, Erickson CA, Weis KF, McGregor MW, Kawaoka Y, et al. Epidermal immunization by a needle-free powder delivery technology: Immunogenicity of influenza vaccine and protection in mice. Nat Med. 2000;6(10):1187–90. doi: 10.1038/80538. [DOI] [PubMed] [Google Scholar]
- 10.Nicolas JF, Guy B. Intradermal, epidermal and transcutaneous vaccination: from immunology to clinical practice. Expert Rev Vaccines. 2008;7(8):1201–14. doi: 10.1586/14760584.7.8.1201. [DOI] [PubMed] [Google Scholar]
- 11.Laurent PE, Bonnet S, Alchas P, Regolini P, Mikszta JA, Pettis R, et al. Evaluation of the clinical performance of a new intradermal vaccine administration technique and associated delivery system. Vaccine. 2007;25(52):8833–42. doi: 10.1016/j.vaccine.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 12.Widera G, Johnson J, Kim L, Libiran L, Nyam K, Daddona PE, et al. Effect of delivery parameters on immunization to ovalbumin following intracutaneous administration by a coated microneedle array patch system. Vaccine. 2006;24(10):1653–64. doi: 10.1016/j.vaccine.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 13.Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK. Microneedle-based vaccines. In: Compans RW, Orenstein OW, editors. Current topics in microbiology and immunology: vaccines for pandemic influenza. Berlin/Heidelberg, Germany: Springer-Verlag; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prausnitz MR. Microneedles for transdermal drug delivery. Adv Drug Del Rev. 2004;56(5):581–7. doi: 10.1016/j.addr.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 15.Bal SM, Caussin J, Pavel S, Bouwstra JA. In vivo assessment of safety of microneedle arrays in human skin. Eur J Pharm Sci. 2008;35(3):193–202. doi: 10.1016/j.ejps.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Gill HS, Denson DD, Burris BA, Prausnitz MR. Effect of microneedle design on pain in human volunteers. Clin J Pain. 2008;24(7):585–94. doi: 10.1097/AJP.0b013e31816778f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holland D, Booy R, De Looze F, Eizenberg P, McDonald J, Karrasch J, et al. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: A randomized controlled trial. J Infect Dis. 2008;198(5):650–8. doi: 10.1086/590434. [DOI] [PubMed] [Google Scholar]
- 18.Alarcon JB, Hartley AW, Harvey NG, Mikszta JA. Preclinical evaluation of microneedle technology for intradermal delivery of influenza vaccines. Clin Vaccine Immunol. 2007;14(4):375–81. doi: 10.1128/CVI.00387-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koutsonanos DG, Martin MdP, Zarnitsyn VG, Sullivan SP, Compans RW, Prausnitz MR, et al. Transdermal influenza immunization with vaccine-coated microneedle arrays. PLoS ONE. 2009;4(3):e4773. doi: 10.1371/journal.pone.0004773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu QY, Zarnitsyn VG, Ye L, Wen ZY, Gao YL, Pan L, et al. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc Natl Acad Sci USA. 2009;106(19):7968–73. doi: 10.1073/pnas.0812652106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton JG. Needle phobia - a neglected diagnosis. J Fam Prac. 1995;41(2):169–75. [PubMed] [Google Scholar]
- 22.Kermode M. Unsafe injections in low-income country health settings: need for injection safety promotion to prevent the spread of blood-borne viruses. Health Promot Int. 2004;19(1):95–103. doi: 10.1093/heapro/dah110. [DOI] [PubMed] [Google Scholar]
- 23.Quan FS, Compans RW, Nguyen HH, Kang SM. Induction of heterosubtypic immunity to influenza virus by intranasal immunization. J Virol. 2008;82(3):1350–9. doi: 10.1128/JVI.01615-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. J Control Release. 2007;117(2):227–37. doi: 10.1016/j.jconrel.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bronaugh RL, Stewart RF, Congdon ER. Methods for in vitro percutaneous absorption studies II Animal models for human skin. Toxicol Appl Pharmacol. 1982;62(3):481–8. doi: 10.1016/0041-008x(82)90149-1. [DOI] [PubMed] [Google Scholar]
- 26.Amorij JP, Huckriede A, Wischut J, Frifflink HW, Hinrichs WLJ. Development of stable influenza vaccine powder formulations: Challenges and possibilities. Pharm Res. 2008 Jun;25(6):1256–73. doi: 10.1007/s11095-008-9559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palladino G, Mozdzanowska K, Washko G, Gerhard W. Virus-neutralizing antibodies of immunoglobulin-G (IgG) but not of IgM or IgA isotypes can cure influenza-virus pneumonia in SCID mice. J Virol. 1995;69(4):2075–81. doi: 10.1128/jvi.69.4.2075-2081.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hobson D, Curry RL, Beare AS, Wardgard A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hygiene. 1972;70(4):767–77. doi: 10.1017/s0022172400022610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Reeth K. Cytokines in the pathogenesis of influenza. Vet Microbiol. 2000;74(1-2):109–16. doi: 10.1016/s0378-1135(00)00171-1. [DOI] [PubMed] [Google Scholar]
- 30.McElhaney JE, Dutz JP. Better influenza vaccines for older people: What will it take? J Infect Dis. 2008;198(5):632–4. doi: 10.1086/590435. [DOI] [PubMed] [Google Scholar]
- 31.Quan FS, Huang CZ, Compans RW, Kang SM. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J Virol. 2007;81(7):3514–24. doi: 10.1128/JVI.02052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snapper CM, Shen Y, Khan AQ, Colino J, Zelazowski P, Mond JJ, et al. Distinct types of T-cell help for the induction of a humoral immune response to Streptococcus pneumoniae. Trends Immunol. 2001;22(6):308–11. doi: 10.1016/s1471-4906(01)01926-3. [DOI] [PubMed] [Google Scholar]
- 33.Stingl G. Dendritic Cells of the Skin. Dermatol Clin. 1990;8(4):673–9. [PubMed] [Google Scholar]
- 34.Hauge S, Madhun A, Cox RJ, Haaheim LR. Quality and kinetics of the antibody response in mice after three different low-dose influenza virus vaccination strategies. Clin Vaccine Immunol. 2007;14(8):978–83. doi: 10.1128/CVI.00033-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belshe RB, Newman FK, Wilkins K, Graham IL, Babusis E, Ewell M, et al. Comparative immunogenicity of trivalent influenza vaccine administered by intradermal or intramuscular route in healthy adults. Vaccine. 2007;25(37-38):6755–63. doi: 10.1016/j.vaccine.2007.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leroux-Roels I, Vets E, Freese R, Seiberling M, Weber F, Salamand C, et al. Seasonal influenza vaccine delivered by intradermal microinjection: A randomised controlled safety and immunogenicity trial in adults. Vaccine. 2008;26(51):6614–9. doi: 10.1016/j.vaccine.2008.09.078. [DOI] [PubMed] [Google Scholar]
- 37.Morse SS. The US pandemic influenza implementation plan at six months. Nat Med. 2007;13(6):681–4. doi: 10.1038/nm1597. [DOI] [PubMed] [Google Scholar]


