Abstract
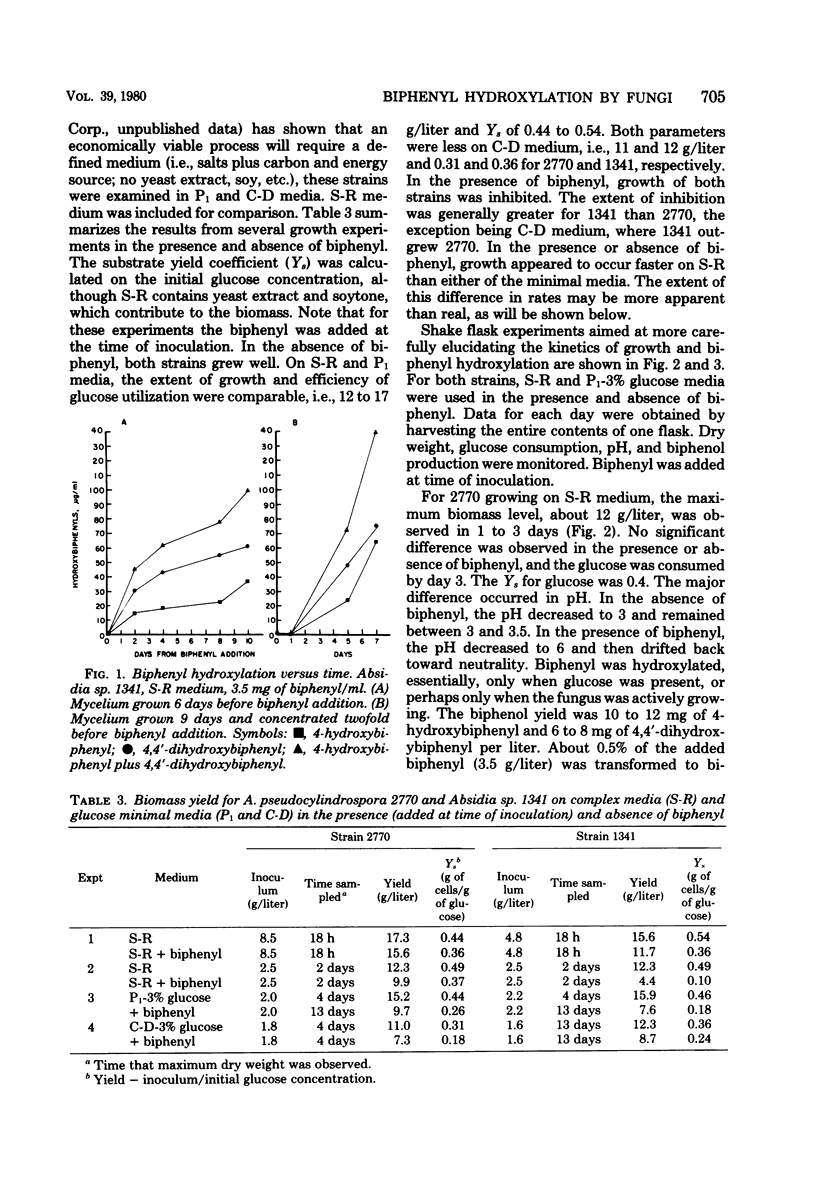
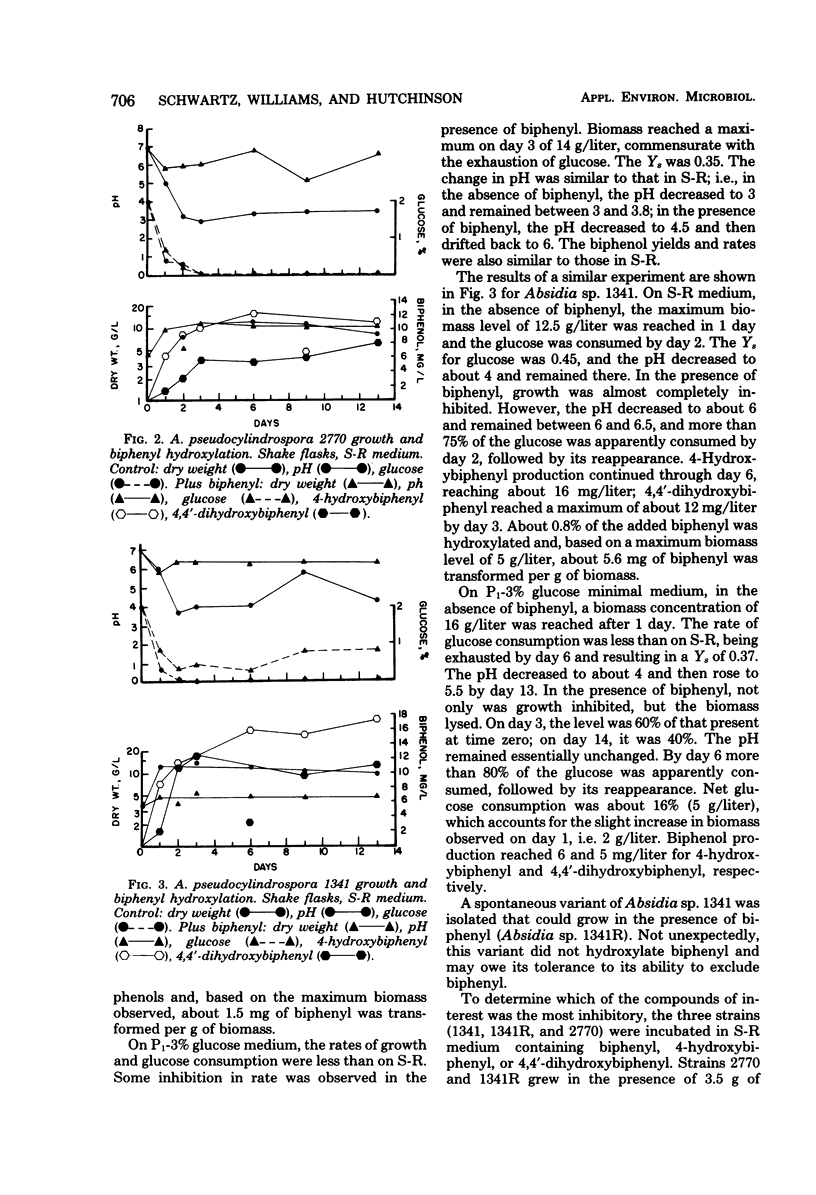
Of 15 species of fungi examined for their ability to hydroxylate biphenyl, 10 produced 4-hydroxybiphenyl. Seven of the 10 also produced 4,4′-dihydroxybiphenyl. The most efficient strains, Absidia pseudocylindrospora NRRL 2770 and Absidia sp. NRRL 1341, were more closely examined to determine their growth characteristics and the kinetics of biphenyl hydroxylation in batch fermentation. In the absence of biphenyl, A. pseudocylindrospora 2770 and Absidia sp. 1341 grew about as rapidly and efficiently in a defined glucose minimal medium as in a complex medium. Substrate yield coefficients for glucose in both media were 0.4 to 0.5 g of biomass per g of glucose, and the specific growth rate was about 0.17 h−1 (doubling time, about 4 h). In this unoptimized system, 10 to 15 g of biomass per liter (dry weight) could be produced, using a defined salt solution and glucose as sole carbon and energy source. In the presence of biphenyl, growth was inhibited, more so for strain 1341 than for strain 2770. However, the specific activity for biphenyl hydroxylation (milligrams of biphenol per gram of biomass) was about 3.5 times greater for strain 1341. Furthermore, biphenyl hydroxylation appeared to require the presence of an oxidizable carbon and energy source (and perhaps growth) to proceed and, at least for strain 1341, hydroxylation seemed to be more efficient in the complex medium.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed M., Focht D. D. Degradation of polychlorinated biphenyls by two species of Achromobacter. Can J Microbiol. 1973 Jan;19(1):47–52. doi: 10.1139/m73-007. [DOI] [PubMed] [Google Scholar]
- Brunner H., Röhr M. Novel system for improved control of filamentous microorganisms in continuous culture. Appl Microbiol. 1972 Sep;24(3):521–523. doi: 10.1128/am.24.3.521-523.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catelani D., Colombi A. Metabolism of biphenyl. Structure and physicochemical properties of 2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoic acid, the meta-cleavage product from 2,3-dihydroxybiphenyl by Pseudomonas putida. Biochem J. 1974 Nov;143(2):431–434. doi: 10.1042/bj1430431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catelani D., Colombi A., Sorlini C., Treccani V. Metabolism of biphenyl. 2-Hydroxy-6-oxo-6-phenylhexa-2,4-dienoate: the meta-cleavage product from 2,3-dihydroxybiphenyl by Pseudomonas putida. Biochem J. 1973 Aug;134(4):1063–1066. doi: 10.1042/bj1341063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge R. H., Cerniglia C. E., Gibson D. T. Fungal metabolism of biphenyl. Biochem J. 1979 Jan 15;178(1):223–230. doi: 10.1042/bj1780223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Matsumura F. Microbial metabolism of polychlorinated biphenyls. Studies on the relative degradability of polychlorinated biphenyl components by Alkaligenes sp. J Agric Food Chem. 1976 Mar-Apr;24(2):251–256. doi: 10.1021/jf60204a002. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Roberts R. L., Wells M. C., Kobal V. M. Oxidation of biphenyl by a Beijerinckia species. Biochem Biophys Res Commun. 1973 Jan 23;50(2):211–219. doi: 10.1016/0006-291x(73)90828-0. [DOI] [PubMed] [Google Scholar]
- Schwartz R. D., McCoy C. J. Epoxidation of 1,7-octadiene by Pseudomonas oleovorans: fermentation in the presence of cyclohexane. Appl Environ Microbiol. 1977 Jul;34(1):47–49. doi: 10.1128/aem.34.1.47-49.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. D. Octene epoxidation by a cold-stable alkane-oxidizing isolate of Pseudomonas oleovorans. Appl Microbiol. 1973 Apr;25(4):574–577. doi: 10.1128/am.25.4.574-577.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. V., Rosazza J. P. Microbial models of mammalian metabolism. Aromatic hydroxylation. Arch Biochem Biophys. 1974 Apr 2;161(2):551–558. doi: 10.1016/0003-9861(74)90338-5. [DOI] [PubMed] [Google Scholar]
- Wiseman A., Gondal J. A., Sims P. 4'-Hydroxylation of biphenyl by yeast containing cytochrome P-450: radiation and thermal stability, comparisons with liver enzyme (oxidized and reduced forms). Biochem Soc Trans. 1975;3(2):278–281. doi: 10.1042/bst0030278. [DOI] [PubMed] [Google Scholar]



