Abstract
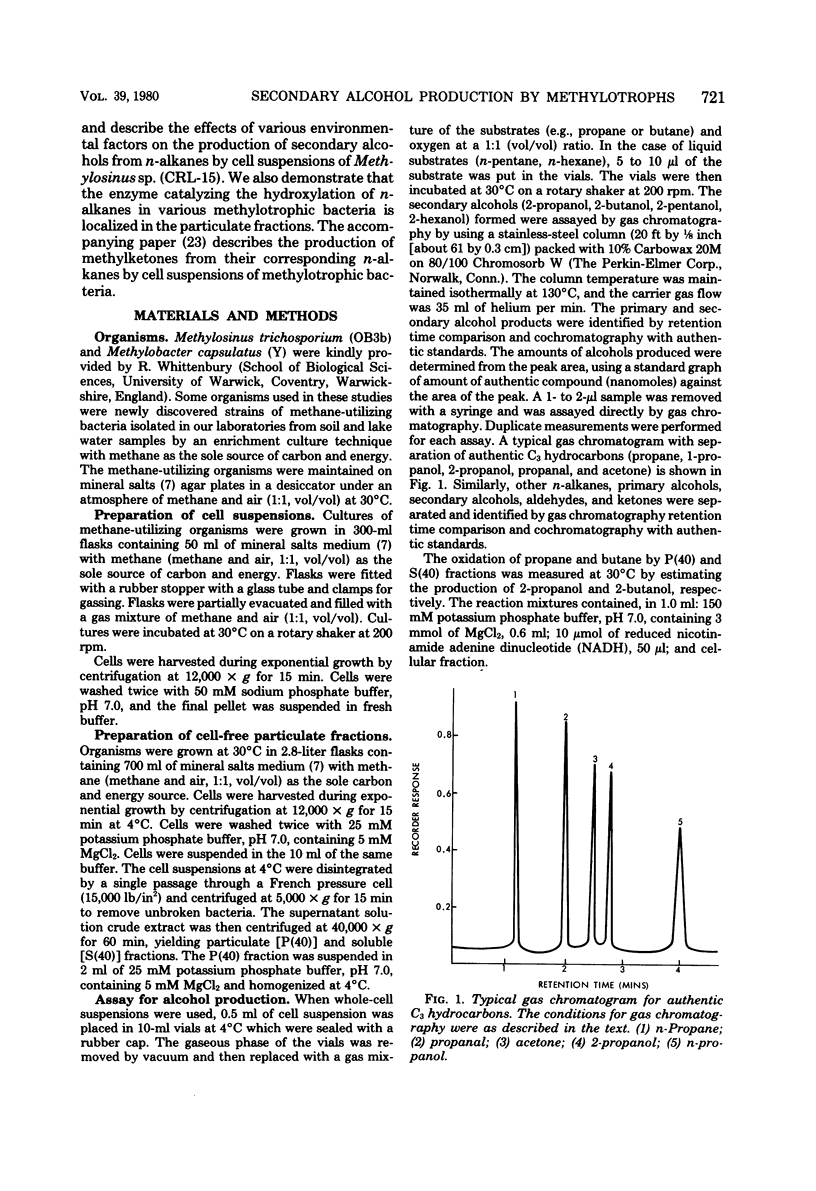
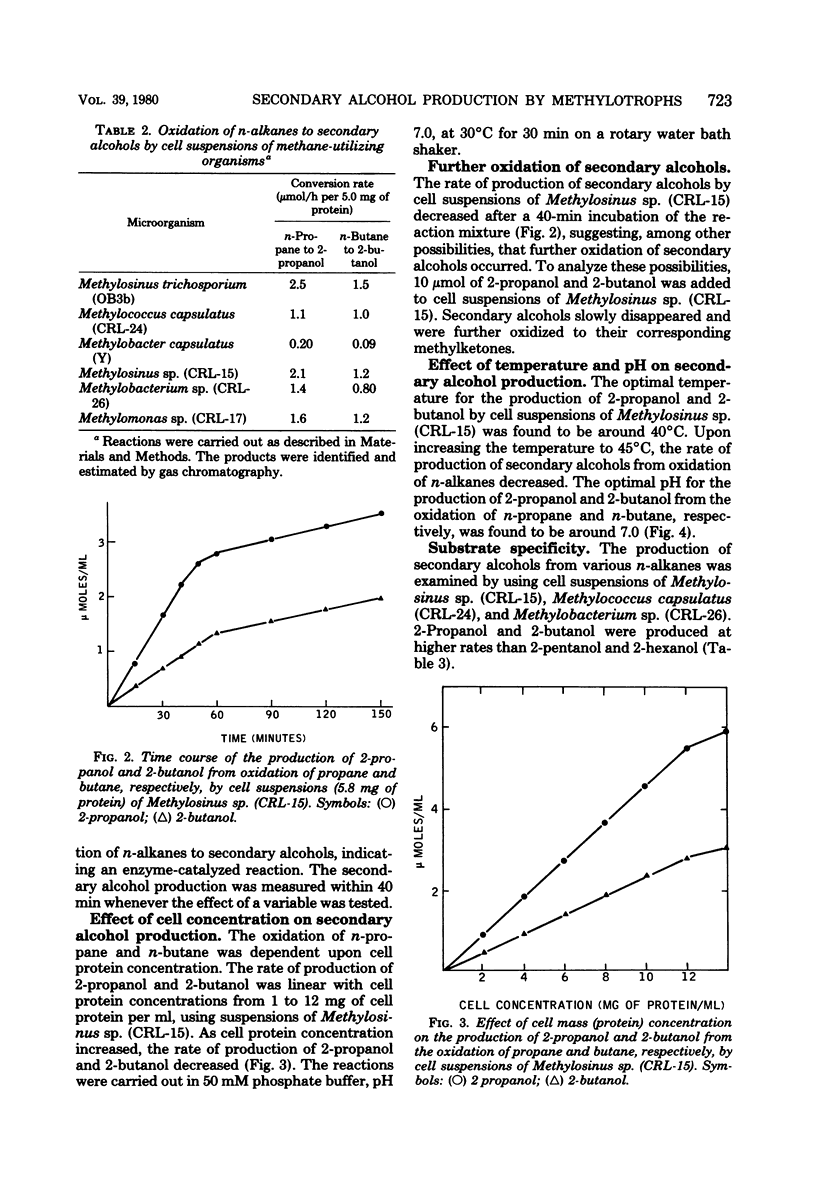
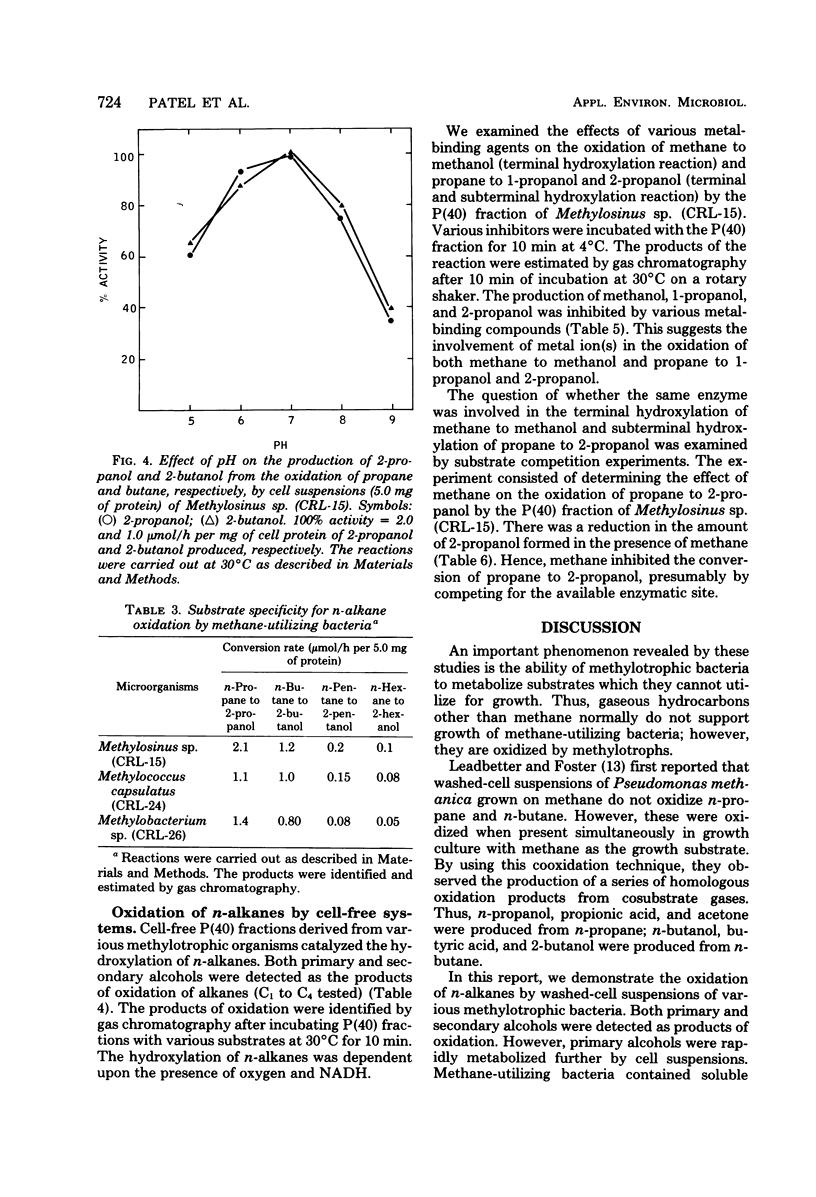
Over 20 new strains of methane-utilizing bacteria were isolated from lake water and soil samples. Cell suspensions of these and of other known strains of methane-utilizing bacteria oxidized n-alkanes (propane, butane, pentane, hexane) to their corresponding secondary alcohols (2-propanol, 2-butanol, 2-pentanol, 2-hexanol). The product secondary alcohols accumulated extracellularly. The rate of production of secondary alcohols varied with the organism used for oxidation. The average rate of 2-propanol, 2-butanol, 2-pentanol, and 2-hexanol production was 1.5, 1.0, 0.15, and 0.08 μmol/h per 5.0 mg of protein in cell suspensions, respectively. Secondary alcohols were slowly oxidized further to the corresponding methylketones. Primary alcohols and aldehydes were also detected in low amounts (rate of production were 0.05 to 0.08 μmol/h per 5.0 mg of protein in cell suspensions) as products of n-alkane (propane and butane) oxidation. However, primary alcohols and aldehydes were rapidly metabolized further by cell suspensions. Methanol-grown cells of methane-utilizing bacteria did not oxidize n-alkanes to their corresponding secondary alcohols, indicating that the enzymatic system required for oxidation of n-alkanes was induced only during growth on methane. The optimal conditions for in vivo secondary alcohol formation from n-alkanes were investigated in Methylosinus sp. (CRL-15). The rate of 2-propanol and 2-butanol production was linear for the 40-min incubation period and increased directly with cell protein concentration up to 12 mg/ml. The optimal temperature and pH for the production of 2-propanol and 2-butanol were 40°C and pH 7.0. Metalchelating agents inhibited the production of secondary alcohols. The activities for the hydroxylation of n-alkanes in various methylotrophic bacteria were localized in the cell-free particulate fractions precipitated by centrifugation between 10,000 and 40,000 × g. Both oxygen and reduced nicotinamide adenine dinucleotide were required for hydroxylation activity. The metal-chelating agents inhibited hydroxylation of n-alkanes by the particulate fraction, indicating the involvement of a metal-containing enzyme system in the oxidation of n-alkanes. The production of 2-propanol from the corresponding n-alkane by the particulate fraction was inhibited in the presence of methane, suggesting that the subterminal hydroxylation of n-alkanes may be catalyzed by methane monooxygenase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN L. R., STRAWINSKI R. J., MCCLESKEY C. S. THE ISOLATION AND CHARACTERIZATION OF METHANOMONAS METHANOOXIDANS BROWN AND STRAWINSKI. Can J Microbiol. 1964 Oct;10:791–799. doi: 10.1139/m64-100. [DOI] [PubMed] [Google Scholar]
- Colby J., Stirling D. I., Dalton H. The soluble methane mono-oxygenase of Methylococcus capsulatus (Bath). Its ability to oxygenate n-alkanes, n-alkenes, ethers, and alicyclic, aromatic and heterocyclic compounds. Biochem J. 1977 Aug 1;165(2):395–402. doi: 10.1042/bj1650395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Zatman L. J. Hexose phosphate synthese and tricarboxylic acid-cycle enzymes in bacterium 4B6, an obligate methylotroph. Biochem J. 1972 Aug;128(5):1373–1376. doi: 10.1042/bj1281373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DWORKIN M., FOSTER J. W. Studies on Pseudomonas methanica (Söhngen) nov. comb. J Bacteriol. 1956 Nov;72(5):646–659. doi: 10.1128/jb.72.5.646-659.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenci T., Strom T., Quayle J. R. Oxidation of carbon monoxide and methane by Pseudomonas methanica. J Gen Microbiol. 1975 Nov;91(1):79–91. doi: 10.1099/00221287-91-1-79. [DOI] [PubMed] [Google Scholar]
- Foster J. W., Davis R. H. A methane-dependent coccus, with notes on classification and nomenclature of obligate, methane-utilizing bacteria. J Bacteriol. 1966 May;91(5):1924–1931. doi: 10.1128/jb.91.5.1924-1931.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C. T., Patel R. N., Laskin A. I., Barnabe N., Marczak I. Identification and purification of a nicotinamide adenine dinucleotide-dependent secondary alcohol dehydrogenase from C1-utilizing microbes. FEBS Lett. 1979 May 1;101(1):179–183. doi: 10.1016/0014-5793(79)81321-6. [DOI] [PubMed] [Google Scholar]
- Johnson P. A., Quayle J. R. Microbial growth on C-1 compounds. 6. Oxidation of methanol, formaldehyde and formate by methanol-grown Pseudomonas AM-1. Biochem J. 1964 Nov;93(2):281–290. doi: 10.1042/bj0930281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEADBETTER E. R., FOSTER J. W. Bacterial oxidation of gaseous alkanes. Arch Mikrobiol. 1960;35:92–104. doi: 10.1007/BF00425597. [DOI] [PubMed] [Google Scholar]
- LEADBETTER E. R., FOSTER J. W. Studies on some methane-utilizing bacteria. Arch Mikrobiol. 1958;30(1):91–118. doi: 10.1007/BF00509229. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Patel R. N., Bose H. R., Mandy W. J., Hoare D. S. Physiological studies of methane- and methanol-oxidizing bacteria: comparison of a primary alcohol dehydrogenase from Methylococcus capsulatus (Texas strain) and Pseudomonas species M27. J Bacteriol. 1972 May;110(2):570–577. doi: 10.1128/jb.110.2.570-577.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. N., Felix A. Microbial oxidation of methane and methanol: crystallization and properties of methanol dehydrogenase from Methylosinus sporium. J Bacteriol. 1976 Oct;128(1):413–424. doi: 10.1128/jb.128.1.413-424.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. N., Hou C. T., Felix A. Microbial oxidation of methane and methanol: crystallization of methanol dehydrogenase and properties of holo- and apomethanol dehydrogenase from Methylomonas methanica. J Bacteriol. 1978 Feb;133(2):641–649. doi: 10.1128/jb.133.2.641-649.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. N., Hou C. T., Felix A. Microbial oxidation of methane and methanol: isolation of methane-utilizing bacteria and characterization of a facultative methane-utilizing isolate. J Bacteriol. 1978 Oct;136(1):352–358. doi: 10.1128/jb.136.1.352-358.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. N., Hou C. T., Felix A. Microbial oxidation of methane and methanol: purification and properties of a heme-containing aldehyde dehydrogenase from Methylomonas methylovora. Arch Microbiol. 1979 Sep;122(3):241–247. doi: 10.1007/BF00411286. [DOI] [PubMed] [Google Scholar]
- Patel R. N., Hou C. T., Laskin A. I., Derelanko P., Felix A. Oxidation of secondary alcohols to methyl ketones by yeasts. Appl Environ Microbiol. 1979 Aug;38(2):219–223. doi: 10.1128/aem.38.2.219-223.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. N., Hou C. T., Laskin A. I., Felix A., Derelanko P. Microbial Oxidation of Gaseous Hydrocarbons: Production of Methylketones from Corresponding n-Alkanes by Methane-Utilizing Bacteria. Appl Environ Microbiol. 1980 Apr;39(4):727–733. doi: 10.1128/aem.39.4.727-733.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. N., Hou C. T., Laskin A. I., Felix A., Derelanko P. Microbial oxidation of gaseous hydrocarbons. II. Hydroxylation of alkanes and epoxidation of alkenes by cell-free particulate fractions of methane-utilizing bacteria. J Bacteriol. 1979 Aug;139(2):675–679. doi: 10.1128/jb.139.2.675-679.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R., Hou C. T., Felix A. Inhibition of dimethyl ether and methane oxidation in Methylococcus capsulatus and Methylosinus trichosporium. J Bacteriol. 1976 May;126(2):1017–1019. doi: 10.1128/jb.126.2.1017-1019.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patt T. E., Cole G. C., Bland J., Hanson R. S. Isolation and characterization of bacteria that grow on methane and organic compounds as sole sources of carbon and energy. J Bacteriol. 1974 Nov;120(2):955–964. doi: 10.1128/jb.120.2.955-964.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbons D. W., Harrison J. E., Wadzinski A. M. Metabolism of single carbon compounds. Annu Rev Microbiol. 1970;24:135–158. doi: 10.1146/annurev.mi.24.100170.001031. [DOI] [PubMed] [Google Scholar]
- Ribbons D. W. Oxidation of C1 Compounds by Particulate fractions from Methylococcus capsulatus: distribution and properties of methane-dependent reduced nicotinamide adenine dinucleotide oxidase (methane hydroxylase). J Bacteriol. 1975 Jun;122(3):1351–1363. doi: 10.1128/jb.122.3.1351-1363.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan B. T., Johnson M. J. Production of bacterial cells from methane. Appl Microbiol. 1971 Mar;21(3):511–515. doi: 10.1128/am.21.3.511-515.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling D. I., Colby J., Dalton H. A comparison of the substrate and electron-donor specificities of the methane mono-oxygenases from three strains of methane-oxidizing bacteria. Biochem J. 1979 Jan 1;177(1):361–364. doi: 10.1042/bj1770361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonge G. M., Harrison D. E., Higgins I. J. Purification and properties of the methane mono-oxygenase enzyme system from Methylosinus trichosporium OB3b. Biochem J. 1977 Feb 1;161(2):333–344. doi: 10.1042/bj1610333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittenbury R., Phillips K. C., Wilkinson J. F. Enrichment, isolation and some properties of methane-utilizing bacteria. J Gen Microbiol. 1970 May;61(2):205–218. doi: 10.1099/00221287-61-2-205. [DOI] [PubMed] [Google Scholar]



