1.0. Introduction
Human hematopoietic cell transplantation (HCT) is now a standard procedure for many patients with hematologic malignancy and genetic disorders. The path from experimental to standard procedure has been difficult, as mortality in the early days was very high and the biology of transplant-related problems was so complex that improvement in outcomes had to wait for deeper scientific knowledge. Liver complications have become far less frequent because we now understand how to prevent and treat most of the serious hepatobiliary problems.(1,2). In supplementary material available on-line, I have provided three color plates of histologic findings, a brief discussion of indications for transplant and how HCT is carried out (Appendix 1), a glossary of common terms (Appendix 2), a list of abbreviations (Appendix 3), and a topic-oriented reading list (Appendix 4).
2.0. Evaluation of liver problems before transplant
2.1. Fungal liver infections
Hepatic fungal infection should be sought in transplant candidates with tender hepatomegaly, fevers, and abnormal liver enzymes, using high resolution computed tomography or magnetic resonance imaging, fungal biomarkers (galactomannan and glucan assays), and liver biopsy. Miliary fungal lesions are too small to be imaged. Fungal liver infection should be controlled before transplant with caspofungin, micafungin, or posaconazole until engraftment, which can then effect resolution of intractable fungal abscesses.(3) Patients without evidence of fungal liver abscesses at baseline routinely receive fluconazole, itraconazole, or voriconazole to prevent liver infections.
2.2. Viral hepatitis in allogeneic HCT donors
Hepatitis B and hepatitis C will be transmitted from viremic donors to transplant recipients.(1) When equally HLA-matched donors are available, a donor not infected by hepatitis viruses should be selected. If the best-matched donor has chronic hepatitis B or C, preventing passage of virus and mitigating the effects of infection in the recipient become priorities. In HBV+ donors, antiviral drugs will reduce viral load prior to harvest of donor cells. However, HBV may persist in donor peripheral blood cells despite clearance from serum.(4) If donor cells are rendered HBV DNA-negative before harvest, passage can be prevented. All recipients of cells from HBsAg positive donors should receive antiviral prophylaxis. HBsAg negative, anti-HBc-positive donors are viremic in fewer than 5% of cases and can be used as donors if their serum and peripheral blood stem cells are HBV DNA-negative. A donor who is naturally anti-HBs-positive is the preferred donor if the recipient is HBsAg-positive or anti-HBc-positive, as adoptive transfer of immunity can effect clearance of HBV from the recipient.(5)
If time permits, treatment of an HCV RNA+ donor to harvest of donor cells may render them less likely to transmit infection.(6) Small molecule antiviral drugs in current development may offer clinical benefit in rendering donors non-viremic, at least temporarily, to allow for harvest of HCV-free donor cells. If virus is transmitted, the acute phase of HCV infection may cause elevated liver enzymes at 2-3 months post-HCT, after recovery of T cell function.(7) Severe hepatitis is rare and the outcome of HCV-infected transplant survivors over 10 years of follow-up is no different than in survivors without HCV infection.(7)
2.3. Chronic liver disease in candidates for HCT
Patients being considered for HCT who have hepatic fibrosis, cirrhosis, or cholestasis, are at increased mortality risk.(7) HCV-infected patients may also have overall poorer survival related to infection. Patients with marginally-compensated cirrhosis (Child-Pugh B or C) should not receive high-dose conditioning regimens and may not be considered suitable candidates for HCT. Patients with Child-Pugh A cirrhosis are at risk for decompensation after HCT even if given a reduced-intensity conditioning regimen.(8) Patients with myelofibrosis and amyloidosis may also evince extensive sinusoidal fibrosis. Hepatitis B-infected HCT recipients are at additional risk for fulminant liver failure if not given antiviral drugs throughout the transplant process.(1) In patients with isolated anti-HBc antibodies, there is a 35% risk of HBV reactivation, usually during prednisone treatment for acute GVHD.(9) Severe hepatitis B reactivation has also been seen in anti-HBc+/anti-HBs+ patients and in those with occult hepatitis B.(10) Anti-viral prophylaxis will prevent almost all cases of fulminant hepatitis B after transplant if begun before the start of conditioning therapy in patients who are viremic (HBV DNA+) or HBs+; patients with latent HBV (anti-HBc+/HBV DNA-) should be monitored with HBV DNA tests after HCT and treated pre-emptively if viremia is detected.(1) Patients with cholestasis at baseline can be successfully transplanted, with subsequent normalization of liver abnormalities.(11)
2.4. Recent liver dysfunction in candidates for HCT
Patients who have had recent liver dysfunction following chemotherapy or radiation therapy in proximity to the start of HCT are also at risk.(12) Imatinib may cause acute hepatocellular necrosis and multiacinar collapse, with eventual healing by focal fibrosis. Gemtuzumab ozogamicin causes sinusoidal liver injury in 3-15% of patients(13); if a patient receives a liver-toxic myeloablative conditioning regimen within 3 months of exposure to high-doses of gemtuzumab ozogamicin, SOS may develop in 15-40% of cases. Therapeutic drug monitoring of components of the conditioning regimens has been used to prevent both liver and systemic toxicity among patients at risk.
2.5. Gallbladder and Bile Duct Stones
HCT candidates with incidental gallstones do not require operative intervention. Patients with symptomatic gallbladder or common duct stones should be considered for cholecystectomy or an endoscopic biliary procedure.
2.6. Iron Overload
HCT candidates with thalassemia, aplastic anemia, chronic leukemia or lymphoma may have marked hepatic iron overload, now readily documented with iron-specific MRI.(14) In patients with extreme iron overload, effective pre-HCT chelation therapy improves post-HCT survival. Excess tissue iron does not appear to increase the toxicity of the conditioning regimen. Severe iron overload has been associated with nonspecific liver dysfunction after transplant.(15) In most patients, quantitation of tissue iron stores and consideration of iron removal can be deferred until after recovery from HCT.
3.0. Hepatobiliary problems from transplant through day 200
Jaundice following HCT remains an ominous prognostic sign, with greatly increased non-relapse mortality in patients whose total serum bililrubin exceeds 4 mg/dL.(16)
3.1. Sinusoidal Obstruction Syndrome (SOS) (Figure 1)
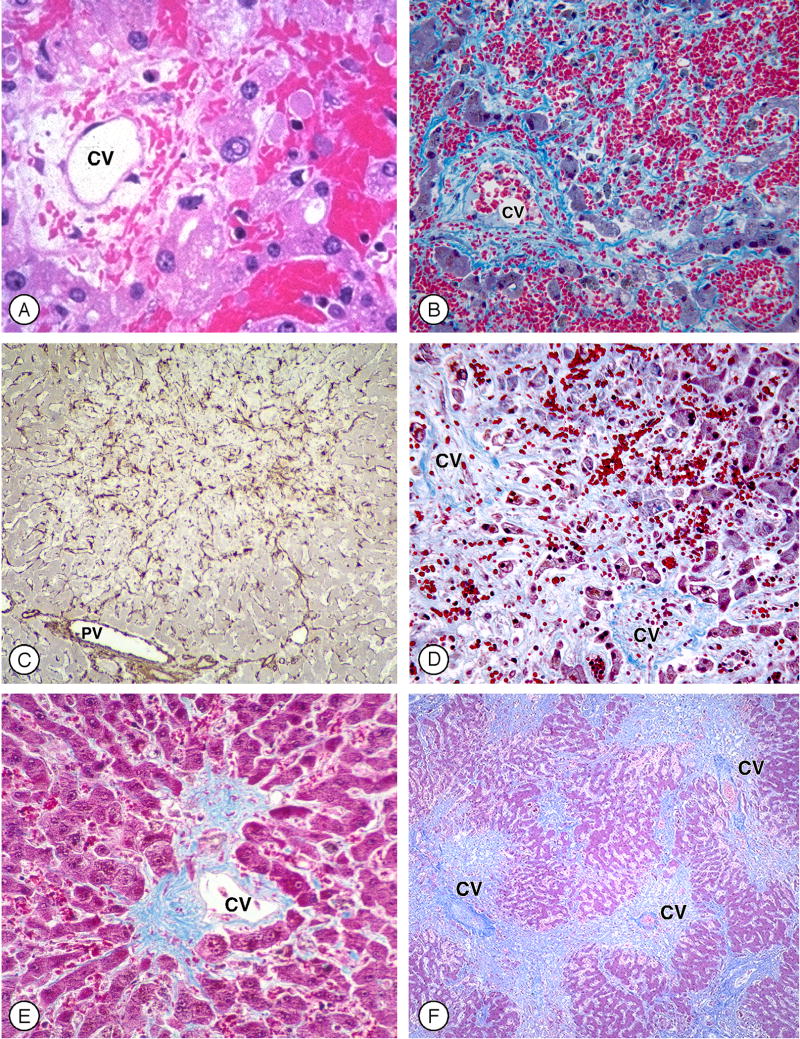
Figure 1. Histology of Sinusoidal Obstruction Syndrome (SOS) after myeloablative hematopoietic cell transplant (Figure 1 can be found in the on-line Supplementary Material).
A. Zone 3 of the liver acinus in an early phase of SOS, with disruption of sinusoidal anatomy, red blood cells extending through the space of Disse, hepatocyte necrosis, and subendothelial edema in a patent central vein (CV) (H&E).
B. Extensive hepatocyte necrosis and dropout, disruption of sinusoids, extravasation of red blood cells throughout zone 3, and subendothelial fibrosis (Masson trichrome).
C. Alpha actin-positive stellate cells within zones 2 and 3 that contain areas of extensive hepatocyte necrosis; periportal hepatocytes are intact (PV, portal vein) (alpha-smooth muscle actin immunohistology).
D. A later phase of SOS, showing extensive collagenization of sinusoids adjacent to two central veins, with hepatocyte dropout and extinction of hepatocyte cords in between the veins (Masson trichrome).
E. Central vein and zone 3 hepatocytes later after transplant, illustrating eccentric phlebosclerosis and collagen deposition in sinusoids (Masson trichrome).
F. Lower power view of confluent fibrosis in and around adjacent central veins, with central to central bridges forming a picture of “reverse” cirrhosis two months after transplant (Masson trichrome).
(Photomicrographs by Howard M. Shulman, M.D.)
Definition
SOS is a syndrome of tender hepatomegaly, fluid retention and weight gain, and elevated serum bilirubin that follows high-dose myeloablative conditioning therapy.(17,18) This syndrome is sometimes called veno-occlusive disease (VOD), but this term is inaccurate, as the liver injury is initiated by damage to hepatic sinusoids and occlusion of hepatic venules is not essential to development of signs and symptoms.(19)
Incidence
SOS is caused by toxins in certain conditioning regimens, thus, the reported incidence varies with the composition and intensity of the conditioning regimen, from zero after most reduced intensity regimens(8) to as high as 50% after cyclophosphamide (CY) 120 mg/kg plus total body irradiation (TBI) >14 Gy. Other important contributors to variability in the incidence of SOS are 1) variations in the metabolism of CY from patient to patient in CY/TBI regimens(20, 21); 2) underlying fibro-inflammatory liver diseases(7); and 3) concomitant use of drugs during and after conditioning therapy that either affect the metabolism of myeloablative drugs (for example, itraconazole) or cause concomitant liver injury (for example, methotrexate, sirolimus, norethisterone) (Table 1). During the 1990s, the overall incidence of SOS among patients at our center was 38% (7% severe) following CY/TBI and 12% (2% severe) following targeted oral busulfan plus CY.(20,22) However, the frequency and severity of SOS have fallen dramatically recently because: 1) doses of TBI >14 Gy are seldom used; 2) fludarabine is replacing CY; 3) patients at risk for SOS are being given conditioning regimens that do not contain either CY or TBI >12 Gy; 4) the incidence of chronic hepatitis C is low; and 5) therapeutic drug monitoring allows personalized dosing of chemotherapy drugs that have variable metabolism. Pediatric patients receiving busulfan/melphalan conditioning regimens remain at risk. A meta analysis suggests that prophylaxis with ursodiol prevents SOS, but the largest randomized trial of ursodiol that specifically tracked SOS as an endpoint found no evidence of protection.(2) It seems likely that many past patients diagnosed as having SOS on the basis of jaundice had mostly cholestatic and not sinusoidal liver injury.
Table 1. Factors that increase the risk of severe sinusoidal liver injury resulting from the conditioning regimen. Refer to Appendix 3 for abbreviations.
| Liver diseases at baseline | Specific conditioning regimens | Concomitant drugs during conditioning therapy |
|---|---|---|
| Inflammatory diseases | Cyclophosphamide-based | Itraconazole |
| Chronic hepatitis B or C | CY 120 mg/kg + TBI (greater risk with higher TBI dosing) | Sirolimus (rapamycin) |
| Non-alcoholic steatohepatitis | BCV (BCNU + CY + VP-16) | Norethisterone |
| Alcoholic hepatitis | BU + CY (greater risk without therapeutic drug monitoring of BU) | |
| Fibrotic diseases | Melphalan-based | |
| Cirrhosis | BU + MEL + thiotepa | |
| Lobular fibrosis | BU + MEL | |
| Extramedullary hematopoiesis with sinusoidal fibrosis | Other regimens | |
| Cholestatic disorders | BU + TBI (greater risk with higher TBI dosing) | |
| Jaundice caused by intrahepatic cholestasis | Gemtuzumab ozogamicin-containing myeloablative regimens | |
| Past history | High-dose radiolabeled antibody myeloablative regimens | |
| Prior SOS from conventional chemotherapy | ||
| Recent exposure to gemtuzumab ozogamicin | ||
| Prior liver irradiation | ||
| Prior myeloablative hematopoietic cell transplant | ||
Clinical presentation and diagnosis
The onset of SOS is heralded by an increase in liver size, right upper quadrant tenderness, renal sodium retention, and weight gain, occurring 10 to 20 days after the start of CY-based cytoreductive therapy and later after other myeloablative regimens. Hyperbilirubinemia follows these signs of portal hypertension by 4-10 days. Portal hypertension, renal and lung dysfunction, and refractory thrombocytopenia strongly suggest SOS.
Measurement of total serum bilirubin is a sensitive test for SOS but not a specific one. Elevations of serum AST/ALT weeks after the clinical onset of SOS reflect ischemic hepatocyte necrosis from sinusoidal fibrosis (Figure 1D).(23) Several plasma proteins have been reported to be abnormally high in patients with SOS (endothelial cell markers, thrombopoietin, pro-inflammatory cytokines, vascular endothelial growth factor, and procollagen peptides); some laboratory tests are abnormally low in patients with SOS (protein C, antithrombin III, and platelet counts) (reviewed in (17)). It is not clear whether any laboratory tests have diagnostic or prognostic utility beyond the clinical criteria of weight gain, jaundice, and hepatomegaly.
Imaging studies of the liver are useful for demonstrating hepatomegaly, ascites, periportal edema, attenuated hepatic venous flow, and gall bladder wall edema consistent with SOS(24), as well as excluding other causes of hepatomegaly and jaundice. Abnormal findings later in the course of SOS may include an enlarged portal vein diameter, slow or reversed flow in the portal vein or its segmental branches, high congestion index, portal vein thrombosis, and increased resistive index to hepatic artery flow. Unfortunately, ultrasound findings very early in the course of SOS—when there is diagnostic uncertainty—do not appear to add to the information provided by clinical criteria.
A transvenous approach that allows both biopsy and hepatic venous pressure measurements is the most accurate diagnostic test.(25,26) A hepatic venous pressure gradient above 10 mm Hg is highly specific for SOS.(25,26) Initial histologic changes are dilation of sinusoids, extravasation of red cells through the space of Disse, necrosis of perivenular hepatocytes, and widening of the subendothelial zone in central veins (Figure 1A and B).(17,19) A finding of “hemorrhage” in zones 2 and 3 of the liver acinus is the result of destruction of sinusoidal endothelium—the initiating injury in SOS. In severe SOS, fragmented hepatocyte cords can be seen, with dislodgement of hepatocytes into both portal and hepatic venules. The later stages of SOS are characterized by activation and proliferation of stellate cells (Figure 1C), extensive collagenization of sinusoids (Figure 1D), and a variable degree of obstruction of venular lumens by collagenized vein walls (Figure 1E), leading to obliteration of sinusoidal blood flow. In severe SOS—if patients survive beyond day +50 post-transplant—a pattern of reverse cirrhosis may develop, with extensive linkage between obliterated central venules by fibrous bridges, collapse, and acinar extinction (Figure 1F). Intensity of collagenization of sinusoids and central veins is correlated with outcome.(19)
Clinical course and prognosis
Complete recovery from SOS occurs in over 70% of patients with just supportive care. Patients with severe SOS seldom die of liver failure, but rather from renal and cardiopulmonary failure.(18,27) For research purposes, a retrospective scoring system classifies SOS as mild (clinically obvious, requires no treatment, resolves completely), moderate (signs and symptoms requiring treatment such as diuretics or pain medications, resolves completely), or severe (requires treatment but does not resolve before death or day +100). Useful prognostic findings include the rapidity with which weight is gained and serum bilirubin rises, development of ascites, renal insufficiency, and hypoxemia.(18,27,28)
Pathogenesis of SOS
Damage to hepatic sinusoids is the proximate cause of SOS; 45% of patients with mild or moderate SOS and 25% of patients with severe SOS did not have occluded hepatic venules at autopsy.(19) Occlusion of central veins of the liver lobule is associated with more severe disease and the development of ascites.
Chemotherapy drugs in conditioning regimens
CY is common to the conditioning regimens with the highest incidence of fatal SOS—CY/TBI, BU/CY, and BCV. The metabolism of CY is highly variable and unpredictable; patients who generate a greater quantity of toxic CY metabolites are more likely to develop fatal SOS.(20) Accurate methods to target the dose of CY to a metabolic endpoint allow personalized CY dosing, significantly reducing liver and kidney injury.(21)
BU is another component of regimens with a high frequency of SOS, but BU itself does not appear to be hepatotoxic.(29,30) BU may contribute to liver injury by inducing oxidative stress, reducing glutathione levels in hepatocytes and sinusoidal endothelial cells (29) and altering CY metabolism.(22) Co-administration of the BU/CY regimen with sirolimus increases the frequency of SOS.
Gemtuzumab ozogamicin may cause sinusoidal liver injury when used to treat patients with AML.(13) The risk of SOS is 15-40% when high-dose gemtuzumab ozogamicin given in proximity ot a CY-based myeloablative regimen. Gemtuzumab may also cause SOS when given for relapsed AML after HCT.
Total Body Irradiation
In combination with CY, there is a clear relationship between the total dose of TBI and the frequency of severe SOS--approximately 1% after CY/TBI 10 Gy, 4-7% after CY/TBI 12-14 Gy, and 20% after CY/TBI >14 Gy.
Intrahepatic coagulation
Some see SOS as a disease of disordered coagulation, in which damage to endothelium in the sinusoids and central veins leads to thrombosis. However, sinusoidal endothelial cells embolize downstream in SOS; heparin and antithrombin III infusions are ineffective in preventing fatal SOS; thrombolytic therapy effects improvement in few patients; and genetic disorders predisposing to coagulation have no associations with SOS. However, thrombosis in the portal vein may result from a hypercoaguable state in patients with severe SOS.
Stellate cells and sinusoidal fibrosis
Procollagen peptides appear in the serum of patients who develop more severe SOS, along with inhibitors of fibrolysis, consistent with the intense fibrosis in sinusoids and venular walls that is common in fatal SOS.(19) Immunohistology for alpha-actin in liver specimens from patients with SOS shows intense staining in sinusoids (Figure 1C).(17)
Genetic factors
Genetically-determined differences in drug metabolism or susceptibility to toxic injury might explain some of the variability in the frequency of SOS. Small case-control studies using single nucleotide polymorphisms have reported associations between SOS and the Carbamyl Phosphate Synthetase 1 c.4340C>A (CPS1), Factor 5 c.1691G>A (FV Leiden), HFE C282Y and glutathione S-transferase (GSTM1 and GSTT1) genes. These associations could not be confirmed in a cohort of 147 Seattle patients receiving a CY/TBI regimen (McDonald GB, unpublished observations).
Prevention of SOS in patients receiving myeloablative therapy
The only certain way to prevent fatal SOS is to avoid damaging hepatic sinusoidal endothelial cells, especially in patients at risk. The two most common sinusoidal toxins are CY and TBI, but other chemotherapy drugs and radiolabeled antibodies have the potential for sinusoidal injury (Table 1).(12,17,20) The challenge for transplant oncologists is to remove liver-toxic drugs from conditioning regimens without sacrificing engraftment or malignancy relapse rates. Prevention of severe sinusoidal liver injury begins with an assessment of the risk in patients with underlying liver disease, for a given myeloablative conditioning regimen (Table 1).
Patients at risk for fatal SOS have several options: 1) conventional therapy that does not involve HCT; 2) a reduced-intensity conditioning regimen(8); 3) a myeloablative regimen that does not contain cyclophosphamide, for example, targeted busulfan-fludarabine for allogeneic(31) or BEAM(32) for autologous HCT; 4) modification of CY-based regimens(21); 5) use of pharmacologic approaches to prevent sinusoidal liver injury. Of the proposed methods for accurate dosing of chemotherapy agents, only therapeutic drug monitoring has been studied in the HCT setting.(12)
If a CY/TBI regimen must be used for a patient at risk for fatal SOS, modifications should be considered for both CY and TBI dosing. The total dose of CY should be 90-110 mg/kg range(21) and TBI doses should not exceed 12 Gy unless there is an oncologic imperative for higher doses.(20) Shielding the liver during TBI will lessen liver injury but leads to relapse of underlying hematological disease. Accurate methods are available to target CY doses to a metabolic endpoint, based on exposure to the CY metabolites 4-hydroxyCY and carboxyethylphosphoramide mustard.(21)
If a BU/CY regimen must be used for a patient at risk for fatal SOS, liver toxicity may be less frequent if CY is given before targeted BU or if dosing of CY is delayed for 1-2 days after completion of BU. BU and phenytoin to prevent BU-related seizures result in increased exposure to toxic CY metabolites when CY is given second in order, compared to giving CY first in order.(22) A lower incidence of SOS has been reported following iv BU/CY, compared to oral BU/CY, when neither BU formulation was adjusted for metabolism. The metabolism of iv BU is variable, with a several-fold range in AUCBU, a problem that can be addressed by therapeutic drug monitoring.(33)
Pharmaceutical prevention of SOS has been achieved in animal models of sinusoidal injury (17) but these strategies (repletion of intracellular GSH or inhibition of matrix metalloproteinase enzymes) have not been studied in the clinical setting. Infusion of defibrotide has been reported to be effective as prophylaxis; preliminary results from a large randomized trial in children reported less liver disease and better outcomes in those receiving defibrotide.(34) Prospective studies have shown no benefit from use of prophylactic heparin or antithrombin III in preventing fatal SOS. A meta analysis suggests that ursodiol may prevent SOS, but SOS was not differentiated from cholestatic liver disease in these studies and a large randomized trial showed no effect of ursodiol on the frequency of SOS.(2)
Treatment of patients with SOS
For >70% of patients with SOS who will recover spontaneously, treatment involves management of sodium and water balance, preservation of renal blood flow, and repeated paracenteses for ascites that is associated with discomfort or pulmonary compromise. Patient with a poor prognosis can be recognized soon after disease onset by steep rises in total serum bilirubin and body weight, serum ALT values >750 U/L, portal pressures over 20 mm Hg, development of portal vein thrombosis, and especially by multiorgan failure requiring dialysis, hemofiltration, or mechanical ventilation.(16,18,23,26-28) There are no satisfactory therapies for severe SOS; the best current results (46% complete response rate, defined as total serum bilirubin <2 mg/dL and resolution of multiorgan failure) are with intravenous defibrotide.(35) Defibrotide, a mixture of porcine oligodeoxyribonucleotides, has antithrombotic and profibrinolytic effects in vitro and in vivo. However, its mechanism of action in the treatment of SOS is not known. The complete recovery of some patients with severe SOS and multi-organ failure suggests that the drug has biologic effects in man.(35)
Numerous other approaches to treatment of severe SOS have been reported (tissue plasminogen activator, intravenous N-acetylcysteine, human antithrombin III concentrate, activated protein C, prostaglandin E1, prednisone, topical nitrate, vitamin E plus glutamine, and use of a liver assist device), but none can be currently recommended. Transhepatic shunts have been placed in patients with SOS to reduce portal pressure and mobilize ascites, but neither serum bilirubin levels nor patient outcomes were improved. Patients with persistent ascites and normal serum bilirubin have undergone successful portosystemic shunts. Peritoneovenous shunts for intractable ascites have been unsuccessful. Successful liver transplants for severe SOS have been reported but in most centers, patients at risk for recurrent malignancy are low-priority candidates for liver transplant. Prevention of sinusoidal injury is likely to be a more effective strategy for improving transplant outcomes than treatment.
3.2. Cholestatic disorders: cholangitis lenta, acute GVHD, and drug-induced liver injury
Prophylactic ursodeoxycholic acid reduces the frequency of cholestasis in general and GVHD-related cholestasis specifically and improves outcomes, compared to placebo.(2)
Cholangitis lenta
Hyperbilirubinemia is common when patients are neutropenic and febrile and have gut mucosal injury from the conditioning regimen. Hepatocyte retention of conjugated bilirubin is mediated by endotoxins, IL6, and TNFα. Although this disorder is often referred to as “cholestasis of sepsis”, it occurs in patients with fever alone and in the presence of localized infection in the lungs and soft tissues.
Acute GVHD (Figure 2)
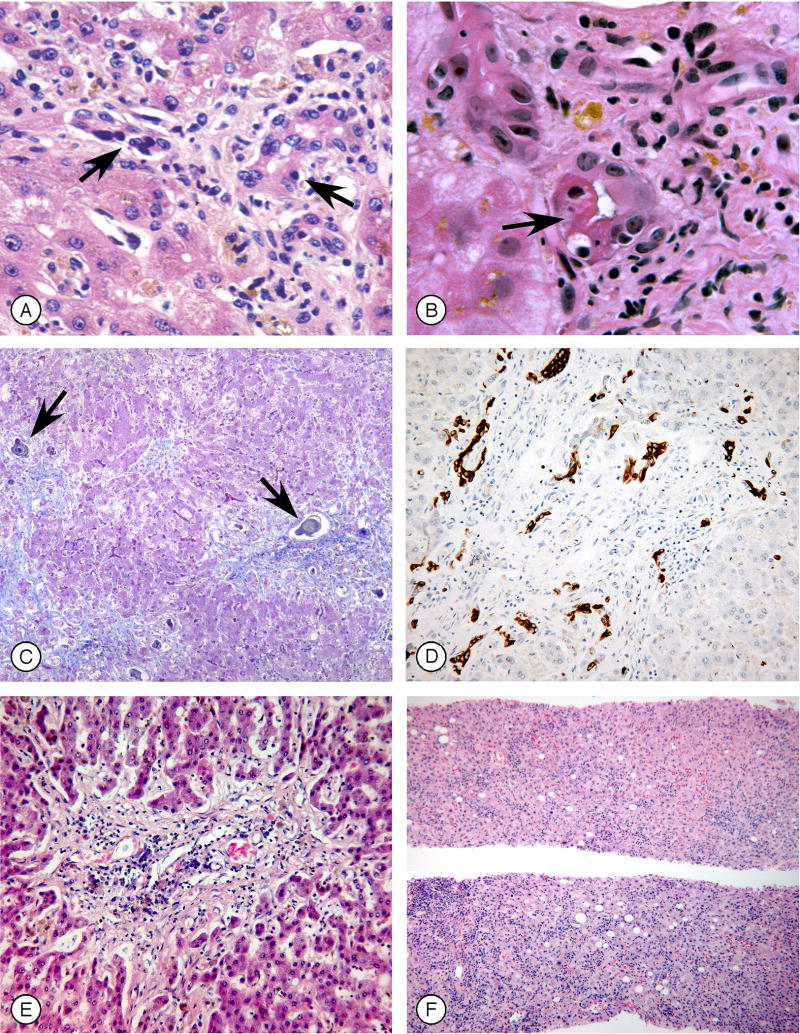
Figure 2. Histology of graft-vs.-host disease (GVHD) involving the liver (Figure 2 can be found in the on-line Supplementary Material).
A. Portal area showing small bile ducts (arrows) with a distorted appearance, lymphocyte infiltration, and epithelial drop-out (H&E).
B. Small bile ducts, showing dysmorphic features, cytoplasmic eosinophilia, apoptosis (arrow), atypical nuclei, and lymphocytic infiltration (H&E).
C. Liver lobules from a patient with severe multisystem acute GVHD, showing fibrotic portal spaces and periportal bile thrombi (arrows) (Masson trichrome).
D. Immunohistochemical stain for cytokeratin 19 in a patient with longstanding liver GVHD, illustrating ductular reaction at the periphery of a portal but without an identifiable interlobular bile duct.
E. A portal space showing absence of recognizable bile duct epithelium in a patient with longstanding refractory chronic GVHD (H&E).
F. Diffuse lobular inflammation, from a patient with a hepatitic onset of GVHD following discontinuation of immunosuppressive drug therapy (H&E).
(Photomicrographs by Howard M. Shulman, M.D.)
Acute GVHD develops in up to 70% of allograft patients, depending on the degree of HLA-match between donor and patient, the intensity of GVHD prophylaxis, and whether T cell are depleted from the donor inoculum. Prophylaxis with ursodiol has greatly decreased the frequency of jaundice after transplant and has altered the clinical phenotype of GVHD.(2) In retrospect, what had been called hepatic GVHD is a mélange of three processes. The first process is jaundice developing in a patient with cutaneous and intestinal GVHD. A blinded study could identify no histologic features characteristic of GVHD when liver biopsies were done within several weeks of the onset of GVHD(36), suggesting that jaundice occurring early in GVHD is related to cholestasis caused by cytokines such as IL-6. The second process is characterized by increases in bilirubin, alkaline phosphatase and GGT, usually in patients with gastrointestinal GVHD, in whom liver biopsies show lymphocytic infiltration of small bile ducts with nuclear pleomorphism, epithelial cell dropout, and cholestasis in zone 3 of the liver acinus (Figure 2).(36) Although the bile duct lesions of GVHD resemble those of primary biliary cirrhosis and some patients have positive anti-mitochondrial antibodies, all AMA-positive samples are false positives and the histology differs (no granulomata or large bile duct lesions are seen in GVHD).(36) Inflammatory infiltrates may be minimal because of immune suppression. Persistent hepatic GVHD and worsening jaundice are associated with ductopenia. The third process in hepatic GVHD is most commonly seen in allograft recipients on minimal immunosuppression or after donor lymphocyte infusion, in whom GVHD presents as an acute hepatitis with marked elevation of serum ALT.(37)
Treatment of acute GVHD is complex and controversial, particularly with regard to treatment of patients who fail to respond to first-line therapy with prednisone (1–2 mg/kg/day). Initial treatment should be based on the risk of a fatal outcome, with more intense immune suppression reserved for patients with the worst prognosis, and conversely, minimal immune suppression for those whose outcome is favorable. Prognosis in patients with GVHD is not related to peak severity of signs and symptoms, but rather to the area under a disease activity curve.(38) In less than 5% of current allograft recipients, acute GVHD is a fatal illness for which there is no effective therapy. Persistent jaundice is an independent predictor of mortality.(16,38)
Drug-induced liver injury
Cyclosporine inhibits canalicular bile transport and commonly causes mild increases in serum bilirubin without an effect on serum ALT or alkaline phosphatase. Tacrolimus less commonly causes cholestasis, except in the setting of toxic blood levels. Many other drugs used after HCT have been associated with liver dysfunction (e.g., trimethoprim-sulfamethoxazole, itraconazole, voriconazole, fluconazole, posaconazole), although drugs are usually not responsible for severe liver injury in this setting.(23)
3.3. Acute hepatocellular injury (Figure 3)
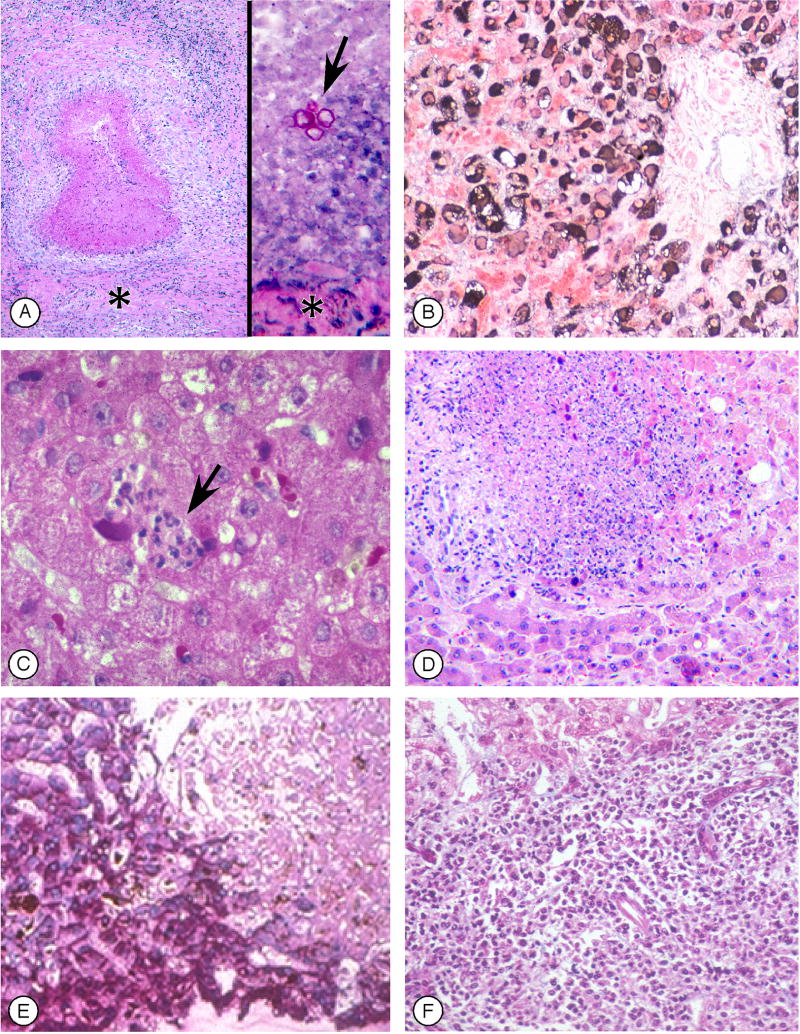
Figure 3. Infections in the liver following hematopoietic cell transplant (Figure 3 can be found in the on-line Supplementary Material).
A. Fungal liver abscesses demonstrating the variability of findings in different samples. On the left, a sterile healing abscess with a necrotic center devoid of fungal elements, surrounded by inflammatory cells and a pseudo-capsule (asterisk) (H&E). On the right, an acute abscess with a small focus of red-staining fungal elements (arrow) in a field of degenerative neutrophils, surrounded by a pseudo-capsule (PAS).
B. Immunohistochemistry for hepatitis B core antigen, in a patient with fulminant hepatitis B after transplant, showing extensive periportal hepatocyte cytoplasmic and some nuclear staining.
C. Focal microabscess (arrow) in the liver lobule caused by cytomegalovirus, in which lymphocytes and neutrophils are seen adjacent to enlarged, brick-red cells containing CMV (H&E)
D. Confluent hepatocyte necrosis caused by Adenovirus infection; in the rim of hepatocytes surrounding the necrotic area are darker “smudged nuclei” typical of Adenovirus (H&E).
E. Confluent hepatcyte necrosis (upper right) caused by Varicella Zoster Virus infection, with absence of PAS staining of necrotic cells.
F. Diffuse infiltration by plasmacytoid cells and immunoblasts with displacement of portal structures, caused by Epstein-Barr Virus lymphoproliferative disease (H&E).
(Photomicrographs by Howard M. Shulman, M.D.)
Elevation of serum aminotransferase enzymes are common and usually in the range of ∼100-500 U/L for AST/ALT.(2,7) Since the advent of antiviral drugs, the frequency of viral infection as a cause of acute elevation of serum ALT/AST after HCT has plummeted.(23) An extreme rise in ALT is now mostly due to a non-infective cause such as zone 3 hepatocyte necrosis in SOS, hypoxic hepatitis, drug-induced liver injury, or a hepatitic presentation of GVHD.(23,37)
Viral infections (Figure 3B-F)
Acute hepatitis caused by HSV, VZV, Adenovirus, and Hepatitis B virus can lead to fatal fulminant hepatic failure after HCT(1) and EBV-lymphoproliferative disease can be rapidly progressive (Figure 3F), whereas hepatic infections caused by CMV and hepatitis C virus are seldom severe.(7) With routine use of prophylactic acyclovir or valacyclovir, acute hepatitis due to HSV and VZV is now rare(23); HHV-6 and HHV-8 reactivation causing hepatitis despite antiviral prophylaxis has been reported. When there is uncertainty about the cause of rising serum ALT levels, DNA blood tests for herpesviruses, Adenovirus, and HBV should be performed. Transvenous measurement of the wedged hepatic venous pressure gradient and biopsy can exclude hepatocyte necrosis caused by SOS and may demonstrate a specific viral diagnosis.(25,26) Acyclovir should be started empirically, particularly if the patient presents with abdominal complaints typical of VZV infection.(39) Adenovirus hepatitis should be suspected if the patient has concomitant pulmonary, renal, bladder, or intestinal symptoms; the most effective treatment is cidofovir when given early.(40) Detection of EBV DNA by PCR in patients receiving immune suppressive drugs has allowed pre-emptive therapy with anti-B cell therapy.
Fulminant hepatitis B may develop during immune reconstitution in patients at risk, but can be prevented with prophylactic antiviral agents.(1,4) If severe hepatitis B reactivation does occur, usually because a diagnosis of HBV was not made prior to HCT(10), antiviral therapy should be initiated immediately. Fulminant hepatitis B has also been reported following premature discontinuation of prophylactic antiviral therapy in oncology patients. Anti-HBV drugs should be not be discontinued until six months after full immune reconstitution.(1) All patients, particularly those with high pre-transplant HBV DNA levels, should be monitored by HBV DNA and serum ALT tests following antiviral drug withdrawal.
Chronic hepatitis C in HCT recipients usually results in asymptomatic elevation of ALT from days +60-120, coinciding with the tapering of immunosuppressive drugs.(7) Severe HCV hepatitis has only rarely been reported; antiviral therapy for HCV is not indicated early after HCT. Fibosing cholestatic hepatitis C is rare after HCT. Therapy directed at chronic HCV infection should be considered once the patient has ceased all immunosuppressive drugs and has no evidence of active GVHD.
Hypoxic hepatitis
Among 6,225 consecutive HCT recipients, 1.4% had AST >1500 U/L; the most common causes were hypoxic hepatitis related to SOS, respiratory failure, and shock syndromes.(23) In SOS, AST increases occurred 2-6 weeks after the onset of liver injury; peak AST was 2,252 U/L and the case fatality rate was 76%. In patients with shock or prolonged hypoxemia, peak serum AST was 3,545 U/L within days, and the case fatality rate was 88%.(23)
Hepatitic presentation of GVHD
Elevations of serum ALT (∼100-300 U/L) are common during the onset of hepatic GVHD during a time when GVHD prophylaxis is being given. In the absence of prophylaxis or after donor lymphocyte infusion, serum ALT may rise rapidly, followed by jaundice, a result of an acute lobular hepatitis and damage to small bile ducts.(37)
Drug-liver injury
Although drug-liver injury is the probable cause of AST/ALT elevation in many cases, attribution to a single drug is mostly guesswork because every patient receives multiple drugs. Isolated AST/ALT elevation has been reported after cyclophosphamide infusions, liposomal amphotericin, trimethoprim-sulfamethoxazole, itraconazole, voriconazole and imatinib.(12,23)
3.4. Fungal and bacterial Infections (Figure 3A)
Antifungal prophylaxis has almost eliminated fungal liver abscesses in HCT recipients. If invasive fungal disease does occur, infection with resistant Candida species or molds is more likely. The sensitivity of imaging tests for detecting miliary fungal lesions after HCT is <30%. Return of neutrophil function after HCT can effect resolution of previously treatment-refractory mold infection.(3) Mycobacterial infection, including activation of latent Bacillus Calmette-Guerin from prior BCG vaccination, may involve the liver in patients receiving prolonged immunosuppressive therapy. Disseminated clostridial infection and gallbladder infection with gas-producing organisms may lead to air in the liver and biliary system.
3.5. Gallbladder and Biliary Disease
Biliary sludge (composed of calcium bilirubinate and crystals of calcineurin inhibitors) may cause transient epigastric pain, nausea, and abnormal serum liver enzymes. Biliary sludge may also cause acute ‘acalculous’ cholecystitis, acute pancreatitis, and bacterial cholangitis. The gallbladder may also become infected by CMV and fungi. Biliary obstruction caused by stones or sludge is rare. Therapeutic ERCP is needed only in patients with clinical evidence of cholangitis or radiologic evidence of persistent biliary obstruction.(41)
3.6. Malignant Disorders
EBV-lymphoproliferative disease is now an infrequent complication because of EBV-DNA surveillance and pre-emptive treatment with rituximab. Presenting signs are sweats, generalized malaise, enlarged tonsils, and cervical lymphadenopathy, with liver infiltration by transformed immunoblasts (Figure 3F) occurring in over 50%, manifest by abnormal serum alkaline phosphatase and massive hepatosplenomegaly.
3.7. Idiopathic hyperammonemia and coma
A lethal but rare syndrome of hyperammonemia and coma has been described after high dose chemotherapy, including conditioning therapy for HCT.(42) Patients present with progressive lethargy, confusion, weakness, incoordination, vomiting, hyperventilation with respiratory alkalosis, and plasma ammonia over 200 μmol/L. The pathogenesis of idiopathic hyperammonemia likely involves the unmasking of a latent genetic disorder similar to ornithine transcarbamylase deficiency.
4.0. Hepatobiliary problems in long-term transplant survivors
Fully-referenced discussions of this topic can be found in two recent textbooks.(43,44)
4.1. Chronic GVHD
About 60% of allograft recipients will develop cgvhd, a process that affects the skin, mouth, eyes, esophagus, muscle, joints, tendons, and vaginal mucosa.(44) Liver abnormalities include a) asymptomatic elevation of serum ALT, AP, and GGT as isolated laboratory abnormalities; b) slowly progressive cholestatic jaundice; and c) acute hepatocellular injury (hepatitic GVHD).(37)
Immunosuppressive therapy is not usually indicated when stable, minor elevation of serum enzymes is the only finding. In contrast, patients with jaundice may have histological findings of cholestatic GVHD -- lymphocytic infiltration of bile ducts, extensive damage to, and loss of, small bile duct epithelial cells, cholestasis, portal fibrosis and piecemeal necrosis.(36,44) Iron overload has been reported to mimic GVHD.(15) Patients who present with steeply rising serum ALT with or without jaundice, present a differential diagnosis of viral infection, drug-injury, and hepatitic GVHD. Hepatitic GVHD is characterized by lobular hepatitis, portal inflammation, and damage to small bile ducts.(37,44) CYP1A2 appears to be a target antigen in GVHD. Acyclovir should be started pending results of viral tests, and if negative, treatment for GVHD started with a calcineurin inhibitor, prednisone 1-2 mg/kg/day, and ursodiol.(37)
Immunosuppressive drug treatment of cGVHD is successful in only half of patients: 50% are able to discontinue therapy after 5 years, 10% require treatment for longer, and 40% die without resolution of cGVHD.(44) The only liver-specific therapy is ursodeoxycholic acid (12-15 mg/kg/day), which results in normalization or improvement in liver enzymes.(2,45) Ductopenic GVHD is potentially reversible if ongoing immunologic destruction of epithelium ceases, but this process may take months before resolution of jaundice.(37) Liver transplantation, including living-donor transplantation from the original hematopoietic cell donor (46), has been performed for patients with intractable hepatic GVHD.
4.2. Chronic hepatitis C
Hepatitis C virus infection in transplant survivors almost always results in chronic hepatitis.(7,47) In the first 10 years post-transplant, there is little liver-related morbidity.(7) However, cirrhosis and end-stage liver disease are now prevalent among patients transplanted before the 1990s. Survivors of allogeneic transplant often have suboptimal platelet and granulocyte counts; the long half-life of pegylated interferon may be associated with rapid falls in these counts. Interferon-based therapy may also activate cGVHD.
4.3. Chronic hepatitis B
The serologic pattern of HBV infection may be atypical in HCT survivors, probably as a consequence of immunosuppression. Clearance of surface antigenemia is particularly likely if the donor was naturally anti-HBs-positive.(1). As HCT survivors are at increased risk of second malignancy or recurrent malignancy, HBV viral status should be reassessed prior to any chemotherapy (particularly rituximab and alemtuzumab) for these cancers.(12)
4.4. Cirrhosis and end-stage liver disease
Cirrhosis and end-stage liver disease related to chronic HCV infection have a ∼35% prevalence among 40-year transplant survivors, with a faster rate of progression than in matched controls.(47,48). Liver transplantation should be considered in any HCT survivor with hepatic decompensation or early hepatocellular carcinoma. Living-donor transplantation from the original hematopoietic cell donor is a consideration in this situation, as minimal immunosuppression is required if the recipient is completely chimeric.(46)
4.5. Acute hepatocellular injury
The differential diagnosis of extreme elevations of serum ALT in an otherwise stable HCT survivor includes VZV or HSV infection, a hepatitic presentation of cGVHD, flares of chronic hepatitis B or C following tapering of immune suppressive drugs, and drug-induced liver injury. After immune suppressive drugs are discontinued, both GVHD and chronic hepatitis C may flare.(7,37)
4.6. Iron overload
Iron overload is particularly severe in thalassemic patients who have undergone HCT but less extreme in patients transplanted for leukemia or lymphoma. After successful HCT, iron accumulation stops and body iron stores fall slowly over time. An elevated serum ferritin level, particularly in patients with cGVHD, chronic viral hepatitis or other causes of liver disease, may not reflect tissue iron stores. The current method of choice is quantitative magnetic resonance imaging with Ferriscan® or T2* MRI.(14) The consequences of extreme iron overload in HCT survivors are primarily those of cardiac, pituitary, and pancreatic endocrine dysfunction. Current recommendations for iron mobilization are based on data from thalassemic patients: Patients with liver iron content >15,000 μg/g dry weight are treated aggressively with both phlebotomy and chelation; when liver iron content is 7,000 – 15,000 μg/g dry weight, phlebotomy is indicated; when liver iron content is under 7,000 μg/g dry weight, treatment is indicated only if there is evidence of liver disease.(49)
4.7. Cancer in the liver
The estimated actuarial incidence of a secondary cancer (melanoma, squamous cell carcinoma, sarcomas, and tumors of the brain, liver, cervix, thyroid, and breast) is 3-4% at 10 years and 10-12% at 15 years following allogeneic transplant.(44) Because of the increased prevalence of chronic hepatitis C in patients transplanted through the 1980s, the risk of hepatocellular carcinoma is particularly elevated in this cohort. Most cases of B-cell lymphoma that develop early post-transplant are related to Epstein-Barr Virus reactivation (Figure 3F), but later development of lymphomas and Hodgkin's diseases has also been described.
4.8. Fungal abscess
After apparently successful antifungal therapy resulting in encapsulization of fungi (Figure 3A), some patients develop recurrent liver abscesses when exposed to prednisone. Non-sterile herbal remedies contaminated by molds may also lead to liver abscesses in HCT survivors.
4.9. Nodular regenerative hyperplasia
Rarely, patients who receive high-dose chemotherapy will develop hepatic nodularity without fibrosis or liver dysfunction. This process is usually clinically silent unless signs of portal hypertension develop. NRH has been described more frequently as a histologic entity after HCT than as a clinical problem.(19)
4.10. Focal nodular hyperplasia
In one series of HCT survivors undergoing liver MRI, incidental FNH lesions were present in 12%.(50) These lesions have characteristic central scars that differentiate them from hepatocellular carcinoma and fungal lesions. The likely cause is sinusoidal injury caused by myeloablative conditioning regimens.
4.11. Gallbladder and biliary disease
Long-term survivors appear to have an increased incidence of gallstones and gallstone complications, probably related to formation of calcium bilirubinate microliths following myeloablative conditioning therapy. Chronic cyclosporine or tacrolimus dosing may also lead to biliary symptoms and acute pancreatitis.
5.0. Summary
Liver problems caused by infection and sinusoidal liver injury in the months following HCT have become less frequent because of preventive and pre-emptive strategies. When patients develop jaundice after transplant, the time to search for treatable causes is early in the course of jaundice, as the risk of mortality rises steeply with small increments of serum bilirubin above normal. Chronic hepatitis C, persistent GVHD, cirrhosis and hepatocellular carcinoma are significant liver problems in the longest-lived survivors of HCT.
Supplementary Material
Acknowledgments
My research cited in this review was supported by these grants from the National Institutes of Health, National Cancer Institute: CA15704 and CA16029. I gratefully acknowledge the work of my distinguished colleague, Dr. Howard Shulman, who provided the photomicrographs.
References
(refer to the on-line Supplementary material for a more comprehensive reading list)
- 1.Lau GKK, Strasser SI, McDonald GB. Hepatitis virus infections in patients with cancer. In: Wingard JR, Bowden RA, editors. Management of Infection in Oncology Patients. London, UK: Martin Dunitz; 2003. pp. 321–342. [Google Scholar]
- 2.Ruutu T, Eriksson B, Remes K, Juvonen E, Volin L, Remberger M, Parkkali T, et al. Ursodeoxycholic acid for the prevention of hepatic complications in allogeneic stem cell transplantation. Blood. 2002;100:1977–1983. doi: 10.1182/blood-2001-12-0159. [DOI] [PubMed] [Google Scholar]
- 3.Marotta G, Tozzi M, Sammassimo S, Defina M, Raspadori D, Gozzetti A, Lauria F. Complete resolution of hepatic aspergillosis after non-myeloablative hematopoietic stem cell transplantation in a patient with acute myeloid leukemia. Hematology. 2005;10:383–386. doi: 10.1080/10245330500141390. [DOI] [PubMed] [Google Scholar]
- 4.Deschenes M, Laneuville P. Pre-emptive use of lamivudine in bone marrow transplantation with chronic hepatitis B virus infection. Hepatology. 2004;39:867–868. doi: 10.1002/hep.20148. [DOI] [PubMed] [Google Scholar]
- 5.Lau GK, Suri D, Liang R, Rigopoulou EI, Thomas MG, Mullerova I, Nanji A, et al. Resolution of chronic hepatitis B and anti-HBs seroconversion in humans by adoptive transfer of immunity to hepatitis B core antigen. Gastroenterology. 2002;122:614–624. doi: 10.1053/gast.2002.31887. [DOI] [PubMed] [Google Scholar]
- 6.Surapaneni SN, Hari P, Knox J, Daniel J, Saeian K. Suppressive anti-HCV therapy for prevention of donor to recipient transmission in stem cell transplantation. American Journal of Gastroenterology. 2007;102:449–451. doi: 10.1111/j.1572-0241.2006.00937.x. [DOI] [PubMed] [Google Scholar]
- 7.Strasser SI, Myerson D, Spurgeon CL, Sullivan KM, Storer B, Schoch HG, Kim S, et al. Hepatitis C virus infection after bone marrow transplantation: A cohort study with 10 year follow-up. Hepatology. 1999;29:1893–1899. doi: 10.1002/hep.510290609. [DOI] [PubMed] [Google Scholar]
- 8.Hogan WJ, Maris M, Storer B, Sandmaier BM, Maloney DG, Schoch G, Wolfrey AE, et al. Hepatic injury after nonmyeloablative conditioning followed by allogeneic hematopoietic cell transplantation: a study of 193 patients. Blood. 2004;103:76–82. doi: 10.1182/blood-2003-04-1311. [DOI] [PubMed] [Google Scholar]
- 9.Picardi M, De Rosa G, Selleri C, Pane F, Rotoli B, Muretto P. Clinical relevance of intrahepatic hepatitis B virus DNA in HBsAg-negative HBcAb-positive patients undergoing hematopoietic stem cell transplantation for hematological malignancies. Transplantation. 2006;82:141–142. doi: 10.1097/01.tp.0000225828.27850.fc. [DOI] [PubMed] [Google Scholar]
- 10.Carpenter PA, Huang ML, McDonald GB. Activation of occult hepatitis B from a seronegative patient after hematopoietic cell transplant: a cautionary tale. Blood. 2002;99:4245–4246. doi: 10.1182/blood-2001-12-0239. [DOI] [PubMed] [Google Scholar]
- 11.Jacobsohn DA, Emerick KM, Scholl P, Melin-Aldana H, O'Gorman M, Duerst R, Kletzel M. Nonmyeloablative hematopoietic stem cell transplant for X-linked hyper-immunoglobulin m syndrome with cholangiopathy. Pediatrics. 2004;113:e122–127. doi: 10.1542/peds.113.2.e122. [DOI] [PubMed] [Google Scholar]
- 12.McDonald GB, Frieze D. A problem-oriented approach to liver disease in oncology patients. Gut. 2008;57:987–1003. doi: 10.1136/gut.2007.131136. [DOI] [PubMed] [Google Scholar]
- 13.McKoy JM, Angelotta C, Bennett CL, Tallman MS, Wadleigh M, Evens AM, Kuzel TM, et al. Gemtuzumab ozogamicin-associated sinusoidal obstructive syndrome (SOS): an overview from the research on adverse drug events and reports (RADAR) project. Leukemia Research. 2007;31:599–604. doi: 10.1016/j.leukres.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Noetzli LJ, Carson SM, Nord AS, Coates TD, Wood JC. Longitudinal analysis of heart and liver iron in thalassemia major. Blood. 2008;112:2973–2978. doi: 10.1182/blood-2008-04-148767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamble RT, Selby GB, Mims M, Kharfan-Dabaja MA, Ozer H, George JN. Iron overload manifesting as apparent exacerbation of hepatic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Biology of Blood & Marrow Transplantation. 2006;12:506–510. doi: 10.1016/j.bbmt.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Gooley TA, Rajvanshi P, Schoch HG, McDonald GB. Serum bilirubin levels and mortality after myeloablative allogeneic hematopoietic cell transplantation. Hepatology. 2005;41:345–352. doi: 10.1002/hep.20529. [DOI] [PubMed] [Google Scholar]
- 17.Deleve LD, Shulman HM, McDonald GB. Toxic injury to hepatic sinusoids: Sinusoidal obstruction syndrome (venocclusive disease) Seminars in Liver Disease. 2002;22:27–41. doi: 10.1055/s-2002-23204. [DOI] [PubMed] [Google Scholar]
- 18.Coppell JA, Richardson PG, Soiffer RJ, Martin PL, Kernan NA, Chen A, Guinan EC, et al. Hepatic Veno-occlusive Disease following Stem Cell Transplantation: Incidence, Clinical Course and Outcome. Biology of Blood & Marrow Transplantation. 2009 doi: 10.1016/j.bbmt.2009.08.024. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shulman HM, Fisher LB, Schoch HG, Henne KW, McDonald GB. Venocclusive disease of the liver after marrow transplantation: Histologic correlates of clinical signs and symptoms. Hepatology. 1994;19:1171–1180. [PubMed] [Google Scholar]
- 20.McDonald GB, Slattery JT, Bouvier ME, Ren S, Batchelder AL, Kalhorn TF, Schoch HG, et al. Cyclophosphamide metabolism, liver toxicity, and mortality following hematopoietic stem cell transplantation. Blood. 2003;101:2043–2048. doi: 10.1182/blood-2002-06-1860. [DOI] [PubMed] [Google Scholar]
- 21.McCune JS, Batchelder AL, Guthrie KA, Witherspoon R, Appelbaum FR, Phillips B, Vicini P, et al. Personalized dosing of cyclophosphamide in the Total Body Irradiation - cyclophosphamide conditioning regimen: A phase II trial in patients with hematologic malignancy. Clinical Pharmacology and Therapeutics. 2009;85:615–622. doi: 10.1038/clpt.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCune JS, Batchelder A, Deeg HJ, Gooley TA, Cole SL, Phillips B, Schoch G, et al. Cyclophosphamide following targeted oral busulfan as conditioning for hematopoietic cell transplantation: pharmacokinetics, liver toxicity, and mortality. Biology of Blood & Marrow Transplantation. 2007;13:853–862. doi: 10.1016/j.bbmt.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Sakai M, Strasser SI, Shulman HM, McDonald SJ, Schoch HG, McDonald GB. Severe hepatocellular necrosis after hematopoietic cell transplant: Incidence, etiology, and outcome. Bone Marrow Transplantation. 2009;44:441–447. doi: 10.1038/bmt.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coy DL, Ormazabal A, Godwin JD, Lalani T. Imaging evaluation of pulmonary and abdominal complications following hematopoietic stem cell transplantation. Radiographics. 2005;25:305–317. doi: 10.1148/rg.252045037. [DOI] [PubMed] [Google Scholar]
- 25.Carreras E, Granena A, Navasa M, Bruguera M, Marco V, Sierra J, Tassies MD, et al. Transjugular liver biopsy in bone marrow transplantation. Bone Marrow Transplant. 1993;11:21–26. [PubMed] [Google Scholar]
- 26.Shulman HM, Gooley T, Dudley MD, Kofler T, Feldman R, Dwyer D, McDonald GB. Utility of transvenous liver biopsies and wedged hepatic venous pressure measurements in sixty marrow transplant recipients. Transplantation. 1995;59:1015–1022. doi: 10.1097/00007890-199504150-00017. [DOI] [PubMed] [Google Scholar]
- 27.Hingorani SR, Guthrie K, Batchelder A, Schoch G, Aboulhosn N, Manchion J, McDonald GB. Acute renal failure after myeloablative hematopoietic cell transplant: incidence and risk factors. Kidney International. 2005;67:272–277. doi: 10.1111/j.1523-1755.2005.00078.x. [DOI] [PubMed] [Google Scholar]
- 28.Bearman SI, Anderson GL, Mori M, Hinds MS, Shulman HM, McDonald GB. Venocclusive disease of the liver: Development of a model for predicting fatal outcome after marrow transplantation. J Clin Oncol. 1993;11:1729–1736. doi: 10.1200/JCO.1993.11.9.1729. [DOI] [PubMed] [Google Scholar]
- 29.DeLeve LD, Wang X. Role of oxidative stress and glutathione in busulfan toxicity in cultured murine hepatocytes. Pharmacology. 2000;60:143–154. doi: 10.1159/000028359. [DOI] [PubMed] [Google Scholar]
- 30.Jenke A, Freiberg-Richter J, Wilhelm S, Freund M, Renner UD, Bornhauser M, Schleyer E, et al. Accidental busulfan overdose during conditioning for stem cell transplantation. Bone Marrow Transplantation. 2005;35:125–128. doi: 10.1038/sj.bmt.1704697. [DOI] [PubMed] [Google Scholar]
- 31.de Lima M, Couriel D, Thall PF, Wang X, Madden T, Jones R, Shpall EJ, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004;104:857–864. doi: 10.1182/blood-2004-02-0414. [DOI] [PubMed] [Google Scholar]
- 32.Puig N, de la Rubia J, Remigia MJ, Jarque I, Martin G, Cupelli L, Sanz GF, et al. Morbidity and transplant-related mortality of CBV and BEAM preparative regimens for patients with lymphoid malignancies undergoing autologous stem-cell transplantation. Leukemia & Lymphoma. 2006;47:1488–1494. doi: 10.1080/10428190500527769. [DOI] [PubMed] [Google Scholar]
- 33.Geddes M, Kangarloo SB, Naveed F, Quinlan D, Chauhry MA, Stewart D, Savoie ML, et al. High busulfan exposure is associated with worse outcomes in a daily IV busulfan and fludarabine allogeneic transplant regimen. Biology of Blood & Marrow Transplantation. 2008;14:220–228. doi: 10.1016/j.bbmt.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 34.Corbacioglu S. The prospective study on the incidence and outcome of VOD with the prophylactic use of defibrotide in paediatric stem cell transplantation has completed enrollment: update on the VOD-DF study. Bone Marrow Transplantation. 2009;43:S34–S35. [Google Scholar]
- 35.Richardson PG, Soiffer RJ, Antin JH, Uno H, Jin Z, Kurtzberg J, Martin PL, et al. Defibrotide for the treatment of severe hepatic veno-occlusive disease and multi-organ failure post stem cell transplantation: a multi-center, randomized, dose-finding trial. Biology of Blood and Marrow Transplantation. doi: 10.1016/j.bbmt.2010.02.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shulman HM, Sharma P, Amos D, Fenster LF, McDonald GB. A coded histologic study of hepatic graft-versus-host disease after human marrow transplantation. Hepatology. 1988;8:463–470. doi: 10.1002/hep.1840080305. [DOI] [PubMed] [Google Scholar]
- 37.Strasser SI, Shulman HM, Flowers ME, Reddy R, Margolis DA, Prumbaum M, Seropian SE, et al. Chronic graft-vs-host disease of the liver: presentation as an acute hepatitis. Hepatology. 2000;32:1265–1271. doi: 10.1053/jhep.2000.20067. [DOI] [PubMed] [Google Scholar]
- 38.Leisenring W, Martin P, Petersdorf E, Regan A, Aboulhosn N, Stern J, Aker S, et al. An acute graft-versus-host disease activity index to predict survival after hematopoietic cell transplantation with myeloablative conditioning regimens. Blood. 2006;108:749–755. doi: 10.1182/blood-2006-01-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yagi T, Karasuno T, Hasegawa T, Yasumi M, Kawamoto S, Murakami M, Uosima N, et al. Acute abdomen without cutaneous signs of varicella zoster virus infection as a late complication of allogeneic bone marrow transplantation: importance of empiric therapy with acyclovir. Bone Marrow Transplantation. 2000;25:1003–1005. doi: 10.1038/sj.bmt.1702340. [DOI] [PubMed] [Google Scholar]
- 40.Neofytos D, Ojha A, Mookerjee B, Wagner J, Filicko J, Ferber A, Dessain S, et al. Treatment of adenovirus disease in stem cell transplant recipients with cidofovir. Biology of Blood & Marrow Transplantation. 2007;13:74–81. doi: 10.1016/j.bbmt.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 41.Alnusair MM, DeMagalhaes-Silverman M, Silverman WB. The role of ERCP in patients with pancreatico-biliary problems in the setting of hematopoietic stem cell transplant. Gastrointestinal Endoscopy. 2006;63:655–659. doi: 10.1016/j.gie.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 42.Frere P, Canivet JL, Gennigens C, Rebeix JP, Fillet G, Beguin Y. Hyperammonemia after high-dose chemotherapy and stem cell transplantation. Bone Marrow Transplantation. 2000;26:343–345. doi: 10.1038/sj.bmt.1702485. [DOI] [PubMed] [Google Scholar]
- 43.Appelbaum FR, Forman SJ, Negrin RS, Blume KG. Thomas' Hematopoietic Cell Transplantation. 4th. Oxford, UK: Wiley-Blackwell Publishing; 2009. [Google Scholar]
- 44.Vogelsang GB, Pavletic SZ. Chronic Graft Versus Host Disease: Interdisciplinary Management. First. New York, NY: Cambridge Univesity Press; 2009. [Google Scholar]
- 45.Fried RH, Murakami CS, Fisher LD, Willson RA, Sullivan KM, McDonald GB. Ursodeoxycholic acid treatment of refractory chronic graft-versus-host disease of the liver. Annals of Internal Medicine. 1992;116:624–629. doi: 10.7326/0003-4819-116-8-624. [DOI] [PubMed] [Google Scholar]
- 46.Shimizu T, Kasahara M, Tanaka K. Living-donor liver transplantation for chronic hepatic graft-versus-host disease. The New England Journal of Medicine. 2006;354:1536–1537. doi: 10.1056/NEJMc052628. [DOI] [PubMed] [Google Scholar]
- 47.Peffault de Latour R, Levy V, Asselah T, Marcellin P, Scieux C, Ades L, Traineau R, et al. Long-term outcome of hepatitis C infection after bone marrow transplantation. Blood. 2004;103:1618–1624. doi: 10.1182/blood-2003-06-2145. [DOI] [PubMed] [Google Scholar]
- 48.Strasser SI, Sullivan KM, Myerson D, Spurgeon CL, Storer B, Schoch HG, Murakami CS, et al. Cirrhosis of the liver in long-term marrow transplant survivors. Blood. 1999;93:3259–3266. [PubMed] [Google Scholar]
- 49.Angelucci E, Muretto P, Lucarelli G, Ripalti M, Baronciani D, Erer B, Galimberti M, et al. Phlebotomy to reduce iron overload in patients cured of thalassemia by bone marrow transplantation. Italian Cooperative Group for Phlebotomy Treatment of Transplanted Thalassemia Patients. Blood. 1997;90:994–998. [PubMed] [Google Scholar]
- 50.Sudour HML, Baumann C, Clement L, Salmon A, Bordigoni P. Focal hepatic hyperplasia following hematopoietic stem cell transplantation. Bone Marrow Transplantation. 2009;43:127–132. doi: 10.1038/bmt.2008.304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.