Introduction
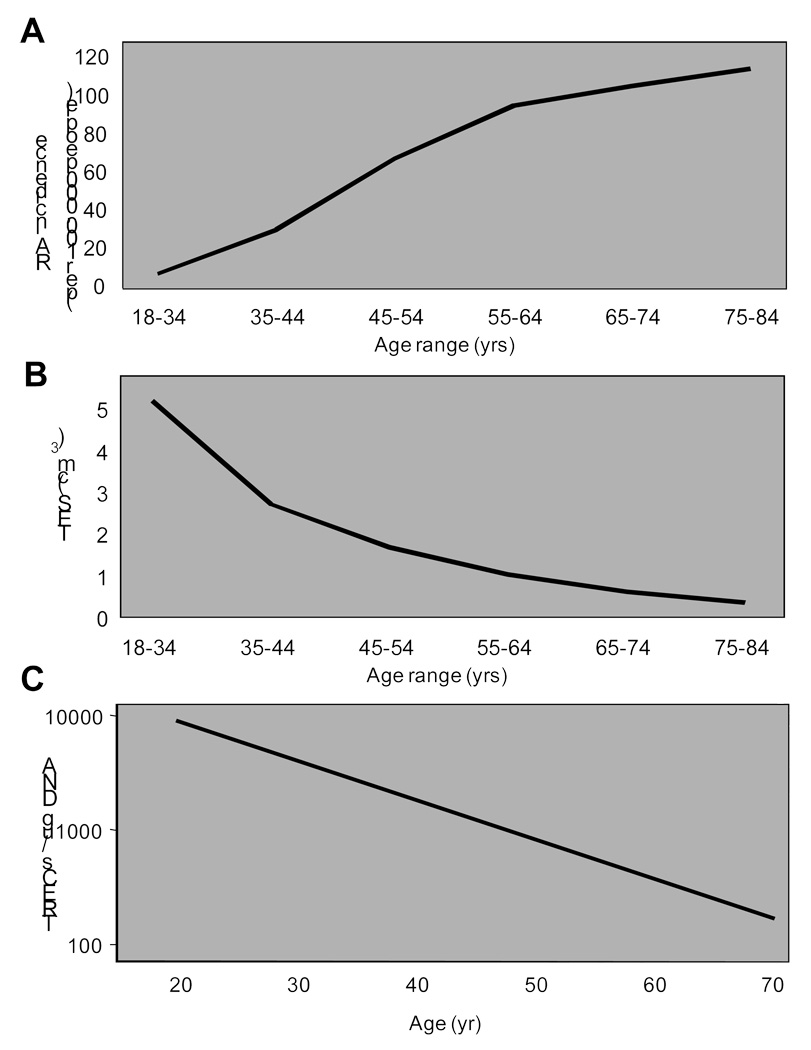
Rheumatoid arthritis is a chronic inflammatory disease that manifests predominantly as synovial inflammation leading to cartilage damage and destruction of the joint infrastructure. Although the joint symptomatology is eventually dominant, the disease is preceded by immune abnormalities that are not joint specific, but systemic and are already apparent many years before onset of the disease (1). The best defined autoimmune phenomena are antibody responses against IgG and against citrullinated peptides, self-antigens or neoantigens that are ubiquitously expressed. While the focus of research in the 1990s has been on identifying a tolerance defect to a joint-specific antigen (reviewed in (2)), the last decade has seen a shift to the model that patients with rheumatoid arthritis have a fundamental breakdown in self-tolerance and that patients are not able to induce or maintain tolerance to neoantigens (3). This breakdown in tolerance occurs in the second half of life suggesting that it is acquired (4). Most patients who develop disease are postmenopausal women; indeed the incidence of the disease continues to rise at least into the seventh decade of life and possibly even beyond that (4, 5). The relationship between RA incidence and age is inverse to that of immunocompetence and age as illustrated in Figure 1 for thymic epithelial space (TES) and frequency of recent thymic emigrants.
Figure 1.
Thymic function and RA incidence – an inverse relationship. The incidence of rheumatoid arthritis (A) is low before menopause and peaks in the seventh to eighth decades of life. Thymic function rapidly declines with age and is minimal after the age of 40 years. Shown are the involution of thymic epithelial space (TES) (B) and the frequencies of recent thymic emigrants as estimated by the concentrations of peripheral T cells with T cell-receptor excision circles (TREC) (C).
From Goronzy JJ, Weyand CM. Rheumatoid arthritis. Immunol Rev 2005;204:62, with permission.
Aging as a Risk Factor for Autoimmunity
The age relationship of RA is different from that of organ-specific autoimmune diseases, such as diabetes mellitus or from systemic lupus erythematosus that peak earlier in life. However, RA does not stand alone in this aspect; age is a major risk factor in many other chronic inflammatory diseases, most notable in giant cell arteritis (6–8). This important role of age in the development of selected autoimmune diseases raises the questions whether immune aging is a contributing factor and tolerance defects are part of the degenerative process of the immune system. Indeed, autoantibodies are a common finding in healthy elderly (9). Of interest, many of these autoantibodies are specific for common autoantigens, such as rheumatoid factor and antinuclear antibodies, while tissue-specific antibodies do not appear to be a normal by-product of immune aging. Studies on the frequency of anti-CCP antibodies with age are not yet available. In general, the age-related rheumatoid factors are low titered, but otherwise not different from the autoantibodies in autoimmune diseases.
The concept that autoimmune disease is a consequence of immune aging is counterintuitive. In general, the aged immune system is less responsive to antigenic challenges; it is more difficult in the elderly to elicit an immune response to an antigen than in young adult (10, 11). As a consequence, vaccine responses decline with age (12–14). Autoantigens, perhaps with the exception of neoantigens, are by definition low affinity antigens because high affinity receptors have been purged from the repertoire by negative selection. How, therefore, can an immune system that is insufficient to generate an adaptive immune response to an exogenous antigen, such as a vaccine, be able to overcome tolerance and generate immune responses to auto- or neoantigens? As always in science, identifying an obvious paradox and overcoming its conundrum provides an opportunity to take a qualitative pivotal step in understanding the mechanisms of a disease.
Immune Aging – What Do We Know
The immunological evidence of immune aging is best illustrated by the increasing incidence and morbidity of infections, the failure to mount vaccine responses and the reactivation of chronic viral infections with age. Epidemiological data suggest that clinical evidence of immune aging is already present, albeit subtle, in the middle-aged adult. Examples include the incidence of herpes zoster reactivation that starts to increase after the age of 50 years (15, 16), the increased hospitalization and mortality rates of influenza Infections that also increase after the age of 50 (17), and also vaccine responses such as the response to hepatitis B vaccination which already starts to decline after the age of 40 years (18). Thus, immune aging is not only a feature of the very elderly, but emerges as a clinical complication already in the middle-aged adult, approximately at the same age when the susceptibility to develop RA increases (4, 6). Immune failure becomes severe in the eighth decade of life in healthy individuals (19, 20). If autoimmunity is a consequence of immune aging, but still requires a functional immune system, one would predict that the incidence of autoimmune diseases will start to dip again in the very elderly.
Immune aging affects both the innate and the adaptive immune system. The innate immune system is constitutively activated in the elderly and the concentration of inflammatory cytokines, in particular IL-6, increases (21). This proinflammatory environment accelerates and complicates numerous degenerative diseases; the classical example is the inflammation in the atherosclerotic plaque that leads to plaque rupture and acute coronary syndromes (22, 23). The mechanisms underlying this innate immune activation are not clear, but possibly are also a consequence of the declining adaptive immune response. Most studies of immune senescence have focused on the adaptive system, and several defects have been described. It has been hypothesized that the defect in T cell immunity is causatively related to the declining thymic generation of new naïve cells (24). By the age of 40 to 50 years, thymic activity is severely limited and the homeostasis of the T cell compartment entirely depends on peripheral mechanisms that regulate the proliferation and survival of naïve and memory T cells. As a consequence, the frequency of naïve T cells declines with age, a phenomenon that is markedly more pronounced in the CD8 than the CD4 compartment (25). The replicative stress associated with immune responses to exogenous antigen, but also due to the homeostatic proliferation to maintain a full peripheral T cell compartment in the absence of thymic production, is associated with decline in telomere lengths, epigenetic changes and accumulation of effector subpopulations, in particular in the CD8 compartment (25–27). Individual T cells are still responsive to stimulation by exogenous antigen. Although signaling defects have been described in elderly T cells, they alone are usually not sufficient to suppress an immune response. It is this environment in which RA and its autoimmune manifestations develop.
Accelerated immune aging in RA
The epidemiologic data clearly show that age is an important risk factor for developing RA. The obvious next question then is what the biological age of a patient with RA is. Is the aging of the adaptive immune system age- appropriate, is it decelerated leading to a better preserved T cell immune responsiveness in an otherwise aging host, or is it accelerated such that immune responses have already declined beyond the actual age of the individual? Early evidence from T cell depletion studies already suggested that RA patients have difficulty in regenerating the immune system (28). In general, therapeutic T cell depletion yielded only moderate benefits, but significant side effects. This was most evident for patients treated with an anti-CD52 antibody (alemtuzumab) that very effectively depletes T cells. Many of these treated patients stayed lymphopenic for a long period of time after treatment, and in those patients who had a sizable recovery of T cells, the population was highly oligoclonal, suggesting that the T cells were derived from a few progenitor cells (28),(29). The same clonally expanded populations that were present in the peripheral blood were also found in the synovial tissue of RA patients who maintained disease activity (30). Longitudinal studies in these patients showed that the lymphopenia in these patients persisted over decades. Of interest, other autoimmune phenomena developed in some of the anti-CD52-treated patients, consistent with the view that tolerance is more difficult to maintain in a lymphopenic host (31).
Subsequent studies have shown that impaired T cell regenerative capacity and evidence for replicative stress in the peripheral T cell compartment are already features in RA patients who have not undergone T cell depletion (32). The frequency of T cell receptor excision circles (TRECs), frequently used as a surrogate measurement for thymic activity, declines in normal healthy individuals between the age of 20 and 65 by about 95% (33, 34). TRECS are episomes generated during T cell receptor rearrangements and are likely to persist in non-proliferating cells, but are not transmitted to all daughter cells during division. TREC frequencies are therefore the net result of two events; generation of new TREC-positive cells from the thymus; and loss of TREC-positive T cells by peripheral proliferation and/or cell loss (35). RA patients have an age-inappropriate decrease in TREC frequencies suggesting that they either have reduced thymic output, increased peripheral T cell apoptosis and proliferation, or a combination of both (32),(36). Obviously, these two processes do not need to be independent because decreased thymic production has to be compensated with increased peripheral proliferation to maintain the size of the compartment (37, 38). Indeed, the peripheral T cell compartment in RA patients exhibits evidence for replicative stress. T cells, like any other cell that proliferates, lose sequence stretches at their chromosomal ends, called telomeres, with each division (39). They also change their phenotype and function, in particular they lose the expression of the costimulatory molecule CD28 (40, 41). When assessed using any or all of these senescence markers, the repertoire of T cells in patients with RA is pre-aged by approximately 20 years. Telomeric ends are shortened, frequencies of CD28-negative T cells are increased and T cell repertoire diversity is contracted, consistent with excessive T cell loss and oligoclonal expansion (32, 42–44). Similar evidence for accelerated immune aging has also been shown for some, but not all, other autoimmune diseases. Most notable is multiple sclerosis which is also associated with reduced TREC numbers and increased frequency of CD28-negative cells. Other chronic inflammatory diseases, such as the spondylarthropathies (which often begin in early adulthood), do not show any evidence for accelerated immune aging (45).
Accelerated Immune Aging- A Primary or Secondary Event?
In any inflammatory disease, the question arises whether observed findings are a primary event involved in the pathogenesis of the disease or a secondary event due to disease-induced inflammation. Several studies have shown that accelerated immune aging is a phenomenon found in patients with early RA and is not influenced by disease duration or treatment (32, 43, 46, 47). Also, in longitudinal studies, the frequency of CD28-negative T cells early in the disease is predictive of severity in joint erosion on follow-up and the frequency of extraarticular disease manifestations in cross-sectional studies (48, 49). These observations have been interpreted in favor of immune aging as a primary event involved in disease pathogenesis and not as a consequence of the presence of inflammatory cytokines. Exceptions to this interpretation do exist, e.g., transcription of the CD28 gene in young T cells can be significantly downregulated by TNF-α (50, 51). The mechanism of TNF-mediated gene repression is the same as that which occurs with replicative T cell aging. Under the influence of TNF, naïve T cells lose the expression of an initiation factor that is necessary to induce CD28 transcription. However, the TNF-mediated CD28 repression is not complete and is readily reversible which is in contrast to the CD28 loss that is seen with replicative senescence or in RA patients. All T cells from patients with RA have reduced cell surface expression of CD28 when measured by flow cytometry while only few have a complete CD28 loss. Full CD28 expression recovers in CD28-low T cells with short-term cultures in vitro or with anti-TNF treatment in vivo. In contrast, CD28 loss in most cases is not reversible with anti-TNF-α. On the contrary, CD28 expression in at least some cells can be restored by another inflammatory cytokine, IL-12 (52).
The most convincing evidence that accelerated immune aging is primary (or at least not exclusively secondary) comes from genetic studies. Increased loss of CD28 on peripheral T cells has been found to be associated with the HLA-DRB1*04 genotype which also predisposes to RA (5, 46, 48, 53, 54). In healthy individuals, the frequencies of CD28-negative cells have been found to be correlated to CMV infection (55). This correlation is maintained in patients with RA suggesting that CMV infection and accelerated aging rather than activity of the rheumatic disease is a key risk factor that leads to senescence of the cell population (45). HLA-DRB1*04 is also associated with accelerated telomeric shortening in healthy controls. Schönland et al have shown that HLA-DRB1*04-positive individuals have shorter telomeres in several hematopoietic stem cell-derived populations including neutrophils and naïve and memory T cells (46). This telomeric shortening was cell-specific for hematopoietic lineages and not found in sperm cells of HLA-DRB1*04-positive donors. The difference in telomere lengths is acquired, since no HLA-related difference was seen in cord blood T cells. These studies led to the intriguing interpretation that the major disease-risk gene for RA, HLA-DRB1*04 predisposes for at least two aging features in normal individuals, telomere shortening in hematopoietic stem cell lineages and loss of CD28 in peripheral T cell memory cell populations. In contrast, the HLA-DR4 haplotype was not associated with reduced frequency of TRECs as a marker of thymic activity and/or peripheral cell death, suggesting that the features of accelerated aging observed in RA patients are at least partially independent and may be additive or even synergistic.
Mechanism of accelerated immune aging in RA
One of the most striking markers of aging in a highly proliferative compartment is telomeric erosion. Telomeres are protein-DNA complexes at the end of eukaryotic chromosomes that protect from fusion and degradation. Telomeric DNA is composed of repeats of G-rich sequences that are packed with a number of DNA-binding proteins involved in protection and repair (56). In the absence of the enzyme telomerase, telomeric sequences are incompletely duplicated; with loss of 40–200 base pairs during each cell division. Telomeric lengths can, therefore, be taken as a marker of the replicative history of individual cells or a population of cells. Critically short telomeres become uncapped and recruit components of the DNA damage repair machinery, such that cells enter replicative senescence or apoptosis. Telomeric erosion is counteracted by an enzyme, telomerase, that is only expressed in selected cell types including stem cells, sperms and lymphocytes (27). Even in stem cells, and certainly in lymphocytes, telomeric repair is incomplete and telomeric erosion occurs progressively with age (46).
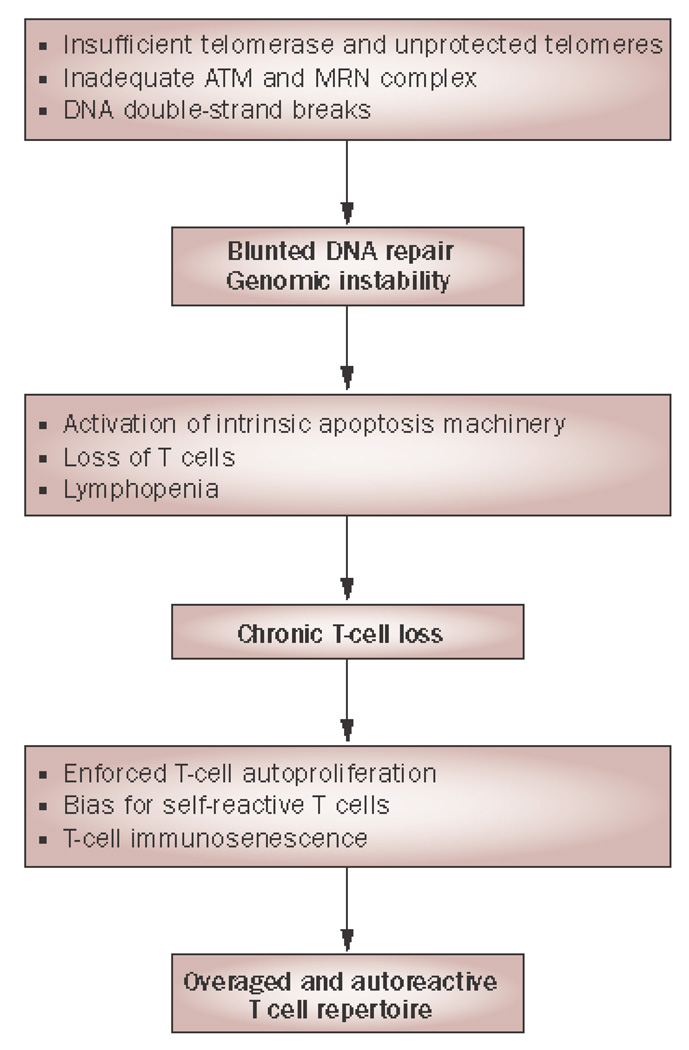
The age-inappropriate accelerated telomeric erosion found in lymphocytes of patients with RA could be caused by several mechanisms, either acting alone or in concert. Telomeric erosion could reflect an increased proliferative history of lymphocytes or their precursor cells (32), or be caused by defective telomerase expression or activity (57, 58); alternatively, telomeric shortening could be the result of excessive DNA damage rather than replication-induced telomeric loss (47, 57). (FIGURE 2) Data on patients with RA indicate that all three of these different mechanisms play a role. Frequencies of hematopoietic stem cells in RA patients are age-inappropriately reduced and their telomeres are already shortened, indicating that the hematopoietic stem cell system is under replicative stress (59). Increased proliferation of peripheral naïve and memory T cells may aggravate this defect. In support of this interpretation, the reduced frequency of TREC-positive cells either indicates reduced thymic activity or accelerated peripheral death of TREC-positive cells; both would increase homeostatic proliferation to maintain the compartment size (19). It is of interest to note that telomeric erosion in hematopoietic stem cells, but not a reduced number of TRECs, is also found in healthy HLA-DRB1*04-positive individuals (32), suggesting that two independent age-related mechanisms contribute to the accelerated immune aging in RA patients, one of them influenced by the disease-associated MHC region.
Figure 2.
DNA instability and autoreactivity in RA. Stability and intactness of DNA and its telomeric ends is critically involved in regulating vitality, proliferative capacity and longevity of lymphocytes. In RA T cell telomerase is insufficiently produced and DNA double-strand breaks are left unrepaired, causing chronic stimulation of cellular checkpoint pathways and DNA repair activity. As a consequence, RA T cells are highly apoptosis sensitive and the T cell pool is inadequately replenished. In the absence of thymic T-cell generation lymphopenia initiates T-cell autoproliferation. Chronic replicative stress exhausts the T cells’ replicative reserve, rendering them senescent. As T-cell renewal is dependent on “tickling” of the T-cell receptor, T cells that recognize autoantigens are preferentially expanded.
Abbreviations: ATM, ataxia telangiectasia mutated; MRN, MRE11-RAD50-NBS1; RA, rheumatoid arthritis.
From Weyand CM, Fujii H, Shao L, Goronzy JJ. Rejuvenating the immune system in rheumatoid arthritis. Nat Rev Rheumatol 2009;5:587, with permission.
Telomerase is a reverse transcriptase composed of a catalytic protein encoded by the hTERT gene and an RNA component encoded by hTERC that contains a sequence complementary to the G-rich telomeric strands (58). hTERC serves as a template for the addition of telomeric repeats at the ends of chromosomes. The telomerase activity appears to be primarily limited by the expression of the catalytic subunit, hTERT. The gene is silent in most somatic cells. Transcriptional and posttranscriptional regulation of hTERT expression is complex and involves epigenetic modifications, overcoming of negative regulatory factors such as tumor suppressors and inhibitory cytokines and hormones, and hTERT phosphorylation and NFκB mediated nuclear translocation. hTERT transcription is induced in naïve, and to a much lesser degree in memory T cells upon T cell receptor-mediated activation (39).
Fuji et al examined telomeric repair mechanisms in naïve CD4 T cells from patients with RA (58). The authors found a blunted induction of telomerase activity due to reduced hTERT transcription, to about half the level that is seen healthy age-matched controls. This reduced telomerase activity in conjunction with homeostatic proliferation could, indeed, be responsible for the telomeric erosion that is seen in patients with RA (58). However, the authors describe a function of telomerase that goes beyond preventing telomeric erosion. Although telomeres in naïve T cells, either in healthy controls or in RA patients, are not critically short, the insufficient telomerase induction in RA T cells after activation was closely correlated with increased cell death during cellular expansion. Mechanistic studies confirmed the Interpretation that telomerase supports cell survival independent of telomeric erosion. Knockdown of hTERT expression in healthy T cells resulted in increased apoptotic cell death through the internal pathway; in contrast, overexpression of hTERT in RA T cells improved their survival. In summary, the reduced induction of telomerase activity in RA patients influences peripheral T cell homeostasis and function by two mechanisms, first, increased susceptibility to undergo apoptosis during proliferation independent of telomeric lengths; and second, progressive telomeric erosion with cell division. Increased apoptosis, consistent with the reduced number of TRECs, increases the need for compensatory replication, initiating a self-perpetuating strain on peripheral T cell homeostatic mechanisms.
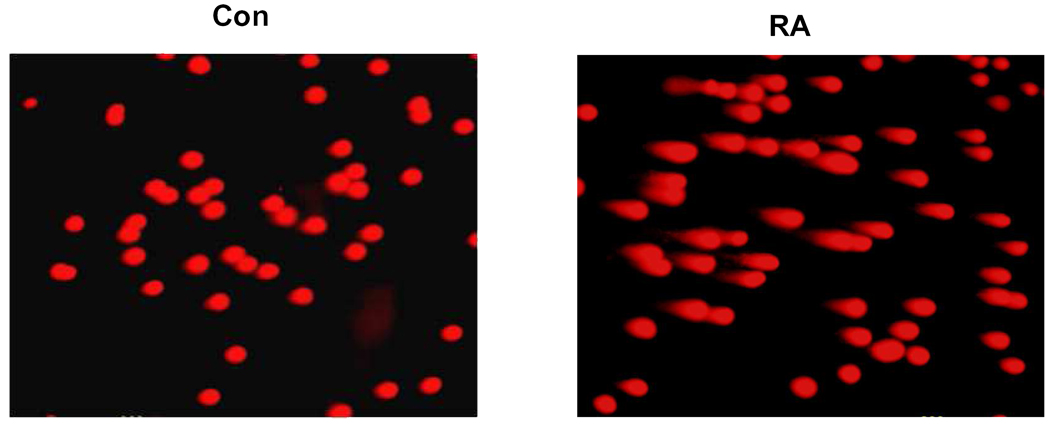
The finding that T cells from RA patients and, in particular, naïve T cells, are susceptible to undergo apoptosis through the intrinsic pathway and that this defect can be, in part, repaired by overexpressing telomerase raises the question about the overall efficacy of DNA repair mechanisms in RA T cells. The genome is constantly exposed to injurious insults that need to be immediately repaired. It can easily be envisioned that cumulative DNA damage contributes to cellular aging, resulting in either senescence or apoptosis. The most lethal DNA lesions are double-stranded breaks that activate the DNA repair complex, including the protein kinase, ataxia telangiectasia mutated (ATM) (60). A sensitive screening assay for DNA damage is the comet assay, DNA electrophoresis at the single cell level. (FIGURE 3) Shao et al compared the tail moments in comet assays, indicative of DNA damage, between age-matched controls and RA T cells, and found increased DNA damage in RA (47). The finding of increased DNA damage in the comet assay was further confirmed by detecting chemically altered DNA with probes reactive to 8-oxoguanin. Several components of the DNA repair complex had reduced expression in RA T cells including several members of the MRN complex that recognize double-stranded breaks, the kinase ATM, and p53. The primary defect appeared to be the decreased expression of ATM because overexpression of ATM in RA T cells restored the expression of the MRN, as well as p53, and rendered RA T cells more resistant to apoptosis (47).
Figure 3.
DNA instability in naïve RA T cells. Unprimed, naïve CD4 T cells isolated from RA patients carry a high load of damaged DNA (right panel, RA). Broken DNA is identified by the comet assay, a single cell gel electrophoresis assay in which DNA fragments leaking from the nucleus form a comet. DNA damage can be quantified for individual cells as a tail moment. Naive CD4 T cells from age-matched control individuals have low levels of tail moments (left panel, Con).
From Weyand CM, Fujii H, Shao L, Goronzy JJ. Rejuvenating the immune system in rheumatoid arthritis. Nat Rev Rheumatol 2009;5:587, with permission.
In summary, several mechanisms come together to accelerate immune aging of T cells and possibly other hematopoietic lineages in patients with RA. Decreased capacity in the reserve of hematopoietic stem cells and shortened telomeres in hematopoietic stem cells appear to be at least in part genetically determined by risk factors that map to HLA-DRB*04, the same genetic region that predisposes to RA. Independent of this is a defect in RA T cells in the maintainance of genomic integrity with age, causing excessive loss of peripheral T cells. Molecular mechanisms include an inability to induce hTERT after stimulation and reduced expression of ATM. Whether the reduced number of TRECs is a consequence of this excessive T cell loss or whether there is also an independent component of accelerated thymic involution remains to be determined. The net effect of these mechanisms is an excessive peripheral loss of T cells that is compensated by homeostatic proliferation to maintain compartment size, leading to the eventual emergence of senescence biomarkers.
Immune Aging and Autoimmunity – Is there a Pathogenetic Link?
The aging immune system is characterized by impaired immune responses to peptide antigens. At first thought, it is, therefore, paradoxical that aging of the immune system should be a risk factor for an increased response to self-antigens and autoimmunity. In one possible model, peripheral regulatory cells are lost with aging resulting in autoreactive responses, even if the individual autoreactive T cell also has decreased responsiveness. A population of natural regulatory cells is generated in the thymus and would, therefore, be susceptible to thymic involution; however, regulatory T cells are also generated from existing T cell populations in the periphery. Data on the frequency and function of regulatory T cells with aging are conflicting and there is no convincing evidence that regulatory function decreases with age. Also, evidence that a deficiency in the number of regulatory T cells is involved in the pathogenesis of RA is, at this time, not convincing, suggesting that RA is not a consequence of failing regulatory T cell function with age.
As described above, one of the prominent defects in RA T cells related to immune aging is the decreased survival and/or decreased production of T cells, resulting into a lymphopenic state that needs to be compensated by homeostatic cytokines. Lymphopenia has been shown to be a major risk factor for autoimmunity in several animal models. Young disease-free NOD mice that are prone to develop diabetes are mildly lymphopenic. Disease development in this strain is dependent on IL-21-mediated homeostatic expansion of islet-specific T cells (61). Similarly, lymphopenia is important for the autoimmune diabetes mellitus in the biobreeding rat (62). Also, unpublished observations from our laboratory show that the SKG mouse is markedly lymphopenic. SKG mice have a mutation in the ZAP70 signaling molecule, causing a defect in T cell activation and in thymic selection. These mice develop a rheumatoid arthritis-like picture at the age of 3–4 months that includes the production of anti-CCP antibodies and rheumatoid factors. Finally, antigen-induced arthritis in normal mouse strains can be age-dependent. Glant et al have shown that proteoglycan-induced arthritis is much more easily induced in middle-aged and old animals rather than in young mice (63). The common denominator of all these models is that lymphopenia is compensated by homeostatic proliferation, suggesting that homeostatically proliferating cells are risk factors for autoimmunity.
How can a defect in T cell homeostasis predispose to autoimmunity and be a disease mechanism for the development of RA? At least three different models come to mind. Increased homeostatic proliferation will peripherally select the repertoire in favor of the survival of T cells with T cell receptors that have a higher affinity for self (64, 65). A repertoire that is biased by peripheral selection may be more difficult to control by peripheral tolerance mechanisms. This model would also explain the observation that autoantibodies in RA are preferentially specific for common self-antigens and neoantigens that may play a particular role in peripheral selection. In this model, one also would expect a contraction in the diversity of the T cell receptor repertoire which, indeed, can be found in patients with RA. A second model is that the homeostatic proliferation is sufficient to induce T cell differentiation and lead to the generation of effector cells. Increased effector frequencies have, in fact, been described in RA (66). Although these effector populations are frequently found in the inflamed synovium, they do not appear to be specific for synovial antigens, and they are also found in peripheral blood. These effector cell populations frequently have lost the CD28 molecule, but have gained other regulatory molecules such as MHC class I recognizing receptors, the fractalkine receptor and increased expression of LFA-1. Such changes facilitate interactions with somatic cells other than professional antigen-presenting cells, and permit these T cells to receive costimulatory signals from the environment in an inflamed tissue such as the synovium (67–71). Lastly, we recently found that RA patients have an altered steady-state equilibrium of the Raf-Mek-ERK signaling pathway (72). This increased ERK-responsiveness was reproduced in T cells from normal individuals by exposure to homeostatic cytokines, but not by proinflammatory cytokines. ERK activation is central in controlling the T cell receptor activation threshold, as active ERK serine phosphorylates Lck and prevents the recruitment of SHP-1 and the early termination of T cell receptor-induced signaling. Indeed, we have shown that the increased ERK responses in RA patients lower the T cell receptor threshold and confer an increased responsiveness of RA T cells to suboptimal stimulation (72). Obviously, these models are not mutually exclusive but can work in concert; patients with RA may have contracted T cell repertoire biased for autoreactive specificities with an accumulation of effector cells that are responsive to suboptimal triggers. In spite of this increased responsiveness, T cell responses to exogenous antigen that require clonal expansion are compromised because of the reduced and shortened survival of proliferating T cells due to a defective DNA damage response (47).
Immune Aging and RA Comorbidities
RA is associated with comorbidities that significantly contribute to morbidity and mortality in this patient population. Of particular interest are cardiovascular manifestations; RA patients have an increased risk to develop accelerated coronary artery disease (73). One possible explanation for this observation is that the chronic inflammatory process associated with RA causes vascular injury and progressive plaque formation. Indeed, plaque inflammation has been identified as a major disease mechanism in coronary artery disease in the general population, not only in patients with autoimmune conditions. The second possible explanation is that accelerated coronary artery disease is a consequence of immune aging, and that RA and coronary artery disease co-occur in a host whose immune system is prematurely aged (74). In support of this hypothesis, evidence for accelerated immune aging is also found in patients with acute coronary symptoms who do not have an inflammatory disease. These patients frequently have an expanded population of T effector cells that have lost the CD28 molecule (75). They contribute to vascular damage by releasing proinflammatory cytokines (76) and also by cytotoxic activity towards vascular endothelial (77) and smooth muscle cells (78–80). Clonally expanded effector T cell populations are found in the inflamed coronary artery plaque (81). These effector cell populations are not necessarily specific for one particular antigen in the vascular environment, but exert their effector function due to a lowered T cell receptor activation threshold and, therefore, a propensity to be stimulated in low affinity recognition processes (82). Expansion of CD28-negative effector cell populations, a hallmark of immune aging, therefore, is a common denominator in the pathogenesis of atherosclerotic coronary artery disease and of RA (83).
Synopsis
Immunological models of RA have to take into account that the disease occurs at an age when immunocompetence is declining and in a host whose immune system shows evidence of accelerated immune aging. By several immune aging biomarkers, the immune system in patients with RA is prematurely aged by more than 20 years. One major pathogenetic mechanism is a defect in telomere maintenance and DNA repair which causes accelerated cell death. These findings in RA are reminiscent of murine autoimmunity models, in which lymphopenia was identified as a major risk factor for autoimmunity. Progress in our understanding how accelerated immune aging is pathogenetically involved in RA may allow development of new therapeutic approaches that go beyond the use of anti-inflammatory agents and could eventually open new avenues for preventive intervention.
Acknowledgments
This work was supported by grant R01 AR 41974, R01 AR 42527, R01 EY 11916, R01 AG 15043, R01 AI 44142, U19 AI 57266, and P01 HL58000 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kokkonen H, Soderstrom I, Rocklov J, Hallmans G, Lejon K, Rantapaa Dahlqvist S. Up-regulation of cytokines and chemokines predates the onset of rheumatoid arthritis. Arthritis Rheum. 2010 Jan 7;62(2):383–391. doi: 10.1002/art.27186. [DOI] [PubMed] [Google Scholar]
- 2.Weyand CM, Goronzy JJ. Pathogenesis of rheumatoid arthritis. Med Clin North Am. 1997 Jan;81(1):29–55. doi: 10.1016/s0025-7125(05)70504-6. [DOI] [PubMed] [Google Scholar]
- 3.Goronzy JJ, Weyand CM. Developments in the scientific understanding of rheumatoid arthritis. Arthritis Res Ther. 2009;11(5):249. doi: 10.1186/ar2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doran MF, Pond GR, Crowson CS, O'Fallon WM, Gabriel SE. Trends in incidence and mortality in rheumatoid arthritis in Rochester, Minnesota, over a forty-year period. Arthritis Rheum. 2002 Mar;46(3):625–631. doi: 10.1002/art.509. [DOI] [PubMed] [Google Scholar]
- 5.Goronzy JJ, Weyand CM. Rheumatoid arthritis. Immunol Rev. 2005 Apr;204:55–73. doi: 10.1111/j.0105-2896.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- 6.Larbi A, Fulop T, Pawelec G. Immune receptor signaling, aging and autoimmunity. Adv Exp Med Biol. 2008;640:312–324. doi: 10.1007/978-0-387-09789-3_21. [DOI] [PubMed] [Google Scholar]
- 7.Weyand CM, Goronzy JJ. Giant-cell arteritis and polymyalgia rheumatica. Ann Intern Med. 2003 Sep 16;139(6):505–515. doi: 10.7326/0003-4819-139-6-200309160-00015. [DOI] [PubMed] [Google Scholar]
- 8.Weyand CM, Goronzy JJ. Medium- and large-vessel vasculitis. N Engl J Med. 2003 Jul 10;349(2):160–169. doi: 10.1056/NEJMra022694. [DOI] [PubMed] [Google Scholar]
- 9.Moulias R, Proust J, Wang A, Congy F, Marescot MR, Deville Chabrolle A, Paris Hamelin A, Lesourd B. Age-related increase in autoantibodies. Lancet. 1984 May 19;1(8386):1128–1129. doi: 10.1016/s0140-6736(84)92547-9. [DOI] [PubMed] [Google Scholar]
- 10.Grubeck-Loebenstein B, Wick G. The aging of the immune system. Adv Immunol. 2002;80:243–284. doi: 10.1016/s0065-2776(02)80017-7. [DOI] [PubMed] [Google Scholar]
- 11.Weng NP. Aging of the immune system: how much can the adaptive immune system adapt? Immunity. 2006 May;24(5):495–499. doi: 10.1016/j.immuni.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Targonski PV, Jacobson RM, Poland GA. Immunosenescence: role and measurement in influenza vaccine response among the elderly. Vaccine. 2007 Apr 20;25(16):3066–3069. doi: 10.1016/j.vaccine.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 13.Jefferson T, Rivetti D, Rivetti A, Rudin M, Di Pietrantonj C, Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet. 2005 Oct 1;366(9492):1165–1174. doi: 10.1016/S0140-6736(05)67339-4. [DOI] [PubMed] [Google Scholar]
- 14.Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med. 2007 Oct 4;357(14):1373–1381. doi: 10.1056/NEJMoa070844. [DOI] [PubMed] [Google Scholar]
- 15.Donahue JG, Choo PW, Manson JE, Platt R. The incidence of herpes zoster. Arch Intern Med. 1995 Aug 7–21;155(15):1605–1609. [PubMed] [Google Scholar]
- 16.Arvin A. Aging, immunity, and the varicella-zoster virus. N Engl J Med. 2005 Jun 2;352(22):2266–2267. doi: 10.1056/NEJMp058091. [DOI] [PubMed] [Google Scholar]
- 17.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003 Jan 8;289(2):179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 18.Looney RJ, Hasan MS, Coffin D, Campbell D, Falsey AR, Kolassa J, Agosti JM, Abraham GN, Evans TG. Hepatitis B immunization of healthy elderly adults: relationship between naive CD4+ T cells and primary immune response and evaluation of GM-CSF as an adjuvant. J Clin Immunol. 2001 Jan;21(1):30–36. doi: 10.1023/a:1006736931381. [DOI] [PubMed] [Google Scholar]
- 19.Goronzy JJ, Weyand CM. T cell development and receptor diversity during aging. Curr Opin Immunol. 2005 Oct;17(5):468–475. doi: 10.1016/j.coi.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 20.Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, Witkowski J, Fulbright J, Weyand CM, Goronzy JJ. The influence of age on T cell generation and TCR diversity. J Immunol. 2005 Jun 1;174(11):7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- 21.Gomez CR, Boehmer ED, Kovacs EJ. The aging innate immune system. Curr Opin Immunol. 2005 Oct;17(5):457–462. doi: 10.1016/j.coi.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Niessner A, Weyand CM. Dendritic cells in atherosclerotic disease. Clin Immunol. Jan;134(1):25–32. doi: 10.1016/j.clim.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weyand CM, Younge BR, Goronzy JJ. T cells in arteritis and atherosclerosis. Curr Opin Lipidol. 2008 Oct;19(5):469–477. doi: 10.1097/mol.0b013e32830bfdc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hakim FT, Memon SA, Cepeda R, Jones EC, Chow CK, Kasten-Sportes C, Odom J, Vance BA, Christensen BL, Mackall CL, Gress RE. Age-dependent incidence, time course, and consequences of thymic renewal in adults. J Clin Invest. 2005 Apr;115(4):930–939. doi: 10.1172/JCI22492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Czesnikiewicz-Guzik M, Lee WW, Cui D, Hiruma Y, Lamar DL, Yang ZZ, Ouslander JG, Weyand CM, Goronzy JJ. T cell subset-specific susceptibility to aging. Clin Immunol. 2008 Apr;127(1):107–118. doi: 10.1016/j.clim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nikolich-Zugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat Rev Immunol. 2008 Jul;8(7):512–522. doi: 10.1038/nri2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodes RJ, Hathcock KS, Weng NP. Telomeres in T and B cells. Nat Rev Immunol. 2002 Sep;2(9):699–706. doi: 10.1038/nri890. [DOI] [PubMed] [Google Scholar]
- 28.Jendro MC, Ganten T, Matteson EL, Weyand CM, Goronzy JJ. Emergence of oligoclonal T cell populations following therapeutic T cell depletion in rheumatoid arthritis. Arthritis Rheum. 1995 Sep;38(9):1242–1251. doi: 10.1002/art.1780380912. [DOI] [PubMed] [Google Scholar]
- 29.Brett S, Baxter G, Cooper H, Johnston JM, Tite J, Rapson N. Repopulation of blood lymphocyte sub-populations in rheumatoid arthritis patients treated with the depleting humanized monoclonal antibody, CAMPATH-1H. Immunology. 1996 May;88(1):13–19. doi: 10.1046/j.1365-2567.1996.d01-650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lorenzi AR, Clarke AM, Wooldridge T, Waldmann H, Hale G, Symmons D, Hazleman BL, Isaacs JD. Morbidity and mortality in rheumatoid arthritis patients with prolonged therapy-induced lymphopenia: twelve-year outcomes. Arthritis Rheum. 2008 Feb;58(2):370–375. doi: 10.1002/art.23122. [DOI] [PubMed] [Google Scholar]
- 31.Jones JL, Phuah CL, Cox AL, Thompson SA, Ban M, Shawcross J, Walton A, Sawcer SJ, Compston A, Coles AJ. IL-21 drives secondary autoimmunity in patients with multiple sclerosis, following therapeutic lymphocyte depletion with alemtuzumab (Campath-1H) J Clin Invest. 2009 Jul;119(7):2052–2061. doi: 10.1172/JCI37878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koetz K, Bryl E, Spickschen K, O'Fallon WM, Goronzy JJ, Weyand CM. T cell homeostasis in patients with rheumatoid arthritis. Proc Natl Acad Sci U S A. 2000 Aug 1;97(16):9203–9208. doi: 10.1073/pnas.97.16.9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kong FK, Chen CL, Six A, Hockett RD, Cooper MD. T cell receptor gene deletion circles identify recent thymic emigrants in the peripheral T cell pool. Proc Natl Acad Sci U S A. 1999 Feb 16;96(4):1536–1540. doi: 10.1073/pnas.96.4.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF, Polis MA, Haase AT, Feinberg MB, Sullivan JL, Jamieson BD, Zack JA, Picker LJ, Koup RA. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998 Dec 17;396(6712):690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 35.van den Dool C, de Boer RJ. The effects of age, thymectomy, and HIV Infection on alpha and beta TCR excision circles in naive T cells. J Immunol. 2006 Oct 1;177(7):4391–4401. doi: 10.4049/jimmunol.177.7.4391. [DOI] [PubMed] [Google Scholar]
- 36.Ponchel F, Morgan AW, Bingham SJ, Quinn M, Buch M, Verburg RJ, Henwood J, Douglas SH, Masurel A, Conaghan P, Gesinde M, Taylor J, Markham AF, Emery P, van Laar JM, Isaacs JD. Dysregulated lymphocyte proliferation and differentiation in patients with rheumatoid arthritis. Blood. 2002 Dec 15;100(13):4550–4556. doi: 10.1182/blood-2002-03-0671. [DOI] [PubMed] [Google Scholar]
- 37.Hazenberg MD, Otto SA, Cohen Stuart JW, Verschuren MC, Borleffs JC, Boucher CA, Coutinho RA, Lange JM, Rinke de Wit TF, Tsegaye A, van Dongen JJ, Hamann D, de Boer RJ, Miedema F. Increased cell division but not thymic dysfunction rapidly affects the T-cell receptor excision circle content of the naive T cell population in HIV-1 infection. Nat Med. 2000 Sep;6(9):1036–1042. doi: 10.1038/79549. [DOI] [PubMed] [Google Scholar]
- 38.Hazenberg MD, Borghans JA, de Boer RJ, Miedema F. Thymic output: a bad TREC record. Nat Immunol. 2003 Feb;4(2):97–99. doi: 10.1038/ni0203-97. [DOI] [PubMed] [Google Scholar]
- 39.Andrews NP, Fujii H, Goronzy JJ, Weyand CM. Telomeres and Immunological Diseases of Aging. Gerontology. 2009 Dec 17; doi: 10.1159/000268620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vallejo AN, Pease LR. The locus-specific enhancer activity of the class I major histocompatibility complex interferon-responsive element is associated with a gamma-interferon (IFN)-inducible factor distinct from STAT1alpha, p48, and IFN regulatory factor-1. J Biol Chem. 1996 Nov 22;271(47):29813–29821. doi: 10.1074/jbc.271.47.29813. [DOI] [PubMed] [Google Scholar]
- 41.Effros RB, Dagarag M, Spaulding C, Man J. The role of CD8+ T-cell replicative senescence in human aging. Immunol Rev. 2005 Jun;205:147–157. doi: 10.1111/j.0105-2896.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt D, Goronzy JJ, Weyand CM. CD4+ CD7- CD28- T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J Clin Invest. 1996 May 1;97(9):2027–2037. doi: 10.1172/JCI118638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt D, Martens PB, Weyand CM, Goronzy JJ. The repertoire of CD4+ CD28- T cells in rheumatoid arthritis. Mol Med. 1996 Sep;2(5):608–618. [PMC free article] [PubMed] [Google Scholar]
- 44.Wagner UG, Koetz K, Weyand CM, Goronzy JJ. Perturbation of the T cell repertoire in rheumatoid arthritis. Proc Natl Acad Sci U S A. 1998 Nov 24;95(24):14447–14452. doi: 10.1073/pnas.95.24.14447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thewissen M, Somers V, Venken K, Linsen L, van Paassen P, Geusens P, Damoiseaux J, Stinissen P. Analyses of immunosenescent markers in patients with autoimmune disease. Clin Immunol. 2007 May;123(2):209–218. doi: 10.1016/j.clim.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 46.Schonland SO, Lopez C, Widmann T, Zimmer J, Bryl E, Goronzy JJ, Weyand CM. Premature telomeric loss in rheumatoid arthritis is genetically determined and involves both myeloid and lymphoid cell lineages. Proc Natl Acad Sci U S A. 2003 Nov 11;100(23):13471–13476. doi: 10.1073/pnas.2233561100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shao L, Fujii H, Colmegna I, Oishi H, Goronzy JJ, Weyand CM. Deficiency of the DNA repair enzyme ATM in rheumatoid arthritis. J Exp Med. 2009 Jun 8;206(6):1435–1449. doi: 10.1084/jem.20082251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goronzy JJ, Matteson EL, Fulbright JW, Warrington KJ, Chang-Miller A, Hunder GG, Mason TG, Nelson AM, Valente RM, Crowson CS, Erlich HA, Reynolds RL, Swee RG, O'Fallon WM, Weyand CM. Prognostic markers of radiographic progression in early rheumatoid arthritis. Arthritis Rheum. 2004 Jan;50(1):43–54. doi: 10.1002/art.11445. [DOI] [PubMed] [Google Scholar]
- 49.Martens PB, Goronzy JJ, Schaid D, Weyand CM. Expansion of unusual CD4+ T cells in severe rheumatoid arthritis. Arthritis Rheum. 1997 Jun;40(6):1106–1114. doi: 10.1002/art.1780400615. [DOI] [PubMed] [Google Scholar]
- 50.Bryl E, Vallejo AN, Weyand CM, Goronzy JJ. Down-regulation of CD28 expression by TNF-alpha. J Immunol. 2001 Sep 15;167(6):3231–3238. doi: 10.4049/jimmunol.167.6.3231. [DOI] [PubMed] [Google Scholar]
- 51.Bryl E, Vallejo AN, Matteson EL, Witkowski JM, Weyand CM, Goronzy JJ. Modulation of CD28 expression with anti-tumor necrosis factor alpha therapy in rheumatoid arthritis. Arthritis Rheum. 2005 Oct;52(10):2996–3003. doi: 10.1002/art.21353. [DOI] [PubMed] [Google Scholar]
- 52.Warrington KJ, Vallejo AN, Weyand CM, Goronzy JJ. CD28 loss in senescent CD4+ T cells: reversal by interleukin-12 stimulation. Blood. 2003 May 1;101(9):3543–3549. doi: 10.1182/blood-2002-08-2574. [DOI] [PubMed] [Google Scholar]
- 53.Chapman A, Stewart SJ, Nepom GT, Green WF, Crowe D, Thomas JW, Miller GG. CD11b+CD28-CD4+ human T cells: activation requirements and association with HLA-DR alleles. J Immunol. 1996 Dec 1;157(11):4771–4780. [PubMed] [Google Scholar]
- 54.Waase I, Kayser C, Carlson PJ, Goronzy JJ, Weyand CM. Oligoclonal T cell proliferation in patients with rheumatoid arthritis and their unaffected siblings. Arthritis Rheum. 1996 Jun;39(6):904–913. doi: 10.1002/art.1780390606. [DOI] [PubMed] [Google Scholar]
- 55.van Leeuwen EM, Remmerswaal EB, Vossen MT, Rowshani AT, Wertheim-van Dillen PM, van Lier RA, ten Berge IJ. Emergence of a CD4+CD28- granzyme B+, cytomegalovirus-specific T cell subset after recovery of primary cytomegalovirus infection. J Immunol. 2004 Aug 1;173(3):1834–1841. doi: 10.4049/jimmunol.173.3.1834. [DOI] [PubMed] [Google Scholar]
- 56.Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 57.Weyand CM, Fujii H, Shao L, Goronzy JJ. Rejuvenating the immune system in rheumatoid arthritis. Nat Rev Rheumatol. 2009 Oct;5(10):583–588. doi: 10.1038/nrrheum.2009.180. [DOI] [PubMed] [Google Scholar]
- 58.Fujii H, Shao L, Colmegna I, Goronzy JJ, Weyand CM. Telomerase insufficiency in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2009 Mar 17;106(11):4360–4365. doi: 10.1073/pnas.0811332106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Colmegna I, Diaz-Borjon A, Fujii H, Schaefer L, Goronzy JJ, Weyand CM. Defective proliferative capacity and accelerated telomeric loss of hematopoietic progenitor cells in rheumatoid arthritis. Arthritis Rheum. 2008 Apr;58(4):990–1000. doi: 10.1002/art.23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lavin MF, Kozlov S, Gueven N, Peng C, Birrell G, Chen P, Scott S. Atm and cellular response to DNA damage. Adv Exp Med Biol. 2005;570:457–476. doi: 10.1007/1-4020-3764-3_16. [DOI] [PubMed] [Google Scholar]
- 61.King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004 Apr 16;117(2):265–277. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 62.Crisa L, Mordes JP, Rossini AA. Autoimmune diabetes mellitus in the BB rat. Diabetes Metab Rev. 1992 Apr;8(1):4–37. [PubMed] [Google Scholar]
- 63.Glant TT, Finnegan A, Mikecz K. Proteoglycan-induced arthritis: immune regulation, cellular mechanisms, and genetics. Crit Rev Immunol. 2003;23(3):199–250. doi: 10.1615/critrevimmunol.v23.i3.20. [DOI] [PubMed] [Google Scholar]
- 64.Goronzy JJ, Weyand CM. Aging, autoimmunity and arthritis: T-cell senescence and contraction of T-cell repertoire diversity - catalysts of autoimmunity and chronic inflammation. Arthritis Res Ther. 2003;5(5):225–234. doi: 10.1186/ar974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goronzy JJ, Weyand CM. Thymic function and peripheral T-cell homeostasis in rheumatoid arthritis. Trends Immunol. 2001 May;22(5):251–255. doi: 10.1016/s1471-4906(00)01841-x. [DOI] [PubMed] [Google Scholar]
- 66.Cope AP. T cells in rheumatoid arthritis. Arthritis Res Ther. 2008;10 Suppl 1:S1. doi: 10.1186/ar2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh K, Colmegna I, He X, Weyand CM, Goronzy JJ. Synoviocyte stimulation by the LFA-1-intercellular adhesion molecule-2-Ezrin-Akt pathway in rheumatoid arthritis. J Immunol. 2008 Feb 1;180(3):1971–1978. doi: 10.4049/jimmunol.180.3.1971. [DOI] [PubMed] [Google Scholar]
- 68.Sawai H, Park YW, Roberson J, Imai T, Goronzy JJ, Weyand CM. T cell costimulation by fractalkine-expressing synoviocytes in rheumatoid arthritis. Arthritis Rheum. 2005 May;52(5):1392–1401. doi: 10.1002/art.21140. [DOI] [PubMed] [Google Scholar]
- 69.Davila E, Kang YM, Park YW, Sawai H, He X, Pryshchep S, Goronzy JJ, Weyand CM. Cell-based immunotherapy with suppressor CD8+ T cells in rheumatoid arthritis. J Immunol. 2005 Jun 1;174(11):7292–7301. doi: 10.4049/jimmunol.174.11.7292. [DOI] [PubMed] [Google Scholar]
- 70.Sawai H, Park YW, He X, Goronzy JJ, Weyand CM. Fractalkine mediates T cell-dependent proliferation of synovial fibroblasts in rheumatoid arthritis. Arthritis Rheum. 2007 Oct;56(10):3215–3225. doi: 10.1002/art.22919. [DOI] [PubMed] [Google Scholar]
- 71.Goronzy JJ, Henel G, Sawai H, Singh K, Lee EB, Pryshchep S, Weyand CM. Costimulatory pathways in rheumatoid synovitis and T-cell senescence. Ann N Y Acad Sci. 2005 Dec;1062:182–194. doi: 10.1196/annals.1358.022. [DOI] [PubMed] [Google Scholar]
- 72.Singh K, Deshpande P, Pryshchep S, Colmegna I, Liarski V, Weyand CM, Goronzy JJ. ERK-dependent T cell receptor threshold calibration in rheumatoid arthritis. J Immunol. 2009 Dec 15;183(12):8258–8267. doi: 10.4049/jimmunol.0901784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Warrington KJ, Kent PD, Frye RL, Lymp JF, Kopecky SL, Goronzy JJ, Weyand CM. Rheumatoid arthritis is an independent risk factor for multi-vessel coronary artery disease: a case control study. Arthritis Res Ther. 2005;7(5):R984–R991. doi: 10.1186/ar1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Crowson CS, Liang KP, Therneau TM, Kremers HM, Gabriel SE. Could accelerated aging explain the excess mortality in patients with seropositive rheumatoid arthritis? Arthritis Rheum. Jan 7;62(2):378–382. doi: 10.1002/art.27194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liuzzo G, Kopecky SL, Frye RL, O'Fallon WM, Maseri A, Goronzy JJ, Weyand CM. Perturbation of the T-cell repertoire in patients with unstable angina. Circulation. 1999 Nov 23;100(21):2135–2139. doi: 10.1161/01.cir.100.21.2135. [DOI] [PubMed] [Google Scholar]
- 76.Liuzzo G, Vallejo AN, Kopecky SL, Frye RL, Holmes DR, Goronzy JJ, Weyand CM. Molecular fingerprint of interferon-gamma signaling in unstable angina. Circulation. 2001 Mar 20;103(11):1509–1514. doi: 10.1161/01.cir.103.11.1509. [DOI] [PubMed] [Google Scholar]
- 77.Nakajima T, Schulte S, Warrington KJ, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM. T-cell-mediated lysis of endothelial cells in acute coronary syndromes. Circulation. 2002 Feb 5;105(5):570–575. doi: 10.1161/hc0502.103348. [DOI] [PubMed] [Google Scholar]
- 78.Niessner A, Sato K, Chaikof EL, Colmegna I, Goronzy JJ, Weyand CM. Pathogen-sensing plasmacytoid dendritic cells stimulate cytotoxic T-cell function in the atherosclerotic plaque through interferon-alpha. Circulation. 2006 Dec 5;114(23):2482–2489. doi: 10.1161/CIRCULATIONAHA.106.642801. [DOI] [PubMed] [Google Scholar]
- 79.Sato K, Niessner A, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM. TRAIL-expressing T cells induce apoptosis of vascular smooth muscle cells in the atherosclerotic plaque. J Exp Med. 2006 Jan 23;203(1):239–250. doi: 10.1084/jem.20051062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pryshchep S, Sato K, Goronzy JJ, Weyand CM. T cell recognition and killing of vascular smooth muscle cells in acute coronary syndrome. Circ Res. 2006 May 12;98(9):1168–1176. doi: 10.1161/01.RES.0000220649.10013.5c. [DOI] [PubMed] [Google Scholar]
- 81.Liuzzo G, Goronzy JJ, Yang H, Kopecky SL, Holmes DR, Frye RL, Weyand CM. Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation. 2000 Jun 27;101(25):2883–2888. doi: 10.1161/01.cir.101.25.2883. [DOI] [PubMed] [Google Scholar]
- 82.Pryshchep S, Goronzy JJ, Parashar S, Weyand CM. Failed Deactivation of the Protein Tyrosine Kinase Lck Amplifies T-Cell Responsiveness in Acute Coronary Syndrome. Circ Res. 2009 Dec 24; doi: 10.1161/CIRCRESAHA.109.206052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pasceri V, Yeh ET. A tale of two diseases: atherosclerosis and rheumatoid arthritis. Circulation. 1999 Nov 23;100(21):2124–2126. doi: 10.1161/01.cir.100.21.2124. [DOI] [PubMed] [Google Scholar]