Abstract
Inflammatory tolerance is the down-regulation of inflammation upon repeated stimuli, which is well-established to occur in peripheral immune cells. However, less is known about inflammatory tolerance in the brain although it may provide an important protective mechanism from detrimental consequences of prolonged inflammation, which appears to occur in many psychiatric and neurodegenerative conditions. Array analysis of 308 inflammatory molecules produced by mouse primary astrocytes after two sequential stimulations with lipopolysaccharide (LPS) distinguished three classes, tolerant, sensitized and unaltered groups. For many of these inflammatory molecules, inhibition of glycogen synthase kinase-3 (GSK3) increased tolerance and reduced sensitization. Focusing on LPS-tolerance in interleukin-6 (IL-6) production, we found that microglia exhibited a strong tolerance response that matched that of macrophages, whereas astrocytes exhibited only partial tolerance. The astrocyte semi-tolerance was found to be regulated by GSK3. GSK3 inhibitors or knocking down GSK3 levels promoted LPS-tolerance and astrocytes expressing constitutively active GSK3 did not develop LPS-tolerance. These findings identify the critical role of GSK3 in counteracting IL-6 inflammatory tolerance in cells of the CNS, supporting the therapeutic potential of GSK3 inhibitors to reduce neuroinflammation by promoting tolerance.
Keywords: neuroinflammation, GSK-3, astrocytes, LPS
(i) Introduction
Tolerance to inflammatory signals, which down-regulates inflammatory molecule production in spite of repeated stimuli, may be a particularly crucial protective mechanism in the brain. Although initial inflammatory reactions, particularly cytokine production by astrocytes and microglia, are crucial for responses to injury, infection, or stress, prolonged neuroinflammation, which can even be induced by psychological stress, damages neuronal function and contributes to many psychiatric and neurodegenerative conditions (Dantzer et al., 2008, Miller et al., 2009). However, little information is available concerning the possibility that tolerance may contribute to regulating the extent and duration of cytokine production in the central nervous system (CNS) (Craft et al., 2005). Lipopolysaccharide (LPS)-preconditioning provides neuroprotection against ischemic brain injury in mouse models of stroke (reviewed in (Kariko et al., 2004)). Furthermore, repeated exposures to LPS are associated with tolerance to mechanical hyperalgesia (Guo and Schluesener, 2006) and with a reduction of tumor necrosis factor (TNF) but not prostaglandin E2 (PGE2) production by primary glia (Shemi and Kaplanski, 2005). This contrasts with the peripheral immune system, where tolerance is well-characterized. For example, it is well-established that pre-conditioning macrophages with LPS desensitizes the cytokine producing machinery to subsequent exposure to LPS (Freudenberg and Galanos, 1990, Ziegler-Heitbrock, 1995, Medvedev et al., 2000, Cavaillon and Adib-Conquy, 2006, Schneider and Ayres, 2008). Opposite to tolerance, some inflammatory molecules become sensitized to multiple exposures to LPS, an outcome that also could contribute to the deleterious effects of long-term or repeated inflammatory stimuli.
Inflammatory cytokine production is promoted by glycogen synthase kinase-3 (GSK3) (Martin et al., 2005, Beurel and Jope, 2009b). GSK3 is a highly conserved Ser/Thr protein kinase that is constitutively active (Cohen and Frame, 2001, Doble and Woodgett, 2003, Jope and Johnson, 2004). GSK3 is predominantly regulated by inhibitory phosphorylation of its two isoforms, serine-9 in GSK3β and serine-21 in GSK3α, which is abrogated in GSK3 knockin mice that express serine-to-alanine mutations in both isoforms (McManus et al., 2005). In this study, we tested if LPS-tolerance or sensitization occurs in astrocytes, and focusing on IL-6 production in response to LPS stimulation, we tested if tolerance is regulated by GSK3.
(ii) Experimental procedures
Cell culture
Primary glia were prepared from cerebral cortex of 1 day old C57Bl/6 mice as described (McCarthy and de Vellis, 1980), and cultured in DMEM/F12 medium supplemented with 10% fetal bovine serum (FBS), 0.3% glucose, 2 mM L-glutamine, 10 U/mL penicillin and 10 µg/mL streptomycin. For separation of astrocytes and microglia, after 10 days of culture the cells were shaken (30 h; 250 rpm), resulting in >99% pure astrocytes as determined by immunostaining with the astrocyte marker glial fibrillary acidic protein (GFAP). After the first hour of shaking, the medium containing microglia cells was collected and microglia were cultured in the same medium as astrocytes. RAW264.7 cells were cultivated as described previously (Beurel and Jope, 2009a). Protein-free E. coli (K235) LPS was prepared as described (Hirschfeld et al., 2000). Cells were left untreated (naive, 0) or stimulated with 100 ng/mL LPS for 24 h (to establish LPS-tolerance) in medium supplemented with 10% FBS, washed twice with warm medium and given fresh media (0/0 or LPS/0) or 10 ng/mL LPS (0/LPS, LPS/LPS) for 1 h or 24 h. Where indicated, cells were treated with 10 µM kenpaullone, 10 µM TDZD-8 (Calbiochem), 10 µ M Chiron99021 (University of Dundee, UK), or 20 mM LiCl (Sigma).
Biochemical methods
The levels of 308 inflammatory proteins were measured with antibody arrays according to the manufacturer’s instructions (Raybiotech). IL-6 levels were measured by ELISA according to the manufacturer’s instructions (eBioscience). For knocking down GSK3 levels, cells were transfected using liposome-mediated transfection reagent LipofectAMINE RNAiMAX (Invitrogen) with 50 nM siRNA according to the manufacturer’s instructions with silencer negative control (Ambion), or GSK3α and GSK3β siRNA (Smart pool; Dharmacon). For overexpressing GSK3, cells were rinsed twice with DMEM/F12 medium without supplements, infected with 50 moi of the designated adenovirus for 30 min, supplemented medium was added for incubation for 36–48 h, and infected cultures were examined for adequate infection efficiency (80%) as assessed by GFP fluorescence after infection with GFP adenovirus. Western blots were carried out as described previously (Beurel and Jope, 2008) using antibodies to phospho-Ser21GSK3α, phospho-Ser9GSK3β, (Cell Signaling Technology), total GSK3α/β (Millipore), and β-actin (Sigma). Statistical significance between groups was evaluated by ANOVA.
(iii) Results
Inflammatory tolerance and sensitization in astrocytes
Array analysis of inflammatory molecules produced by mouse primary astrocytes was used to identify those that display adaptive responses to repeated LPS exposure and to determine which are regulated by GSK3. Cells were treated according to the paradigm of Foster et al. (2007) that was developed to examine LPS-tolerance in macrophages. This involved comparing the production of inflammatory molecules stimulated by 10 ng/mL LPS for 24 hr in astrocytes that either had been preincubated for 24 hr in media with no addition (0/LPS group) or with 100 ng/mL LPS (LPS/LPS group) (Figure 1A). With both conditions, to test if GSK3 regulated adaptive responses to repeated stimulation with LPS, during the first incubation, additional cells were treated with 20 mM lithium to fully inhibit GSK3 (Klein and Melton, 1996, Stambolic et al., 1996), followed by thoroughly washing out the lithium before the second exposure to LPS. Of the 308 inflammatory molecules assessed on the array, 125 were reliably increased in media from LPS-treated astrocytes (Figure 1B). Of these, 13% displayed LPS-tolerance, defined as molecules that were produced by <50% during the second LPS stimulation (LPS/LPS) compared to the amounts produced by a single LPS stimulation (0/LPS) (Figure 2; white area in center circle). Opposite to LPS-tolerance, 34% of the inflammatory molecules displayed sensitization after repeated LPS administration, defined as >50% increased production by the second LPS stimulation compared with a single LPS stimulation (Figure 2; shaded area in center circle), and 53% were unaffected by pretreatment with LPS.
Figure 1.
Inflammatory molecule tolerance and sensitivity to repeated LPS exposures: Modulation by inhibition of GSK3 by lithium. (A) Scheme of tolerance paradigm in which cells were untreated (0), or stimulated with 100 ng/mL LPS for 24 h without or with GSK3 inhibitors, thoroughly washed with media, and reincubated for 24 h with or without 10 ng/mL LPS. (B) Mouse primary astrocytes were treated as described in (A) with or without the GSK3 inhibitor LiCl (20 mM). Supernatants from 2 experiments were pooled and analyzed by cytokine array detecting 308 molecules in duplicates. Four groups of six dark spots are positive control standards. The box indicates the changes observed with IL-6. Data are representative of 2 independent experiments.
Figure 2.
Classification of inflammatory molecule responses to repeated LPS stimulation. From the array data described in Figure 1, the ratio of LPS/LPS to 0/LPS was calculated for each inflammatory molecule. Classifications of inflammatory molecule production caused by repeated stimulation with LPS were defined as follows: "tolerance" was a ratio of <0.5 (>50% reduction), "sensitization" was a ratio of >1.5 (>50% increase), and "no change" indicates a ratio between 0.5 and 1.5. The center circle represents the distribution of molecules in the absence of lithium in the "tolerance", "sensitization" and "no change" groups. Each of these three groups was subclassified according to the effects of lithium exposure during the pretreatment phase and these are shown in the circles in the periphery. For the original "no change" group, in the presence of lithium if the ratio was (a) <0.5 they were classified as "tolerance", (b) > 1.5 they were classified as "sensitization", and (c) 0.5 – 1.5 they were classified as "no change". For the original "tolerance" group, in the presence of lithium if the ratio was (a) still <0.5 and at least 0.1 lower than without lithium they were classified as "more tolerance", (b) still <0.5 and equal or greater than the ratio without lithium they were classified as "no change", and (c) >0.5 they were classified as "blocked tolerance". For the original "sensitization" group, in the presence of lithium if the ratio was (a) <0.5 they were classified as "tolerance", (b) 0.5 – 1.5 they were classified as "blocked sensitization", (c) still >1.5 they were classified as "no change".
GSK3 regulates inflammatory tolerance and sensitization in astrocytes
GSK3 was found to regulate many of the adaptive responses to repeated LPS exposure. Of the tolerant molecules, lithium pretreatment increased the tolerance of 19% (Figure 2, white area in upper right circle), lithium pretreatment blocked tolerance for half of the tolerant molecules (shaded area in upper right circle), and 31% were unaffected by lithium (dark area in upper right circle). Sensitization may be a critical response involved in heightened dysfunction in the brain associated with unabated neuroinflammation, and remarkably, inhibition of GSK3 with lithium during the first LPS exposure dampened the production of 63% of the sensitized inflammatory molecules (Figure 2; shaded area in upper left circle). Lithium also promoted tolerance of eight inflammatory molecules that otherwise demonstrated a sensitization response to repeated LPS treatment (Figure 2, white area in upper left circle). The group of inflammatory molecules least affected by lithium were those that were unaffected by pretreatment with LPS (the "No Change" group in the center circle). These results demonstrate that GSK3 contributes to the tolerance and sensitization processes of several inflammatory molecules in astrocytes exposed to repeated LPS exposure, indicating that GSK3 can influence the repertoire of inflammatory molecules that are produced after repeated exposure to LPS. Considering these differential responses to LPS with inhibition of GSK3, it is evident that GSK3 does not act only at the level of the initial LPS-induced activation of the toll-like receptor 4 (TLR4) signaling pathway, but must act after dispersal of the signal to differentially modulate the expression of inflammatory molecules.
IL-6 production displays LPS-tolerance in astrocytes, macrophages, and microglia
Since IL-6 is involved in many neuroinflammatory conditions and promotion of tolerance to LPS would provide a mechanism to reduce neuroinflammation (Cerciat et al., Lee et al., Sironi et al., 1993, Bull et al., 2009), we examined in greater detail the regulation by GSK3 of LPS-tolerance in IL-6 production. As indicated by array analysis (Figure 1), astrocytes preincubated with LPS produced significantly less IL-6 upon restimulation with LPS than did astrocytes not pre-exposed to LPS (Figure 3A), demonstrating a phenotype of semi-tolerance. Preconditioning of astrocytes with LPS for up to 96 h induced semi-tolerance (Figure 3B), and astrocyte semi-tolerance to LPS persisted for at least 96 h after the initial 24 h stimulation (data not shown). However, more complete tolerance was achieved by two or more sequential 24 h treatments with 100 ng/mL LPS (Figure 3C). In contrast to astrocytes, complete LPS-tolerance occurred after a single LPS stimulus in macrophage-derived RAW264.7 cells, as very little IL-6 was produced by a second application of 10 ng/mL LPS following a first tolerance-inducing exposure to 100 ng/mL LPS (Figure 3D), consistent with previous studies (Foster et al., 2007). Microglia, which in common with macrophages are bone marrow derived haematopoietic cells, also developed complete LPS-tolerance in IL-6 production after an initial LPS stimulation (Figure 3E). The LPS semi-tolerance characteristic of astrocytes provides a model system to study the bi-directional modulation of LPS-tolerance.
Figure 3.
LPS-tolerance in IL-6 production differs in astrocytes, RAW264.7 cells and microglia. (A) Primary mouse astrocytes, (D) RAW264.7 cells or (E) primary mouse microglia were pretreated with 100 ng/mL LPS for 24 h, washed, and restimulated with 10 ng/mL LPS for 24 h. Media from the final 24 h were analyzed for IL-6 by ELISA. (B) Primary mouse astrocytes were pretreated with 100 ng/mL LPS for the indicated times, washed, and restimulated with 10 ng/mL LPS for 24 h. (C) Primary mouse astrocytes were pretreated with 100 ng/mL LPS for one (1×), two (2×) or three (3×) 24 h intervals, with washes between intervals, and restimulated with 10 ng/mL LPS for 24 h. Proliferation of astrocytes resulted in greater production of IL-6 by 10 ng/mL LPS treatment without LPS-pretreatment in the 2× and 3× samples. Values represent mean ± SEM (n=3–6; *p<0.05, ANOVA).
GSK3 regulates LPS-tolerance of IL-6 production in astrocytes
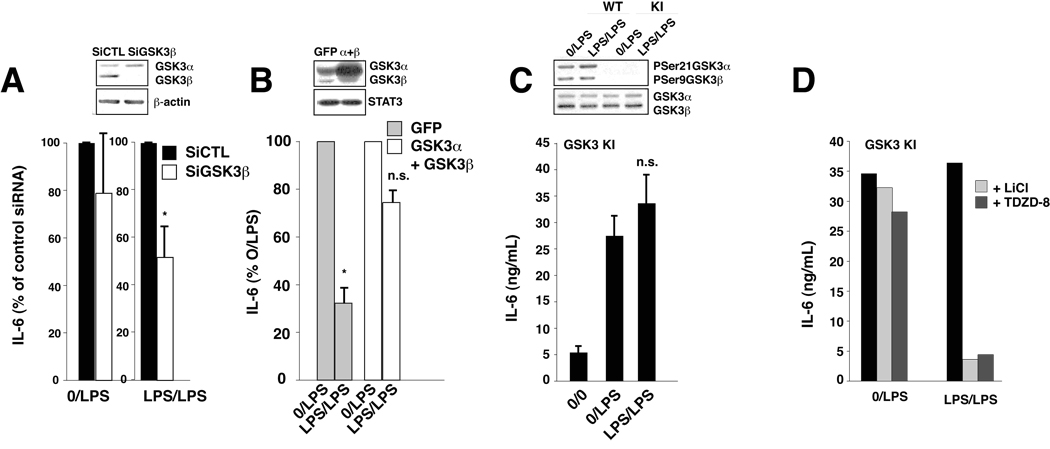
To examine the involvement of GSK3 in LPS-tolerance of IL-6 production, astrocytes were pretreated with or without LPS in the absence or presence of GSK3 inhibitors, the inhibitors were then washed out, and the response to LPS was measured. Pretreatment with GSK3 inhibitors kenpaullone (Leost et al., 2000), Chiron99021 (Wagman et al., 2004), or TDZD-8 (Martinez et al., 2002) in the absence of LPS had no effect on the subsequent LPS-induced production of IL-6 (Figure 4A). However, all three GSK3 inhibitors greatly increased the development of tolerance when present with LPS in the initial incubation (Figure 4B). Incubation with the three GSK-3 inhibitors for the last one hour of the initial LPS treatment had no effect on subsequent LPS-induced IL-6 production (Figure 4C), demonstrating that more than 1 hr exposure to GSK3 inhibitors in the presence of LPS was required for the inhibitors to promote tolerance. Lithium is the most widely used GSK3 inhibitor (Klein and Melton, 1996, Stambolic et al., 1996), and like the other GSK3 inhibitors, lithium pretreatment with LPS during the initial treatment also promoted LPS-tolerance in IL-6 production (Figure 5A) and this did not occur if lithium was only present for the last one hour of the pretreatment (Figure 5B). Unlike the other GSK3 inhibitors, pretreatment with lithium alone, in the absence of LPS (0/LPS condition), tended to reduce the subsequent LPS-induced production of IL-6 (Figure 5A). Besides directly inhibiting GSK3, lithium treatment also indirectly inhibits GSK3 through an increase in the inhibitory serine-phosphorylation of GSK3 (Jope 2003), as shown in Figure 5C (right lane), but there was no residual increased serine-phosphorylation of GSK3 in the samples pretreated with lithium followed by a second incubation (Figure 5C lanes 4–6 compared with lanes 1–3). Nonetheless, the time necessary for inhibitory serine-phosphorylation of GSK3 to dissipate during the second incubation may have contributed to the lower production of IL-6 in samples pretreated with lithium followed by stimulation with LPS. Since LPS-tolerance was 100% in microglia and RAW264.7, the lack of IL-6 produced in response to a second LPS stimulus was not modulated by GSK3 inhibitors (data not shown). These results demonstrate that inhibition of GSK3 promotes the induction of LPS-tolerance on IL-6 production, indicating that active GSK3 counteracts LPS-tolerance. Comparing GSK3 levels in microglia and astrocytes revealed that astrocytes contain 362 ± 40% (n=3) greater levels of GSK3β, the predominant isoform, than microglia (Figure 5D), raising the possibility that elevated GSK3 in astrocytes provides a stronger signal to counteract LPS tolerance compared with microglia. This was further indicated by the increased inhibitory serine-phosphorylation of GSK3β in the tolerant condition in microglia, but not in astrocytes (Figure 5D). Molecular means were also used to define the role of GSK3 in LPS-tolerance in IL-6 production by astrocytes. Knocking down GSK3β levels with siRNA promoted LPS-tolerance, as indicated by greater reduction of IL-6 production following pretreatment with LPS than in control siRNA cells (Figure 6A). Conversely, overexpression of active mutants of GSK3 that cannot be inhibited by serine-phosphorylation, S21AGSK3α and S9AGSK3β, blocked the induction of LPS-tolerance for IL-6 production in astrocytes (Figure 6B). This was further examined by measuring the production of IL-6 in astrocytes isolated from GSK3 knockin mice (McManus et al., 2005). These mice express GSK3α and GSK3β at normal levels but with serine-to-alanine mutations at serine-21 in GSK3α and serine-9 in GSK3β to abrogate inhibitory serine-phosphorylation of GSK3 (Figure 4C), the major target for inhibition of GSK3, so GSK3 is constitutively maximally active, but within its normal physiological range. In astrocytes from GSK3 knockin mice there was a complete loss of LPS-tolerance, as IL-6 production was the same with or without a preconditioning LPS treatment (Figure 6C). LPS-tolerance was restored in GSK3 knockin astrocytes by treatment with GSK3 inhibitors (Figure 6D). Altogether, these results demonstrate that GSK3β counteracts LPS-tolerance in the production of IL-6 by mouse primary astrocytes.
Figure 4.
GSK3 inhibitors enhance LPS-tolerance. (A, B) Mouse primary astrocytes were pretreated without (0) or with 100 ng/mL LPS (LPS) with or without GSK3 inhibitors 10 µM kenpaullone, 10 µM Chiron99021, or 10 µM TDZD-8 for 24 h, washed, and restimulated with 10 ng/mL LPS for 24 h and media from the second incubation were analyzed for IL-6 by ELISA. Values are shown as a percent of IL-6 production in the absence of GSK3 inhibitors. (C) Experiments were carried out as in A and B but GSK3 inhibitors were added only for the last 1 h of the first stimulation with LPS, and IL-6 was measured in the media after washing and restimulation with 10 ng/mL LPS for 24 h. (mean ± SEM; n=3–4; *p<0.05, ANOVA).
Figure 5.
Lithium enhances LPS-tolerance. (A) Mouse primary astrocytes were pretreated with 100 ng/mL LPS with or without the GSK3 inhibitor 20 mM LiCl for 24 h, or (B) LiCl was added for either 24 h or for the last 1 h of the first stimulation with LPS, cells were washed and restimulated with 10 ng/mL LPS for 24 h (mean ± SEM; n=3; *p<0.05, ANOVA). (C) Mouse primary astrocytes were treated as in (B) and were immunoblotted for phosphoSer21GSK3α, phosphoSer9GSK3β, and total GSK3α/β (representative of 3 independent experiments). (D) After treatments as indicated, immunoblotting was used to measure the total level and serine-phosphorylation of GSK3α and GSK3β in mouse primary microglia (3.75 µg protein) and mouse primary astrocytes (1.5 µg protein). Results are representative of three independent measurements. The level of GSK3β in astrocytes was 362 ± 40% (n=3) the level in microglia in the 0/0 treated samples (taking into account the different amounts of protein loaded for each cell type, which was necessary to obtain accurate immunoblots of GSK3β in each cell type measured on the same gel).
Figure 6.
GSK3 counteracts LPS-tolerance in astrocytes. (A) Mouse primary astrocytes were treated with control siRNA (SiCtl) or siRNA for GSK3β for 48 h (values are shown as a percent of IL-6 production in the absence of GSK3 knockdown), or (B) control GFP or constitutively active S21A-GSK3α and S9A-GSK3β (α+β) were expressed in astrocytes for 48 h, followed by pretreatment with 100 ng/mL LPS for 24 h and restimulated with 10 ng/mL LPS for 24 h. Media were analyzed for IL-6 by ELISA (bar graph) or cell lysates were immunoblotted for GSK3α and GSK3β and β-actin or STAT3 loading controls (top panels) (n=3–5). (C, D) Mouse primary astrocytes isolated from GSK3 knockin mice (KI) or wild-type mice (WT) were pretreated with 100 ng/mL LPS (C) without or (D) with GSK3 inhibitors, 20 mM LiCl or 10 µM TDZD-8, for 24 h and restimulated with 10 ng/mL LPS for 24 h. Media were analyzed for IL-6 by ELISA. Cell lysates were immunoblotted for phosphoSer21GSK3α, phosphoSer9GSK3β, and total GSK3α/β (C, n=3, D, n=2). Values represent mean ± SEM (*p<0.05, ANOVA).
(iv) Discussion
The brain appears to be particularly sensitive to inflammation, as neuroinflammation has been implicated in deleterious outcomes in many neurological and psychiatric diseases (Miller et al., 2009). This may be due to the long lifetimes of neurons and likely partially underlies the barriers that evolved to isolate the brain from the life-sustaining peripheral inflammatory responses that are required for pathogen resistance (Rivest, 2009). However, a further protective mechanism could develop to minimize the deleterious effects of repeated inflammation by down-regulating inflammatory responses, i.e., the development of inflammatory tolerance. Here we found a wide range of responses to repeated LPS stimulation of astrocytes, including tolerance, sensitization, and no changes in response, indicating that the inflammatory environment of the brain following repeated episodes of inflammation is much different than following a single episode, and that much remains to be learned about inflammatory responses in the brain following repeated stimulation. Our survey of inflammatory molecules regulated by lithium treatment demonstrated broad effects, showing that inhibition of GSK3 can not be simply classified as producing an anti-inflammatory outcome but inflammatory molecules must be examined on an individual basis.
We focused on the regulation of tolerance in IL-6 production by GSK3 because IL-6 has been associated with several neurodegenerative and psychiatric diseases, including impairing higher functions such as cognition (Campbell et al., 1997, Marsland et al., 2008, Dugan et al., 2009). Microglia, like related macrophages, displayed a nearly complete tolerance in LPS-induced IL-6 production. This extends to LPS tolerance previous evidence of cross-tolerance that has been attributed to microglia in the brain between apparently unrelated noxious stimuli, such as LPS and ischemia (Shemi and Kaplanski, 2005, Guo and Schluesener, 2006). For IL-6 production astrocytes display a response of semi-tolerance, which allowed experimental manipulations to determine that active GSK3 counteracts tolerance and inhibition of GSK3 promoted more full tolerance. Interestingly, the relative magnitudes of tolerance in microglia and astrocytes inversely correlated with GSK3 levels in each cell type, as GSK3 expression levels were ~4-fold higher in astrocytes than microglia. Although further mechanistic insight remains a goal for further studies, previously reported regulatory actions of GSK3 on transcription factors that control IL-6 production, such as NF-κB and STAT3 (Hoeflich et al., 2000, Steinbrecher et al., 2005, Beurel and Jope, 2008) may contribute to the regulation of LPS tolerance by GSK3. Regardless of the mechanism, it is evident that inhibiting GSK3 not only reduces initial production of inflammatory molecules in the periphery and the brain (Martin et al., 2005, Beurel and Jope, 2009b, Yuskaitis and Jope, 2009), it also promotes IL-6 tolerance to repeated inflammatory stimuli.
The discovery that GSK3 inhibition promotes LPS-tolerance in IL-6 production besides participating in resistance to endotoxin shock (Martin et al., 2005) reveals new mechanisms of actions of GSK3 in the inflammatory process in the brain. Equally intriguing is the discovery that many inflammatory molecules are sensitized upon repeated LPS exposure, with greater production following an initial LPS stimulus. This characteristic raises the possibility that this sensitization process contributes to the difficulty in surmounting chronic neuroinflammation, although GSK3 appears to be a feasible target for controlling inflammatory sensitization as well as promoting tolerance to certain inflammatory molecules.
ACKNOWLEDGEMENTS
Supported by a grant from the National Institutes of Health (MH38752) and a Young Investigator Award from NARSAD. We thank Dr. S. M. Michalek for the LPS.
Abbreviations
The abbreviations used are:
- GFAP
glial fibrillary acidic protein
- GSK3
glycogen synthase kinase-3
- IL-6
interleukin-6
- LiCl
lithium chloride
- LPS
lipopolysaccharide
- TNFα
tumor necrosis factor-α.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Beurel E, Jope RS. Differential regulation of STAT family members by glycogen synthase kinase-3. J Biol Chem. 2008;283:21934–21944. doi: 10.1074/jbc.M802481200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Jope RS. Glycogen synthase kinase-3 promotes the synergistic action of interferon-gamma on lipopolysaccharide-induced IL-6 production in RAW264.7 cells. Cell Signal. 2009a;21:978–985. doi: 10.1016/j.cellsig.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Jope RS. Lipopolysaccharide-induced interleukin-6 production is controlled by glycogen synthase kinase-3 and STAT3 in the brain. J Neuroinflammation. 2009b;6:9. doi: 10.1186/1742-2094-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull SJ, Huezo-Diaz P, Binder EB, Cubells JF, Ranjith G, Maddock C, Miyazaki C, Alexander N, Hotopf M, Cleare AJ, Norris S, Cassidy E, Aitchison KJ, Miller AH, Pariante CM. Functional polymorphisms in the interleukin-6 and serotonin transporter genes, and depression and fatigue induced by interferon-alpha and ribavirin treatment. Mol Psychiatry. 2009;14:1095–1104. doi: 10.1038/mp.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IL, Stalder AK, Chiang CS, Bellinger R, Heyser CJ, Steffensen S, Masliah E, Powell HC, Gold LH, Henriksen SJ, Siggins GR. Transgenic models to assess the pathogenic actions of cytokines in the central nervous system. Mol Psychiatry. 1997;2:125–129. doi: 10.1038/sj.mp.4000225. [DOI] [PubMed] [Google Scholar]
- Cavaillon JM, Adib-Conquy M. Bench-to-bedside review: endotoxin tolerance as a model of leukocyte reprogramming in sepsis. Crit Care. 2006;10:233–240. doi: 10.1186/cc5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerciat M, Unkila M, Garcia-Segura LM, Arevalo MA. Selective estrogen receptor modulators decrease the production of interleukin-6 and interferon-gamma-inducible protein-10 by astrocytes exposed to inflammatory challenge in vitro. Glia. 58:93–102. doi: 10.1002/glia.20904. [DOI] [PubMed] [Google Scholar]
- Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2:769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- Craft JM, Watterson DM, Van Eldik LJ. Neuroinflammation: a potential therapeutic target. Expert Opin Ther Targets. 2005;9:887–900. doi: 10.1517/14728222.9.5.887. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugan LL, Ali SS, Shekhtman G, Roberts AJ, Lucero J, Quick KL, Behrens MM. IL-6 mediated degeneration of forebrain GABAergic interneurons and cognitive impairment in aged mice through activation of neuronal NADPH oxidase. PLoS One. 2009;4:e5518. doi: 10.1371/journal.pone.0005518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447:972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- Freudenberg MA, Galanos C. Bacterial lipopolysaccharides: structure, metabolism and mechanisms of action. Int Rev Immunol. 1990;6:207–221. doi: 10.3109/08830189009056632. [DOI] [PubMed] [Google Scholar]
- Guo LH, Schluesener HJ. Acute but not chronic stimulation of glial cells in rat spinal cord by systemic injection of lipopolysaccharide is associated with hyperalgesia. Acta Neuropathol. 2006;112:703–713. doi: 10.1007/s00401-006-0135-z. [DOI] [PubMed] [Google Scholar]
- Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol. 2000;165:618–622. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3β in cell survival and NF-κB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Kariko K, Weissman D, Welsh FA. Inhibition of toll-like receptor and cytokine signaling--a unifying theme in ischemic tolerance. J Cereb Blood Flow Metab. 2004;24:1288–1304. doi: 10.1097/01.WCB.0000145666.68576.71. [DOI] [PubMed] [Google Scholar]
- Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci USA. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Sparatore A, Del Soldato P, McGeer E, McGeer PL. Hydrogen sulfide-releasing NSAIDs attenuate neuroinflammation induced by microglial and astrocytic activation. Glia. 58:103–113. doi: 10.1002/glia.20905. [DOI] [PubMed] [Google Scholar]
- Leost M, Schultz C, Link A, Wu YZ, Biernat J, Mandelkow EM, Bibb JA, Snyder GL, Greengard P, Zaharevitz DW, Gussio R, Senderowicz AM, Sausville EA, Kunick C, Meijer L. Paullones are potent inhibitors of glycogen synthase kinase-3beta and cyclin-dependent kinase 5/p25. Eur J Biochem. 2000;267:5983–5994. doi: 10.1046/j.1432-1327.2000.01673.x. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Gianaros PJ, Abramowitch SM, Manuck SB, Hariri AR. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol Psychiatry. 2008;64:484–490. doi: 10.1016/j.biopsych.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A, Alonso M, Castro A, Perez C, Moreno FJ. First non-ATP competitive glycogen synthase kinase 3 beta (GSK-3beta) inhibitors: thiadiazolidinones (TDZD) as potential drugs for the treatment of Alzheimer's disease. J Med Chem. 2002;45:1292–1299. doi: 10.1021/jm011020u. [DOI] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, Marquez R, Alessi DR. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. Embo J. 2005;24:1571–1583. doi: 10.1038/sj.emboj.7600633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedev AE, Kopydlowski KM, Vogel SN. Inhibition of lipopolysaccharide-induced signal transduction in endotoxin-tolerized mouse macrophages: dysregulation of cytokine, chemokine, and toll-like receptor 2 and 4 gene expression. J Immunol. 2000;164:5564–5574. doi: 10.4049/jimmunol.164.11.5564. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivest S. Regulation of innate immune responses in the brain. Nat Rev Immunol. 2009;9:429–439. doi: 10.1038/nri2565. [DOI] [PubMed] [Google Scholar]
- Schneider DS, Ayres JS. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat Rev Immunol. 2008;8:889–895. doi: 10.1038/nri2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemi D, Kaplanski J. Involvement of PGE2 and TNF-alpha in LPS-tolerance in rat glial primary cultures. Prostaglandins Leukot Essent Fatty Acids. 2005;73:385–389. doi: 10.1016/j.plefa.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Sironi M, Bianchi M, Riganti F, Ghezzi P. Suppression of interleukin-6 production in endotoxin tolerance in a mouse glioma cell line: reversal by phorbol ester. Lymphokine Cytokine Res. 1993;12:39–43. [PubMed] [Google Scholar]
- Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6:1664–1668. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- Steinbrecher KA, Wilson W, 3rd, Cogswell PC, Baldwin AS. Glycogen synthase kinase 3β functions to specify gene-specific, NF-κB-dependent transcription. Mol Cell Biol. 2005;25:8444–8455. doi: 10.1128/MCB.25.19.8444-8455.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagman AS, Johnson KW, Bussiere DE. Discovery and development of GSK3 inhibitors for the treatment of type 2 diabetes. Curr Pharm Des. 2004;10:1105–1137. doi: 10.2174/1381612043452668. [DOI] [PubMed] [Google Scholar]
- Yuskaitis CJ, Jope RS. Glycogen synthase kinase-3 regulates microglial migration, inflammation, and inflammation-induced neurotoxicity. Cell Signal. 2009;21:264–273. doi: 10.1016/j.cellsig.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock HW. Molecular mechanism in tolerance to lipopolysaccharide. J Inflamm. 1995;45:13–26. [PubMed] [Google Scholar]