Abstract
Misalignment between the timing of sleep and the circadian pacemaker has been linked to depression symptoms. This study sought to extend earlier findings by comparing sleep and circadian markers in healthy controls and individuals with major depression. Two markers of circadian misalignment correlated with depression severity in the depressed group.
Keywords: Circadian rhythms, depression, dim light melatonin onset (DLMO), core body temperature, sleep
1. Introduction
Decades of accumulating evidence of sleep and circadian abnormalities in mood disorders have engendered a wide range of explanatory hypotheses. These varied perspectives have focused on factors including sleep processes (e.g., deficiency in slow wave sleep and slow wave activity), neurotransmitter systems (e.g., a cholinergic-aminergic imbalance), or the behavioral-environmental interface (e.g., social rhythm irregularities leading to disruptions in physiological circadian rhythms (reviewed in Benca et al., 1997, Boivin, 2000, Riemann et al., 2001, Germain and Kupfer, 2008, among others). Still another early hypothesis, the internal coincidence hypothesis (Wehr et al., 1979) posited that sleeping at the wrong biological time, with relatively advanced circadian phase, is depressogenic. While none of these competing explanations has emerged as conclusive, recent evidence suggests that hypotheses linking misalignment between the timing of sleep and the endogenous circadian pacemaker to depressive symptomatology may deserve further consideration.
Several recent studies have operationalized circadian misalignment by measuring the interval between the dim light melatonin onset (DLMO), the most robust indicator of the timing of the central pacemaker, and the time of midsleep. In healthy samples on typical sleep/wake schedules, DLMO occurs approximately two to three hours before sleep onset (Benloucif et al., 2008). In a sample of Seasonal Affective Disorder patients, the phase angle difference (PAD), or interval, between the timing of DLMO and that of midsleep was associated with more severe depression. The strongest relationship was observed for phase-delayed individuals (i.e., relatively later DLMOs resulting in shorter PADs) (Lewy et al., 2006). In a group of women with Major Depressive Disorder, the PAD in delayed individuals correlated with depression severity (Emens et al., 2009a). Preliminary findings also suggest a parallel association between the PAD and subclinical depressed mood in healthy individuals (Emens et al., 2009b). In contrast to the original internal coincidence hypothesis, both of these studies suggest that the timing of the circadian clock is delayed rather than advanced relative to the sleep/wake cycle.
These data suggest that circadian misalignment may be relevant to depressive symptomatology across mood disorders, and perhaps also to healthy individuals. However, the published findings have not been independently replicated. Furthermore, less attention has been paid to the respective phase angles between circadian processes other than sleep, phase angles that may reveal other evidence of internal desynchrony. We sought to determine if the respective phase angles between DLMO, core body temperature minimum (CBTmin), and mid-sleep differed between healthy controls and individuals with major depressive disorder (MDD), and if these phase angles correlated with depression severity. Based on previous findings, we hypothesized that: 1) depressed individuals would be phase-delayed, with shorter DLMO-midsleep and longer midsleep-CBTmin phase angles relative to those of the controls (see Figure 1); and 2) the extent of phase-delay would correlate with the severity of depression. We included measures of both global and anhedonic depression given recent evidence that the circadian system may be particularly involved in regulation of reward processes (McClung, 2007, Murray et al., 2009). Finally, we also explored group differences in the DLMO-CBTmin phase angle as an additional and novel measure of circadian desynchrony.
Figure 1.
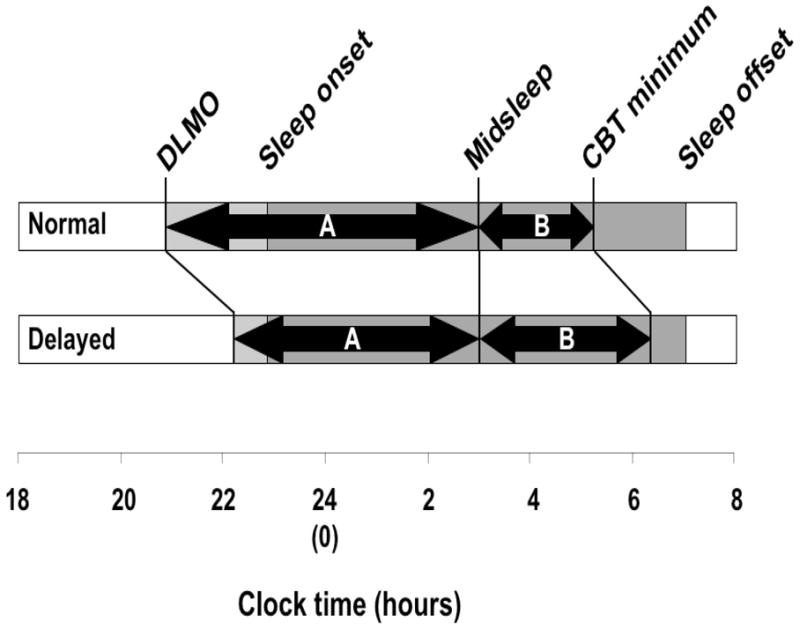
A schematic of relevant phase angles. The two-headed arrows below represent the predicted DLMO-midsleep (A) and midsleep-CBT minimum (B) phase angles under normal and delayed conditions. The DLMO-CBT minimum phase angle was omitted given the lack of an a priori hypothesis about how it would differ from the normal condition.
2. Methods
Subjects participated in a study investigating circadian, electrophysiological, and cognitive changes around sleep onset in patients with MDD and healthy controls. Inclusion criteria for the patient group included meeting criteria for recurrent unipolar MDD, with a current endogenous depressive episode as assessed by the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (First et al., 2002). Patients were required to score >16 on the 17-item Hamilton Rating Scale for Depression (Hamilton, 1967) and >7 on the Raskin Severity of Depression Scale. Psychiatric exclusion criteria included 1) antidepressant, anxiolytic, or antipsychotic medication within the past two weeks (four weeks for fluoxetine); 2) suicidal ideation; 3) any other psychiatric illness, except for comorbid anxiety disorders which clearly follow the onset of MDD; 4) substance abuse or dependence within the past 2 ½ years; 5) personality disorders assessed by the Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) (First et al., 1997); 6) sleep disorders (except for insomnia or hypersomnia related to MDD) identified by history and one night of screening polysomnography (PSG); 7) irregular sleep schedules as indicated by weekly variation of >3 hours in bed time, or sleep duration <5.5 or >10 hours per night. Medical exclusion criteria included 1) any significant or unstable medical illness, including anemia, cardiovascular, renal, hepatic, neurologic, or endocrine disorders; 2) current pregnancy; 3) smoking >20 cigarettes per day. Control participants had no current or past diagnosis of any major psychiatric disorder according to the SCID and SCID-II Interview Schedules, were medication-free, and met the same medical and sleep exclusion criteria indicated for MDD group. All patients and control participants gave informed consent prior to participation. The study was approved by the University of Pittsburgh Institutional Review Board.
A total of 42 participants signed consent and underwent screening procedures; 18 MDD and 19 controls met all of the above inclusion criteria. For the present analyses, participants with missing or unusable data in the variables of interest were also excluded, resulting in samples of 14 adults with current MDD (11 females, M=39.72 years, range=22–57 years old) and 13 healthy controls (7 female, M=39.80 years, range=20–59 years old). All participants were drug-free during the overnight sleep studies, which were conducted in the General Clinical Research Center (GCRC) at the University of Pittsburgh. All participants slept at their habitual sleep hours for three consecutive nights (following a screening night) in the GCRC; data were collected between approximately 7:00 PM and one hour after wake-up time. Sleep studies included bilateral occipital, central, and frontal EEG leads. Additional electrodes were placed to measure bilateral electrooculogram and submental EMG. Standard silver or gold plated electrodes were affixed with tape and/or collodion. Core body temperature (CBT) and melatonin data were collected concomitant with PSG data on the 3nd and 4rd night; data was retained from whichever night produced the most usable data. Core body temperature was measured with a flexible rectal thermistor inserted 10 cm into the rectum. The thermistor was connected either to a portable temperature collection device (Minilogger). Blood samples (3 cc each) were collected under dim light conditions through an indwelling venous catheter connected to IV tubing every 15 minutes from 7 PM until one hour after wake-up time.
Sleep variables (sleep onset, sleep midpoint, and sleep offset) did not differ across the three nights; thus the average was used in analyses. The CBT minimum was visually-determined by inspection of each individual’s data by a single blinded rater (BPH). Plasma melatonin concentrations were measured via RIA (ALPCO Ltd., Windham, NH); DLMOs were assessed using a 10 pg/ml threshold (Lewy et al., 1999). Three phase angles (see Figure 1) were calculated between DLMO, the CBT minimum, and mean PSG-based mid-sleep time (halfway between sleep onset and offset). The severity of global depression was assessed the day prior to the first sleep study using the 17-item Hamilton Depression Rating Scale (HAM-D) and the Beck Depression Inventory (BDI). Anhedonic depression was assessed using a previously-validated subscale based on three items of the BDI (Joiner et al., 2003). The Morningness-Eveningness Questionnaire (MEQ; Horne and Östberg, 1976) was also administered to assess morningness-eveningness, the preference for morning or evening activities. Spearman’s correlations and two-tailed t-tests were run in SPSS 17.0 (SPSS Inc.). Given the exploratory nature and small sample size of the study, we report p-values < 0.10.
3. Results
The patient group had a mean HAM-D score (± SD) of 21.0 ± 4.07 (range=16–29) and a mean total BDI score of 25.15 ± 7.15 (range=12–36); the control group had mean HAM-D and BDI scores below 1. Compared to controls, depressed patients had a lower mean score (greater eveningness) on the MEQ (t(14)= 3.40, p=0.004), and later mean sleep onset (t(24)=−4.27, p<0.001) and sleep midpoint (t(24)=−2.60, p=0.02), but did not differ on clock times of sleep offset or CBT minimum. The depressed group’s mean DLMO was numerically later than that of the control group, but this difference was not significant (t(19)=−1.78, p=0.09). Contrary to our hypothesis, the groups did not differ on any of the three phase angles. Within-group variability of all three phase angles tended to be greater among the MDD group, but was significantly different only for midsleep-CBTmin (F=7.63, p=0.01; DLMO-midsleep: F=3.60, p=0.07; DLMO-CBTmin: F=3.10, p=0.09).
Consistent with hypotheses, the patients’ midsleep-CBTmin phase angles showed significant correlations with HAM-D and BDI-Anhedonia scores (rs=0.67, p=0.03; rs=0.62, p=0.03), indicating that CBT minima relatively delayed with respect to sleep were associated with greater depression and anhedonic depression. However, this pattern was not observed in DLMO-midsleep phase angle data (rs =0.18, p=ns; rs =−0.07, p=ns). Finally, the patients’ DLMO-CBTmin phase angles showed significant correlations with HAM-D and BDI-Anhedonia scores (rs=0.82, p=0.006; rs=0.83, p=0.006). None of the phase angles showed significant correlations with the total BDI scores (midsleep-CBTmin; rs=0.41, p=ns, DLMO-midsleep: rs=−0.19, p=ns; DLMO-CBTmin: rs=0.57, p=0.09).
4. Discussion
Findings from this exploratory study replicate the observed association between circadian misalignment and depression severity in patients with MDD. Specifically, a larger phase angle difference between midsleep and the core body temperature minimum was associated with greater depression. We also found preliminary evidence of an association between depressive severity and the phase angle between DLMO and CBT minimum. Both of these phase angles correlated with the severity of anhedonic depression from the BDI and global depression measured by the HAM-D. The patients showed a greater tendency towards eveningness, more delayed sleep onsets and sleep midpoints, as well as more delayed DLMOs compared to healthy individuals. Finally, although the groups did not differ as predicted in the mean magnitude of the three phase angles, the MDD group did show a greater heterogeneity than healthy individuals across the three phase angles.
We did not find the predicted association between depression severity and the DLMO-midsleep phase angle, referred to by Emens and colleagues (2009a) as the PAD. This could be due to the sample sizes of both studies, to differences in the samples, or to measurement error. Not only were all of the patients in the prior report female, but they were on a variety of antidepressants, which have been reported to impact circadian phase (Morin, 1999), while our patients were free of psychotropic medications. In addition, measurement error due to inter-lab differences in the melatonin assay might also account for the discrepancy in findings.
The observed association between the DLMO-CBTmin phase angle and depression severity must be interpreted with caution due to the small sample size and limited statistical power. Nonetheless, the finding may indicate the importance of particular temporal alignments between the melatonin and temperature rhythms, thus supporting a role of internal desynchrony in depressive symptoms. Melatonin and CBT rhythms are both tightly coupled to the central pacemaker and thus considered to maintain a stable phase relationship (i.e., be phase-locked) under normal conditions (Wyatt et al., 1999). Hypothetically, factors downstream of the central pacemaker could disrupt this internal synchrony in depressed patients.
The lack of group differences in the mean phase angles is consistent with the non-statistical comparison made in Emens and colleagues (2009a), who noted the similarity of their depressed group’s mean DLMO-midsleep phase angle to that of historical controls. The greater heterogeneity in phase angles among the patients may indicate the presence of multiple depressive phenotypes or more simply a vulnerability to circadian disturbance among the patients. Alternatively, “social jet lag” may be present in some portion of the sample—the heterogeneity may reflect varying degrees of concordance between self-selected sleep-wake schedules and the underlying biological timing (Wittman et al., 2006).
The correlations between measures of circadian misalignment and the BDI-Anhedonic subscale are consistent with accumulating evidence of the circadian system’s role in the regulation of reward. This cross-method support comes from both the human and animal literatures. In humans, this includes evidence of a circadian rhythm in a psychophysiological measure of reward responsiveness, evidence that self-reported reward responsiveness accounts for the aforementioned association between eveningness and depression, and an association between the degree of circadian misalignment and extent of substance abuse (Hasler et al., 2008, Murray et al., 2009, Hasler et al., in press). The animal literature has demonstrated circadian modulation of both drug- and sex-related reward, identified circadian rhythmicity in brain areas associated with reward, and shown that mutations or polymorphisms of the circadian genes in the neurons of these regions result in increased appetitive behavior (McClung, 2007, Hampp et al., 2008, Webb et al., 2009)
Limitations of our study include small sample size and limited statistical power. For instance, in a larger sample, the moderate correlations observed with total BDI might be statistically significant. The study was also limited by its cross-sectional design, which raises questions regarding the stability of these alignments and precludes any determination of causation. Longitudinal approaches will be required to investigate the relationships of circadian alignment to depression across multiple timepoints, preferably in both currently depressed and remitted individuals. The exclusion of patients with comorbid sleep disorders and/or irregular sleep schedules may have affected the present findings, although including patients with more extreme circadian misalignment (i.e., widening the range of PAD values) could plausibly lead to stronger associations with depression severity. Despite these limitations, the findings support the notion that circadian misalignment is a correlate of symptom severity, and may be a potentially pathogenic process, in a subsample of depressed individuals.
Beyond the realm of MDD, the findings may be relevant to reports of depression in Delayed Sleep Phase Disorder (Rahman et al., 2009), and to the oft-reported association between eveningness and depression (e.g., Drennan et al., 1991, Chelminski et al., 1999, Hasler et al., in press). Delayed circadian phase appears to underlie the preference for eveningness in at least a portion of individuals (Mongrain et al., 2006). Future investigations into whether realigning patients’ circadian phase and sleep timing leads to reductions in symptoms may provide novel therapeutic targets.
Acknowledgments
This work was supported by National Institutes of Health Grant R01- MH24652 and University of Pittsburgh General Clinical Research Center Grant RR00056 to DJK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benca RM, Okawa M, Uchiyama M, Ozaki S, Nakajima T, Shibui K, Obermeyer WH. Sleep and mood disorders. Sleep Medicine Reviews. 1997;1:45–56. doi: 10.1016/s1087-0792(97)90005-8. [DOI] [PubMed] [Google Scholar]
- Benloucif S, Burgess HJ, Klerman EB, Lewy AJ, Middleton B, Murphy PJ, Parry BL, Revell VL. Measuring melatonin in humans. Journal of Clinical Sleep Medicine. 2008;4:66–9. [PMC free article] [PubMed] [Google Scholar]
- Boivin DB. Influence of sleep-wake and circadian rhythm disturbances in psychiatric disorders. Journal of Psychiatry & Neuroscience. 2000;25:446–58. [PMC free article] [PubMed] [Google Scholar]
- Chelminski I, Ferraro F, Petros T, Plaud J. An analysis of the “eveningness-morningness” dimension in “depressive” college students. Journal of Affective Disorders. 1999;52:19–29. doi: 10.1016/s0165-0327(98)00051-2. [DOI] [PubMed] [Google Scholar]
- Drennan M, Klauber M, Kripke D, Goyette L. The effects of depression and age on the Horne-Ostberg morningness-eveningness score. Journal of Affective Disorders. 1991;23:93–98. doi: 10.1016/0165-0327(91)90096-b. [DOI] [PubMed] [Google Scholar]
- Emens J, Lewy A, Kinzie JM, Arntz D, Rough J. Circadian misalignment in major depressive disorder. Psychiatry Research. 2009a;168:259–61. doi: 10.1016/j.psychres.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Emens J, Lewy AJ, Rough JN, Songer JB. Sub-clinical dysphoria correlates with phase-delayed circadian misalignment in healthy individuals. Sleep. 2009b;32:A355–356. [Google Scholar]
- First MB, Gibbon M, Spitzer RL. Structured clinical interview for DSM-IV axis II personality disorders (SCID-II) American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- First MB, Spitzer RC, Gibbon M, Williams JBW. Biometrics Research. New York State Psychiatric Institute; New York: 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I) [Google Scholar]
- Germain A, Kupfer DJ. Circadian rhythm disturbances in depression. Human Psychopharmacology. 2008;23:571–85. doi: 10.1002/hup.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. The British Journal of Social and Clinical Psychology. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Hampp G, Ripperger JA, Houben T, Schmutz I, Blex C, Perreau-Lenz S, Brunk I, Spanagel R, Ahnert-Hilger G, Meijer JH, Albrecht U. Regulation of monoamine oxidase A by circadian-clock components implies clock influence on mood. Current Biology. 2008;18:678–83. doi: 10.1016/j.cub.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Hasler BP, Allen JJB, Sbarra DA, Bootzin RR, Bernert RA. Morningness-eveningness and depression: Preliminary evidence for the role of BAS and positive affect. Psychiatry Research. doi: 10.1016/j.psychres.2009.06.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Bootzin RR, Cousins JC, Fridel K, Wenk GL. Circadian phase in sleep-disturbed adolescents with a history of substance abuse: a pilot study. Behavioral Sleep Medicine. 2008;6:55–73. doi: 10.1080/15402000701796049. [DOI] [PubMed] [Google Scholar]
- Horne JA, Östberg O. A self-assesment questionnaire to determine morningness-eveningness. International Journal of Chronobiology. 1976;4:97–110. [PubMed] [Google Scholar]
- Joiner TE, Brown JS, Metalsky GI. A test of the tripartite model’s prediction of anhedonia’s specificity to depression: patients with major depression versus patients with schizophrenia. Psychiatry Research. 2003;119:243–50. doi: 10.1016/s0165-1781(03)00131-8. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Cutler NL, Sack RL. The endogenous melatonin profile as a marker for circadian phase position. Journal of Biological Rhythms. 1999;14:227–36. doi: 10.1177/074873099129000641. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Lefler BJ, Emens JS, Bauer VK. The circadian basis of winter depression. Proceedings of the National Academy of Science U S A. 2006;103:7414–9. doi: 10.1073/pnas.0602425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcclung CA. Circadian genes, rhythms and the biology of mood disorders. Pharmacology & Therapeutics. 2007;114:222–232. doi: 10.1016/j.pharmthera.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongrain V, Carrier J, Dumont M. Circadian and homeostatic sleep regulation in morningness-eveningness. Journal of Sleep Research. 2006;15:162–6. doi: 10.1111/j.1365-2869.2006.00532.x. [DOI] [PubMed] [Google Scholar]
- Morin LP. Serotonin and the regulation of mammalian circadian rhythmicity. Annals of Medicine. 1999;31:12–33. doi: 10.3109/07853899909019259. [DOI] [PubMed] [Google Scholar]
- Murray G, Nicholas CL, Kleiman J, Dwyer R, Carrington MJ, Allen NB, Trinder J. Nature’s clocks and human mood: the circadian system modulates reward motivation. Emotion. 2009;9:705–16. doi: 10.1037/a0017080. [DOI] [PubMed] [Google Scholar]
- Rahman SA, Kayumov L, Shapiro CM. Antidepressant action of melatonin in the treatment of Delayed Sleep Phase Syndrome. Sleep Medicine. 2009;11:131–6. doi: 10.1016/j.sleep.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Riemann D, Berger M, Voderholzer U. Sleep and depression--results from psychobiological studies: an overview. Biological Psychology. 2001;57:67–103. doi: 10.1016/s0301-0511(01)00090-4. [DOI] [PubMed] [Google Scholar]
- Webb IC, Baltazar RM, Wang X, Pitchers KK, Coolen LM, Lehman MN. Diurnal variations in natural and drug reward, mesolimbic tyrosine hydroxylase, and clock gene expression in the male rat. Journal of Biological Rhythms. 2009;24:465–76. doi: 10.1177/0748730409346657. [DOI] [PubMed] [Google Scholar]
- Wehr TA, Wirz-Justice A, Goodwin FK, Duncan W, Gillin JC. Phase advance of the circadian sleep-wake cycle as an antidepressant. Science. 1979;206:710–3. doi: 10.1126/science.227056. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: Misalignment of biological and social time. Chronobiology International. 2006;23:497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- Wyatt JK, Cecco ARD, Czeisler CA, Dijk DJ. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 1999;277:R1152–1163. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]


