Abstract
Changes in brain-derived neurotrophic factor (BDNF) expression have been implicated in the etiology of psychiatric disorders. To investigate pathological mechanisms elicited by perturbed BDNF signaling, we examined mutant mice with central depletion of BDNF (BDNF2L/2LCk-cre). A severe impairment specific for the serotonin 2A receptor (5-HT2A) in prefrontal cortex was described previously in these mice. This is of much interest, as 5-HT2A receptors have been linked to neuropsychiatric disorders and in anxiety-related behavior. Here we further characterized the serotonin receptor alterations triggered by BDNF depletion. 5-HT2A (3H-MDL100907) and 5-HT1A (3H-WAY100635) receptor autoradiography revealed site-specific alterations in BDNF mutant mice. They exhibited lower 5-HT2A receptor binding in frontal cortex but increased binding in hippocampus. Additionally, 5-HT1A receptor binding was decreased in hippocampus of BDNF mutants, but unchanged in frontal cortex. Molecular analysis indicated corresponding changes in 5-HT2A and 1A mRNA expression but normal 5-HT2C content in these brain regions in BDNF2L/2LCk-cre mice. We investigated whether the reduction in frontal 5-HT2A receptor binding was reflected in reduced functional output in two 5-HT2A-receptor mediated behavioral tests, the head-twitch response (HTR) and the ear-scratch response (ESR). BDNF2L/2LCk-cre mutants treated with the 5-HT2A receptor agonist DOI showed a clearly diminished ESR but no differences in HTR compared to wildtypes. These findings illustrate the context-dependent effects of deficient BDNF signaling on the 5-HT receptor system and 5-HT2A-receptor functional output.
Keywords: BDNF, serotonin, 5-HT2A, 5-HT1A, 5-HT2C, behavior
Brain-derived neurotrophic factor (BDNF) belongs to the family of neurotrophins and signals through the TrkB receptor. It is important for neuronal survival and differentiation during development and synaptic plasticity in adulthood. In clinical studies, reductions in BDNF levels have been linked to various psychiatric disorders including schizophrenia, bipolar disorder and major depression (Kozisek et al., 2008). Studies involving rodent models of BDNF deficiency also lend support to a chief role of BDNF in the control of affective behavior. For example, BDNF+/− mice exhibited hyper aggressiveness, hyperphagia, excessive weight gain (Lyons et al., 1999; Kernie et al., 2000) and diminished responses to antidepressant treatment (Deltheil et al., 2008). In BDNF2L/2LCk-cre mice, BDNF is depleted in all brain regions, except cerebellum. The depletion begins during the first post-natal week and is completed around P21, when BDNF mRNA levels in hippocampus, cortex and hypothalamus range from very low to non-detectable (Chan et al., 2006; Rios et al., 2001). The adult conditional BDNF mutants have similar behavioral alterations and show increases in depressive and anxiety-like behavior (Rios et al., 2001; Chan et al., 2006).
Alterations in the serotonin receptor system might underlie some of the behavioral changes observed in BDNF2L/2LCk-cre mice. Deficient BDNF levels result in significant perturbations in serotonin receptor expression (Lyons et al., 1999) and in impairment of 5-HT2A-mediated neurotransmission in the prefrontal cortex (Rios et al., 2006). 5-HT2AR is a post-synaptic G-protein coupled receptor widely expressed in the CNS. It is located on glutamatergic and GABAergic neurons, and expressed at high densities in pyramidal neurons in frontal cortex, amygdala and hippocampus (for review, see Leysen, 2004). Importantly, changes in 5-HT2AR binding have been associated with schizophrenia, Alzheimer’s disease and depression (Naughton et al., 2000). Moreover, a recent study showed that cortical depletion of 5-HT2ARs in mice resulted in decreased anxiety-like behavior (Weisstaub et al., 2006). Together, the collective data support an important role of 5-HT2AR in the regulation of affective behavior.
To further delve into the nature of the alterations in serotonin receptor system in the BDNF2L/2LCk-cre mutant mice and the impact on receptor functionality, we conducted an analysis of various 5-HT receptors in the hippocampus and frontal cortex of these mice. Autoradiography and RT-qPCR were used to quantify differences in receptor binding and expression, respectively, in these brain regions. Besides 5-HT2ARs, we also examined 5-HT1A and 5-HT2C receptors. 5-HT1ARs have been implicated in depression and anxiety phenotypes (for review, see Savitz et al., 2009 and Akimova et al. 2009) and exert effects opposite to those of 5-HT2ARs (Yuen et al., 2008).. 5-HT2CRs, for their part, resemble 5-HT2A receptors in terms of functional activity and cellular distribution (Leysen et al., 2004). Levels of serotonin transporter were also measured in BDNF mutant mice to ascertain whether the alterations in the postsynaptic 5-HT receptors could be due to primary changes in serotonergic projection. Finally, we assessed 5-HT2A-functional output in vivo by measuring DOI (DOI, ((±)-2,5-Dimethoxy-4-iodoamphetamine hydrochloride) induced head-twitch and ear-scratch responses in BDNF2L/2LCk-cre mice. This was followed by an analysis of DOI-induced neuronal activation, measured by c-Fos protein induction, in frontal cortex and hippocampus. We found that central depletion of BDNF resulted in site-specific alterations in 5-HT receptor binding and expression. Furthermore, a deficit in 5-HT2A-functional output was evident in BDNF mutants. The cumulative data indicate an important effect of BDNF depletion on serotonin receptor levels and 5-HT2A functional output.
Experimental Procedures
The following procedures were in accordance with the guidelines from the Animal Care and Use Committee at Tufts University and with the NIH Guide for the Care and Use of Laboratory Animals. Every effort was made to minimize the number of animals.
Animals
BDNF2L/2LCk-Cre mutants were generated as previously described (Rios et al., 2001). Briefly, mice containing floxed Bdnf alleles were crossed with with transgenic mice in which expression of cre recombinase was driven by the α-calcium/calmodulin protein kinase II (CamKII-cre93 mice). Animals used in the studies described here were in a mixed C57Bl6 and 129 background and wild type littermates used as controls to avoid problems interpreting the data due to differences in background. Animals were 10–14 weeks of age and the groups were age- and sex matched. All mice were individually housed in the Tufts University Behavioral Core Facility, habituated to the reverse 12-h light/dark cycle for a minimum of 1 week and had free access to water and standard chow. All of the procedures described here were approved by the Institutional Animal Care and Use Committee at Tufts University and were in compliance with the NIH guide for the care and use of laboratory animals.
Drugs and reagents
The 5-HT2A agonist DOI ((±)-2,5-Dimethoxy-4-iodoamphetamine hydrochloride)(Sigma-Aldrich, St. Louis, MO, USA) was administered intraperitoneally in a volume of 10 μL/g body weight. The dose used in the studies was 2.5 mg/kg. Saline was used as vehicle and injected in a corresponding volume in wild-types.
Western blotting
After cervical dislocation, the brain was quickly removed and hippocampus and frontal cortex dissected out (n=7–8 per group), homogenized and sonicated in ice-cold protein extraction buffer (Pierce, Rockford, IL) and protein inhibitor cocktail (Pierce). The protein concentration was measured with modified Lowry method (DC Protein Assay, BioRad Laboratories, Herlev, Denmark). Samples were protein-matched and dissolved in 2 × SDS (sodium dodecyl sulphate) sample buffer (125 mM Tris-HCl, pH 6.8, 4% SDS, 20% glycerol, 10% 2-mercaptoethanol), heated for 5 min, 100°C and run on 6–15 % SDS-polyacrylamide gel (60 minutes, 150 V, RT). The proteins were transferred onto a PVDF membrane (300 mA, 50 min, RT, semi-dry transfer, BioRad Laboratories, Herlev, Denmark) and the membrane was blocked for unspecific binding with 5% non-fat dry milk (1 h, RT) and then incubated overnight at 4°C with either primary antibody (β-actin, 1:10.000, Sigma; BDNF, 1:1000, sc-546, Santa Cruz, CA; 5-HT2C, 1:1000, Abcam, MAP2, 1:2000, M4403, Sigma; synaptophysin, 1:5000, MAB368, Millipore, MA, PSD-95, 1:2000, Millipore; β-tubulin III, 1:10.000, Sigma, MO). Washed 3×10 min in TBS, blocked with 5% BSA in TBS for 1 h at room temperature (RT), incubated with horseradish peroxidase-linked secondary antibody (GαM or GαR, DAKO Cytomation, Glostrup, Denmark) diluted at 1:5000 in blocking solution for 1 h at RT, and finally developed with ECL detection kit (Amersham Biosciences, RPN2106PL). Band intensity was quantified using the NIH Image J software. For each sample, the optical density of each band was normalized by dividing with the optical density of the corresponding band of β-tubulin III or β-actin.
Autoradiography
To determine 5-HT2A receptor binding in conditional BDNF knock-out mice and wild-types, animals (n=4 per group) were decapitated and brains were quickly dissected out and stored at −80°C until sectioning. Brains were cut in 10 μm sections and mounted on gelatine-coated slides and stored at −80°C until further processing. 5-HT2A autoradiography was performed using 3H-MDL100907 [R(+)-α-(2,3-dimethoxyphenyl)-1-[2-(4-fluorphenyl)-ethyl]-4-piperidin-methanol] (specific activiy; 64 Ci/mmol, a gift from C. Halldin, Karolinska Institute, Stockholm) and non-specific binding was determined using 10 μM ketanserin tartrate ([3-[2-[4-(4-fluorobenzoyl)-1-piperidinyl]-ethyl]-2,4[1H,3H]quinazolinedione tartrate)(Tocris Cookson Ldt, Bristol, UK). For 5-HT1A autoradiography we used 3H-WAY100635 (GE Healthcare, UK) and measured non-specific binding with 10 μM 5-HT (Sigma-Aldrich, Copenhagen, Denmark). Briefly, sections were allowed to thaw for 1 hr at room temperature (RT) and then pre-incubated at with 50 mM Tris-HCl (Sigma), pH 7.4 containing 0.01% ascorbic acid (Sigma) and 10 μM pargyline hydrochloride (N-methyl-N-2. propynylbenzylamine hydrochloride)(Research Biochemicals International, MA, USA) for 30 minutes at RT under constant gentle shaking. Sections were then incubated for 60 minutes at RT using the same buffer containing 0.4 nM of 3H-MDL100907 (1.5 nM of 3H-WAY100635 for 5-HT1A binding). Following incubation, slides were washed 2 × 20 sec in ice-cold 50 mM Tris-HCl, pH 7.4, and 1× 20 sec in ice-cold dH2O, dried for 1 hr under a gentle stream of air.
The SERT autoradiography protocol was modified from Thomsen and Helboe (2003). Briefly, sections were pre-incubated for 30 minutes in 50 mM Tris-HCl, 120 mM NaCl, 5 mM KCl, pH 7.4 and subsequently incubated with 2.0 nM [3H]-escitalopram (donated by H. Lundbeck A/S, Copenhagen, Denmark) for 60 minutes diluted in the same buffer. Nonspecific binding was determined in the presence of 10 μM paroxetine. After incubation, sections were washed for 3 × 2 minutes in 50 mM Tris-HCl, 120 mM NaCl, 5 mM KCl, pH 7.4 (4°C) and 1 × 20 seconds in ice-cold dH2O.
All sections were placed at 4°C overnight in a fixator containing paraformaldehyde vapour and then put in an excicator box for 3 hrs before slides were together with 3H-microscales (GE Healthcare, UK) exposed to a BAS-TR2040 Imaging Plate (Science Imaging Scandinavia AB, Nacka, Sweden) for 14 days at 4°C. Finally, the imaging plate was scanned on a BAS-2500 scanner (Fujifilm Europe GmbH, Düsseldorf, Germany) and specific and non-specific binding was determined in frontal cortex and hippocampus using ImageJ (NIH) and expressed as fmol/mg tissue equivalents (TE).
RT-qPCR
For total RNA extraction the mice were sacrificed by cervical dislocation and the brain quickly removed and frontal cortex and hippocampus were dissected on ice (n=7). Samples were frozen at −80 °C before homogenization. Total RNA was isolated with TRIzol Reagent (Sigma) according to the manufacturer’s directions. The RNA samples were dissolved in RNase-free water and quantified with UV-spectrophotometry at 260 nm.
Extracted RNA was reverse transcribed into cDNA using the procedure of the supplier (ImProm-II™ reverse transcription system, Promega, Madison, WI). The experimental RNA solution was combined with oligo(dT)15 primers and heated at 70°C for 5 min. The reverse transcription reaction mixture contained of 20% ImProm-II™ 5x reaction buffer, 6 mM MgCl2, 0.5 mM dNTP mix and 20 units RNase inhibitor. The reverse transcription reaction was performed at 42°C for 60 min, followed by heating at 70 °C for 15 min.
The real-time PCR reactions were performed by adding the sample cDNA to a reaction mixture consisting of 1x Brilliant II SYBR green mastermix (Stratagene, La Jolla, CA) and 15 pmol of each primer (DNA technology, Aarhus, Denmark) and adjusting the volume to 20 μl with DNase free water (Invitrogen, Carlsbad, CA). PCR was performed with a 10 minutes preincubation at 94°C followed by 40 cycles of 30 seconds at 94°C, 45 seconds at 60°C and 90 seconds at 72 °C (Roche Light Cycler 480, Roche, Indianapolis, IN). The real-time PCR method was validated by using serially diluted cDNA to establish a standard curve. To quantify the gene expression profile of each sample, the efficiency of each standard curve was determined from its slope and comparative threshold according to the manufacturer’s instructions. For each sample, the amount of targeted mRNA was normalized to that of the reference gene β-tubulin III. Primers for mouse 5-HT1A, 5-HT2A receptors and β-tubulin III were designed for real-time PCR. The sequence of the specific primers designed based on gene bank data were as follows: 5-HT2A: F: 5-ccgcttcaactccagaaccaaagc-3, R: 5-cttcgaatcatcctgtacccgaa-3; 5-HT2C: F: 5-taatggtgaacctgggcactgcgg-3, R: 5-taaaagtgtcagttactatagctgc, 5-HT1A, F: 5-ctgtttatcgccctggatg-3, R: 5-atgagccaagtgagcgagat-3; β-tubulin III, F: 5-ataggggccaagttctgggaggtc-3, R: 5-ctgggcacatacttgtgagaggag-3
Behavioral studies
The head-twitch response (HTR) and ear-scratch response (ESR) scoring was performed as previously reported (Darmani 2001) with minor modifications. In brief, animals (n=7 per group) were kept in their standard home cage (15 × 24 cm), but enrichment material was removed during the test period. Animals were injected with either DOI (2.5 mg/kg, i.p.) or corresponding volume of saline 5 min prior to testing. The mice were video taped for 20 min and subsequently HTR and ESR were scored. One week later, the experiment was repeated so animals receiving DOI on the first test day, were given saline and vice versa.
Immunohistochemistry
Wildtype and cBDNF KOs mice were injected either saline or DOI (2.5 mg/kg) and after 1 hr, transcardially perfused with 20 ml of cold saline followed by 50 ml of 4% paraformaldehyde (PFA). Brains were immediately removed, postfixed in 4% PFA overnight at 4°C, cryoprotected in a 30% sucrose solution, and frozen in mounting media (Tissue-Tek, Torrance, CA) until further use. 40 μM coronal sections representing frontal cortex and hippocampus were obtained and c-fos immunohistochemistry was performed as previously described (Unger et al., 2007). In brief, endogenous peroxidase activity was quenched in free-floating sections with 0.5% H2O2 in PBS for 15 min, permeabilized with 0.5% Triton X-100 in PBS for 20 min, and blocked in 10% normal horse serum for 30 min at RT. Sections were then incubated for 48 h at 4°C with a rabbit primary antibody against c-fos (1:50,000; Ab5; Oncogene Science, Cambridge, MA), followed by washes and incubation with a biotinylated secondary antibody (1:200) and an avidin – biotin peroxidise complex as per the manufacturers instructions (ABC, Vector Elite Kit; Vector Laboratories, Burlingame, CA). Sections were then incubated with substrate solution (0.025% diaminobenzidine/0.15% nickel ammonium sulfate/0.0036% H2O2 in 0.05 M Tris buffer) for 5 min, mounted on slides and left to dry for 2 hrs before placing cover slips. c-fos positive neurons were quantified in frontal cortex and hippocampus using the computer assisted stereological toolbox software (CAST) module (Visiopharm, Denmark).
Statistical analysis
All analyses were performed using GraphPad 5. Data are presented as mean ± SEM if not otherwise stated. The data were analyzed using Student’s t-test if comparing the two groups, and two-way ANOVA with Bonferoni as post hoc test, when comparing the groups with more than one variable. The level of statistical significance was set to P < 0.05
Results
Total depletion of BDNF in frontal cortex and hippocampus of BDNF mutants
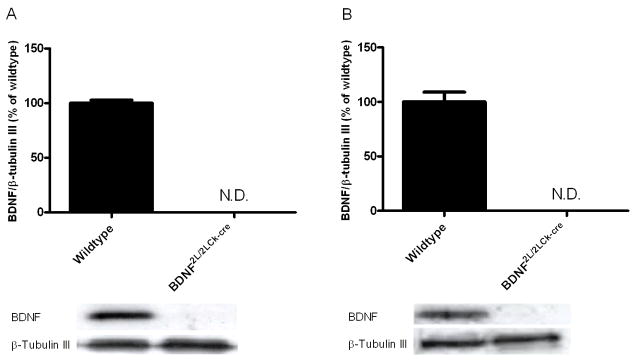
To confirm that adult BDNF2L/2LCk-cre mutant mice were depleted of BDNF protein in our brain regions of interest, we measured levels of BDNF in the mutant frontal cortex and hippocampus. Whereas BDNF protein was clearly detectable by Western blotting in wildtype mice, BDNF levels in BDNF mutants were below detectable levels (Fig. 1A and B). These results demonstrate that BDNF2L/2LCk-cre mice are depleted of BDNF protein in the frontal cortex and hippocampus.
Figure 1.
BDNF protein levels in frontal cortex (A) and hippocampus (B) in wildtype and BDNF2L/2LCk-cre mice. BDNF levels were not detectable in frontal cortex and hippocampus in the BDNF mutant mice using western blot, while wildtype levels corresponded to levels seen in other mice strains. BDNF levels were related to β-tubulin III levels (n=7). Representative bands for BDNF (14 kDa) and β-tubulin III (55 kDa) are shown below the corresponding column. N.D.: not detectable. Error bars indicate SEM.
5-HT2A and 5-HT1A receptor binding and expression is altered in BDNF mutant mice
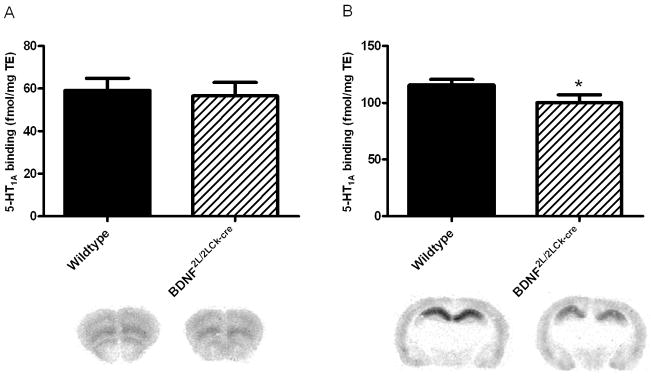
The number of 5-HT2A and 5-HT1A receptors in frontal cortex and hippocampus was analyzed using autoradiography applying highly selective tritium-labeled ligands (3H-MDL100907 and 3H-WAY100635, respectively). 5-HT2A binding in frontal cortex of BDNF2L/2LCk-cre mice was lower when compared to wildtype littermates (45.6 ± 1.7 vs. 35.2 ± 1.6 fmol/mg/TE, respectively)(P = 0.03)(Fig. 2A). However, in hippocampus, 5-HT2A receptor binding was increased by 31% in BDNF mutants (12.5 ± 0.7 vs. 16.4 ± 1.2 fmol/mg/TE, respectively)(P = 0.02)(Fig. 2B). In frontal cortex 5-HT1A receptor binding was similar in the two groups (59.15 ± 2.9 (WT) vs 56.55 ± 3.2 (BDNF mutants) Fig. 3A), while in hippocampus there was a significant decrease in 5-HT1A receptor binding in BDNF mutants compared to wildtypes (115.7 ± 2.5 vs. 100.2 ± 3.5; Fig 3B) (P = 0.01).
Figure 2.
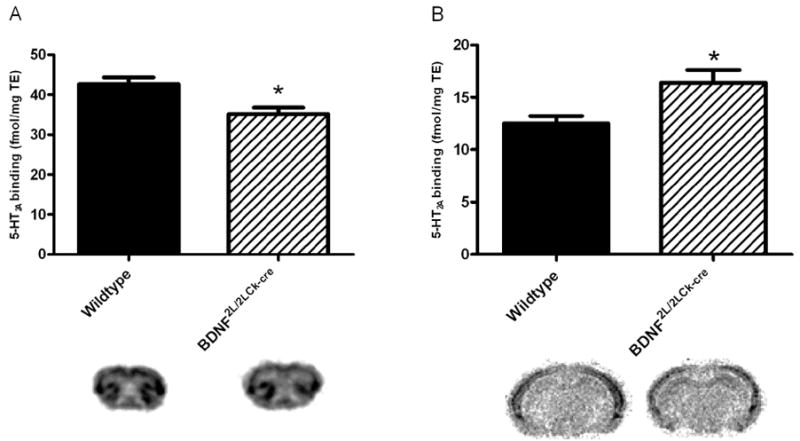
5-HT2A receptor binding (3H-MDL-100907) in BDNF2L/2LCk-cre mice and wildtype littermates in frontal cortex (A) and dorsal hippocampus (B). There was a significant decrease in binding in frontal cortex in BDNF2L/2LCk-cre mice vs wildtypes, while the opposite was observed for dorsal hippocampus. (n=4, Student’s t-test, *P < 0.05). Representative autoradiograms are shown below the corresponding column. Error bars indicate SEM
Figure 3.
5-HT1A receptor binding (3H-WAY100635) in BDNF2L/2LCk-cre mice and wildtype littermates in frontal cortex (A) and dorsal hippocampus (B). There was a significant decrease in binding in dorsal hippocampus in BDNF2L/2LCk-cre mice vs wildtypes, while no change was observed in frontal cortex. (n=4, Student’s t-test, *P < 0.05). Representative autoradiograms are shown below the corresponding column. Error bars indicate SEM
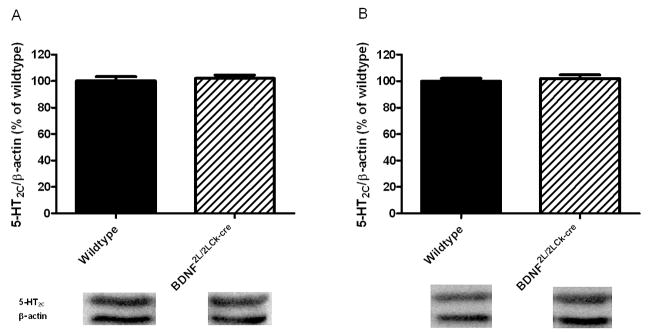
The effect of perturbed BDNF signaling on 5-HT1A and 5-HT2A receptors was further evaluated by gene expression studies. Using RT-qPCR, we observed a decrease in 5-HT2A mRNA levels in the frontal cortex of BDNF2L/2LCk-cre mutants that corresponded to the observed deficit in receptor binding (Fig. 4A). However, the increase in 5-HT2A receptor binding in the BDNF mutant hippocampus was not associated with changes in 5-HT2A mRNA levels (Fig 4D). Additionally, there was a trend towards a significant decrease in 5-HT1A mRNA expression in the BDNF mutant hippocampus but it did not reach statistical significance (P = 0.08) (Fig 4E). No differences in 5-HT1A mRNA levels were observed in the mutant frontal cortex (Fig. 4B). We also investigated whether transcript or protein levels of the 5-HT2C receptor were altered in BDNF mutant animals. No between-group differences were observed in frontal cortex or hippocampus (Figs. 4C, 4F and 5). The cumulative data indicate that the changes in 5-HT2A and 5-HT1A receptor binding in BDNF mutants differ between brain regions and are apparently inversely regulated in hippocampus.
Figure 4.
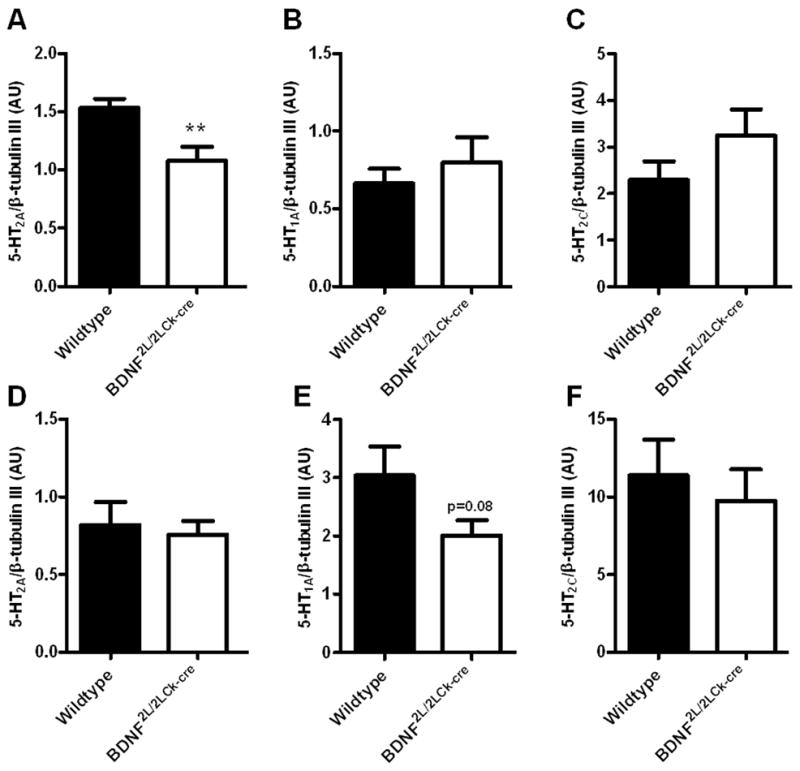
RT-qPCR on 5-HT2A (A, D), 5-HT1A (B, E) and 5-HT2C (C, F) mRNA in frontal cortex (upper panel) and hippocampus (lower panel) of wildtype and BDNF2L/2LCk-cre mice. There was a significant lower 5-HT2A mRNA expression in BDNF2L/2LCk-cre mice in frontal cortex compared to wildtypes (A), while no differences could be observed in hippocampus (D)(n=7–8, Student’s t-test, **P < 0.01). For the 5-HT1A receptor, there was a tendency towards a decrease in expression in BDNF2L/2LCk-cre mice compared to wildtypes (E)(P = 0.08) for hippocampus, while no change was observed in frontal cortex (B). For the 5-HT2C receptor we did not find any differences in expression of mRNA between BDNF2L/2LCk-cre mice and wildtypes. (AU, arbitrary units, Error bars indicate SEM)
Figure 5.
5-HT2C receptor protein levels in wildtype and BDNF2L/2LCk-cre mice in frontal cortex (A) and hippocampus (B). No changes in protein levels of this serotonin receptor could be observed (n=7–8, Student’s t-test). Representative bands for 5-HT2C (55 kDa) and β-actin (40 kDa) are shown below the corresponding column. Error bars indicate SEM
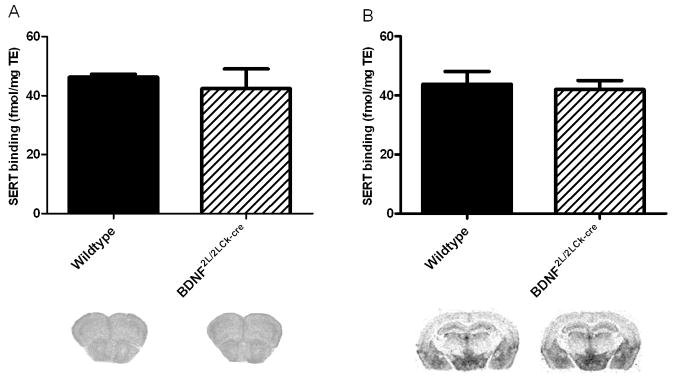
SERT binding is normal in BDNF2L/2LCk-cre mutant mice
To determine whether the observed alterations in 5-HT receptor binding and expression were related to upstream changes in the serotonin transporter (SERT), we used 3H-escitalopram autoradiography to quantify SERT binding sites. This analysis revealed that there were no differences in SERT binding between wildtypes and BDNF mutants either in frontal cortex (Fig. 6A) or hippocampus (Fig. 6B).
Figure 6.
SERT binding performed with 3H-escitalopram in frontal cortex (A) and hippocampus (B). We did not detect any differences in SERT binding between wildtypes and BDNF2L/2LCk-cre mice. (n=4, Student’s t-test). Representative autoradiograms are shown below the corresponding column. Error bars indicate SEM
BDNF depletion leads to altered behavioral responses to 5-HT2A receptor stimulation
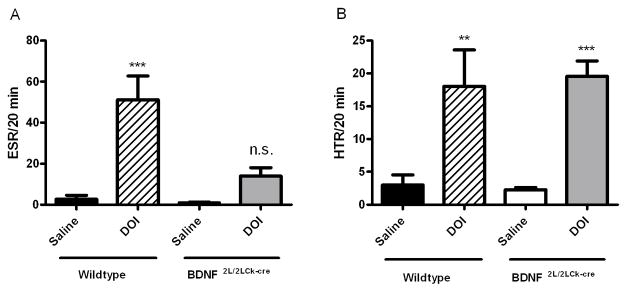
Next, we tested the effect of the altered 5-HT2A receptor expression in BDNF2L/2LCk-cre mutant mice on 5-HT2A-mediated behaviors. For this, we measured the ESR and HTR following saline or DOI (2.5mg/kg, i.p.) administration. There was a significant interaction of genotype and drug treatment in the ESR (F, 12.39 and P = 0.002, two-way ANOVA)(Fig. 7A). DOI induced a significant increase in ESR in wildtypes compared to their saline-treated counterparts (WT saline: 3 ± 1.5; WT DOI: 18 ± 5.5 head-twitches/20 min, Bonferroni post hoc test, P < 0.001). In contrast, DOI did not induce an increase in ESR in BDNF2L/2LCk-cre mice compared to saline-treated mutants (Fig. 7A). However, DOI treatment induced similar levels of HTR in wild type and BDNF mutant mice (two-way ANOVA) (Fig. 7B). These results indicate that the 5-HT2A mediated functional output measured by ESR is diminished in the BDNF mutant mice.
Figure 7.
Ear-Scratch Reponse (ESR)(A) and Head-Twitch Reponse (HTR)(B) in BDNF mutant mice and wildtype littermates after administration of DOI (2.5 mg/kg, i.p.) or saline. The registration of ESR and HTR was started 5 min after administration and continued for 20 min. Bars: Black: wildtype, saline; Crossed: wildtype, DOI 2.5 mg/kg, i.p.; White: BDNF2L/2LCk-cre mice, saline; Gray: BDNF2L/2LCk-cre mice (n=7, two-way ANOVA, Bonferroni post test, n.s.=not significant, **P < 0.01, ***P < 0.001. Error bars indicate SEM
Neuronal activation following 5-HT2A receptor stimulation in BDNF mutant mice
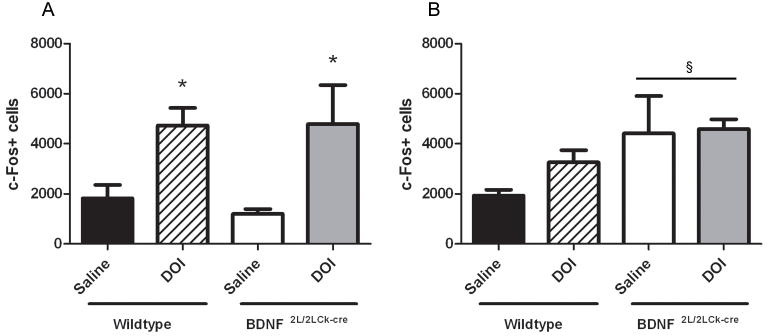
To further assess possible functional deficits in BDNF2L/2LCk-cre mutants related to 5-HT2A receptor alterations, we measured neuronal activation by c-fos immunoreactivity in frontal cortex and hippocampus 1 hr after a single DOI or saline injection. DOI elicited similar increases in number of c-Fos positive cells in frontal cortex in both wildtypes (saline: 1820; DOI: 4731 c-Fos+ cells; P<0.05) and BDNF mutants (saline: 1198; DOI: 4783 cells; P<0.05)(Fig. 8A)(two-way ANOVA, Bonferroni post hoc). In hippocampus, DOI did not induce a significant increase in c-Fos+ cells in either wild types (saline:1930; DOI: 3257 cells), or BDNF mutants (saline: 4420; DOI: 4593 cells)(Fig. 8B). However, there was an effect of genotype indicating that the cell activation response following 5-HT2AR-mediated cell activation is higher in hippocampus of the BDNF mutants compared to wildtypes (P = 0.04). This difference between genotypes was mainly driven by high baseline activation in the BDNF mutants. Collectively, these data indicate that the 5-HT2A receptor changes in frontal cortex are not reflected in 5-HT2AR-mediated cell activation, while the hippocampal cell activation is higher for the BDNF mutants.
Figure 8.
c-Fos immunopositive cells in frontal cortex (A) and hippocampus (B). A single DOI (2.5 mg/kg) injection significantly induced c-Fos immunoreactive cells compared to a single saline injection in frontal cortex in both wildtypes and BDNF2L/2LCk-cre mice (A). There was a genotype effect in hippocampus (§) indicating higher cell activation response BDNF2L/2LCk-cre mice compared to wildtypes (B)(n=4, two-way ANOVA, Bonferroni post hoc test, *P < 0.05. §P<0.05. Error bars indicate SEM
Expression of synaptic markers in hippocampus and frontal cortex of BDNF2L/2LCk-cre mutant mice is normal
To examine the degree of synaptic integrity, we measured protein levels of synaptophysin (pre-synaptic), PSD-95 (post-synaptic) and MAP2 (dendritic) using western blotting. We found no change in protein levels between wildtypes and BDNF mutants neither in frontal cortex nor in hippocampus (Fig. 9)
Figure 9.
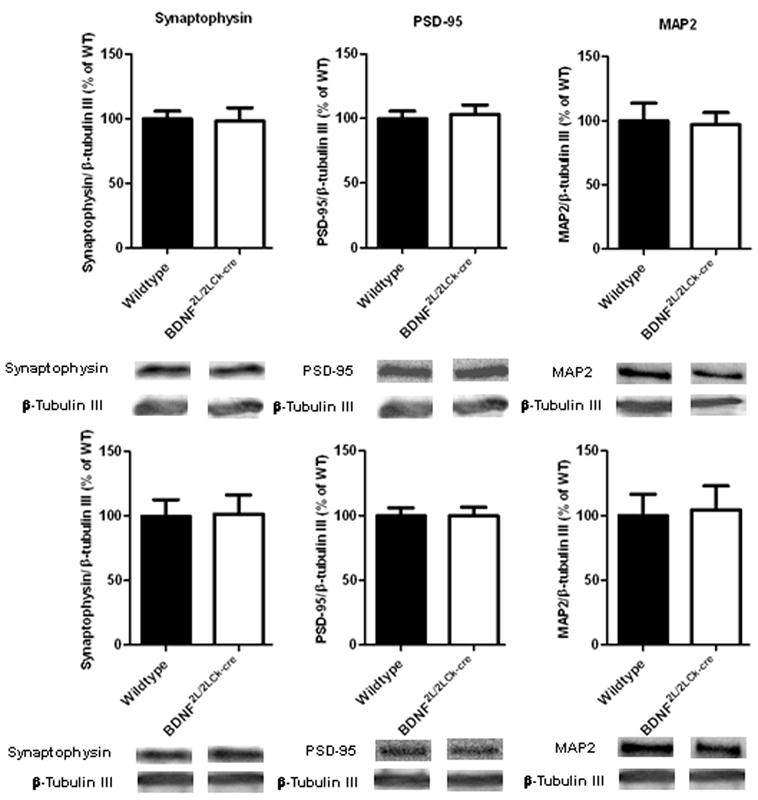
Analysis of protein levels using western blotting for synaptophysin (38 kDa), PSD-95 (95 kDa) and MAP2 (250 kDa). Upper panel: frontal cortex, lower panel: hippocampus. BDNF mutants have normal contents of the synaptic markers synaptophysin and PSD-95 and the dendritic marker MAP2 when compared to wildtypes. β-tubulin III (55 kDa) was used as loading control. (n=7–8, Student’s t-test). Error bars indicate SEM
Discussion
We hypothesized that alterations in the 5-HT system might underlie the behavioral alterations triggered by deficient BDNF signaling in the brain. One test of this hypothesis was to ascertain the viability of pivotal components of this neurotransmitter system in limbic regions of brain associated with anxiety and mood. We focused our investigations on 5-HT2A, 5-HT2C and 5-HT1A receptors because human and animal model studies have linked them to behaviors known to be altered in BDNF mutant mice including anxiety-like behavior (Naughton et al., 2000; Heisler et al., 2007; Akimova et al., 2009). Our results confirm the previously described differences in cortical 5-HT2A levels in BDNF mutant mice and reveal additional alterations in the 5-HT receptor system elicited by depleted BDNF stores. The decreased 5-HT2A receptor content observed in frontal cortex of BDNF2L/2LCk-cre mice using western blotting was also reflected in lower receptor binding in this area. Previously, we reported a 50–60% decrease in 5-HT2A receptor protein levels in frontal cortex (Rios et al., 2006), and in the present paper we found a ~20% decrease in receptor binding in this region. Since the analyses were not conducted on the same tissue, the quantitative outcomes cannot be directly compared. Differences in the receptor detection methods utilized could, however, explain eventual discrepancies. Total protein measurements reflect the entire pool of 5-HT2A receptors, while receptor autoradiography reflects the binding of the antagonist radioligand to the intact receptor binding pocket. A special characteristic of the 5-HT2A receptor is its high turnover rate, the largest receptor fraction being located intracellularly (Cornea-Hebert et al., 1999; Gray & Roth, 2001). Our results suggest that in BDNF mutant animals, it is primarily the intracellular fraction of receptors not available for ligand binding that is diminished. This is supported by the mRNA quantification results showing that indeed the rate of receptor synthesis is decreased in BDNF mutant mice.
Contrary to our observations in frontal cortex, we found that there was an increase in 5-HT2AR binding in the BDNF mutant hippocampus. This is in accordance with our in vivo findings from BDNF+/− mice, where we also found increased hippocampal 5-HT2AR protein levels (Trajkovska et al., 2009). The inverse observation, namely that exposure of BDNF to hippocampal cultures results in 5-HT2AR downregulation is in also line with this (Trajkovska et al., 2009). The in vivo studies of BDNF2L/2LCk-cre mice described here indicate that the effects of BDNF on 5-HT2A receptor levels are context-dependent.
We extended our analysis to other 5-HT receptors to ascertain whether the effects of BDNF were restricted to the 5-HT2A receptor. We found that 5-HT2C receptor expression in frontal cortex and hippocampus of BDNF mutants was normal. We also measured expression and binding of the 5-HT1A receptor in these brain regions. The 5-HT1A receptor co-localizes with the 5-HT2A receptor in dendrites in prefrontal cortex (Feng et al., 2001). Central BDNF depletion did not impact 5-HT1A receptor binding in frontal cortex, suggesting that the lower 5-HT2A receptor binding was a result from structural reorganization or retraction of dendrites. In support of this assertion, we found that expression of synaptophysin, a synaptic marker and of MAP2, a dendritic marker, were normal in BDNF mutants. In contrast to our findings in frontal cortex, 5-HT1A receptor binding was decreased in the BDNF mutant hippocampus. Furthermore, we showed previously that 5-HT1A receptor protein levels were unchanged in dorsal raphe nucleus (DRN) in BDNF2L/2LCk-cre mutants (Rios et al., 2006). The primary role of the 5-HT1ARs in DRN is as pre-synaptic autoreceptors, while in hippocampus they mainly exist as post-synaptic heteroreceptors (Riad et al., 2001). Therefore, the data shown here and previous findings provide additional examples of the context-dependent effects of BDNF. An intriguing study by Hensler et al. (2007) found no changes in hippocampal 5-HT1A receptor binding using a tetracycline-inducible model of BDNF depletion (Hensler et al., 2007). It is important to note that whereas in those mutants BDNF was depleted in adulthood, in the mutant mice described here BDNF was gradually depleted during the first post-natal weeks (Rios et al., 2001). Therefore, the 5-HT1A receptor alterations reported here might reflect the long-term effect of BDNF depletion, the effects of perturbing BDNF signaling during a critical developmental period or a compensatory response to the 5-HT2A receptor changes also observed in these mice. A “functional antagonism” between these two receptors was proposed previously as a mechanism to circumvent challenges on the serotonin system and restore proper neurotransmission (Yuen et al., 2008). Nonetheless, this alteration in 5-HT1A receptors might contribute to the complex behavioral phenotype of these mice.
By examining 5-HT2A-mediated behavioral output, we showed that the decrease in 5-HT2A receptor levels was accompanied by a diminished functional response to pharmacological stimulation of this receptor in vivo. DOI-induced ESR was significantly attenuated in BDNF mutants, indicating a reduction in the pool of functional 5-HT2A receptors that mediate this response. Interestingly, the DOI-induced HTR was not affected in the mutants. This was surprising as it is well established that both HTR and ESR are mediated by the 5-HT2A receptor (Gonzalez-Maeso et al., 2007; Willins & Meltzer, 1997). Accordingly, both HTR and ESR were completely abrogated in 5-HT2A receptor knock-out mice (Gonzalez-Maeso et al., 2007, suppl.mat). We suggest that the ESR is more sensitive to small changes in 5-HT2A receptor signaling, and that the 20% decrease in 5-HT2A receptor binding in frontal cortex is too small to be reflected in the HTR. Indeed, the ESR was demonstrated to be 30 times more sensitive than HTR (Darmani, 2001). Alternatively, the HTR and ESR might be mediated by different brain circuits that are differentially affected by perturbed BDNF signaling. Unfortunately, little is known regarding the exact networks underlying the HTR and ESR. In a thorough study, Willins and Meltzer (1997) delivered DOI to different regions of the rat brain and established that HTR could only be induced when the medial prefrontal cortex was activated. The ESR was not examined as this response had not been described in rats. If other brain areas besides the medial prefrontal cortex such as the hippocampus are involved in the ESR, the observed increase in hippocampal 5-HT2AR binding in the BDNF mutants might exacerbate the diminished 5-HT2A frontal cortex output leading to impaired ESR. In support of this model, a previous study demonstrated a marked DOI-induced increase in motor-movements (head-bobs), which can be compared to HTR and ESR in rodents, in rabbits with increased 5-HT2A receptor binding in the CA1 region of the hippocampus (Dave et al., 2004). Future studies with intracerebral site-directed injections of 5-HT2A agonists in mice are needed to test this hypothesis.
Even though activation of the 5-HT2A receptor signaling pathway is necessary for both ESR and HTR, it has been suggested that other receptor systems can modulate the response. For example, Darmani and colleagues showed that HTR and ESR induced by DOI can both be inhibited by cannabinoids (e.g., CP55,940), but not to the same degree. This indicates that there are different thresholds for each response which can be modulated through other receptors. Other data suggest that 5-HT2A mediated responses can be modulated by activation of the 5-HT1A and 5-HT2C receptors (Willins & Meltzer, 1997). Interestingly, we found that there was a significant decrease in hippocampal 5-HT1A receptor binding in BDNF mutants. However, it remains to be elucidated whether this change is sufficient to impact the ESR and/or the HTR. Since BDNF depletion was not associated with changes in 5-HT2C receptor expression, it is unlikely that deficits in this pathway contribute to the diminished ESR in BDNF mutants.
To further evaluate 5-HT2A receptor functionality in the BDNF2L/2LCk-cre mutant mice, we quantified DOI-induced c-Fos expression as a measure of 5-HT2A-mediated neuronal activation. In spite of decreased frontal 5-HT2A-receptor binding, BDNF mutants had the same DOI-induced c-Fos expression in frontal cortex, as the wildtype mice. However, in hippocampus, BDNF mutants had a different cell activation response. This was partly driven by high levels of c-fos+ cells in the hippocampus following the saline injection, suggesting that mutants might have elevated baseline levels of neuronal activity in this region at baseline. This may be mediated through the observed increase in 5-HT2A receptors in hippocampus in these mice. Alternatively, BDNF mutants might have a higher basal level of arousal and are easily stressed when handled and accordingly, the saline injection per se may elicit a c-Fos response in this brain region.
In summary, we demonstrated that BDNF depletion results in context-dependent changes in 5-HT2A and 5-HT1A binding and expression. Furthermore, we showed that alterations in 5-HT2A levels have functional consequences as illustrated by the diminished DOI-induced ESR in BDNF mutants. The diverse regulation of these receptors in BDNF2L/2LCk-cre mice underlines the complexity of neurotrophin and serotonin signaling interaction.
Acknowledgments
German A. Calderon, Hans-Jørgen Jensen and Christine B. Janssens are acknowledged for great technical assistance. This work was supported by the Lundbeck Foundation, the Augustinus Foundation, the EC-FP6-project DiMI, LSHB-CT-2005- 512146 and the University of Copenhagen. MR was supported by NIH/NIDDK (DK073311) and NIH/NIMH (MH67817) research grants.
Abbreviations
- BDNF
Brain-derived neurotrophic factor
- CA1
cornu ammonis 1 of hippocampus
- DOI
(±)-2,5-Dimethoxy-4-iodoamphetamine hydrochloride
- DRN
dorsal raphe nucleus
- ESR
ear-scratch response
- 5-HT
serotonin
- 5-HT1AR
5-HT receptor 1A
- 5-HT2AR
5-HT receptor 2A
- 5-HT2CR
5-HT receptor 2C
- HTR
head-twitch response
- MAP2
microtubule associated protein 2
- SERT
serotonin transporter
- TrkB
tropomysoin-related kinase B
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akimova E, Lanzenberger R, Kasper S. The serotonin-1A receptor in anxiety disorders. Biol Psychiatry. 2009;66:627–635. doi: 10.1016/j.biopsych.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Chan JP, Unger TJ, Byrnes J, Rios M. Examination of behavioral deficits triggered by targeting Bdnf in fetal or postnatal brains of mice. Neuroscience. 2006;142:49–58. doi: 10.1016/j.neuroscience.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Cornea-Hebert V, Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol. 1999;409:187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Darmani NA. Cannabinoids of diverse structure inhibit two DOI-induced 5-HT(2A) receptor-mediated behaviors in mice. Pharmacol Biochem Behav. 2001;68:311–317. doi: 10.1016/s0091-3057(00)00477-9. [DOI] [PubMed] [Google Scholar]
- Dave KD, Fernando GS, Quinn JL, Harvey JA, Aloyo VJ. Serotonin 5-HT2A receptors in the CA1 field of the hippocampus mediate head movements in the rabbit. Psychopharmacology (Berl) 2004;176:287–295. doi: 10.1007/s00213-004-1887-6. [DOI] [PubMed] [Google Scholar]
- Deltheil T, Guiard BP, Cerdan J, David DJ, Tanaka KF, Reperant C, Guilloux JP, Coudore F, Hen R, Gardier AM. Behavioral and serotonergic consequences of decreasing or increasing hippocampus brain-derived neurotrophic factor protein levels in mice. Neuropharmacology. 2008;55:1006–1014. doi: 10.1016/j.neuropharm.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Feng J, Cai X, Zhao J, Yan Z. Serotonin receptors modulate GABA(A) receptor channels through activation of anchored protein kinase C in prefrontal cortical neurons. J Neurosci. 2001;21:6502–6511. doi: 10.1523/JNEUROSCI.21-17-06502.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley- Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Gray JA, Roth BL. Paradoxical trafficking and regulation of 5-HT(2A) receptors by agonists and antagonists. Brain Res Bull. 2001;56:441–451. doi: 10.1016/s0361-9230(01)00623-2. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Zhou L, Bajwa P, Hsu J, Tecott LH. Serotonin 5-HT(2C) receptors regulate anxiety-like behavior. Genes Brain Behav. 2007;6:491–496. doi: 10.1111/j.1601-183X.2007.00316.x. [DOI] [PubMed] [Google Scholar]
- Hensler JG, Advani T, Monteggia LM. Regulation of serotonin-1A receptor function in inducible brain-derived neurotrophic factor knockout mice after administration of corticosterone. Biol Psychiatry. 2007;62:521–529. doi: 10.1016/j.biopsych.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 2000;19:1290–1300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozisek ME, Middlemas D, Bylund DB. Brain-derived neurotrophic factor and its receptor tropomyosin-related kinase B in the mechanism of action of antidepressant therapies. Pharmacol Ther. 2008;117:30–51. doi: 10.1016/j.pharmthera.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Leysen JE. 5-HT2 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:11–26. doi: 10.2174/1568007043482598. [DOI] [PubMed] [Google Scholar]
- Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, Wihler C, Koliatsos VE, Tessarollo L. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci U S A. 1999;96:15239–15244. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughton M, Mulrooney JB, Leonard BE. A review of the role of serotonin receptors in psychiatric disorders. Hum Psychopharmacol. 2000;15:397–415. doi: 10.1002/1099-1077(200008)15:6<397::AID-HUP212>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Riad M, Watkins KC, Doucet E, Hamon M, Descarries L. Agonist-induced internalization of serotonin-1a receptors in the dorsal raphe nucleus (autoreceptors) but not hippocampus (heteroreceptors) J Neurosci. 2001;21:8378–8386. doi: 10.1523/JNEUROSCI.21-21-08378.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios M, Fan G, Fekete C, Kelly J, Bates B, Kuehn R, Lechan RM, Jaenisch R. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15:1748–1757. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- Rios M, Lambe EK, Liu R, Teillon S, Liu J, Akbarian S, Roffler-Tarlov S, Jaenisch R, Aghajanian GK. Severe deficits in 5-HT2A -mediated neurotransmission in BDNF conditional mutant mice. J Neurobiol. 2006;66:408–420. doi: 10.1002/neu.20233. [DOI] [PubMed] [Google Scholar]
- Savitz J, Lucki I, Drevets WC. 5-HT(1A) receptor function in major depressive disorder. Prog Neurobiol. 2009;88:17–31. doi: 10.1016/j.pneurobio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen C, Helboe L. Regional pattern of binding and c-Fos induction by (R)- and (S)-citalopram in rat brain. Neuroreport. 2003;14:2411–2414. doi: 10.1097/00001756-200312190-00024. [DOI] [PubMed] [Google Scholar]
- Trajkovska V, Santini MA, Marcussen AB, Thomsen MS, Hansen HH, Mikkelsen JD, Arneberg L, Kokaia M, Knudsen GM, Aznar S. BDNF downregulates 5-HT(2A) receptor protein levels in hippocampal cultures. Neurochem Int. 2009;55:697–702. doi: 10.1016/j.neuint.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Unger TJ, Calderon GA, Bradley LC, Sena-Esteves M, Rios M. Selective deletion of Bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. J Neurosci. 2007;27:14265–14274. doi: 10.1523/JNEUROSCI.3308-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisstaub NV, Zhou M, Lira A, Lambe E, Gonzalez-Maeso J, Hornung JP, Sibille E, Underwood M, Itohara S, Dauer WT, Ansorge MS, Morelli E, Mann JJ, Toth M, Aghajanian G, Sealfon SC, Hen R, Gingrich JA. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science. 2006;313:536–540. doi: 10.1126/science.1123432. [DOI] [PubMed] [Google Scholar]
- Willins DL, Meltzer HY. Direct injection of 5-HT2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats. J Pharmacol Exp Ther. 1997;282:699–706. [PubMed] [Google Scholar]
- Yuen EY, Jiang Q, Chen P, Feng J, Yan Z. Activation of 5-HT2A/C receptors counteracts 5-HT1A regulation of n-methyl-D-aspartate receptor channels in pyramidal neurons of prefrontal cortex. J Biol Chem. 2008;283:17194–17204. doi: 10.1074/jbc.M801713200. [DOI] [PMC free article] [PubMed] [Google Scholar]