Abstract
Improgan, the prototype compound of a novel class of non-opioid analgesic drugs derived from histamine antagonists, attenuates thermal and mechanical nociception in rodents following intracerebroventricular administration. Improgan does not bind to known opioid, histamine or cannabinoid receptors, and its molecular target has not been identified. It is known however that improgan acts directly in the periaqueductal gray and the rostral ventromedial medulla to produce its antinociceptive effects, and that inactivation of the rostral ventromedial medulla prevents the antinociceptive effect of improgan given intracerebroventricularly. Here we used in vivo single-cell recording in lightly anesthetized rats to show that improgan engages pain-modulating neurons in the medulla to produce antinociception. Following improgan administration, OFF-cells, which inhibit nociception, became continuously active and no longer paused during noxious stimulation. The increase in OFF-cell firing does not represent a non-specific neuroexcitant effect of this drug, since ON-cell discharge, associated with net nociceptive facilitation, was depressed. NEUTRAL-cell firing was unaffected by improgan. The net response of RVM neurons to improgan is thus comparable to that evoked by μ-opioids and cannabinoids, well known RVM-active analgesic drugs. This common basis for improgan, opioid, and cannabinoid antinociception in the RVM supports the idea that improgan functions as a specific analgesic agent.
Keywords: pain-modulation, brainstem, rostral ventromedial medulla, ON-cells, OFF-cells
Improgan (Li et al., 1996), a derivative of the histamine receptor antagonist cimetidine, produces highly effective antinociception following intracerebroventricular (icv) administration. Extensive testing with several rodent pain models has shown improgan to have the preclinical profile of an effective analgesic (Li et al., 1997; Hough, 2004; Nalwalk et al., 2004). Improgan antinociception is not mediated by known opioid or histamine receptors (Hough et al., 2000b; Mobarakeh et al., 2003), and screening at over 100 sites has not revealed the improgan molecular target (Hough, 2004). Recent studies suggest that improgan may act through an endocannabinoid mechanism (Nalwalk et al., 2006; Gehani et al., 2007), but the drug does not have measurable affinity for known cannabinoid receptors (Hough et al., 2006). Several new improgan congeners with enhanced potency and/or brain-penetrating properties have been described (Hough et al., 2005; Hough et al., 2006; Hough et al., 2007).
The neural basis for improgan's antinociceptive action remains unknown. Opioids and many non-opioid analgesic drugs relieve pain, in part, by activating descending control systems. The best studied opioid-sensitive descending system has critical links in the periaqueductal gray and rostral ventromedial medulla (RVM). In recent extensive mapping studies, improgan was shown to act in both sites (Hough et al., 2001; Nalwalk et al., 2004; Hough et al., 2009). Further, the antinociceptive effect of icv improgan is abolished by functional lesions of the RVM, showing an essential requirement for activation of RVM neurons in the antinociceptive actions of improgan (Nalwalk et al., 2004). The present report describes the effects of improgan given icv on physiologically identified RVM neurons.
OFF-cells and ON-cells are two physiological classes of pain-modulating neurons that have been identified in the RVM. ON-cells are now known to facilitate, and OFF-cells to suppress nociception (Heinricher and Ingram, 2008; Heinricher et al., 2009). Focal application of μ-opioid and cannabinoid agonists in the RVM has a differential effect on the firing of these two classes, causing OFF-cells to become continuously active and suppressing ON-cell discharge (Heinricher et al., 1994; Meng et al., 1998; Meng and Johansen, 2004). Activation of OFF-cells is required for opioid analgesia; the parallel suppression of ON-cell firing may contribute but is not necessary for antinociception (Heinricher and Tortorici, 1994; Heinricher et al., 1999). The present experiments were therefore designed to determine whether improgan produces its behavioral antinociceptive effect by a non-selective excitant effect in the RVM or whether, like opioids and cannabinoids, improgan activates RVM OFF-cells while suppressing the firing of ON-cells.
Experimental Procedures
Animals, surgical preparation and nociceptive testing
All experimental procedures followed the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain, and were approved by the Institutional Animal Care and Use Committee at Oregon Health & Science University. Male Sprague-Dawley rats (Taconic, 275–325 g) were maintained under isoflurane anesthesia throughout the experiments, which generally required three to five hours. After initial induction (4% isoflurane in humidified O2, 1.5 L/min), isoflurane concentration was reduced to 3% and the rats were placed in a stereotaxic instrument for surgical preparation (requiring no more than 20–30 min). Small craniotomies and openings in the dura were made to allow stereotaxic placement of electrodes in the RVM and infusion cannulae in the lateral ventricle. Following surgery, the animals were maintained with 1.00–1.25% isoflurane, a concentration that allows a stable baseline tail flick latency, and is sufficient to prevent signs of discomfort. With this approach, animals did not show spontaneous movement, and noxious stimulation such as pinch elicited only a brief withdrawal reflex. Body temperature was maintained at approximately 37 °C by use of a circulating water pad. A drug protocol was begun after a stabilization period of at least 30 min, and isoflurane concentration was not altered during the protocol.
Tail flick (TF) latency was used as described previously as a measure of nociceptive responsiveness (Barbaro et al., 1989; Neubert et al., 2004). Heat was applied using a feedback-controlled projection lamp (Boehme Electronics, Portland, OR) focused on the ventral surface of the tail. Each trial consisted of a linear increase in temperature at approximately 1.8 °C/s from a holding temperature of 34 °C until the reflex occurred or to a maximum of 53 °C (cut-off) at approximately 10.6 s. Trials were carried out at 5-min (baseline) or 10-min (post-injection) intervals throughout the experiment (the 10-min intertrial interval was used following the icv injection to reduce the possibility of damage to the tail tissue in animals that were non-responsive following improgan treatment). The holding temperature obviates any concern that apparent effects on reflex latency could be attributed to changes in skin temperature.
RVM Recording
A gold- and platinum-plated stainless steel recording microelectrode (FHC, Bowdoinham, ME or Microprobe, Gaithersburg, MD) was inserted into the RVM for extracellular single-unit recording using surface landmarks. RVM neurons were classified as previously described (Fields et al., 1983; Barbaro et al., 1989). Spike waveforms were monitored and stored for off-line analysis (CED Spike 2, Cambridge, UK) to ensure that the unit under study was unambiguously discriminated throughout the experiment. OFF-cells were characterized by an abrupt pause in ongoing activity beginning just prior to the occurrence of the TF. ON-cells were identified by a sudden burst of activity beginning just prior to the occurrence of the TF. Both OFF- and ON-cells typically responded to noxious pinch at various locations on the body, with inhibition and excitation, respectively. NEUTRAL-cells were identified by no change in activity associated with the TF. Because the reflex-related ON-cell activation is not normally detectable when the neuron is already spontaneously active, and because a reflex-related pause can be seen only during active periods, we attempted to test neurons during both active and silent periods in the characterization phase.
Protocol and data analysis
Improgan base (Hough et al., 2000a) was dissolved in dilute HCl, neutralized to pH 5.5 to 6, and diluted with saline. Improgan or saline vehicle was injected into the left lateral ventricle (8 μl, coordinates relative to bregma: −0.8 anterior-posterior, 1.5 lateral, 3.3 ventral to surface). Only one protocol was performed per animal.
Effects of improgan on TF latency and RVM cell activity were determined. Following three pre-drug baseline TF trials (5-, 10- and 15-min time points), improgan (580 nmol) or pH-matched saline was infused into the lateral ventricle over a period of approximately 4 min. TF latency and cell activity were then monitored for an additional 70 min.
Up to four cell parameters were analyzed in the different experiments so that we could determine the effects of improgan on both ongoing (i.e., spontaneous) activity and reflex-related responses of RVM neurons. 1) Ongoing activity. Because OFF-cells and ON-cells often show irregular alternations between periods of silence and activity, cell activity integrated over the 30 s prior to each withdrawal trial was used as an index of overall ongoing firing. 2) ON-cell TF-related burst. Average firing rate in the 3 s period beginning 0.5 s before the TF was recorded for all TF trials. For trials in which the TF was inhibited by improgan, this parameter was calculated around the mean pre-drug TF latency. This approach, rather than counting the number of spikes or duration of the reflex-related burst, was necessary because a burst as such can only be identified in cases in which the neuron happens to have been inactive at the time of heat onset. 3) OFF-cell pause. Reflex- or heat-related inhibition was calculated as the firing rate in the 3 s beginning 0.5 s before the reflex (or mean pre-drug reflex latency in cases with no flick) expressed as a percentage of firing rate in the 10 s immediately before heat onset (“Pause index”). In addition, duration of the reflex-related pause was determined for those trials that fell at a time when the OFF-cell was active at the time of heat onset and for which there was a tail flick. 4) Cycling. The proportion of time that a given cell was considered to be in an “active” or “silent” period was defined as described previously (Barbaro et al., 1989). Briefly, an active period was defined as any epoch lasting at least 2 s with a minimum of 1 spike/s, and a silent period as any epoch of at least 2 s without any cell activity. The proportion of time in which each OFF-cell was “active” or “silent” was then calculated for the pre-drug and post-treatment periods. Cell data are presented only for those experiments in which we were able to follow and reliably identify the cell under study for the entire protocol.
Data are presented as mean + SEM. The average of the three baseline trials was compared with post-improgan time points. Wilcoxon's signed ranks or Mann-Whitney U tests were used for statistical analysis of firing rates. ANOVA with repeated measures (followed by Dunnett's test for comparison with baseline or Bonferroni test for comparisons between groups) or a t-test for correlated means was used to compare pre- and post-drug TF latencies and pause parameters. Fisher's exact test was used to compare the proportion of animals that failed to respond to heat following administration of improgan vs. saline. p < 0.05 was considered statistically significant.
Histology
At the conclusion of the experiments, recording sites were marked with an electrolytic lesion, and lateral ventricle infusion sites by injection of pontamine sky blue dye. Animals were euthanized with an overdose of isoflurane, and perfused intracardially with physiological saline followed by 10% formalin. Recording and infusion sites were histologically verified. The RVM was defined as the nucleus raphe magnus and adjacent reticular formation at the level of the facial nucleus. Recording sites were located within the RVM as in previous publications from this laboratory (Heinricher and Tortorici, 1994; Heinricher and Roychowdhury, 1997).
Results
Effects of icv improgan on TF latency and RVM cell activity in lightly anesthetized animals
TF latency
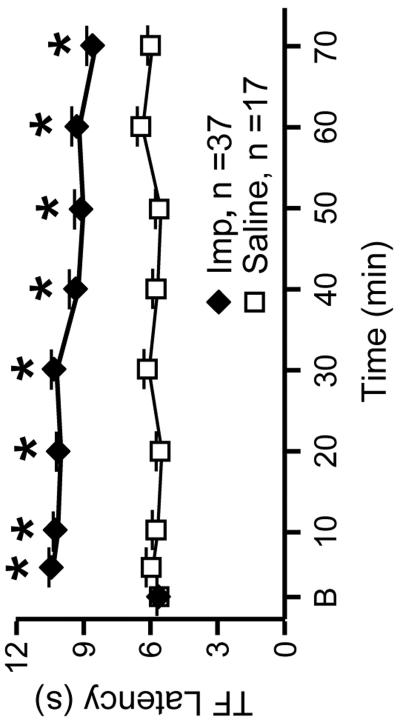
As demonstrated previously in awake animals using a variety of nociceptive tests (Li et al., 1997; Hough, 2004; Nalwalk et al., 2004), icv administration of improgan produced powerful antinociception as measured by an increase in the latency of the heat-evoked TF response (Fig. 1). The increase in latency was evident at the first trial following completion of the icv injection, and was maintained for over an hour. The TF was completely inhibited (at least two trials) in 33 of 37 improgan-treated animals (89.2%). The duration of TF inhibition averaged 43.2 ± 3.7 min. TF latency in saline-treated animals was unchanged over the full course of the experimental protocol (Fig. 1), demonstrating both the stability of the anesthetic state in our preparation, and that latency was not affected by repeated testing. The TF was not inhibited on any trial following saline administration in any animal (0%, proportion of animals with complete inhibition of the TF was significantly less in saline-treated compared to improgan-treated animals, p < 0.001).
Fig. 1. Nociceptive latencies before and after icv injection of improgan (Imp, 580 nmol) or saline vehicle (8 μl).
Time is min after completing icv injection, B represents an average of the three pre-injection baseline trials. Data are mean + SEM. *p < 0.05 compared to pre-drug baseline, ANOVA followed by Dunnett's test.
Improgan effects on RVM cell activity
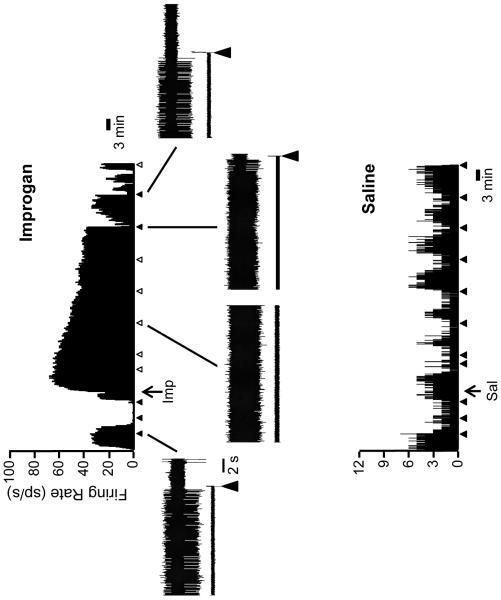
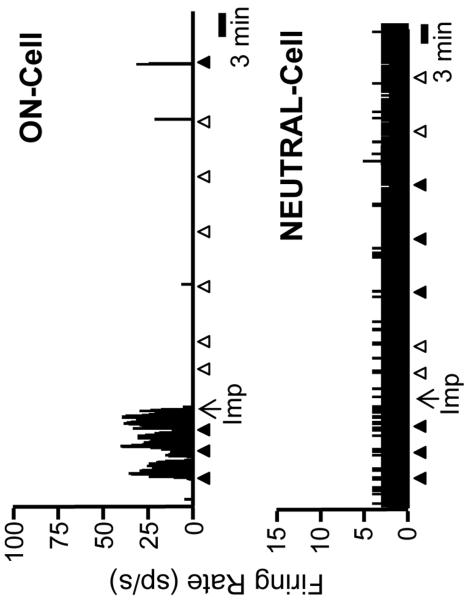
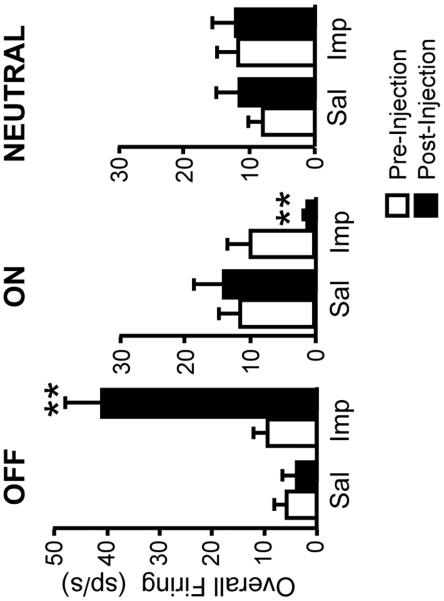
ON- and OFF-cells have long been known to display periodic fluctuations in ongoing activity in lightly anesthetized animals (Barbaro et al., 1989; Heinricher et al., 1989). As shown in the examples of Figs. 2 and 3, improgan given icv altered the ongoing firing of both OFF-cells and ON-cells: OFF-cells entered a prolonged period of continuous firing, ON-cells shifted into an inactive state. NEUTRAL-cell firing was unchanged following icv injection of improgan. On average (Fig. 4), overall OFF-cell firing following improgan administration was increased to over 400% of predrug baseline (n = 11), whereas ON-cell firing was depressed to 15.6 ± 7.7% of baseline (n = 14). NEUTRAL-cell firing was not significantly changed following improgan (110.9 ± 18.9% of baseline, n = 10). Saline had no effect on activity of any cell class (6 OFF-cells, 7 ON-cells, 4 NEUTRAL-cells).
Fig. 2. Firing of typical OFF-cells before and after icv improgan and saline vehicle.
Top: Ratemeter record shows ongoing activity of an OFF-cell before and after improgan injection (at arrow below trace, Imp). Triangles indicate TF trials. Filled triangles indicate a withdrawal, open triangles no behavioral response within 10.6 s cut-off period. Examples of the reflex-related pause are shown on an expanded time base for selected trials (baseline, during TF inhibition, and following recovery).
Below: Ratemeter record shows ongoing activity of an OFF-cell before and after saline injection (at arrow below trace, Sal). Triangles indicate TF trials, with withdrawal throughout the protocol.
Ratemeter bins: 1 s. Sp/s: number of spikes per second.
Fig. 3. Firing of typical ON-cell and NEUTRAL-cell before and after icv improgan (Imp).
Ongoing activity of an ON-cell and NEUTRAL-cell before and after improgan injection (at arrows below traces, Imp). Triangles indicate TF trials. Filled triangles indicate a withdrawal, open triangles no behavioral response within 10.6 s cut-off period. 1 s bins. Sp/s: number of spikes per second.
Fig. 4. Overall activity in pre-drug baseline compared with that following injection of saline (Sal) or improgan (Imp).
Post-injection values are average of the three trials in the 5–20 min post-injection interval. **p < 0.01 compared to baseline, Wilcoxon's signed ranks test. (Improgan: 11 OFF-cells, 14 ON-cells, 10 NEUTRAL-cells. Saline: 6 OFF-cells, 7 ON-cells, 4 NEUTRAL-cells).
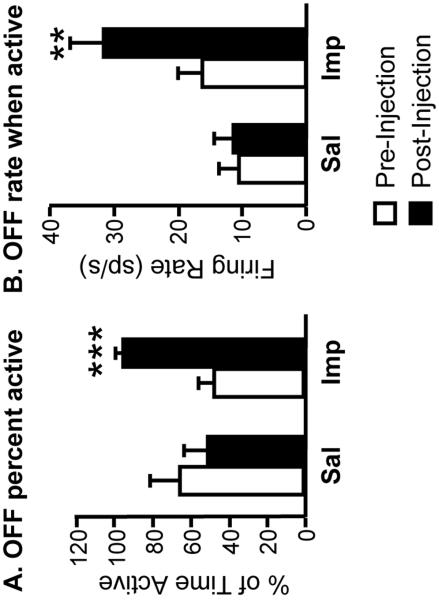
The increase in OFF-cell ongoing discharge following improgan administration was due to changes in two aspects of cell activity. First, there was an increase in the proportion of time that these cells spent in the active phase (Fig. 5A). Indeed, the OFF-cells under study fired without interruption for an average of 40 min (2429 ± 603 s) beginning with the improgan injection, comparable to the average duration of TF inhibition (43.2 ± 3.7 min, see previous section). By contrast, the average duration of the longest single active period of these same cells in the pre-drug baseline was significantly shorter, only 174.9 ± 26.4 s (p = 0.003, t-test for correlated means). Second, the firing rate of OFF-cells during active periods was significantly increased following improgan but not saline (Fig. 5B).
Fig. 5. Modulation of OFF-cell firing pattern by improgan.
Increase in overall activity of OFF-cells (see Fig. 4) following icv improgan (Imp) reflected both an increase in the proportion of time active (A) and the firing rate during active periods (B). Neither parameter was altered following saline (Sal) injection. ***p < 0.001, t-test for correlated means (percent active), **p < 0.01 Wilcoxon's signed ranks test (firing rate during active periods). Same OFF-cells as in Fig. 4.
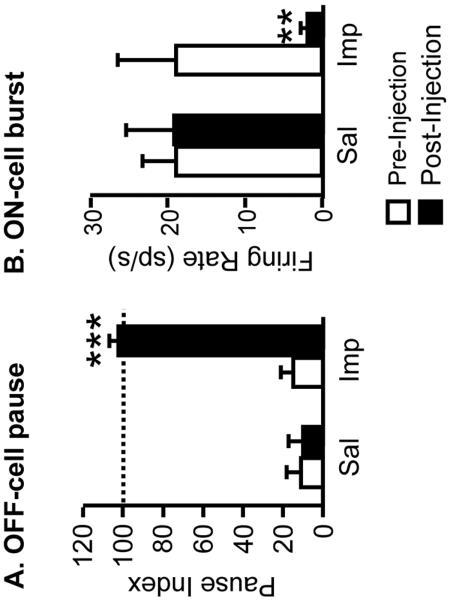
Finally, improgan eliminated the reflex-related OFF-cell pause and ON-cell burst. Thus, OFF-cells did not slow during tail heat trials following improgan (Fig. 6A). Similarly, ON-cells were not activated during tail heating after improgan (Fig. 6B). Neither the burst nor the pause was changed following saline injection (Fig. 6).
Fig. 6. Modulation of reflex-related changes in firing by improgan.
Effect of icv injection of saline (Sal) and improgan (Imp) on the OFF-cell pause (A) and ON-cell burst (B). Pause index: firing rate at time of the reflex (or at mean pre-drug reflex latency for trials when tail flick was inhibited following improgan) as percent of firing immediately prior to heat onset. A pause index of 100 would indicate no reflex- or heat-related slowing. Burst: firing rate in spikes/s at time of reflex (or at mean pre-drug reflex latency if no flick). ***p < 0.001 compared to pre-drug baseline period, t-test for correlated means (pause index); ** p < 0.01 compared to pre-drug baseline period, Wilcoxon's signed ranks test (ON-cell burst). Same OFF- and ON-cells as in Fig. 4.
Discussion
Improgan given icv in awake behaving animals produces potent non-opioid antinociception in a variety of rodent models of pain (Li et al., 1996; Li et al., 1997). Although a chemical congener of the H2 receptor antagonist cimetidine, improgan does not bind known histamine or opioid receptors (Li et al., 1997; Hough et al., 2000a; Mobarakeh et al., 2003), and its molecular target remains to be discovered. In the present experiments, we focused on a circuit-level explanation of improgan action in the RVM, since microinjection mapping studies demonstrate that the RVM supports improgan antinociception (Hough et al., 2001; Nalwalk et al., 2004; Hough et al., 2009), and because blocking RVM activity interferes with the antinociceptive effect of improgan given icv (Nalwalk et al., 2004).
The antinociceptive action of the RVM is acknowledged to be an active process, reflecting recruitment of an antinociceptive outflow from this region. This is inferred from demonstrations that non-selective excitation of RVM neurons, using electrical stimulation or glutamate microinjection, is antinociceptive (Zorman et al., 1981; Sandkühler and Gebhart, 1984; Aimone and Gebhart, 1986; Lovick, 1986; Jensen and Yaksh, 1989), whereas inactivation of all neurons in the region (e.g., by electrolytic lesion or local anesthetic infusion) does not produce antinociception, and may even lead to hyper-responsiveness (Proudfit, 1980; Young et al., 1984; Lovick, 1985). One possible explanation for the antinociceptive action of improgan could therefore be that this compound has a non-specific neuroexcitant effect in the RVM. The present experiments demonstrate that this is not the case. Rather, improgan administration leads to specific and differential effects on two physiologically defined classes of RVM neurons, activating anti-nociceptive OFF-cells and suppressing the firing of pro-nociceptive ON-cells.
Our data do not by themselves demonstrate that icv-administered improgan targets RVM OFF- or ON-cells directly. The observed changes in RVM neuronal activity could be secondary to an action of the drug at a distant site, and reflect, rather than contribute to, antinociception. However, in extensive mapping studies, the only brain regions found to support improgan antinociception were the periaqueductal gray and RVM (Nalwalk et al., 2004; Hough et al., 2009). Further, pharmacological inactivation of the RVM interferes with the antinociceptive action of improgan given icv (Hough et al., 2001). It is thus reasonable to suggest that the changes in RVM neuronal activity seen here reflect an action of the drug within the RVM itself and/or in the periaqueductal gray.
The methods used here also do not permit any conclusions as to whether the observed effects of improgan on RVM cell activity are mediated by pre-or post-synaptic actions. However, both μ-opioids and cannabinoids are known to have pre-synaptic actions in the RVM, inhibiting GABA transmission (Pan et al., 1990; Vaughan et al., 1999). This disinhibitory mechanism is thought to contribute to μ-opioid activation of OFF-cells, although NMDA-mediated excitation acting through positive feedback also plays a role (Heinricher et al., 1992; Heinricher et al., 1994; Heinricher et al., 1999; Heinricher et al., 2001b). ON-cells, by contrast, are directly inhibited by μ-opioids (Heinricher et al., 1992). Comparison of the present results with those from iontophoretic application of improgan would be helpful in determining direct vs. indirect effects of this compound on identified ON- and OFF-cells. In vitro studies will be needed to dissect membrane mechanisms of improgan action.
Parallels with opioid and cannabinoid actions in the RVM, and a potential circuit for improgan's effects
The RVM is well known to support both μ-opioid and cannabinoid antinociception. The actions of these agents in the RVM have been characterized at multiple levels: cell membrane, functionally identified neurons, circuits, and behavior. For both drug classes, behavioral antinociception is associated with activation of OFF-cells and suppression of ON-cell firing (Heinricher et al., 1994; Meng and Johansen, 2004). We show here that improgan, like μ-opioids and cannabinoids, causes activation of OFF-cells and suppression of ON-cell firing, with block of the reflex-related OFF-cell pause and ON-cell burst. Thus, improgan shares a common neural substrate with these agents to produce behavioral antinociception. The antinociceptive actions of improgan are likely due to the ability of this drug to abolish the OFF-cell pause, as previously shown for μ-opioids (Heinricher et al., 1999; Heinricher et al., 2001a).
Improgan antinociception is reversed by several CB1 antagonists (Hough et al., 2002; Salussolia et al., 2007), although improgan lacks affinity for CB1 or other known cannabinoid receptors (Hough et al., 2002). One possible circuit that would account for these behavioral findings as well as the changes in OFF-cell firing seen here would be that improgan activates OFF-cells directly, which in turn causes release of an endocannabinoid. This would be an example of “depolarization-induced suppression of inhibition” (Freund et al., 2003; Lovinger, 2008). There is evidence for retrograde endocannabinoid signaling in a number of brain regions, including the midbrain periaqueductal gray (Lau and Vaughan, 2008; Drew et al., 2009), which like the RVM is known to support improgan antinociception (Nalwalk et al., 2004). However, a retrograde endocannabinoid-mediated inhibition of GABAergic inhibitory synaptic currents has not to date been demonstrated in the RVM.
Explaining the suppression of ON-cell firing by improgan is less straightforward with this model. One possibility is that the changes in ON-cell discharge following improgan administration are secondary to changes in OFF-cell activity, i.e., that OFF-cells inhibit ON-cells (directly or indirectly) or that OFF-cells depress nociceptive drive to ON-cells, thus producing disfacilitation. An inhibitory connection from OFF-cells to ON-cells is possible and consistent with the anti-phase relationship between spontaneous firing of these two classes and with the fact that the OFF-cell pause precedes the ON-cell burst (Barbaro et al., 1989; Cleary et al., 2008). Such a connection has not, however, been demonstrated either physiologically or anatomically. An endocannabinoid mechanism is also possible, as ON-cell firing is reduced by cannabinoid agonists (Heinricher et al., 1994; Meng and Johansen, 2004).
Studies using in vitro approaches and direct iontophoretic application of improgan to functionally identified RVM neurons will be needed to clarify direct and indirect actions of improgan on OFF- and ON-cells in the RVM.
Conclusion
Previous work from Hough and colleagues has demonstrated an antinociceptive action of improgan in a variety of pain tests in awake behaving animals (Li et al., 1996; Li et al., 1997; Bannoura et al., 1998; Nalwalk et al., 2004; Nalwalk et al., 2005; Gehani et al., 2007; Hough et al., 2009). The present studies extend this earlier work to anesthetized animals. These experiments further demonstrate that the novel non-opioid antinociceptive agent improgan shares a common neural substrate with opioids and cannabinoids in the RVM, activating OFF-cells and suppressing ON-cell firing.
Acknowledgements
Supported by grants from NIDA, DA03816 (LBH) and DA022492 (MMH).
Abbreviations
- icv
intracerebroventricular
- RVM
rostral ventromedial medulla
- TF
tail flick
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Aimone LD, Gebhart GF. Stimulation-produced spinal inhibition from the midbrain in the rat is mediated by an excitatory amino acid neurotransmitter in the medial medulla. J Neurosci. 1986;6:1803–1813. doi: 10.1523/JNEUROSCI.06-06-01803.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannoura MD, Nalwalk JW, Tang Y, Carlile M, Leurs R, Menge WM, Timmerman H, Hough LB. Absence of antinociceptive tolerance to improgan, a cimetidine analog, in rats. Brain Res. 1998;814:218–221. doi: 10.1016/s0006-8993(98)01024-5. [DOI] [PubMed] [Google Scholar]
- Barbaro NM, Heinricher MM, Fields HL. Putative nociceptive modulatory neurons in the rostral ventromedial medulla of the rat display highly correlated firing patterns. Somatosens Mot Res. 1989;6:413–425. doi: 10.3109/08990228909144684. [DOI] [PubMed] [Google Scholar]
- Cleary DR, Neubert MJ, Heinricher MM. Are opioid-sensitive neurons in the rostral ventromedial medulla inhibitory interneurons? Neuroscience. 2008;151:564–571. doi: 10.1016/j.neuroscience.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew GM, Lau BK, Vaughan CW. Substance P drives endocannabinoid-mediated disinhibition in a midbrain descending analgesic pathway. J Neurosci. 2009;29:7220–7229. doi: 10.1523/JNEUROSCI.4362-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL, Bry J, Hentall I, Zorman G. The activity of neurons in the rostral medulla of the rat during withdrawal from noxious heat. J Neurosci. 1983;3:2545–2552. doi: 10.1523/JNEUROSCI.03-12-02545.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Gehani NC, Nalwalk JW, Razdan RK, Martin BR, Sun X, Wentland M, Abood ME, Hough LB. Significance of cannabinoid CB1 receptors in improgan antinociception. J Pain. 2007;8:850–860. doi: 10.1016/j.jpain.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinricher MM, Barbaro NM, Fields HL. Putative nociceptive modulating neurons in the rostral ventromedial medulla of the rat: firing of on- and off-cells is related to nociceptive responsiveness. Somatosens Mot Res. 1989;6:427–439. doi: 10.3109/08990228909144685. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Ingram SL. The brainstem and nociceptive modulation. In: Bushnell MC, Basbaum AI, editors. The Senses, A Comprehensive Reference, Vol 5, Pain. Academic Press; San Diego: 2008. pp. 593–626. [Google Scholar]
- Heinricher MM, McGaraughty S, Farr DA. The role of excitatory amino acid transmission within the rostral ventromedial medulla in the antinociceptive actions of systemically administered morphine. Pain. 1999;81:57–65. doi: 10.1016/s0304-3959(98)00271-1. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, McGaraughty S, Tortorici V. Circuitry underlying antiopioid actions of cholecystokinin within the rostral ventromedial medulla. J Neurophysiol. 2001a;85:280–286. doi: 10.1152/jn.2001.85.1.280. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Morgan MM, Fields HL. Direct and indirect actions of morphine on medullary neurons that modulate nociception. Neuroscience. 1992;48:533–543. doi: 10.1016/0306-4522(92)90400-v. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Morgan MM, Tortorici V, Fields HL. Disinhibition of off-cells and antinociception produced by an opioid action within the rostral ventromedial medulla. Neuroscience. 1994;63:279–288. doi: 10.1016/0306-4522(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Roychowdhury S. Reflex-related activation of putative pain facilitating neurons in rostral ventromedial medulla (RVM) depends upon excitatory amino acid transmission. Neuroscience. 1997;78:1159–1165. doi: 10.1016/s0306-4522(96)00683-5. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Schouten JC, Jobst EE. Activation of brainstem N-methyl-7D-aspartate receptors is required for the analgesic actions of morphine given systemically. Pain. 2001b;92:129–138. doi: 10.1016/s0304-3959(00)00480-2. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: specificity, recruitment and plasticity. Brain Res Rev. 2009;60:214–225. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinricher MM, Tortorici V. Interference with GABA transmission in the rostral ventromedial medulla: disinhibition of off-cells as a central mechanism in nociceptive modulation. Neuroscience. 1994;63:533–546. doi: 10.1016/0306-4522(94)90548-7. [DOI] [PubMed] [Google Scholar]
- Hough LB. Improgan-like analgesics: A family of compounds derived from histamine antagonists. Med Chem Res. 2004;13:78–87. [Google Scholar]
- Hough LB, de Esch IJ, Janssen E, Phillips J, Svokos K, Kern B, Trachler J, Abood ME, Leurs R, Nalwalk JW. Antinociceptive activity of chemical congeners of improgan: optimization of side chain length leads to the discovery of a new, potent, non-opioid analgesic. Neuropharmacology. 2006;51:447–456. doi: 10.1016/j.neuropharm.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Hough LB, Menge WM, van de Stolpe AC, Nalwalk JW, Leurs R, De Esch IJ. Antinociceptive activity of furan-containing congeners of improgan and ranitidine. Bioorg Med Chem Lett. 2007;17:5715–5719. doi: 10.1016/j.bmcl.2007.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough LB, Nalwalk JW, Chen Y, Schuller A, Zhu Y, Zhang J, Menge WM, Leurs R, Timmerman H, Pintar JE. Improgan, a cimetidine analog, induces morphine-like antinociception in opioid receptor-knockout mice. Brain Res. 2000a;880:102–108. doi: 10.1016/s0006-8993(00)02776-1. [DOI] [PubMed] [Google Scholar]
- Hough LB, Nalwalk JW, Leurs R, Menge WM, Timmerman H. Antinociceptive activity of derivatives of improgran and burimamide. Pharmacol Biochem Behav. 2000b;65:61–66. doi: 10.1016/s0091-3057(99)00187-2. [DOI] [PubMed] [Google Scholar]
- Hough LB, Nalwalk JW, Leurs R, Menge WM, Timmerman H. Significance of GABAergic systems in the action of improgan, a non-opioid analgesic. Life Sci. 2001;68:2751–2757. doi: 10.1016/s0024-3205(01)01080-3. [DOI] [PubMed] [Google Scholar]
- Hough LB, Nalwalk JW, Lu Q, Shan Z, Svokos K, Wentland MP, Montero MJ. Antinociceptive, brain-penetrating derivatives related to improgan, a non-opioid analgesic. Eur J Pharmacol. 2005;522:38–46. doi: 10.1016/j.ejphar.2005.08.040. [DOI] [PubMed] [Google Scholar]
- Hough LB, Nalwalk JW, Stadel R, Timmerman H, Leurs R, Paria BC, Wang X, Dey SK. Inhibition of improgan antinociception by the cannabinoid (CB)1 antagonist N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (SR141716A): lack of obligatory role for endocannabinoids acting at CB1 receptors. J Pharmacol Exp Ther. 2002;303:314–322. doi: 10.1124/jpet.102.036251. [DOI] [PubMed] [Google Scholar]
- Hough LB, Svokos K, Nalwalk JW. Non-opioid antinociception produced by brain stem injections of improgan: Significance of local, but not cross-regional, cannabinoid mechanisms. Brain Res. 2009;1247:62–70. doi: 10.1016/j.brainres.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TS, Yaksh TL. Comparison of the antinociceptive effect of morphine and glutamate at coincidental sites in the periaqueductal gray and medial medulla in rats. Brain Res. 1989;476:1–9. doi: 10.1016/0006-8993(89)91529-1. [DOI] [PubMed] [Google Scholar]
- Lau BK, Vaughan CW. Muscarinic modulation of synaptic transmission via endocannabinoid signalling in the rat midbrain periaqueductal gray. Mol Pharmacol. 2008;74:1392–1398. doi: 10.1124/mol.108.045872. [DOI] [PubMed] [Google Scholar]
- Li BY, Nalwalk JW, Barker LA, Cumming P, Parsons ME, Hough LB. Characterization of the antinociceptive properties of cimetidine and a structural analog. J Pharmacol Exp Ther. 1996;276:500–508. [PubMed] [Google Scholar]
- Li BY, Nalwalk JW, Finkel JM, Glick SD, Hough LB. SKF92374, a cimetidine analog, produces mechanical and thermal antinociception in the absence of motor impairment. Analgesia. 1997;3:15–20. [Google Scholar]
- Lovick TA. Ventrolateral medullary lesions block the antinociceptive and cardiovascular responses elicited by stimulating the dorsal periaqueductal grey matter in rats. Pain. 1985;21:241–252. doi: 10.1016/0304-3959(85)90088-0. [DOI] [PubMed] [Google Scholar]
- Lovick TA. Analgesia and the cardiovascular changes evoked by stimulating neurones in the ventrolateral medulla in rats. Pain. 1986;25:259–268. doi: 10.1016/0304-3959(86)90101-6. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Presynaptic modulation by endocannabinoids. Handb Exp Pharmacol. 2008;184:435–477. doi: 10.1007/978-3-540-74805-2_14. [DOI] [PubMed] [Google Scholar]
- Meng ID, Johansen JP. Antinociception and modulation of rostral ventromedial medulla neuronal activity by local microinfusion of a cannabinoid receptor agonist. Neuroscience. 2004;124:685–693. doi: 10.1016/j.neuroscience.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Meng ID, Manning BH, Martin WJ, Fields HL. An analgesia circuit activated by cannabinoids. Nature. 1998;395:381–383. doi: 10.1038/26481. [DOI] [PubMed] [Google Scholar]
- Mobarakeh JI, Nalwalk JW, Watanabe T, Sakurada S, Hoffman M, Leurs R, Timmerman H, Silos-Santiago I, Yanai K, Hough LB. Improgan antinociception does not require neuronal histamine or histamine receptors. Brain Res. 2003;974:146–152. doi: 10.1016/s0006-8993(03)02572-1. [DOI] [PubMed] [Google Scholar]
- Nalwalk JW, Svokos K, Hough LB. Cannabinoid-improgan cross-tolerance: Improgan is a cannabinomimetic analgesic lacking affinity at the cannabinoid CB1 receptor. Eur J Pharmacol. 2006;549:79–83. doi: 10.1016/j.ejphar.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Nalwalk JW, Svokos K, Leurs R, Hough LB. Absence of 5-HT3 and cholinergic mechanisms in improgan antinociception. Pharmacol Biochem Behav. 2005;80:505–510. doi: 10.1016/j.pbb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Nalwalk JW, Svokos K, Taraschenko O, Leurs R, Timmerman H, Hough LB. Activation of brain stem nuclei by improgan, a non-opioid analgesic. Brain Res. 2004;1021:248–255. doi: 10.1016/j.brainres.2004.06.066. [DOI] [PubMed] [Google Scholar]
- Neubert MJ, Kincaid W, Heinricher MM. Nociceptive facilitating neurons in the rostral ventromedial medulla. Pain. 2004;110:158–165. doi: 10.1016/j.pain.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Pan ZZ, Williams JT, Osborne PB. Opioid actions on single nucleus raphe magnus neurons from rat and guinea-pig in vitro. J Physiol. 1990;427:519–532. doi: 10.1113/jphysiol.1990.sp018185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfit HK. Reversible inactivation of raphe magnus neurons: effects on nociceptive threshold and morphine-induced analgesia. Brain Res. 1980;201:459–464. doi: 10.1016/0006-8993(80)91053-7. [DOI] [PubMed] [Google Scholar]
- Salussolia CL, Nalwalk JW, Hough LB. Improgan-induced hypothermia: a role for cannabinoid receptors in improgan-induced changes in nociceptive threshold and body temperature. Brain Res. 2007;1152:42–48. doi: 10.1016/j.brainres.2007.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandkühler J, Gebhart GF. Relative contributions of the nucleus raphe magnus and adjacent medullary reticular formation to the inhibition by stimulation in the periaqueductal gray of a spinal nociceptive reflex in the pentobarbital-anesthetized rat. Brain Res. 1984;305:77–87. doi: 10.1016/0006-8993(84)91121-1. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, McGregor IS, Christie MJ. Cannabinoid receptor activation inhibits GABAergic neurotransmission in rostral ventromedial medulla neurons in vitro. Br J Pharmacol. 1999;127:935–940. doi: 10.1038/sj.bjp.0702636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EG, Watkins LR, Mayer DJ. Comparison of the effects of ventral medullary lesions on systemic and microinjection morphine analgesia. Brain Res. 1984;290:119–129. doi: 10.1016/0006-8993(84)90741-8. [DOI] [PubMed] [Google Scholar]
- Zorman G, Hentall ID, Adams JE, Fields HL. Naloxone-reversible analgesia produced by microstimulation in the rat medulla. Brain Res. 1981;219:137–148. doi: 10.1016/0006-8993(81)90273-0. [DOI] [PubMed] [Google Scholar]