Abstract
Poor control of postural muscles is a primary impairment in cerebral palsy (CP), yet core trunk and hip muscle activity has not been thoroughly investigated. Frequency analysis of electromyographic (EMG) signals provides insight about the intensity and pattern of muscle activation, correlates with functional measures in CP, and is sensitive to change after intervention. The objective of this study was to investigate differences in trunk and hip muscle activation frequency in children with CP compared to children with similar amounts of walking experience and typical development (TD). EMG data from 31 children (15 with CP, 16 with TD) were recorded from 16 trunk and hip muscles bilaterally. A time-frequency pattern was generated using the continuous wavelet transform and instantaneous mean frequency (IMNF) was calculated at each interval of the gait cycle. Functional principal component analysis (PCA) revealed that IMNF was significantly higher in the CP group throughout the gait cycle for all muscles. Additionally, stride-to-stride variability was higher in the CP group. This evidence demonstrated altered patterns of trunk and hip muscle activation in CP, including increased rates of motor unit firing, increased number of recruited motor units, and/or decreased synchrony of motor units. These altered muscle activation patterns likely contribute to muscle fatigue and decreased biomechanical efficiency in children with CP.
Keywords: cerebral palsy, muscle, trunk, frequency, gait
Cerebral palsy (CP) is the most common neuromuscular disorder in children with an increasing prevalence [Blair and Stanley, 1997; Odding et al., 2006; Yeargin-Allsopp, 2007], high economic cost [Honeycutt et al., 2004] and negative impact on quality of life [Livingston et al., 2007; Majnemer et al., 2007; Minter et al., 2007]. CP is characterized by impairment in the development of movement and posture attributed to disturbances that occurred in the developing fetal or infant brain [Rosenbaum et al., 2007].
Poor control of trunk postural muscles is a primary impairment in CP [Davis et al., 2007; Rosenbaum et al., 2007] which causes compensation by other muscles to assist in maintaining upright posture. Compensation by accessory muscles to aid in posture reduces their effectiveness in functioning as primary movers of the extremities [Nicholson et al., 2001]. Evidence to support this notion includes observations that children with CP have greater ambulatory ability when the distal limb musculature is primarily affected, and proximal limb musculature is less affected [Lauer et al., 2007b; Policy et al., 2001]. Additionally, proximal limb muscles, such as those of the hip, are critical for maintaining upright mobility. Compared to knee and ankle muscles, the strength of the hip abductors explained the largest variance in gait and motor function in CP [Ross and Engsberg, 2007]. Given their importance for ambulatory ability, it is surprising that activation patterns of trunk and hip muscles during walking have not been previously investigated in individuals with CP. We have reported muscle activation timing information from this study (manuscript in review) and report the frequency analysis here.
Electromyographic (EMG) analysis is a critical component in the examination of gait in individuals with CP. Several methods are used to determine relative levels of muscle activation. The analysis of EMG signal amplitude is a common method, but is influenced by anthropometrics, precise electrode placement, and body tissue impedance. Additionally, normalization techniques typically require individuals to perform a maximal volitional contraction, which is not reliable in young children because of limited cognitive capabilities, and may not truly represent maximal contraction in individuals with neurologic impairment [Gueth et al., 1984]. Frequency analysis of EMG signals using the continuous wavelet transform allows the dynamic EMG signal to be decomposed into its individual frequency components as a function of time. The frequency of an EMG signal contains information about the pattern of muscle fiber activation and is influenced by the shapes and conduction velocities of the motor unit action potentials within the recording area, with minimal effect from anthropometrics and body tissue impedance [Hermens et al., 1992]. Using wavelet transformation can also effectively overcome the potential problem of non-stationarity of the EMG signals that may affect analysis in the frequency domain [Frigo and Crenna, 2009].
These time-frequency characteristics of the EMG signal have been shown to be elevated in the leg muscles and to correlate with functional measures in CP [Lauer et al., 2007b; Wakeling et al., 2007]. Additionally, EMG frequency characteristics were sensitive to muscle function changes after surgical intervention in CP when EMG timing information did not change [Lauer et al., 2007a]. Lastly, an attractive feature of EMG frequency analysis is that it does not require the participant to generate a maximal force contraction.
Individuals with CP use compensatory movements as a result of decreased postural control, poor coordination, muscle weakness and spasticity. These abnormal motor patterns are reinforced with repetition [Hlustik et al., 2004; Nudo, 2003; Winchester et al., 2005], over time and throughout development. Therefore, it is critical to examine muscle behavior in individuals with CP during the development of these compensatory postural and movement patterns.
Early walkers are rarely included as research participants, in part because of limited cognition and tolerance to the instrumentation involved in gait analysis. The immaturity of movement patterns in early walkers also complicates data interpretation. One characteristic of immature gait is greater stride-to-stride variability than mature adult gait [Stolze et al., 1998]. Stride-to-stride variability should be reported in addition to mean values in order to better characterize immature walking patterns prior to the development of fixed maladaptive patterns. The variability of muscle activity has not been investigated during the early years of walking in individuals with CP, and may provide insight on the extent of disordered muscle activation. The objective of this study was to investigate differences in the time-frequency characteristics of the trunk and hip muscles during the early stages of walking in children with CP compared to children with similar amounts of walking experience and typical development (TD). It was hypothesized that children with CP would demonstrate elevated mean frequency of trunk muscle activation, and increased stride-to-stride variability in mean frequency.
METHODS
Participants
Thirty-four children were enrolled in this study, 18 with CP and 16 with TD. Participants with CP were recruited through the CP clinic at Shriners Hospital for Children in Philadelphia, PA and through other local rehabilitation facilities. Participants with TD were recruited from siblings of the participants with CP, children of people known to the investigators, and from a local day care center. All procedures were approved by the IRB of Temple University Hospital (for Shriners Hospital) and also the IRBs of additional data collection sites as needed. Parental consent was obtained prior to participation. Assent of a minor was obtained from any participant 7 years of age or older.
Inclusion and exclusion criteria are listed in Table 1. Children with CP were able to walk with a hand held assistive device if it did not restrict movement of the trunk or pelvis. The selection of months of walking experience rather than age as a primary inclusion criterion was chosen based on reports that experience is a stronger predictor of walking and balance skill than age in early walkers [Adolph et al., 2003; Sundermier et al., 2001]. The onset of walking was operationally defined as the age in months at which an infant or child was able to take at least 3 continuous independent steps on a consistent basis [Roncesvalles et al., 2001; Wu et al., 2007]. Walking experience, in months, was calculated as the difference in the participant’s current age and the age of onset of walking.
Table 1.
Participant inclusion and exclusion criteria
| Inclusion | Exclusion |
|---|---|
|
|
Procedures
EMG
Surface EMG data from trunk, gluteal, and thigh muscles were acquired using a 16-channel recording system (Myomonitor III, Delsys Inc., Boston, MA) with preamplified silver-silver chloride, single differential, parallel bar surface electrodes that were 1.0 cm in length with a 1.0 cm interelectrode distance. The system had an input impedance of >1015Ω // 0.2pF, a common mode rejection ratio of −92 dB, signal-to-noise ratio of 1.5 μV, and a pre-amplifier gain 1000 V/V ±1%. EMG data were collected at 1200 Hz, preamplified with a gain of 10, and filtered with a high pass filter of 20 Hz and low pass filter of 450 Hz.
EMG data were collected from 8 muscles bilaterally: trapezius (middle), erector spinae (longissimus), rectus abdominis, external oblique, gluteus maximus, gluteus minimus, quadriceps femoris (rectus femoris), and semitendinosus. The rectus femoris and semitendinosus were chosen in addition to the trunk and hip muscles because these muscles anatomically cross the hip joint, and they have been extensively investigated in CP, allowing for comparison of data to existing literature. Sensor placement for the abdominal muscles was determined using the methods described by Ng et al [Ng et al., 1998]. Sensor placement for all other muscles (back, gluteal, and thigh) was determined in accordance with SENIAM recommendations [Hermens et al., 2000].
The skin areas were cleaned with alcohol and the sensors were affixed to the skin with a double-sided adhesive interface (Delsys Inc., Boston, MA). The electrodes were further secured using hypoallergenic tape or a flexible, non-adhesive wrap encircling the waist and thighs (Coflex-NL®, Andover Healthcare, Inc.). Self-adhesive reference electrodes (Axelgaard Manufacturing Co., Ltd., Lystrup, Denmark) were placed on the skin over the patella bilaterally. The children were able to watch a video or interact with a research assistant during sensor placement to increase tolerance and compliance.
A volitional contraction of each muscle was elicited to verify placement. The children were asked to perform specific movements to elicit the corresponding specific muscle contractions, such as leaning backward in sitting to activate the rectus abdominis muscles, and lifting one leg to activate the contralateral gluteus medius muscles. For synchronous data collection during walking trials, the EMG system was triggered by the instrumented walkway.
Walking trials
Children walked barefoot down an instrumented walkway (GAITRite®, CIR Systems, Havertown, PA) at a self-selected pace. Three to 5 trials, each consisting of one walk down the walkway with at least 4 consecutive footfalls, were collected depending on participant tolerance to testing procedures and fatigue. Footfall information was collected at 30 Hz. All walking trials were videotaped.
Start and stop targets were placed on the floor approximately 5 feet beyond either end of the instrumented walkway to minimize acceleration or deceleration in the data capture space. During the walking trials, the EMG preamplification unit that is typically worn on a backpack was carried behind all participants by an assistant so as not to add additional weight, which could affect muscle activity in the smaller children. Children had the opportunity to sit in between walking trials to minimize fatigue.
Data analysis
Video footage and raw EMG signals of each trial were reviewed by the principal investigator to determine the most appropriate gait cycles to select for analysis. Gait cycles from the first walking trial were not included to avoid possible effects of gait alteration due to familiarization with the new activity. The next ten gait cycles (5 left, 5 right) that were observed to represent each individual’s typical walking (e.g child was not distracted by a person or noise, did not stop walking mid-trial, and was not moving arms toward an object), and were free of artifact in the raw EMG signal, were selected for analysis to avoid any possible effects of fatigue from later trials.
The 10 selected gait cycles were extracted from the EMG files using the time-synchronized marker data (initial foot contact) collected from the instrumented walkway. EMG data were processed using custom-written programs in MATLAB software (The Mathworks Inc., Natick, MA). All signals were normalized to 1000 points, representing the gait cycle from 0 to 100% in 0.1% increments.
A time-frequency pattern for each gait cycle was generated using the continuous wavelet transform (CWT) as described by Lauer and colleagues [Lauer et al., 2007b]. The CWT describes a series of mathematical techniques that can be used to analyze a complex time series signal with variable power or magnitude in a wide range of frequencies, while preserving the original timing. The output of the CWT analysis is a scalogram, which is a three-dimensional representation of the analysis where time (% gait cycle) is on the x-axis, frequency (scale) is on the y-axis, and power (magnitude) is on the z-axis. The reduction of the three-dimensional scalogram to a time–frequency curve was performed by calculating the mean frequency for each gait cycle interval using the following equation:
| (1) |
where P (t,f) represents the range of powers at a given frequency at each interval of the gait cycle, and f represents the frequency range of the EMG signal. The calculation of the mean frequency at each time interval, referred to as the instantaneous mean frequency curve (IMNF), was selected as the representative value of the frequency spectra across time because mean frequency has been used in the past to characterize muscle fatigue and activation level [Karlsson et al., 2003]. Thus, by calculating the mean frequency at each time interval, a representative curve of muscle activity over time is generated. For reporting of IMNF in each group, an average IMNF curve was calculated from the individual IMNF curves for each participant.
A functional principal component analysis (fPCA) was completed for each muscle using the IMNF curves from all selected gait cycles in order to identify regions of difference in the gait cycle curves. The PCA is a mathematical least squares maximization procedure that transforms a large number of correlated variables into a smaller number of uncorrelated variables called principal components (PC). The fPCA performs the same operation, converting a large number of correlated variables into a smaller number of principal components. However, the ‘variable’ is a relationship of data points, or mathematical function (in this case, the individual IMNF curves), not a single data point as in a multivariate PCA [Ramsay and Silverman, 1997]. Each individual IMNF curve is then assigned a weight for each PC (in this case, the PCs are regions of the gait cycle where variability in the data set was identified). The value of the weight describes the degree of agreement or disagreement between the individual IMNF curve and the group variance identified by that particular PC for each muscle. This allows for differences across an entire curve to be captured in a small subset of principal components.
To assess if the regions of variability identified by the fPCA were statistically different between the CP and TD groups, the PC weights were averaged for each muscle in each group and tested using a Welch statistic to determine if the means between the groups were equal. Because the variances may be unequal, the Welch statistic is preferred to the F-statistic and is considered a more robust test [Hartung et al., 2002].
To assess consistency from stride to stride, individual stride-to-stride standard deviations (SD) in mean frequency were calculated for the gait cycle as a function of time for each muscle and for each participant based on the 10 analyzed gait cycles (5 left, 5 right). Average stride-to-stride variability was then calculated for each muscle across the gait cycle for each group. Statistical analyses of the variability curves were performed using a functional analysis of variance (fANOVA) model [Ramsay and Silverman, 1997]. A Fourier basis function of 20 expansion terms was fit to each curve, imposing a roughness penalty to ensure smoothness of the function up to the second derivative. A linear model was constructed using the following formula:
| (2) |
where g indicated the group, m is the number of functions representative of that group, μ is the grand mean function across all the functions, α are the specific effects on the function of being within a group g, and ε is the unexplained variation specific to the mth curve. The terms for each of the general linear models was determined using a least squares fit. To be able to define a set of terms unique to each group, an additional constraint was placed that Σ αg(%) = 0 for all gait cycle increments. Overall differences in variability were assessed using the F-ratio function, based on the F-statistic, calculated for each interval of the gait cycle.
RESULTS
Data from 3 children in the CP group were excluded due to 1 having questionable diagnosis of CP, and 2 who were unable to walk without assistance from an investigator during the testing session. Data for the remaining 31 children were used for analysis. Walking experience did not differ between groups (p=0.969). As expected, because of a later onset of walking in the CP group and matching by walking experience, the TD group was older and heavier than the CP group. Of the children with CP, 7 were classified as GMFCS level II and 8 were level III. Three walked without assistive devices, 9 used posterior rolling walkers, 1 used bilateral forearm crutches, and 2 used unilateral forearm crutches. Demographic and anthropometric data are provided in Table 2.
Table 2.
Participant demographic and anthropometric characteristics
| Number (n) |
Onset of walking (months) |
Walking experience (months) |
Gender | Age (months) |
Weight (kg) |
Height (m) |
||
|---|---|---|---|---|---|---|---|---|
| TD | Mean (SD) | 16 | 11.7 (3.1) | 28.6 (19.6) | 9F, 7M | 39.7 (19.5) | 15.1 (3.9) | 0.97 (0.13) |
| Range | 8.0-20.0 | 1.0-58.0 | 13.0-67.5 | 10.0-21.9 | 0.75-1.18 | |||
|
| ||||||||
| CP | Mean (SD) | 15 | 34.8 (10.2)* | 28.4 (17.0) | 5F, 10M | 63.1 (23.2)* | 19.6 (5.9)* | 1.06 (0.14) |
| Range | 18.0-55.0 | 2.0-60.0 | 25.0-108.0 | 10.9-31.2 | 0.83-1.32 | |||
|
| ||||||||
| Total | Mean (SD) | 31 | 22.9 (13.8) | 28.5 (18.1) | 14F, 17M | 51.0 (24.1) | 17.3 (5.4) | 1.10 (0.14) |
| Range | 8.0-55.0 | 1.0-60.0 | 13.0-108.0 | 10.0-31.2 | 0.75-1.32 | |||
TD=typically developing, CP=cerebral palsy, SD=standard deviation, M=male, F=female, kg=kilograms, m=meters
indicates significant difference from TD group (p<0.05)
The principal component output for the gluteus maximus is shown as an example in Figure 1. The positive variance direction is shown for all PCs (which, in this example, represents the CP group for PCs 1, 2, and 4, and the TD group for PC 3). The group not shown for each PC (TD for 1, 2, 4 and CP for 3) demonstrates equal variance in the opposite direction. When combined, the major regions of variance identified (the 4 PCs) encompass the entire gait cycle, with the CP group demonstrating variance in the direction of higher mean frequency for each PC. This pattern of the 4 PCs encompassing the entire gait cycle was consistent for each muscle.
Figure 1.
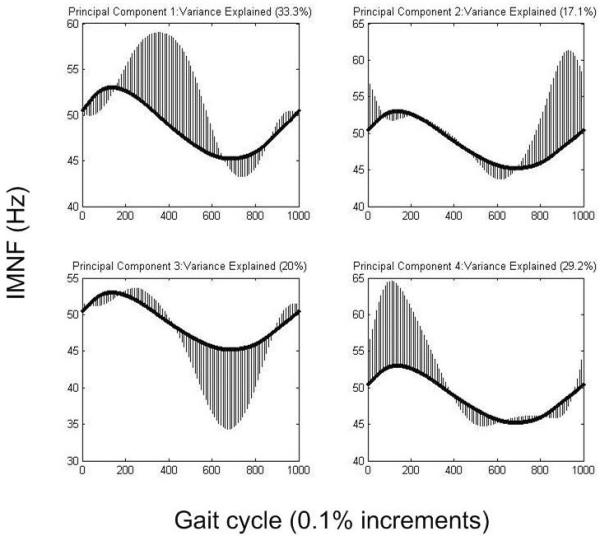
The principal component output for the gluteus maximus, with 99.6% of the variance explained. The dark curve in each graph is the mean instantaneous mean frequency for all participants, and is the same in each graph. The shaded areas indicate the regions of variability in the entire data set identified by each principal component. Principal components 1, 2, and 4 show the direction of variance for the cerebral palsy (CP) group and principal component 3 shows the direction of variance for the typical developing (TD) group. The group not shown for each principal component demonstrates equal variance in the opposite direction.
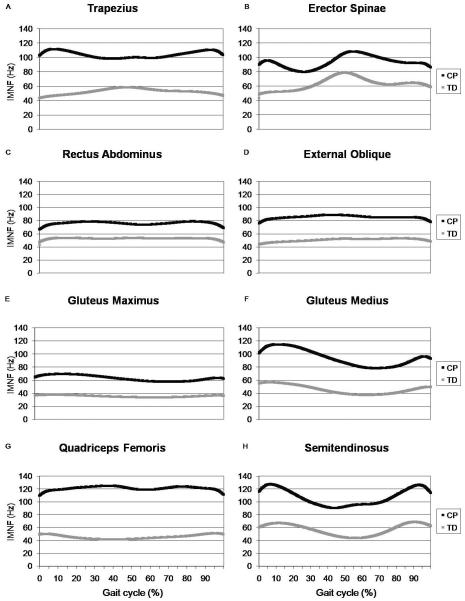
Average IMNF curves for each muscle are shown in Figure 2. The first four PCs accounted for 97.4% of the variability in the erector spinae IMNF curves, 98.4% of the variability for the semitendinosus, 98.6% for the rectus abdominus, 98.8% for the trapezius and external abdominal oblique, 98.9% for the gluteus medius, 99.3% for the quadriceps femoris, and 99.6% for the gluteus maximus. All four PCs were significantly different between the TD and CP groups for each muscle (p<0.001). Examination of the specific regions of variability identified by each PC revealed that the CP group demonstrated higher IMNF than the TD group throughout the gait cycle for all muscles because the PCs encompassed the entire gait cycle for each muscle.
Figure 2.
Instantaneous mean frequency (IMNF) mean curves across the gait cycle for children with typical development (TD) and cerebral palsy (CP) for the trapezius (A), erector spinae (B), rectus abdominus (C), external oblique (D), gluteus maximus (E), gluteus medius (F), quadriceps femoris (G), and semitendinosus (H).
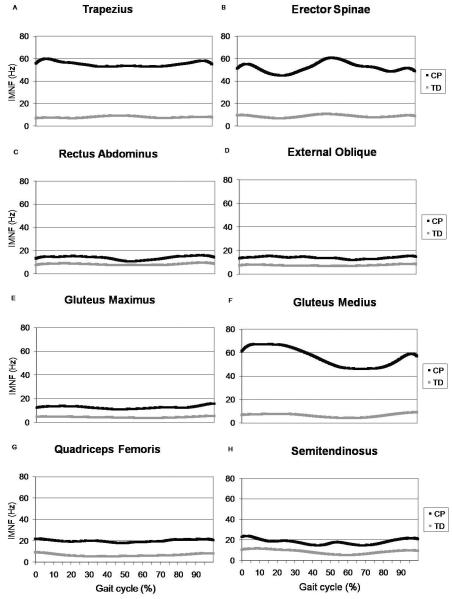
Stride-to-stride variability curves are presented in Figure 3. The F(2,57) critical value was 3.16 for an alpha level of 0.05. Individual variability was statistically higher in the CP group for all muscles across the gait cycle.
Figure 3.
Stride-to-stride variability in instantaneous mean frequency (IMNF) across the gait cycle for children with typical development (TD) and cerebral palsy (CP) for the trapezius (A), erector spinae (B), rectus abdominus (C), external oblique (D), gluteus maximus (E), gluteus medius (F), quadriceps femoris (G), and semitendinosus (H).
DISCUSSION
This study reports trunk and hip muscle activity during the early stages of walking in children with CP compared to children with TD. Mean frequency of muscle activation during walking was higher and more variable from stride-to-stride throughout the gait cycle for the CP group than TD for all 8 muscles investigated. The higher mean frequency in the CP group suggests altered patterns of muscle activation and motor unit recruitment. Higher IMNF can result from increased rates of motor unit firing, increased number of recruited motor units, or decreased synchrony of motor units [Hermens et al., 1992]. This is consistent with literature suggesting excessive and dyscoordinated muscle activity in CP [Unnithan et al., 1996b; van der Heide and Hadders-Algra, 2005]. Lam et al have suggested that higher EMG median frequency contributes to muscle fatigue in children with CP [Lam et al., 2005]. Additionally, excessive muscle activity is related to decreased biomechanical efficiency, with muscle cocontraction explaining a significant amount of variability in the energy cost of walking in children with CP [Unnithan et al., 1996a]. The markedly increased stride-to-stride variability in muscle activation patterns in the CP group may further imply discoordination and immaturity of muscle behavior, because greater variability in other gait measures is characteristic of immature walking patterns [Hausdorff et al., 1999].
Wakeling et al [Wakeling et al., 2007] reported mean frequency of lower extremity muscles for 36 children and adolescents with TD and 17 with spastic diplegic CP. The mean frequency for the rectus femoris in the CP group is consistent with that obtained in this study. The CP mean frequency reported for the semimembranosus is slightly higher than that obtained in the current study for the semitendinosus muscle. The TD data for both of these muscles in the current study is lower than that reported for the control group in Wakeling’s study, which may be a result of the older age of the control group in that study (mean age 10.8 years), but requires further investigation.
Similarly, the IMNF values are consistent with those reported by Lauer and colleagues for the medial hamstrings and quadriceps in the CP group, and at the low end of the range for the TD group [Lauer et al., 2007b]. The control group in Lauer’s study had the same mean age as the control group in Wakeling’s study (10.8 years), which is older than the control group in the current study. Higher mean frequency in children with CP has also been reported during recumbent cycling [Lauer et al., 2008]. To our knowledge, no previous studies have investigated mean frequency characteristics of the trunk or gluteal muscles in children with TD or with CP.
White and colleagues investigated trunk muscle activity during walking in 38 able-bodied adults [White and McNair, 2002]. While they considered the amplitude of the EMG signal, not the frequency, the patterns of increased EMG amplitude in the rectus abdominus, external oblique and erector spinae were similar to patterns of increased frequency in both groups for the current study. This indicates that the timing of muscle activation bursts alone may not be sufficient to examine muscle behavior in individuals with neurological impairments, who may demonstrate increased activation during similar periods of the gait cycle, but have an altered level of activation throughout the walking cycle.
Rose and colleagues have reported a predominance of Type I (slow-twitch) muscle fibers in children with spastic diplegic CP compared to nearly equivalent distribution of Type I and II fibers in the control group [Rose et al., 1994]. They also observed neuromuscular activation deficits that suggest an inability to recruit higher threshold motor units (Type II, fast) due to an inability to produce adequate torque levels [Rose and McGill, 2005]. The results of our study, in consideration of these previous findings, suggest that individuals with CP over-use the Type I muscle fibers and under-use the Type II fibers, with asynchronous activation, in the trunk muscles. However, considerable caution must be taken in the interpretation of surface EMG signals to infer specific neural control strategies, as articulated by Farina and colleagues [2004].
The results of this study may not be generalizable to children with greater or lesser severity of CP or to other children with neurological disorders. Additionally, the data from the trapezuis muscle should be interpreted with caution due to the use of an assistive device in the majority (12/15) of the children with CP. Use of an assistive device alone may have contributed to greater activation of the TZ in the CP group, because the shoulders were engaged during forward movement and bearing weight through the assistive device. This issue is difficult to avoid when studying early walkers with CP. According to the GMFCS classification,[Palisano et al., 1997] only children classified as level I (the least impaired) begin to walk without the use of any assistive device. Therefore, to study any children with greater severity of CP during the early years of walking, the use of walking aids must be allowed.
Postural muscle training during the development of upright postural control may be an effective time to intervene and change these compensatory movements, prior to years of reinforcing poor trunk movement patterns. Core muscle control training has been effectively applied to several other clinical populations [Britnell et al., 2005; Norris and Matthews, 2008; Willson et al., 2005], but has not been systematically investigated in children with neurological disorders. If Type II fiber under-activation is in fact a primary factor in altered EMG patterns in CP, interventions to increase trunk muscle force production, such as core muscle strengthening, may assist in the activation of Type II fibers. Similarly, electrical stimulation may be a possible intervention because it can generate higher force contractions than volitional contractions, and stimulates Type II fibers first or in conjunction with Type I fibers, in contrast to the orderly recruitment of Type I fibers first during volitional contractions [Chou et al., 2008]. Additionally, efforts to investigate muscle activation during other functional movements should continue in order to fully understand the development of abnormal movement patterns in young children with CP.
This study demonstrates increased mean frequency in the trunk and hip muscles in early walkers with CP. Stride-to-stride variability in mean frequency was also increased in CP. The increased muscle activation and decreased synchrony in the trunk muscles are consistent with muscle behavior of the lower extremities in CP. The clinical implications of this work are that postural muscle training interventions should be investigated in CP to encourage the development of more effective and stable trunk muscle activation patterns in these children.
ACKNOWLEDGEMENTS
The authors thank Steve Capella and Jenny Lee for assistance with data collection, and Diana Deshefy, DPT, Samuel Pierce, PT, PhD and Erin Sheeder, DPT for assistance with participant recruitment. This work was funded by a Clinical Research Grant to Dr. Prosser from the Section on Pediatrics, American Physical Therapy Association, NINDS R03NS048875 to Dr. Lauer, and NICHD R01HD043859 to Dr. Lee. This research was also supported in part by the Intramural Research Program of the NIH Clinical Center. Portions of this work were presented at the 2009 Annual Meeting of the American Society of Biomechanics.
Biographies

Laura A. Prosser, PT, PhD
Dr. Prosser is a post-doctoral fellow in the Functional & Applied Biomechanics section in the Rehabilitation Medicine Department at the National Institutes of Health Clinical Center. She completed her B.S. in health science and Master’s in physical therapy at the University of Scranton, and her Ph.D. at Temple University. Her clinical experience is in pediatric neurorehabilitation. Her research interests include the investigation of movement patterns and the effects of therapeutic intervention on movement patterns and functional ability in children with neurological conditions. Dr. Prosser’s thesis work was funded by the American Physical Therapy Association Section on Pediatrics and was awarded the Temple University College of Health Professions Dean’s Award for Excellence.

Samuel C.K. Lee, PhD, PT
Dr. Lee is an Assistant Professor in the Department of Physical Therapy at the University of Delaware and holds a joint appointment in Delaware’s Biomechanics and Movement Science Program. Additionally, Dr. Lee is a Research Associate and Scientific Staff at Shriners Hospitals for Children in Philadelphia, PA. Sam received his BS in biomedical engineering from Boston University; and both his Masters in physical therapy and Ph.D. in applied physiology at the University of Delaware. Dr. Lee’s major research interests involve using neuromuscular electrical stimulation both for rehabilitation and for functional electrical stimulation applications. His current work is focused on enhancing muscle strength, fitness and function in children with cerebral palsy and spinal cord injuries. Dr. Lee’s work has been funded by the National Institutes of Health, Shriners Hospitals for Children, American Physical Therapy Association and Foundation for Physical Therapy. His work was nominated for the 2005 Gayle G. Arnold Award for Excellence in the Care of Children with CP from the American Academy of Cerebral Palsy and Developmental Medicine. In 2002, Dr. Lee and his colleagues were awarded the Chattanooga Research Award by the APTA for the best research article published in the Physical Therapy Journal. Dr. Lee has also served on the APTA Section on Pediatrics Research Summit and on the Program Committee for the APTA Section on Research.

Mary F. Barbe, PhD
Dr. Barbe is a Professor at Temple University with joint appointments in the Departments of Physical Therapy and Anatomy and Cell Biology. She received her B.A. in Biology from the University of North Carolina at Greensboro and a PhD in Anatomy from the Bowman Gray School of Medicine at Wake Forest University. She completed an NIH post-doctoral fellowship in developmental neuroscience research at Medical College of Pennsylvania. Dr. Barbe uses an animal model of cumulative trauma disorder to examine motor behavioral and pathophysiological changes associated with repetitive, forceful upper limb motor tasks. Her grant funding sources include the Foundation for Physical Therapy, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Institute of Occupational Safety and Health, Temple University, and Shriners Hospitals. Dr. Barbe is a member of Temple University’s One Million Dollar Research Award Club and has received the Excellence in Teaching Award from the Temple University College of Health Professions in 1994 and 2007.

Ann F. Van Sant, PT, PhD, FAPTA
Dr. Van Sant is a Professor in the Department of Physical Therapy at Temple University and a Catherine Worthingham Fellow of the American Physical Therapy Association. Dr. Van Sant received her BS in physical therapy from Russell Sage College, her MS in physical therapy from Medical College of Virginia of Virginia Commonwealth University and PhD in motor development from the University of Wisconsin-Madison. Dr. Van Sant’s major research interests are in describing the movement patterns used to perform fundamental skills such as rising from the floor and rising from bed in individuals who are developing typically and those with movement disorders. Dr. Van Sant’s work has been funded by the Foundation for Physical Therapy and the National Institute on Disability & Rehabilitation Research. She is chair of the Research Committee of International Organization of Physical Therapists in Pediatrics, a subsection of the World Confederation of Physical Therapy, and Editor-in-Chief of Pediatric Physical Therapy.

Richard T. Lauer, PhD
Dr. Lauer is an Assistant Professor in the Departments of Physical Therapy and Computer and Electrical Engineering at Temple University, and co-director of the Motor Performance Assessment Laboratory (MPAL). Dr. Lauer received his BBE from the Catholic University of America, and his MS and PhD in biomedical engineering from Case Western Reserve University. His research interests are in understanding muscle function in children and adults with cerebral palsy, and the use of functional electrical stimulation for movement and exercise. He is a member of the American Society of Mechanical Engineers, the American Society of Biomechanics, and a Senior Member of the Institute of Electrical and Electronics Engineers. His work has been funded by the National Institute of Neurological Disorders and Stroke, and the Cerebral Palsy Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Blair E, Stanley FJ. Issues in the classification and epidemiology of cerebral palsy. MMDD Research Reviews. 1997:3184–93. [Google Scholar]
- Chou LW, Kesar TM, Binder-Macleod SA. Using customized rate-coding and recruitment strategies to maintain forces during repetitive activation of human muscles. Physical Therapy. 2008;88(3):363–75. doi: 10.2522/ptj.20070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MF, Worden K, Clawson D, Meaney J, Duncan B. Confirmatory Factor Analysis in Osteopathic Medicine: Fascial and Spinal Motion Restrictions as Correlates of Muscle Spasticity in Children With Cerebral Palsy. Journal of the American Osteopathic Association. 2007:107226–32. [PubMed] [Google Scholar]
- Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG. J Appl Physiol. 2004;96(4):1486–95. doi: 10.1152/japplphysiol.01070.2003. [DOI] [PubMed] [Google Scholar]
- Frigo C, Crenna P. Multichannel SEMG in clinical gait analysis: a review and state-of-the-art. Clin Biomech (Bristol, Avon) 2009;24(3):236–45. doi: 10.1016/j.clinbiomech.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Gueth V, Steinhausen D, Abbink F. The investigation of walking of patients with cerebral palsy by the electromyogram (EMG) using surface electrodes. Electromyography and Clinical Neurophysiology. 1984;24(3):225–40. [PubMed] [Google Scholar]
- Hartung J, Argac D, Makambi K. Small sample properties of tests on homogeneity in one-way ANOVA and meta-analysis. Stat Pap. 2002:43197–235. [Google Scholar]
- Hausdorff JM, Zemany L, Peng C, Goldberger AL. Maturation of gait dynamics: stride-to-stride variability and its temporal organization in children. Journal of Applied Physiology. 1999;86(3):1040–7. doi: 10.1152/jappl.1999.86.3.1040. [DOI] [PubMed] [Google Scholar]
- Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. Journal of Electromyography and Kinesiology. 2000;10(5):361–74. doi: 10.1016/s1050-6411(00)00027-4. [DOI] [PubMed] [Google Scholar]
- Hermens HJ, v.Bruggen TAM, Batten CTM, Rutten WIC, Boom HBK. The Median Frequency of the Surface EMG Power Spectrum in Relation to Motor Unit Firing and Action Potential Properties. Journal of Electromyography and Kinesiology. 1992;2(1):15–25. doi: 10.1016/1050-6411(92)90004-3. [DOI] [PubMed] [Google Scholar]
- Honeycutt A, Dunlap L, Chen H, Al Homsi G. Economic costs associated with mental retardation, cerebral palsy, hearing loss, and vision impairment --- United States, 2003. MMWR Weekly. 2004;53(03):57–9. [PubMed] [Google Scholar]
- Karlsson JS, Ostlund N, Larsson B, Gerdle B. An estimation of the influence of force decrease on the mean power spectral frequency shift of the EMG during repetitive maximum dynamic knee extensions. Journal of Electromyography and Kinesiology. 2003;13(5):461–8. doi: 10.1016/s1050-6411(03)00063-4. [DOI] [PubMed] [Google Scholar]
- Lam WK, Leong JC, Li YH, Hu Y, Lu WW. Biomechanical and electromyographic evaluation of ankle foot orthosis and dynamic ankle foot orthosis in spastic cerebral palsy. Gait & Posture. 2005;22(3):189–97. doi: 10.1016/j.gaitpost.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Lauer RT, Johnston TE, Smith BT, Lee SC. Lower extremity muscle activity during cycling in adolescents with and without cerebral palsy. Clinical Biomechanics (Bristol, Avon) 2008;23(4):442–9. doi: 10.1016/j.clinbiomech.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer RT, Smith BT, Shewokis PA, McCarthy JJ, Tucker CA. Time-frequency changes in electromyographic signals after hamstring lengthening surgery in children with cerebral palsy. Journal of Biomechanics. 2007a;40(12):2738–43. doi: 10.1016/j.jbiomech.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Lauer RT, Stackhouse CA, Shewokis PA, Smith BT, Tucker CA, McCarthy J. A time-frequency based electromyographic analysis technique for use in cerebral palsy. Gait & Posture. 2007b;26(3):420–7. doi: 10.1016/j.gaitpost.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Ng JK, Kippers V, Richardson CA. Muscle fibre orientation of abdominal muscles and suggested surface EMG electrode positions. Electromyography and Clinical Neurophysiology. 1998;38(1):51–8. [PubMed] [Google Scholar]
- Odding E, Roebroeck ME, Stam HJ. The epidemiology of cerebral palsy: incidence, impairments and risk factors. Disability and Rehabilitation. 2006;28(4):183–91. doi: 10.1080/09638280500158422. [DOI] [PubMed] [Google Scholar]
- Policy JF, Torburn L, Rinsky LA, Rose J. Electromyographic test to differentiate mild diplegic cerebral palsy and idiopathic toe-walking. Journal of Pediatric Orthopaedics. 2001;21(6):784–9. [PubMed] [Google Scholar]
- Ramsay JO, Silverman BW. Applied Functional Data Analysis. Springer; New York, NY: 1997. [Google Scholar]
- Rose J, Haskell WL, Gamble JG, Hamilton RL, Brown DA, Rinsky L. Muscle pathology and clinical measures of disability in children with cerebral palsy. J Orthop Res. 1994;12(6):758–68. doi: 10.1002/jor.1100120603. [DOI] [PubMed] [Google Scholar]
- Rose J, McGill KC. Neuromuscular activation and motor-unit firing characteristics in cerebral palsy. Dev Med Child Neurol. 2005;47(5):329–36. doi: 10.1017/s0012162205000629. [DOI] [PubMed] [Google Scholar]
- Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, Dan B, Jacobsson B. A report: the definition and classification of cerebral palsy April 2006. Developmental Medicine and Child Neurology Supplement. 2007:1098–14. [PubMed] [Google Scholar]
- Ross SA, Engsberg JR. Relationships between spasticity, strength, gait, and the GMFM-66 in persons with spastic diplegia cerebral palsy. Archives of Physical Medicine and Rehabilitation. 2007;88(9):1114–20. doi: 10.1016/j.apmr.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Stolze H, Kuhtz-Buschbeck JP, Mondwurf C, Johnk K, Friege L. Retest reliability of spatiotemporal gait parameters in children and adults. Gait & Posture. 1998;7(2):125–30. doi: 10.1016/s0966-6362(97)00043-x. [DOI] [PubMed] [Google Scholar]
- Unnithan VB, Dowling JJ, Frost G, Bar-Or O. Role of cocontraction in the O2 cost of walking in children with cerebral palsy. Medicine and Science in Sports and Exercise. 1996a;28(12):1498–504. doi: 10.1097/00005768-199612000-00009. [DOI] [PubMed] [Google Scholar]
- Unnithan VB, Dowling JJ, Frost G, Volpe AB, Bar-Or O. Cocontraction and phasic activity during GAIT in children with cerebral palsy. Electromyography and Clinical Neurophysiology. 1996b;36(8):487–94. [PubMed] [Google Scholar]
- van der Heide JC, Hadders-Algra M. Postural muscle dyscoordination in children with cerebral palsy. Neural Plasticity. 2005;12(2-3):197–203. doi: 10.1155/NP.2005.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeling J, Delaney R, Dudkiewicz I. A method for quantifying dynamic muscle dysfunction in children and young adults with cerebral palsy. Gait & Posture. 2007;25(4):580–9. doi: 10.1016/j.gaitpost.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Yeargin-Allsopp M. Prevalence of cerebral palsy in three areas of the USA: a multisite collaboration. Developmental Medicine and Child Neurology Supplement. 2007;49(111):23. [Google Scholar]