Abstract
Patients with basal ganglia (BG) pathology are consistently found to be impaired on rule-based category learning tasks in which learning is thought to depend upon the use of an explicit, hypothesis-guided strategy. The factors that influence this impairment remain unclear. Moreover, it remains unknown if the impairments observed in patients with degenerative disorders such as Parkinson's disease (PD) are also observed in those with focal BG lesions. In the present study, we tested patients with either focal BG lesions or PD on two categorization tasks that varied in terms of their demands on selective attention and working memory. Individuals with focal BG lesions were impaired on the task in which working-memory demand was high and performed similarly to healthy controls on the task in which selective-attention demand was high. In contrast, individuals with PD were impaired on both tasks, and accuracy rates did not differ between on- and off-medication states for a subset of patients who were also tested after abstaining from dopaminergic medication. Quantitative, model-based analyses attributed the performance deficit for both groups in the task with high working-memory demand to the utilization of suboptimal strategies, whereas the PD-specific impairment on the task with high selective-attention demand was driven by the inconsistent use of an optimal strategy. These data suggest that the demands on selective attention and working memory affect the presence of impairment in patients with focal BG lesions and the nature of the impairment in patients with PD.
Keywords: decision-making, putamen, neostriatum, strategy, explicit, classification
The role of the basal ganglia (BG) in category learning has been the subject of considerable study. Patients with BG pathology such as Parkinson's disease have been found to be impaired on category learning tasks, but the underlying nature of the deficit has not been well-characterized. Two consistent findings stand out in this literature. First, BG dysfunction impairs learning on rule-based, category-learning tasks – i.e., categorization tasks where learning entails the use of an explicit, hypothesis-guided strategy (see Ashby & Maddox, 2005; Price et al., 2009; C.A. Seger, 2008 for reviews). Second, the magnitude of this impairment is related to the demands on selective attention (Filoteo et al., 2007; Filoteo, Maddox, Ing et al., 2005).
The results of these neuropsychological studies fit well with a number of neurocomputational models that emphasize the role of the BG in category learning (e.g., Ashby et al., 1998; M. J. Frank, 2005; Moustafa & Gluck, in press). For instance, the COVIS model of Ashby and colleagues posits that a hypothesis-testing system that involves working memory and cognitive control processes is specialized to mediate learning in rule-based tasks. In the current instantiation of the model, the caudate nucleus plays a critical role in maintaining the current rule and dopamine facilitates the selection and modification of rules in response to corrective feedback.
The neuropsychological evidence in support of BG-based computational models of category learning comes, predominantly, from studies involving patients with Parkinson’s disease (PD). An alternative approach is to evaluate the performance of individuals with focal lesions of the BG. While the number of such studies is small, the results have shown that these patients are impaired on rule-based categorization tasks (Ell et al., 2006; Keri et al., 2002; Swainson & Robbins, 2001). No studies, however, have directly compared the performance of patients with focal BG lesions and patients with PD on the same set of rule-based, category learning tasks. One goal of the present study was to systematically investigate the performance of patients with focal basal ganglia lesions, comparing them to patients with PD on rule-based categorization tasks. Given the importance of dopamine in neurocomputational models of rule-based category learning, we also investigated the extent to which PD patient performance is dependent upon dopaminergic medication.
Comparing multiple models of BG dysfunction has several advantages compared to focusing on a single patient group. Degenerative disorders such as PD are not pure models of BG dysfunction. Although the dopamine depletion that results from PD is thought to occur earlier and be most extensive in the BG, prefrontal dopamine is also reduced in PD (Agid et al., 1987). Furthermore, PD directly affects other neurotransmitter systems as well as other subcortical regions (e.g., Braak et al., 2003). Focal BG lesions provide a model in which the pathology can be more precisely characterized. This also entails its own costs: the pathology is limited to a single hemisphere, raising the possibility that the intact hemisphere might prove sufficient for performance or compensate for the damaged basal ganglia. In addition, the size and location of the damage will vary across participants. Nonetheless, testing different models of BG dysfunction allows an assessment of whether task-specific impairments are a general feature of BG dysfunction or, alternatively, associated with one form of pathology.
In the present paper, we focus on the effect of BG dysfunction on rule-based, category-learning tasks that vary in terms of their demands on selective attention. More specifically, the tasks vary in the extent to which they require the participant to ignore irrelevant information (i.e., decisional selective attention, see Maddox et al., 2002). Consider, for example, stimuli that vary continuously along two dimensions. A categorization task with high demands on selective attention would require the participant to attend to a relevant stimulus dimension and ignore an irrelevant stimulus dimension as is the case with the unidimensional task in Figure 1A. Optimal performance on this task requires learning the decision criterion on dimension 1 while ignoring irrelevant variation on dimension 2. In contrast, the conjunction task in Figure 1B places low demands on selective attention because both dimensions are relevant for successful performance.
Figure 1.
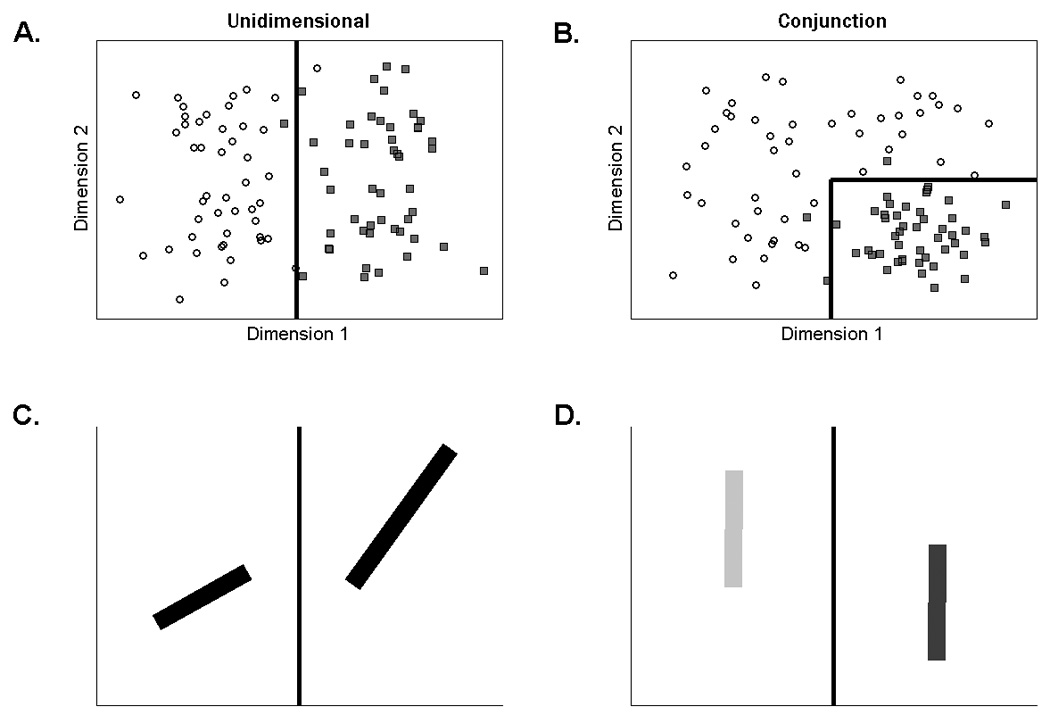
Scatterplot of the stimuli in the A) unidimensional and B) conjunction tasks. Each point represents a single stimulus. Category A exemplars are plotted as black circles and Category B as gray squares. The solid lines are the optimal decision boundaries. In order to minimize carry-over effects between the tasks, two sets of stimuli (counterbalanced across the two tasks) were used: lines varying across trials in length and orientation, or lines varying in brightness and vertical position. Example stimuli from the unidimensional task for C) lines varying in length and orientation and D) lines varying in brightness and vertical position.
In addition to varying the demands on selective attention, the unidimensional and conjunction tasks may also vary in terms of the demand on working memory (Maddox et al., 2004). Successful performance on the unidimensional task requires the participant to learn a single decision criterion. In contrast, successful performance on the conjunction task requires the participant to learn two decision criteria. Thus, relative to the unidimensional task, the conjunction task is thought to place greater demand on working memory because of the increased number of decision criteria (e.g., Filoteo et al., 2007).
The current literature reveals a mixed picture in terms of a comparison between the effects of PD and focal BG lesions on rule-based categorization tasks. As shown in previous studies, PD patients are impaired on unidimensional, categorization tasks, perhaps due to a deficit in selective attention (Ashby et al., 2003; Filoteo et al., 2007; Filoteo, Maddox, Ing et al., 2005). In contrast, they perform similar to matched-controls on conjunction tasks (Filoteo et al., 2007). Focal BG lesion patients have been shown to be impaired on a four category version of the conjunction task (i.e., the stimuli in the four quadrants in Figure 1B were assigned to four contrasting categories, Ell et al., 2006); thus, we might predict that they would also be impaired on the current conjunction task. This population has not been tested on a unidimensional categorization task, and the existing empirical literature precludes a strong prediction given the heterogeneity in methodology and results across previous studies. Current neurocomputational models, in contrast, predict a more general pattern of impairment resulting from PD and focal BG lesions (e.g., Ashby et al., 1998; M. J. Frank, 2005; Moustafa & Gluck, in press).
The PD literature is further complicated by the fact that performance on many cognitive tasks is modulated by the participants' dopaminergic medication state (e.g., Cools et al., 2001; Jahanshahi et al., 2010). Given the prominent role of dopamine in neurocomputational models of rule-based category learning dopaminergic medications would be expected to influence learning on rule-based tasks. In COVIS, for example, dopamine is critical for rule selection and switching. The ability to flexibly implement rules should be important for rule-based categorization: for example, an initial hypothesis may need to be altered based on feedback. These considerations led us to evaluate the effects of dopaminergic medication on rule-based category learning tasks by testing a subset of PD patients in both on and off medication states.
Method
Participants and Design
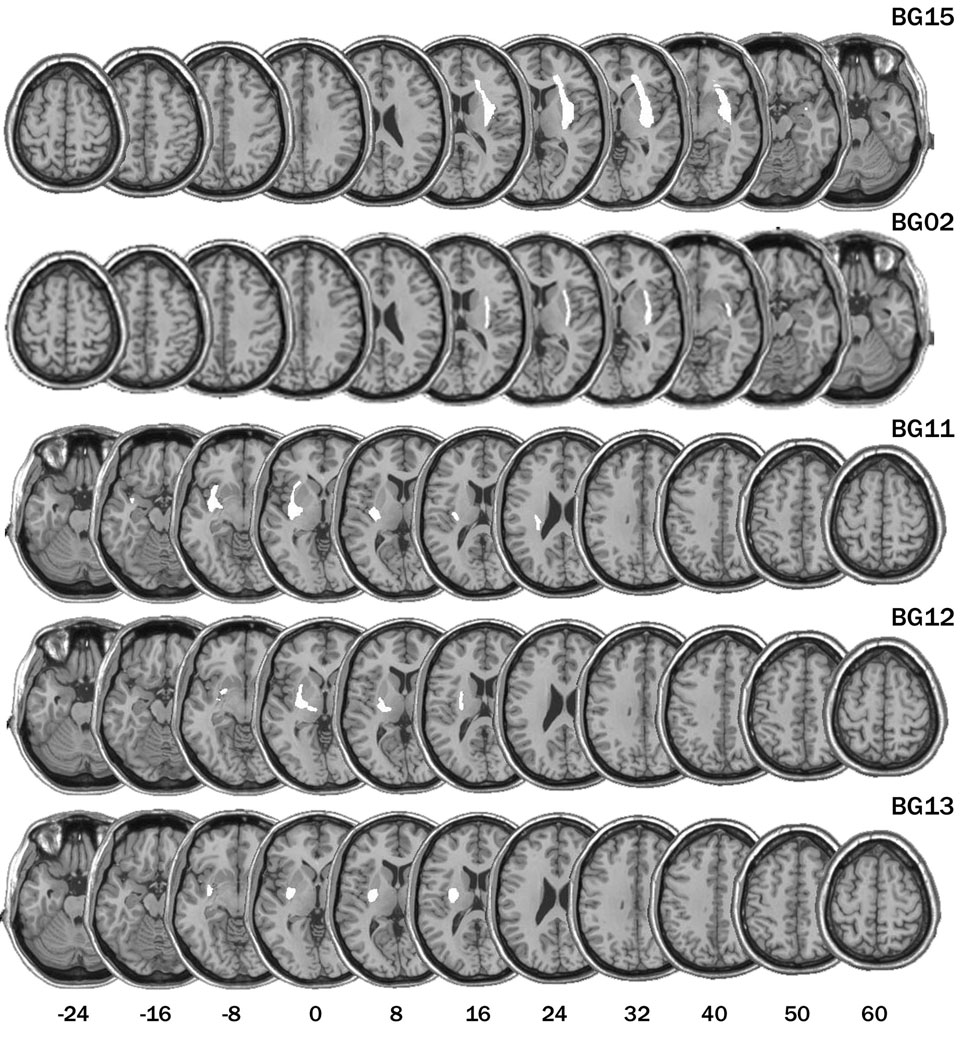
Six patients (one female) with unilateral damage to the BG resulting from stroke were tested. The patients were recruited from the VA Medical Center in Martinez, CA. The lesion was restricted to the left side for four of the patients and to the right side in the other two patients. Lesion reconstructions for five of the patients are presented in Figure 2. We were unable to obtain access to a digital copy of the scan for one patient (BG01). The pathology was centered in the BG, with evidence of putamen involvement in all six patients. The lesion also included the caudate for one patient (BG01). The lesions extended into white matter (internal, external, and extreme capsules) for some of the patients, insular cortex in one patient (BG11), and thalamic nuclei in two patients (BG01, BG12). Testing was conducted at least 12 months after the time of stroke, and for most of the patients many years post-stroke (average interval = 6.7 years, SD=8.1). Five of the six BG patients participated in a prior study on a related topic (Ell et al., 2006).
Figure 2.
Lesion reconstruction (in white) for five of the patients with focal lesions of the basal ganglia, presented on 11 axial slices corresponding to Talairach coordinates of −24, −16, −8, 0, 8, 16, 24, 32, 40, 50, and 60 mm. The striatum (putamen and caudate) is present in sections −8 through 24; the globus pallidus in sections −8 through 16. Figures were generated with the MRIcro software package (Rorden & Brett, 2000) using procedures described in (Brett et al., 2001). We were unable to obtain access to a digital copy of the scan for one patient, BG01.
Seventeen patients (seven female) with idiopathic PD were tested. The patients were recruited by referrals from neurologists or through Parkinson’s support groups. Nine of the PD patients were tested in California and eight in Maine. The patients had been diagnosed an average of 7.4 years (SD = 4.8) prior to testing. Disease severity based on Hoehn and Yahr ratings (Hoehn & Yahr, 1967) averaged 1.6 (SD =.7) with 15 of the 17 patients at stages 1 or 2 (on the five-point scale). Disease severity was also evaluated with the motor subscale of the Unified Parkinson’s Disease Rating Scale (UPDRS - Fahn et al., 1987) and averaged 24.9 (SD = 7.4) on the 0 – 108 point scale.
At the time of the experiment, sixteen of the PD patients were taking daily doses of L-dopa and/or dopamine receptor agonist medications. One PD patient was not taking any medication. Several of the PD patients were taking additional medications: Amantadine (n=1), MAO-B inhibitor (n=1), COMT inhibitor (n=4), anticholinergic (n=1). Ten of the 17 PD patients were tested, in separate sessions, both on and off their medications. For the off session, the participant abstained from all medication for at least 18 hours prior to testing. This time interval is commonly used in investigations of the effects of medication withdrawal (Cools et al., 2003; M.J. Frank et al., 2004; Kehagia et al., 2009; Shohamy et al., 2006) and is well beyond the half-life of the medications (Cedarbaum, 1987; Dingemanse et al., 1995; Holm & Spencer, 1999; Kompoliti et al., 2002). For the patients tested on and off medication, the order of the two sessions was counterbalanced and the sessions were separated by a minimum of two weeks.
A control group (n=23, 6 female) was recruited from the communities surrounding the University of California, Berkeley and the University of Maine (see Table 1). None of the controls reported a history of neurological or psychiatric disorders and were selected to span the range of the patients in terms of age and education (see Table 1). Given the possibility that the BG and PD patient groups would differ on any number of demographic variables, separate groups of control participants were recruited for comparison to each patient group. Analysis of the demographic variables from the patient and control groups, however, did not reveal any substantial group differences. Thus, for simplicity, the control participants were combined into a single group and the results below are presented as a single experiment.
Table 1.
Participant Demographic Information and Neuropsychological Assessment
| CO | BG | PD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | rUD | rCJ | M | SD | rUD | rCJ | M | SD | rUD | rCJ | |
| Age (years) | 65.1 | 7.5 | −.31 | −.10 | 61.2 | 11.5 | −.11 | −.66 | 63.7 | 10.7 | .01 | −.39 |
| Education (years) | 15.3 | 2.4 | −.03 | .23 | 14.0 | 3.2 | −.02 | .24 | 14.6 | 2.9 | −.23 | −.17 |
| IQ* | 122.9 | 4.6 | .17 | .26 | 112.0 | 9.8 | .52 | .25 | 122.3 | 9.7 | .11 | −.32 |
| Spatial Span backward (raw) | 7.5 | 1.6 | −.03 | .19 | 6.8 | 2.6 | −.09 | .40 | 6.9 | 1.9 | −.37 | −.21 |
| Digit Span backward (raw)† | 8.0 | 2.2 | −.04 | .28 | 6.3 | 1.8 | .45 | .27 | 6.4 | 1.9 | .49 | −.32 |
| CWI: Inhibition (s) | 34.5 | 11.3 | 0 | −.06 | 45.4 | 14.9 | −.05 | −.67 | 39.4 | 15.3 | −.55‡ | −.09 |
| CWI: Switching + Inhibition (s) | 38.0 | 16.8 | −.18 | −.29 | 42.8 | 11.7 | .17 | −.68 | 47.4 | 18.1 | −.08 | −.02 |
CO – control participants; BG – basal ganglia patients; PD – Parkinson’s disease patients; IQ – premorbid verbal IQ estimated using the NART; CWI – Color-Word interference subtest from the DKEFS (see text for details on score calculation); rUD – correlation estimated using accuracy (averaged over blocks) on the unidimensional task; rCJ – correlation estimated using accuracy (averaged over blocks) on the conjunction task.
IQ: significant one-way ANOVA [F (2, 28) = 5.21, p < .05] driven by lower scores for the BG patients relative to the PD patients and controls (p’s < .05).
Digit span backward: significant one-way ANOVA [F (2, 42) = 3.63, p < .05] driven by lower scores for the PD patients relative to controls (p < .05) and marginally lower scores for the BG patients relative to controls (p = .07).
CWI: inhibition: significant correlation (p <.05).
The study protocol was approved by the institutional review boards of the VA Medical Center in Martinez, University of California, Berkeley, and the University of Maine. Neither the patients nor controls had any signs of dementia (as indicated by the Mini Mental State Exam, all scores > 28 - Folstein et al., 1975) or symptoms of clinical depression (as assessed by the Beck Depression Inventory - Beck et al., 1996). All participants reported 20/20 vision or vision corrected to 20/20.
Neuropsychological Assessment
A battery of neuropsychological tests was used to assess different aspects of cognitive function in both patients and controls. We added the National Adult Reading Test (NART - Nelson, 1982) to the battery after testing had commenced, desiring a tool that could provide an estimate of pre-morbid verbal intelligence. Given this change in method, we obtained NART data for 13 PD patients, all 6 focal BG patients, and 22 controls.
In rule-based tasks, learning is assumed to be highly dependent upon working memory and executive function (see Ashby et al., 1998; Ashby & Maddox, 2005 for reviews). Thus, neuropsychological tests were included to assess these processes. The digit span subtest (backward) of the Wechsler Adult Intelligence Scale – Third Edition (Wechsler, 1997a) and the spatial span subtest (backward) of the Wechsler Memory Scale – Third Edition (Wechsler, 1997b) provided an index of working memory. Executive functions were evaluated with the Color-Word Interference (CWI) subtest from the Delis-Kaplan Executive Function System (DKEFS - Delis et al., 2001)1. The CWI comprises four subtests. The first two are baseline measures of the time to name a list of colors and the time to read a list of color words. The third is a modified version of the traditional Stroop task (Stroop, 1935), designed to assess the role of response conflict and inhibitory processes when naming the ink color of dissonant color words (e.g., the word “green” in red ink). The fourth subtest incorporates a task switching component in which participants are asked to alternate (irregularly) between naming the ink color and reading the word. We used the third (i.e., inhibition) and fourth (i.e., switching + inhibition) subtests as indices of executive functioning. Inhibition scores, and switching + inhibition scores, were computed by subtracting the average time to complete the two baseline subtests. Higher numbers indicate a greater cost, or reduced executive functioning.
The motor subscale of the UPDRS and a maximum-rate tapping task were used as indices of the effect of medication withdrawal on motor functioning in eight of the ten patients tested both on and off their medications. On the tapping task, participants were instructed to tap as fast as possible with the index finger on a response key. The trial was initiated when the participant made the first keypress and continued until 31 taps were recorded. At the end of each trial, feedback was provided indicating the mean intertap interval (ITI) and the standard deviation of the ITIs. This procedure was repeated six times for each hand. An average tapping score was calculated for each participant (separately for each hand) by computing the mean ITI for the last five trials and averaging the ITIs across trials. The experimenter monitored performance to ensure that scores were not artificially inflated by the failure to activate the response key.
Categorization Tasks
The participants were tested on the unidimensional and conjunction tasks in the same session. The order of the categorization tasks was counterbalanced across participants. In order to minimize carry-over effects between the tasks, two sets of stimuli (counterbalanced across the two tasks) were used (Figure 1). One set involved lines that varied in length and orientation; the other set involved lines that varied in brightness and vertical position. Length was defined in pixels. Orientation was defined as the counterclockwise rotation in degrees from horizontal. Brightness was defined as the intensity in RGB units. Vertical position was defined as the vertical location in pixels of the center of the lines. For the length-orientation stimuli, length was relevant and orientation irrelevant for the unidimensional task. For the conjunction task with these stimuli, the quadrant assigned to category B was high on length and low on orientation, with all other stimuli assigned to category A. For the brightness-position stimuli, brightness was relevant and position irrelevant for the unidimensional task and the quadrant assigned to category B was high on position and low on brightness for the conjunction task2.
Ninety-six stimuli were used in the unidimensional and conjunction tasks, with 48 assigned to each of the two response categories. To create these structures, we used the randomization technique introduced by Ashby and Gott (1988). Each category was defined as a bivariate normal distribution with a mean and a variance on each dimension, and by a covariance between dimensions. The exact parameter values were taken from previous work (Ell et al., 2006; Maddox et al., 2004). To generate the stimuli for the unidimensional task, 24 pseudo-random samples (x, y) were drawn from the distribution for each of the four quadrants. For the length-orientation stimuli, the length range was selected to roughly match the range of visual angles used in previous work and the orientation range was selected to equate the discriminability of changes in perceived length to changes in perceived orientation (Ashby et al., 1999). For the brightness-position stimuli, the RGB intensity of the stimulus ranged from 75 to 225 (of a possible range of 0 to 255 in RGB units) and the vertical position range was selected such that the optimal position criterion was above the center of the monitor. These values were again based on pilot work in which we sought to equate discriminability of the two dimensions.
Each stimulus was presented on a black background and subtended a visual angle ranging from 0.7 to 7.3 degrees at a viewing distance of approximately 60 cm. The stimuli were generated and presented using the Psychophysics Toolbox extensions (Brainard, 1997; Pelli, 1997) for MATLAB. The stimuli were displayed on either a 15” CRT with 1024 × 768 pixel resolution in a dimly lit room or on a laptop LCD of the same resolution when testing was conducted in the participants' home. In the latter case, the stimuli were scaled to equate the visual angle.
On each trial, a single stimulus was presented and the participant was instructed to make a category assignment by pressing one of two response keys (labeled ‘A’ or ‘B’) with either the left or right index finger. Participants were instructed that their goal was to learn the categories by trial-and-error. Participants were informed that there were two equally likely categories and that the best possible accuracy was 95% (i.e., optimal accuracy). The instructions emphasized accuracy and there was no response time limit. After responding, feedback was provided. When the response was correct, the word “CORRECT” appeared in green and was accompanied by a 1 s, 500 Hz tone; when incorrect, the word “WRONG” appeared in red and was accompanied by a 1 s, 200 Hz tone. The screen was then blanked for 500 ms prior to the appearance of the next stimulus. In addition to trial-by-trial feedback, summary feedback was given at the end of each 96-trial block, indicating overall accuracy for that block.
A standard keyboard was used to collect responses. The keyboard characters ‘s’ and ‘l’ were assigned to categories ‘A’ and ‘B’, respectively. Following, previous work (Ell et al., 2006; Maddox et al., 2004), the response mappings were fixed across participants. We did not expect performance to vary between the two hands given that the response requirements were minimal (e.g., speed was not emphasized) and that all of the patients had no overt difficulty producing the finger movements. Indeed, error rates did not differ as a function of the hand used to respond in the current study.
Each participant completed three blocks of 96 trials, with the presentation order of the stimuli randomized within each block. After completing one of the two categorization tasks with one set of stimuli (e.g., the unidimensional task with lines varying in length and orientation), the participant completed neuropsychological testing, followed by the other categorization task with the other set of stimuli (e.g., the conjunction task with lines varying in brightness and position). As noted above, the order of the two categorization tasks and the categorization task-stimulus set pairings were counterbalanced across participants. Each session lasted approximately 2.5 hours, including neuropsychological testing and multiple breaks.
Results
Accuracy-Based Analyses: Patients vs. Controls
The learning curves for the unidimensional task suggest a late-training impairment for the PD patients and no indication of impairment for the focal BG patients (Figure 3A)3. Consistent with this observation, a 3 block × 3 group mixed ANOVA revealed a significant block × group interaction [F (3.04, 59.36) = 3.09, p = .03, MSE = 70.11, ηp2 = .14] that was driven by decreased accuracy for the PD patients relative to controls during the final training block (p = .02) 4. The PD patients did not perform significantly worse than the focal BG patients during the final block (p = .33). The main effect of block was significant reflecting the general increase in accuracy with training for all groups [F (1.52, 59.36) = 20.90, p < .01, MSE = 70.11, ηp2 = .35]. Neither the main effect of group [F (2, 39) = .4, p = .68, MSE = 389.78, ηp2 = .02] nor the other pairwise comparisons (p’s > .33) were significant.
Figure 3.
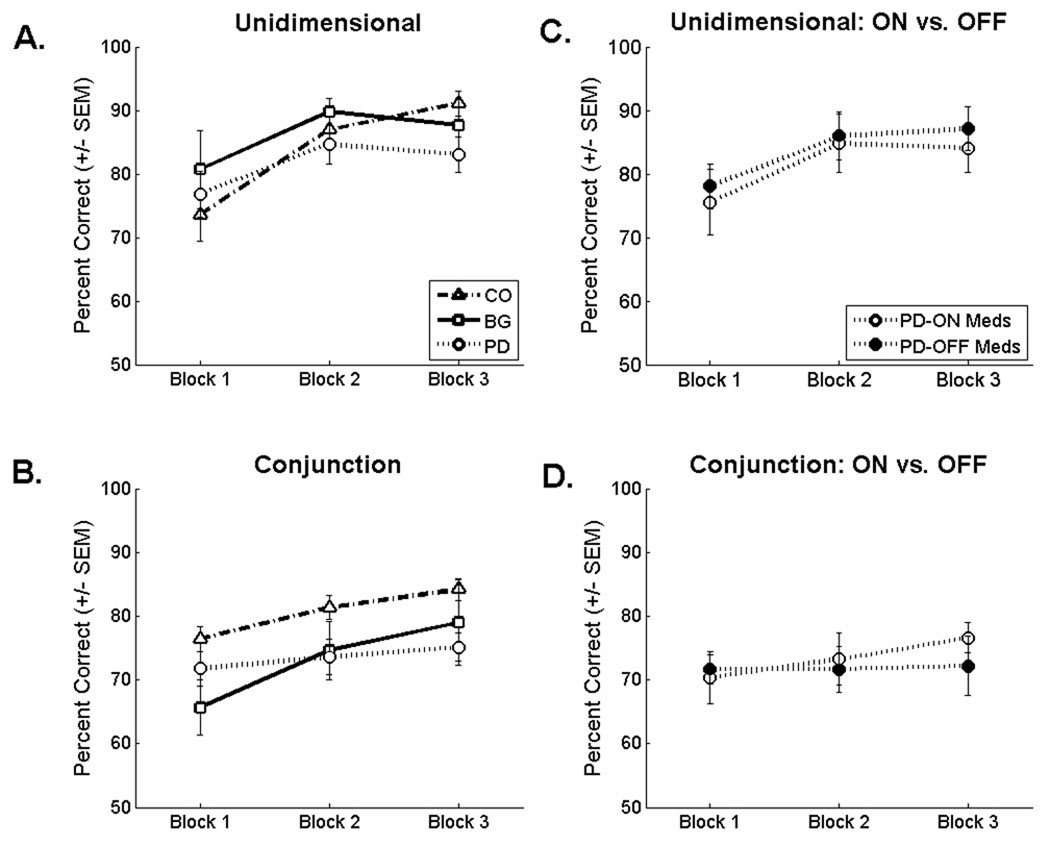
Average accuracy (+/− SEM) for the controls (CO), the basal ganglia lesion patients (BG), and the Parkinson’s disease patients (PD) on the A) unidimensional and B) conjunction tasks. Average accuracy for the subset of PD patients tested both on and off their medications on the C) unidimensional and D) conjunction tasks.
The learning curves for the conjunction task suggest that both patient groups were impaired throughout training relative to controls (Figure 3B). Consistent with this observation, a 3 block × 3 group mixed ANOVA revealed a significant main effect of group [F (2, 41) = 3.68, p = .03, MSE = 236.69, ηp2 = .15] that was driven by lower accuracy (averaged across blocks) for the PD patients (M = 73.52, SE = 2.22) and focal BG patients (M = 73.08, SE = 3.63) relative to controls (M = 80.66, SE = 1.89). The comparison of the PD group and controls was significant (p = .02); the comparison of the focal BG group and controls was only marginally significant (p = .07). The main effect of block was significant reflecting the general increase in accuracy with training for all groups [F (2, 82) = 18.96, p < .01, MSE = 29.56, ηp2 = .32]. The block × group interaction was not significant by traditional standards [F (4, 82) = 2.06, p = .09, MSE = 29.56, ηp2 = .09]. To directly test the hypothesis that the BG patients would have an impairment early in training as would be predicted from our previous work (Ell et al., 2006) and related findings of a pronounced early-training dependence on the BG in rule-based tasks (e.g., Knowlton et al., 1996; Pasupathy & Miller, 2005),,we conducted a planned comparison of the focal BG patients and controls during the first training block. This analysis revealed a significant impairment for the BG group (p = .02).
It is important to consider whether the pattern of impairment in the two patient groups can be attributed to differences in task difficulty. We assessed this by examining the data from the control participants. Nineteen controls contributed data for both tasks (i.e., were not outliers on either task – see footnote 3). A 3 block × 2 task within-subjects ANOVA conducted on the data from these 19 participants did not reveal a significant effect of task [F (1, 22) = .003, p = .96, MSE = 545.45, ηp2 = 0]. The block × task interaction was marginally significant [F (1.21, 26.57) = 3.47, p = .07, MSE = 81.98, ηp2 = .14], but control accuracy on the two tasks did not significantly differ for any block (p’s > .41). Further evidence that the tasks were of similar difficulty is given by the fact that 11 of the 23 controls had higher average accuracy on the conjunction task and 12 had higher average accuracy on the unidimensional task.
We also asked if there was evidence of a difference in task difficulty in the response time data. Consistent with the accuracy data, an analysis of the response time data (response times were calculated for each participant by computing the median response time across trials) provided no support for the task difficulty hypothesis. A 3 block × 2 task within-subjects ANOVA indicated that neither the main effect of task [F (1, 22) = .07, p = .79, MSE = 181271.05, ηp2 = .07] nor the task × block interaction [F (2, 44) = 1.56, p = .22, MSE = 27970.58, ηp2 = 07] was significant [main effect of block: F (2, 44) = 10.93, p < .001, MSE = 56436.82, ηp2 = .33]. The analysis of the RT data, however, is limited given that there was no response deadline.
Accuracy-Based Analyses: Medication Effects for PD patients
The learning curves for the subset of PD patients tested both on and off their dopaminergic medication suggests that abstaining from dopaminergic medication had a negligible effect on categorization accuracy (Figures 3C and 3D). Separate 3 block × 2 medication state repeated-measures ANOVAs conducted on the two tasks showed no difference of medication state on either the unidimensional task [main effect of medication state: F (1, 8) = .15, p = .71, MSE = 439.62, ηp2 = .02; medication state × block interaction: F (1.33, 10.65) = .12, p = .80, MSE = 6.82, ηp2 = .02; main effect of block: F (1.25, 10.01) = 14.58, p < .01, MSE = 49.25, ηp2 = .65] or the conjunction task [main effect of medication state: F (1, 9) = .12, p = .73, MSE = 307.94, ηp2 = .01; medication state × block interaction: F (2, 18) = .91, p = .42, MSE = 43.8, ηp2 = .09; main effect of block: F (2, 18) = 3.01, p < .01, MSE = 18.64, ηp2 = .25].5
Surprisingly, the patients did not show dramatic changes in symptomology following 18 hours of medication withdrawal. Their score on the motor subscale of the UPDRS [MON = 23.9, SEON = 2.5; MOFF = 28.1, SEOFF = 3; t(7) = 1.4, p = .2, SE = 3] was slightly elevated. Similar modest, and non-significant, increases in ITI were observed on the tapping task for both the right [MON = 247.3, SEON = 19.5; MOFF = 253.8, SEOFF = 20.3; t(7) = 1, p = .4, SE = 6.6] and left [MON = 273.1, SEON = 20.9; MOFF = 279.3, SEOFF = 24.9; t(7) = .6, p = .5, SE = 9.9] hands.
Model-Based Analyses
The analysis of the accuracy data revealed a selective impairment of the BG patients on the conjunction task and a more general impairment for the PD patients on both tasks. To further explore the basis of these impairments, we used model-based analyses to evaluate different ways in which the patients might have difficulty on rule-based tasks. For example, a failure of selective attention on the unidimensional task might result in a decision strategy that was sensitive to both stimulus dimensions. Similarly, a failure to attend to both dimensions on the conjunction task would result in a decision strategy overly sensitive to a single dimension. Alternatively, a learning impairment may be driven by the inconsistent application of an optimal strategy. The following analyses represent a quantitative approach to evaluating these hypotheses.
Three different types of models were evaluated, each based on a different assumption concerning the participant's strategy (see the Appendix for a more detailed description of the models and fitting procedure). Rule-based models assume that the participant either attends selectively to one dimension (unidimensional classifiers; e.g., if the line is long, respond B; otherwise respond A) or makes independent decisions about the stimulus on both dimensions (conjunctive classifiers; e.g., if the line is long and low in angle Respond B; otherwise respond A). For the unidimensional task, there were two versions of the unidimensional classifier, one assuming participants used the optimal decision strategy in Figure 1A (optimal classifier) and one assuming participants used a unidimensional classifier with a suboptimal intercept on the relevant dimension (unidimensional classifier). Similarly, for the conjunction task there were two versions of the conjunctive classifier: one assuming participants used the optimal conjunctive classifier in Figure 1B (optimal classifier) and one assuming participants used a conjunctive classifier with suboptimal intercepts on the two stimulus dimensions (conjunctive classifier). Information-integration models (linear and minimum-distance classifiers) assume that the participant combines the stimulus information from both dimensions prior to making a categorization decision. Finally, random-responder models assume that the participant guesses.
These models make no detailed processing assumptions in the sense that a number of different process based accounts are compatible with each of the models (e.g., Ashby, 1992a; Ashby & Waldron, 1999). Thus, the modeling described in this section provides a formal vehicle to test hypotheses about the decision strategies used by participants, and gain insight into the underlying deficits observed in the patient groups. For example, for the unidimensional task, if either the conjunctive classifier or information-integration models provide a better fit than the unidimensional classifier, then we would have evidence of a failure of selective attention. For the PD patients, all model-based analyses were limited to the data to the session in which the patients were on medication given the lack of an effect of medication withdrawal.
On the unidimensional task, the majority of the data sets were best-fit by the optimal classifier and all but one participant was best fit by a model assuming selective attention (optimal and unidimensional classifiers, Figure 4A). Thus, both patient groups were able to attend selectively to the relevant stimulus dimension. Moreover, the late-training impairment observed for the PD patients was not driven by a pure failure of selective attention. Rather, the PD impairment was attributed to the inconsistent use of this strategy. This could arise from an increase in trial-by-trial variability in the representation and/or application of the decision criterion (i.e., internal noise)6. Consistent with the hypothesis of increased decision criterion variability, the average noise parameter estimate was higher on block 3 for the PD patients than the controls (Figure 5A) [t (33) = 3.2, p < .01, SE = .13]. In addition, increased noise was associated with decreased accuracy as evidenced by a significant negative correlation between the estimate of internal noise and block 3 accuracy [r (16) = −.54, p<.05].
Figure 4.
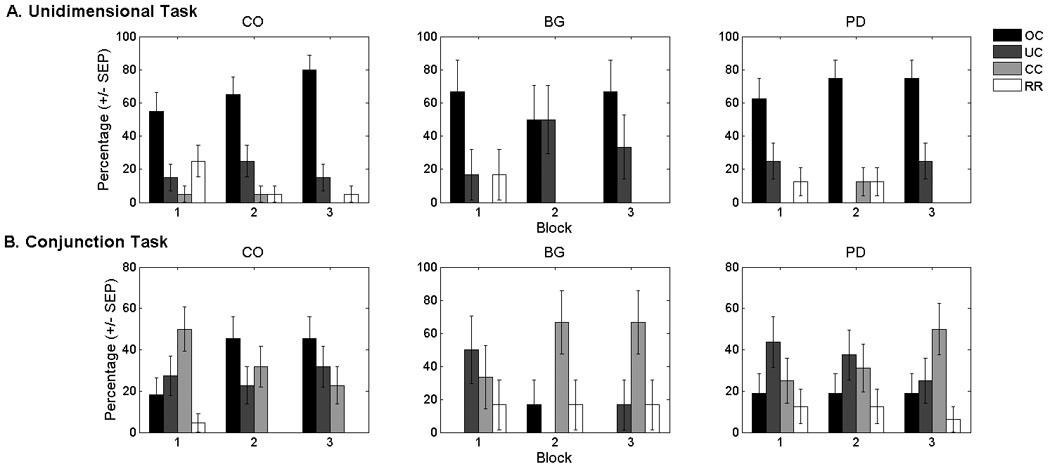
Percentage of participants in the (A) unidimensional and (B) conjunction tasks whose data were best fit by the optimal classifier (OC), the suboptimal unidimensional classifier (UC), the suboptimal conjunctive classifier (CC), or a model assuming that participants were responding randomly (RR). None of the data sets were best fit by the information-integration models. The models provided a reasonable account of these data as indexed by the average (over blocks and participants) percent of responses accounted for by the best-fitting model: unidimensional task: CO (M = 89.02, SD = 9.4), BG (M = 88.7, SD = 8.1), PD (M = 83.9, SD = 11.1); conjunction task: CO (M = 85.2, SD = 7.1), BG (M = 80.7, SD = 10.6), PD (M = 81.0, SD = 6.9). The best-fitting models accounted for a far greater percentage of the responses than would be predicted by chance (i.e., 50% of responses accounted for) for all groups. CO: control participants; BG: basal ganglia lesion patients; PD: Parkinson’s disease patients.
Figure 5.
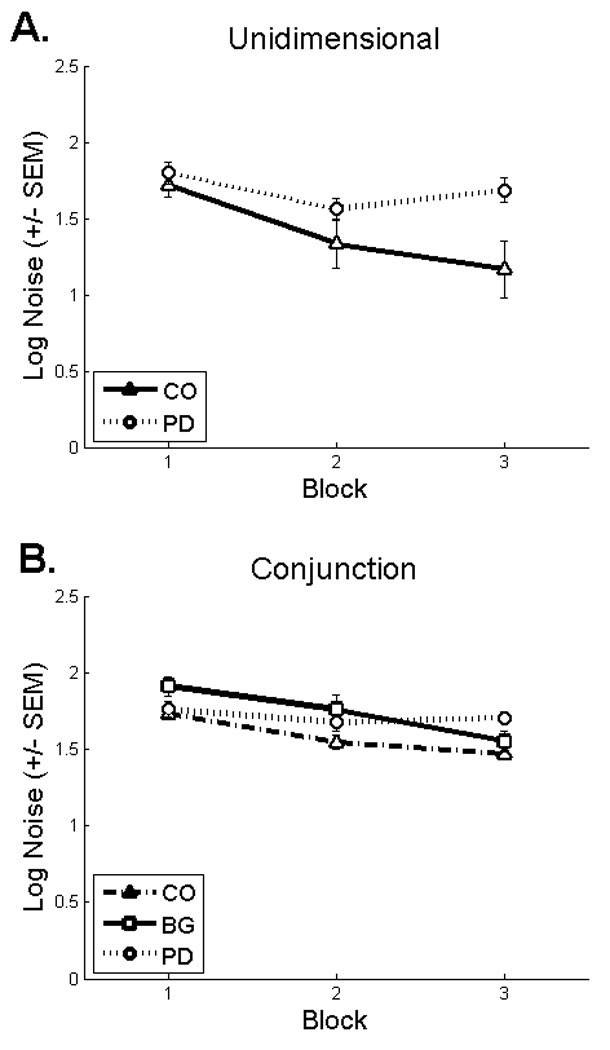
Average criterial noise estimates (+/− SEM) from the best-fitting model (excluding random responders) for the A) unidimensional and B) conjunction tasks. These data have been log transformed to correct for a positive skew in the sample distributions. PD: Parkinson’s disease patients; CO: control participants; BG: basal ganglia lesion patients.
On the conjunction task, the majority of controls were best fit by the conjunctive classifier during block 1, but this pattern shifted in favor of the optimal classifier during blocks 2 and 3 (Figure 4B). During block 1, only 33% of the focal BG patients were best fit by a model assuming a conjunctive strategy (i.e., optimal and conjunctive classifiers) as compared to 68% of controls. As would be expected, the BG patients who were best fit by the unidimensional classifier or responding randomly averaged low accuracy (Mblock1 = 66.8%, SEblock1 = 5.4). Moreover, criterial noise estimates were larger for the BG patients relative to controls during block 1 [t (24) = 2.32, p < .05, SE = .08], but not block 3 [t (25) = .99, p = .33, SE = .08] (Figure 5B). The noise estimates were negatively correlated with accuracy during block 1[r (5) = −.87, p=.05]. Although limited by a small sample size, these data suggest that the impairment for the focal BG group during block 1 was driven by the inefficient use of non-optimal strategies.
Similar to the BG patients, only 44% of the PD patients were best fit by a model assuming a conjunctive strategy during block 1 (i.e., conjunctive and optimal classifiers, Figure 4B). By block 3, however, a similar percentage of PD patients and controls were best fit by a model assuming a conjunctive strategy. During block 3, the majority of controls were best fit by the optimal classifier whereas the majority of PD patients were best fit by the conjunctive classifier and performed similarly to the group average for all PD patients (M = 72.3, SE = .6). Criterial noise estimates were also higher for PD patients than controls during block 3 [t (35) = 4.46, p < .001, SE = .05], but not block 1 [t (33) = .51, p = .62, SE = .06] or block 2 [t (34) = 1.77, p = .09, SE = .07] (Figure 5B). Importantly, however, the increased noise during block 3 did not appear to have any functional significance as neither noise estimates from the best-fitting model [r (15) = −.28, p = .31] nor noise estimates from the subset of patients best fit by models assuming a conjunctive strategy [r (16) = .02, p = .95] were significantly correlated with accuracy. In short, these data suggest that the PD impairment on the conjunction task was driven primarily by the use of suboptimal decision strategies.
Relationship between Accuracy on Categorization Tasks and Demographic, Neuropsychological, and Neuropathological Variables
A summary of the demographic and neuropsychological variables is given in Table 1. Omnibus analyses of these data were conducted using separate one-way ANOVAs evaluated at a criterion of p = .05 (uncorrected) (see Table 1). There was a significant group difference on IQ that was driven by lower IQ for the focal BG patients relative to the controls and PD patients. There was also a significant group difference on digit span (backward) that was driven by an impairment for the PD patients relative to controls and a marginally significant impairment for the focal BG patients relative to controls. None of the remaining variables significantly differed across groups (p’s > .17).
To investigate the relationship between the demographic and neuropsychological variables and category learning, correlations were computed with accuracy (averaged over blocks) on the unidimensional and conjunction tasks evaluated at a criterion of p = .05 (uncorrected) (see Table 1). Lower inhibition scores on the CWI (indicating better inhibition) were associated with higher accuracy on the unidimensional task for the PD patients suggesting that those patients that were better able to inhibit a pre-potent response were more accurate on a categorization task requiring the inhibition of irrelevant information. None of the other correlations were significant.
For the focal BG patients, lesion volume was weakly related to accuracy on the conjunction task [averaged over blocks: r(6) = −.36, p = .55; block 1: r(6) = −.2, p = .75]. Average accuracy on the conjunction task was similar for the two patients with right-sided lesions (M = 72.86, SE = 2.03) compared to the four with left-sided lesions (M =73.19, SE = 7.32).
For the PD patients, increasing disease severity (i.e., UPDRS) was associated with decreased accuracy on the unidimensional task with the correlation being significant for block 3 accuracy [averaged over blocks: r(16) = −.44, p = .09; block 3: r(16) = −.56, p < .05]. In contrast, there was no association between disease severity and accuracy on the conjunction task [averaged over blocks: r(16) = .08, p = .76; block 3: r(16) = .09, p = .75]. There was a trend for PD patients with bilateral involvement (block 3: n = 8, M = 78.2, SEM = 4.3) to perform worse than patients with only unilateral involvement (block 3: n = 8, M = 87.9, SEM = 2.6) on the unidimensional task, but this difference was only marginally significant [t (14) = 1.94, p = .07, SE = 5.0]. PD patients with bilateral (averaged over blocks: n = 7, M = 72.1, SEM = 2.8) involvement performed comparably to PD patients with unilateral involvement (averaged over blocks: n = 9, M = 74.6, SEM = 10.6) on the conjunction task [t (14) = .54, p = .6, SE = 4.7]. PD patients with bilateral involvement also performed worse on the inhibition [t (14) = 2.13, p = .05, SE = 6.93] and inhibition+switching [t (14) = 2.13, p = .05, SE = 8.2] subtests of the CWI test.
General Discussion
Converging lines of evidence are consistent with the hypothesis that the basal ganglia play an important role in rule-based category learning (Ashby & Maddox, 2005; Price et al., 2009; C.A. Seger, 2008). However, a comparison of neuropsychological studies suggests that the pattern of impairment may differ across patient models of BG dysfunction (Ell et al., 2006; Filoteo et al., 2007). The present study addressed this issue by testing patients with focal lesions of the BG due to stroke and patients with PD on an identical set of tasks. The individuals with focal BG lesions were impaired on the conjunction task and performed similar to controls on the unidimensional task. In contrast, the PD patients were impaired on both tasks, although a model-based analysis suggests that the source of the PD impairment differed across the two tasks.
Consistent with our previous work involving a four-dimensional, conjunction task (Ell et al., 2006), patients with focal BG lesions were impaired on the two-dimensional, conjunction task used in the present study. In both studies, the impairment was only present early in training. This stands in contrast to the finding that the BG patients performed similar to matched controls on the unidimensional task. The results of the model-based analyses suggest that the selective early impairment of the BG patients on the conjunction task was driven primarily by the inefficient use of suboptimal decision strategies.
A more general impairment on both tasks was observed for the PD patients. The results of the model-based analyses suggest that the impairment on the two tasks occurred for different reasons. The impairment on the unidimensional task was manifest late in training and was attributed to instability in the setting of the decision criterion. In contrast, the consistent impairment on the conjunction task was driven by the use of suboptimal strategies. Furthermore, accuracy on the unidimensional task, but not the conjunction task, was associated with increased disease severity and a decreased ability to inhibit pre-potent responses. We did not observe any consistent change in performance in the PD patients when they were tested off medication.
Selective Attention, Working Memory, and Rule-Based Categorization
Our selection of the conjunction and unidimensional tasks was motivated by consideration of the demands these tasks place on selective attention (Ashby & Townsend, 1986; Maddox, 1992; Maddox et al., 2002). To perform optimally on the conjunction task, the participant must attend to the stimulus value on both dimensions. As such, this task places low demands on selective attention; selectively attending to one dimension at the expense of the other would impair performance. In contrast, optimal performance on the unidimensional task requires that the participant attend to the stimulus value on only the task-relevant dimension. As such, this task places a high demand on selective attention.
The conjunction and unidimensional tasks may also differ in their demand on working memory (Maddox et al., 2004). To perform optimally the participant must learn two decision criteria in the conjunction task whereas the participant need only learn a single decision criterion in the unidimensional task. Consistent with this hypothesis, many studies have shown that learning multiple criteria on different dimensions is more difficult than learning one criterion on a single dimension (Maddox et al., 2004; Salatas & Bourne, 1974; Shepard et al., 1961), although it is unclear if this difference can be attributed to differences in working memory demand. Furthermore, the relationship between working memory and the present tasks is not straightforward. While increasing the number of decision criteria may tax working memory, this increase is at least partially offset by splitting the decision criteria across multiple stimulus dimensions (Ell et al., 2009).
Intuitively, the conjunction task would appear more difficult due to the increased complexity of the optimal decision strategy; thus, one might argue that the observed dissociation for the focal lesion group is related to difficulty rather than a failure to attend to both dimensions. While we can not rule out this possibility, the performance of the control participants was not consistent with a difficulty hypothesis. Accuracy, as well as response time did not differ in a consistent manner between tasks. Moreover, previous studies involving patients with BG dysfunction have observed selective impairment on easier rule-based tasks (Ashby et al., 2003; Filoteo et al., 2007)
On the unidimensional task, the focal BG patients performed similar to matched controls but the PD patients were impaired, at least late in training. The PD impairment was not driven by a failure of selective attention (e.g., the use of a two-dimensional classifier). Instead, the deficit was more subtle, being attributed to an increase in variability in the representation of the decision criterion. This increased variability was associated with decreased categorization accuracy. Interestingly, those PD patients who were better able to inhibit pre-potent responses (as assessed by the CWI subtest of the DKEFS) were more accurate on a categorization task requiring the inhibition of irrelevant information. Thus, it would appear that variation in selective attention ability was relevant for the PD deficit, even if they were able to selectively attend to the relevant dimension in the categorization task.
Both patient groups were impaired on the conjunction task. Our model-based analyses indicate that the impairment for the focal lesion group was driven by the use and inconsistent application of suboptimal decision strategies. This pattern is consistent with a previous study involving focal BG patients (5 of 6 were tested in the present study, Ell et al., 2006). One departure from Ell et al. is that, in the present study, a subset of BG patients were best fit by the unidimensional classifier (i.e., they ignored one of the stimulus dimensions). We attribute this to differences in the category structure. Ell et al. used a four-category, conjunction task where the most accurate unidimensional strategy would result in only 25% correct. In the present paper, we used a two-category, conjunction task where the most accurate unidimensional strategy would result in 75% correct. The PD impairment on the conjunction task was also attributed to the use of suboptimal decision strategies. Moreover, for the PD patients, variation in criterial noise was not predictive of overall accuracy.
While the focal BG group demonstrated an impairment during the first phase of testing with the conjunction task, their performance was normal across all blocks on the unidimensional task. This finding may appear to be at odds with previous reports of impairment of focal BG lesion patients on the WCST, a unidimensional task with many, discrete-valued dimensions (Benke et al., 2003; Keri et al., 2002; Pickett et al., 1998). It is unlikely that the discrepant findings are due to methodological differences between the WCST and the unidimensional task as the present sample of focal BG lesion patients were not impaired on the WCST (see footnote 1).
PD patients, on the other hand, are consistently impaired on unidimensional tasks and this impairment is robust to methodological differences (Ashby et al., 2003; Filoteo et al., 2007; Filoteo, Maddox, Ing et al., 2005; Price, 2006). In contrast to the present results, Filoteo and colleagues (2007) found that PD patients performed similar to matched controls on two conjunction tasks, suggesting that the PD impairment may be restricted to rule-based tasks with high selective-attention demand. The methodology in the present study is very similar to that used by Filoteo et al., with the exception of the specific stimulus dimensions. In the present study, two stimulus sets were used: lines varying across trials in length and orientation, and rectangles varying across trials in brightness and position. Filoteo et al. used Gabor filters (i.e., sine-wave gratings weighted by a circular Gaussian filter that vary across trials in spatial frequency and orientation). PD patients experience a number of visual processing deficits (Davidsdottir et al., 2005) with reduced contrast sensitivity functions (e.g., Bodis-Wollner et al., 1987) being one of the more prominent impairments. Although visual processing deficits should have a negative impact on all of the stimulus sets, Gabor filters would appear to be particularly susceptible given the importance of contrast in resolving spatial frequency differences (e.g., Blakemore & Campbell, 1969). Thus, it seems unlikely that the discrepant results are due to methodological differences.
Although our results suggest that the PD impairment on rule-based tasks may be more general than previously thought, the neuropsychological data argue against a general cognitive deficit. Relative to controls (and the focal BG patients), the PD patients were not impaired on measures of IQ, spatial working memory, or executive function. Of course, these tasks do not test learning per se, but rather component processes that are thought to be important for learning. Thus we cannot rule out the possibility that the PD patients have a more general learning deficit that might be driven by the online use of these component processes.
Basal Ganglia Contributions to Rule-Based Categorization
The focal BG and PD groups differ in a number of substantive ways. The former have suffered an acute neurological episode, have damage limited to one side, and the pathology is relatively focal. The latter have had an on-going degenerative process, generally bilateral symptoms, and pathology that may be more diffuse. Assuming the BG contribute to rule-based categorization, one might suppose that the PD patients would demonstrate a more general deficit than patients with focal BG lesions. Indeed, our data are consistent with this hypothesis.
The focal BG group, although small in number, does provide some insight into the contribution of different subregions of the BG in rule-based categorization. The current results suggest that the impairment on the conjunction task, the task hypothesized to place relatively high demands on working-memory demand (Filoteo et al., 2007), may be related to putamen damage. Putamen dysfunction is observed early in PD (Brooks & Piccini, 2006; Kish et al., 1988) and this nucleus showed the greatest overlap of pathology in our sample of focal BG lesion patients. Converging lines of evidence point to a role for the putamen in rule-based tasks. In neuroimaging studies, activation levels in the putamen have been associated with working memory maintenance (Chang et al., 2007), the manipulation of information during retrieval (Dodds et al., 2009), and feedback processing during rule-based categorization (Monchi et al., 2001; C. A. Seger & Cincotta, 2006). Moreover, putamen activity is positively correlated with working memory load (Chang et al., 2007). The conjunction task may place greater demand on working memory processes than the unidimensional task given the need to combine information from two dimensions.
The observation that only the PD patients were impaired on the unidimensional task suggests three possible hypotheses concerning the neuroanatomical locus of impairment on selective-attention-demanding, categorization tasks. First, it may be related to pathology in other basal ganglia nuclei. For instance, dopamine depletion in the caudate nucleus may be critical. Consistent with this hypothesis, previous studies involving focal BG lesion patients on rule-based tasks with high selective-attention demand, had shown that the impairment was associated with pathology in the caudate nucleus (e.g., Swainson & Robbins, 2001).
Second, selective-attention impairments may require bilateral pathology in the basal ganglia. Consistent with this argument, there was a trend for PD patients with bilateral involvement to perform worse on the unidimensional task than PD patients with unilateral involvement. In addition, bilateral patients had more difficulty inhibiting a pre-potent response and with task switching.
Third, the PD impairment might arise from dysfunction in structures outside the basal ganglia. For instance, although cortical dopamine depletion is thought to be less severe and occur in the later stages of the disease (Agid et al., 1987), it is impossible to rule out the hypothesis that the PD deficits are related to prefrontal dysfunction in our sample of mild-to-moderate PD patients. Indeed, as might be expected if the PD impairment on the unidimensional task were related to disruption of processing in prefrontal cortex, the patients demonstrated a significant) correlation between disease severity and accuracy on the unidimensional task. Although there were no group differences in measures of executive functioning that are commonly associated with frontal function, the ability to inhibit a pre-potent response was related to accuracy on the unidimensional task. Testing patients with focal prefrontal lesions on unidimensional and conjunction tasks will be important for clarifying the respective contributions of the basal ganglia and prefrontal cortex to rule-based categorization.
Interestingly, we did not observe any consistent change in performance in the PD patients when they were tested after abstaining from their medication for at least 18 hours (M = 20.1 hrs, SD = 3). Although based upon a null result, these data suggest that rule-based category learning may not be dependent upon global dopamine levels. This interpretation, however, is complicated by the observation that patients also showed very mild and non-reliable changes in motor performance after abstaining from their medication.
It is important to interpret these data within the broader context of neurocomputational models of category learning. Particularly relevant is the COVIS model of category learning proposed by Ashby and colleagues. According to COVIS, learning in rule-based tasks requires the maintenance of decision strategies in working memory, the selection of novel rules, and the ability to switch attention among competing rules (Ashby et al., 1998). In theory, basal ganglia dysfunction may have interfered with any of these sub-processes. The increased criterial noise that was observed for the PD patients on the unidimensional task and BG patients on the conjunction task suggests, however, that the impairment was driven by impaired maintenance or an increased propensity to switch attention from one rule to another. Although speculative, this hypothesis does tie in with conjectures on how the basal ganglia contribute to rule-based processing in a variety of other domains such as working memory (Ashby et al., 2005; Lawrence et al., 2000), executive functioning (Cools, 2006; Crone et al., 2006; Owen et al., 1993), and language use (Longworth et al., 2005; Teichmann et al., 2005; Ullman, 2004).
One caveat to point out, though, is that, COVIS focuses on the caudate nucleus as the critical BG component for rule-based learning, a hypothesis motivated by the neuroimaging literature (e.g., Filoteo, Maddox, Simmons et al., 2005; Hikosaka et al., 1989; Rao, 1997; C. A. Seger & Cincotta, 2006). The one patient in our sample whose lesion also included the caudate performed normally on the unidimensional task (Macross blocks = 86.5%), but was severely impaired on the conjunction task (Macross blocks = 53.1%). The present results suggest that the role of the putamen in rule-based categorization may need to be re-evaluated. As noted above, the putamen has been associated with many of the component processes thought to be critical for rule-based tasks. Alternatively, the putamen may influence processing within the caudate nucleus via striatal cell bridges (Martin, 1996) or other local networks within the basal ganglia (e.g., striato-nigral-striatal projections, see Haber, 2003). Another hypothesis is that the putamen may be involved in resolving competition between multiple learning systems engaged during categorization (Ashby et al., 1998).
Conclusions
Patients with BG lesions demonstrated an early-training impairment on a rule-based task in which the demands on working memory demand were high, but not on a rule-based task that required selectively attending to one dimension. In contrast, the PD patients were impaired on both tasks, although the cause of this impairment, as inferred from a model-based analysis, differed for the two tasks. The PD impairment on the task with high working memory demand was driven by the use of suboptimal decision strategies. In contrast, the impairment on the task with high selective-attention demand was driven by the inconsistent application of an appropriate decision strategy. These data suggest that demands on selective attention and working memory influence the presence of impairment in patients with focal BG lesions and the nature of the impairment in patients with PD. Moreover, these data highlight the value of comparing multiple models of BG dysfunction.
Acknowledgments
This research was supported by the National Institutes of Health (NS047884 to SWE and DA02066 and NS040813 to RBI). The authors thank the patients and their caregivers for their participation and ongoing commitment to research. Thanks to Ed Drasby, Donatella Scabini, Leslie Shupenko, and William Stamey for their assistance in the recruitment and/or assessment of the patients. Thanks to Andrea Jang for assistance with data collection and to Matthew Brett, Mark D’Esposito, and Robert Knight for their assistance with the analysis of the MRI scans.
Appendix
To get a more detailed description of how participants categorized the stimuli, a number of different decision bound models (Ashby, 1992a; Maddox & Ashby, 1993) were fit separately to the data for each participant from every block. Decision bound models are derived from general recognition theory (Ashby & Townsend, 1986), a multivariate generalization of signal detection theory (Green & Swets, 1966). It is assumed that, on each trial, the percept can be represented as a point in a multidimensional psychological space and that each participant constructs a decision bound to partition the perceptual space into response regions. The participant determines which region the percept is in, and then makes the corresponding response. While this decision strategy is deterministic, decision bound models predict probabilistic responding because of trial-by-trial perceptual and criterial noise (Ashby & Lee, 1993).
The appendix briefly describes the decision bound models. For more details, see Ashby (1992a) or Maddox and Ashby (1993). The classification of these models as either rule-based or information-integration models is designed to reflect current theories of how these strategies are learned (e.g., Ashby et al., 1998) and has received considerable empirical support (see Ashby & Maddox, 2005; Maddox & Ashby, 2004 for reviews).
Rule-Based Models
Unidimensional Classifier (UC)
This model assumes that the stimulus space is partitioned into two regions by setting a criterion on one of the stimulus dimensions. Two versions of the UC were fit to these data. For example, for the line stimuli, one version assumes that participants attended selectively to length and the other version assumes participants attended selectively to orientation. The UC has two free parameters, one corresponds to the decision criterion on the relevant dimension and the other corresponds to the variance of internal (perceptual and criterial) noise (σ2). For the unidimensional task, a special case of the UC, the Optimal Unidimensional Classifier, assumes that participants use the unidimensional decision bound that maximizes accuracy. This special case has one free parameter (σ2)
Conjunctive Classifier (CC)
A more appropriate rule-based strategy in the conjunction task is a conjunction rule involving separate decisions about the stimulus value on the two dimensions with the response assignment based on the outcome of these two decisions (Ashby & Gott, 1988). The CC assumes that the participant partitions the stimulus space into four regions in a manner consistent with the optimal decision strategy. For example, for the line stimuli, the CC would assume that individuals assigned a stimulus to category B if it was high in length and low in orientation (i.e., the lines are long and shallow); otherwise the stimulus would be assigned to category A. The CC has three free parameters: the decision criteria on the two dimensions and a common value of σ2 for the two dimensions. The Optimal Conjunctive Classifier assumes that participants use decision bounds that maximize accuracy. This special case has one free parameter (σ2)
Information-Integration Model
The Linear Classifier (LC)
This model assumes that a linear decision bound partitions the stimulus space into two regions. The LC differs from the CC in that the LC does not assume decisional selective-attention (Ashby & Townsend, 1986). This produces an information-integration decision strategy because it requires linear integration of the perceived values on the stimulus dimensions. The LC has three parameters, slope and intercept of the linear bound, and σ2.
The Minimum Distance Classifier (MDC)
This model assumes that there are a number of units representing a low-resolution map of the stimulus space (Ashby & Waldron, 1999; Ashby, Waldron, Lee, & Berkman, 2001; Maddox, Filoteo et al., 2004). On each trial, the participant determines which unit is closest to the perceived stimulus and produces the associated response. The version of the MDC tested here assumed four units because the category structures were generated from four multivariate normal distributions. Because the location of one of the units can be fixed, and because a uniform expansion or contraction of the space will not affect the location of the minimum-distance decision bounds, the MDC has six free parameters (five determining the location of the units and σ2).
Random Responder Models
Equal Response Frequency (ERF)
This model assumes that participants randomly assign stimuli to the two response frequencies in a manner that preserves the category base rates (i.e., 50% of the stimuli in each category). This model has no free parameters.
Biased Response Frequency (BRF)
This model assumes that participants randomly assign stimuli to the two response frequencies in a manner that matches the participant’s categorization response frequencies (i.e., the percentage of stimuli in each category is computed from the observed response frequencies). This model has no free parameters.
Model Fitting
The model parameters were estimated using maximum likelihood (Ashby, 1992b; Wickens, 1982) and the goodness-of-fit statistic was
where N is the sample size, r is the number of free parameters, and L is the likelihood of the model given the data (Schwarz, 1978). The BIC statistic penalizes a model for poor fit and for extra free parameters. To find the best model among a set of competitors, one simply computes a BIC value for each model, and then chooses the model with the smallest BIC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The Wisconsin Card Sorting Task (WCST - Berg, 1948; Heaton et al., 1993) and Trail-Making (TM) subtest from the DKEFS were included as additional measures of executive function for the BG and PD patients, respectively. The difference in neuropsychological test batteries between the two patient groups is the result of the original design of two, patient-specific experiments. The BG patients did not significantly differ from control participants on the WCST [number of categories: t (11) = .56, p = .59, SE = 1.33; perseverative errors: t (11) = 1.12, p = .29, SE = 6.46; set-loss errors: t (11) = .36, p = .72, SE = .55] nor was performance on the WCST significantly associated with average accuracy on the conjunction task [number of categories: r (5) = .51, p = .38; perseverative errors: r (5) = −.58, p = .31; set-loss errors: r (5) = .11, p = .87]. Similarly, the PD patients did not significantly differ from control participants on the TM test [set shifting: t (31) = 1.52, p = .14, SE = 21.49] nor was performance on the TM test significantly associated with average accuracy on the unidimensional [set shifting: r (15) = −.11, p = .70] or conjunction tasks [set shifting: r (15) = .08, p = .77].
Pilot testing with healthy young controls revealed no difference in task difficulty as a function of stimulus type. There was a trend in both experiments for the patients and controls to perform worse with the rectangles varying in brightness and position. Importantly, the pattern of data for the patients in both experiments was present regardless of stimulus type.
On the unidimensional task, one PD patient and three control participants performed much worse than the average for their respective group means (> 2SD difference on overall accuracy and during the final block). These four participants were excluded from the analyses of these data. This PD patient was also tested OFF medication and was also excluded from the analysis of the effect of medication. On the conjunction task, one PD patient and one control were outliers and were excluded from the analyses of these data.
A Huynh-Feldt correction for violation of the sphericity assumption has been applied to this, and subsequent, mixed ANOVAs (when appropriate). Sidak multiple comparison correction used for these and all subsequent post hoc tests.
Counterbalancing medication state across the two testing sessions successfully minimized the impact of order effects as the difference in average accuracy (across blocks and participants) did not vary across testing sessions [Unidimensional: t(8) = −.31, p = .76, SE = 5.73; : t(9) = −.85, p = .42, SE = 4.39]. In addition, the use of different stimulus sets successfully minimized carry over effects between testing sessions as the correlations in average accuracy between testing sessions were small and non-significant [Unidimensional: r(9) = −.16, p = .69; Conjunction: r(10) = .1, p = .77].
All of the models include a free parameter to reflect the combined trial-by-trial variability in perceptual and criterial noise (see the Appendix for details). Given that the duration of stimulus presentation was unlimited, it is reasonable to assume that this internal noise primarily reflects variability in the decision criteria.
References
- Agid Y, Ruberg M, Dubois B, Pillon B. Anatomoclinical and biochemical concepts of subcortical dementia. In: Stahl SM, Iversen SD, Goodman EC, editors. Cognitive Neurochemistry. Oxford: Oxford University Press; 1987. pp. 248–271. [Google Scholar]
- Ashby FG. Multidimensional models of categorization. In: Ashby FG, editor. Multidimensional models of perception and cognition. Hillsdale, NJ: Erlbaum; 1992a. [Google Scholar]
- Ashby FG. Multivariate probability distributions. In: Ashby FG, editor. Multidimensional models of perception and cognition. Hillsdale: Lawrence Erlbaum Associates, Inc.; 1992b. pp. 1–34. [Google Scholar]
- Ashby FG, Alfonso-Reese LA, Turken AU, Waldron EM. A neuropsychological theory of multiple systems in category learning. Psychological Review. 1998;105:442–481. doi: 10.1037/0033-295x.105.3.442. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Ell SW, Valentin VV, Casale MB. FROST: A distributed neurocomputational model of working memory maintenance. Journal of Cognitive Neuroscience. 2005;17:1728–1743. doi: 10.1162/089892905774589271. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Gott RE. Decision rules in the perception and categorization of multidimensional stimuli. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1988;14:33–53. doi: 10.1037//0278-7393.14.1.33. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Lee WW. Perceptual variability as a fundamental axiom of perceptual science. In: Masin SC, editor. Foundations of percpetual theory. Amsterdam: Elsevier; 1993. pp. 369–399. [Google Scholar]
- Ashby FG, Maddox WT. Human category learning. Annual Review of Psychology. 2005;56:149–178. doi: 10.1146/annurev.psych.56.091103.070217. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Noble S, Filoteo JV, Waldron EM, Ell SW. Category Learning Deficits in Parkinson's Disease. Neuropsychology. 2003;17:115–124. [PubMed] [Google Scholar]
- Ashby FG, Queller S, Berretty PM. On the dominance of unidimensional rules in unsupervised categorization. Perception & Psychophysics. 1999;61:1178–1199. doi: 10.3758/bf03207622. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Townsend JT. Varieties of perceptual independence. Psychological Review. 1986;93:154–179. [PubMed] [Google Scholar]
- Ashby FG, Waldron EM. The nature of implicit categorization. Psychonomic Bulletin & Review. 1999;6:363–378. doi: 10.3758/bf03210826. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer R, Brown G. Beck Depression Inventory - Second edition manual. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Benke T, Delazer M, Bartha L, Auer A. Basal ganglia lesions and the theory of frontosubcortical loops: Neuropsychological findings in two patients with left caudate lesions. Neurocase. 2003;9:70–85. doi: 10.1076/neur.9.1.70.14374. [DOI] [PubMed] [Google Scholar]
- Berg EAA. A simple objective test for measuring flexibility in thinking. Journal of General Psychology. 1948;39:15–22. doi: 10.1080/00221309.1948.9918159. [DOI] [PubMed] [Google Scholar]
- Blakemore C, Campbell FW. On the existence of neurones in the human visual system selectively sensitive to the orientation and size of retinal images. Journal of Physiology. 1969;203:237–260. doi: 10.1113/jphysiol.1969.sp008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodis-Wollner I, Marx MS, Mitra S, Bobak P, Mylin L, Yahr M. Visual dysfunction in Parkinson's disease: Loss in spatiotemporal contrast sensitivity. Brain. 1987;110:1675–1698. doi: 10.1093/brain/110.6.1675. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rüb U, De Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurbiology of Aging. 2003;24:197–210. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Brett M, Leff AP, Rorden C, Ashburner J. Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage. 2001;14:486–500. doi: 10.1006/nimg.2001.0845. [DOI] [PubMed] [Google Scholar]
- Brooks DJ, Piccini P. Imaging in Parkinson's disease: the role of monamines in behavior. Biological Psychiatry. 2006;59:908–918. doi: 10.1016/j.biopsych.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Cedarbaum JM. Clinical pharmacokinetics of anti-parkinsonian drugs. Clinical Pharmacokinetics. 1987;3:141–178. doi: 10.2165/00003088-198713030-00002. [DOI] [PubMed] [Google Scholar]
- Chang C, Crottaz-Herbette S, Menon V. Temporal dynamics of basal ganglia response and connectivity during verbal working memory. Neuroimage. 2007;34:1253–1269. doi: 10.1016/j.neuroimage.2006.08.056. [DOI] [PubMed] [Google Scholar]
- Cools R. Dopaminergic modulation of cognitive function: implications for L-DOPA treatment in Parkinson's disease. Neuroscience and Biobehavioral Reviews. 2006;30:1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or impaired cognitive function in Parkinson's disease as a function of dopaminergic medication and task demands. Cerebral Cortex. 2001;11:1136–1143. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. L-Dopa medication remediates cognitive inflexibility, but increases impulsivity in patients with Parkinson's disease. Neuropsychologia. 2003;41:1431–1441. doi: 10.1016/s0028-3932(03)00117-9. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue SE, Bunge SA. Neural evidence for dissociable components of task-switching. Cerebral Cortex. 2006;16:475–486. doi: 10.1093/cercor/bhi127. [DOI] [PubMed] [Google Scholar]
- Davidsdottir S, Cronin-Golomb A, Lee A. Visual and spatial symptoms in Parkinson's disease. Vision Research. 2005;45:1285–1296. doi: 10.1016/j.visres.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Functioning System. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Dingemanse J, Jorga K, Zurcher G, Schmitt M, Sedek G, Da Prada M, Van Brummelen P. pharmacokinetic-pharmacodynamic interaction between the COMT inhibitor tolcapone and single-dose levodopa. British Journal of Clinical Pharmacology. 1995;40:253–262. doi: 10.1111/j.1365-2125.1995.tb05781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds CM, Clark L, Dove A, Regenthal R, Baumann F, Bullmore E, Robbins TW, Müller U. The dopamine D2 receptor antagonist sulpiride modulates striatal BOLD signal during the manipulation of information in working memory. Psychopharmacology. 2009;207:35–45. doi: 10.1007/s00213-009-1634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ell SW, Ing AD, Maddox WT. Criterial noise effects on rule-based category learning: The impact of delayed feedback. Attention, Perception, & Psychophysics. 2009;71:1263–1275. doi: 10.3758/APP.71.6.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ell SW, Marchant NL, Ivry RB. Focal putamen lesions impair learning in rule-based, but not information-integration categorization tasks. Neuropsychologia. 2006;44:1737–1751. doi: 10.1016/j.neuropsychologia.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton R . Members of the UPDRS Development Committee. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent developments in Parkinson's disease. Vol. 2. Florham Park, NH: Macmillan Health Care Information; 1987. pp. 153–163.pp. 293–304. [Google Scholar]
- Filoteo JV, Maddox WT, Ing AD, Song DD. Characterizing rule-based category learning deficits in patients with Parkinson's disease. Neuropsychologia. 2007;45:305–320. doi: 10.1016/j.neuropsychologia.2006.06.034. [DOI] [PubMed] [Google Scholar]
- Filoteo JV, Maddox WT, Ing AD, Zizak V, Song DD. The impact of irrelevant dimensional variation on rule-based category learning in patients with Parkinson's disease. Journal of the International Neuropsychological Society. 2005;11:503–513. doi: 10.1017/S1355617705050617. [DOI] [PubMed] [Google Scholar]
- Filoteo JV, Maddox WT, Simmons AN, Ing AD, Cagigas XE, Matthews S, Paulus MP. Cortical and subcortical brain regions involved in rule-based category learning. NeuroReport. 2005;16:111–115. doi: 10.1097/00001756-200502080-00007. [DOI] [PubMed] [Google Scholar]
- Folstein M, Folstein SE, McHugh PR. "Mini-Mental State" a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frank MJ. Dynamic dopamine modulation in the basal ganglia:A neurocomputational account of cognitive deficits in medicated and non-medicated Parkinsonism. Journal of Cognitive Neuroscience. 2005;17:51–72. doi: 10.1162/0898929052880093. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Seeberger LC, O'Reilly RC. By carrot or by stick: Cognitive reinforcement learning in Parkinsonism. Science. 2004;306:1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal detection theory and psychophysics. New York: Wiley; 1966. [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. Journal of Chemical Neuroanatomy. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Test manual. Odessa, FL: Psychological Assessment Resources, Inc.; 1993. [Google Scholar]
- Hikosaka O, Sakamoto M, Sadanari U. Functional properties of monkey caudate neurons III. Activities related to expectation of target and reward. Journal of Neurophysiology. 1989;61:814–831. doi: 10.1152/jn.1989.61.4.814. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: Onset, progression, and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Holm KJ, Spencer CM. Entacapone: a review of its use in Parkinson's disease. Drugs. 1999;58:159–177. doi: 10.2165/00003495-199958010-00017. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Wilkinson L, Gahir H, Dharminda A, Lagnado DA. Medication impairs probabilistic classification learning in Parkinson's disease. Neuropsychologia. 2010;48:1096–1103. doi: 10.1016/j.neuropsychologia.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Kehagia AA, Cools R, Barker RA, Robbins TW. Switching between abstract rules reflects disease severity but not dopaminergic status in Parkinson's disease. Neuropsychologia. 2009;47:1117–1127. doi: 10.1016/j.neuropsychologia.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Keri S, Beniczky S, Voros E, Janka Z, Benedek G, Vecsei L. Dissociation between attentional set shifting and habit learning: a longitudinal case study. Neurocase. 2002;8:219–225. doi: 10.1093/neucas/8.3.219. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Shannak K, Hornykiewicz O. Uneven patterns of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. New England Journal of Medicine. 1988;318:876–880. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Kompoliti K, Adler CH, Raman R, Pincus JH, Leibowitz MT, Ferry JJ, Blasucci L, Caviness JN, Leurgans S, Chase WM, Yones LC, Tan E, Carvey P, Goetz CG. Gender and pramipexole effects on levodopa pharmacokinetics and pharmacodynamics. Neurology. 2002;58:1418–1422. doi: 10.1212/wnl.58.9.1418. [DOI] [PubMed] [Google Scholar]
- Lawrence AD, Watkins LHA, Sahakian BJ, Hodges JR, Robbins TW. Visual object and visuospatial cognition in Huntington's disease: implications for information processing in corticostriatal circuits. Brain. 2000;123:1349–1364. doi: 10.1093/brain/123.7.1349. [DOI] [PubMed] [Google Scholar]
- Longworth CE, Keenan SE, Barker RA, Marslen-Wilson WD, Tyler LK. The basal ganglia and rule-governed language use: evidence from vascular and degenerative conditions. Brain. 2005;128:584–596. doi: 10.1093/brain/awh387. [DOI] [PubMed] [Google Scholar]
- Maddox WT. Percepetual and decisional separability. In: Ashby FG, editor. Multidimensional models of perception and cognition. Hillsdale, NJ: Lawrence Erlbaum Associates; 1992. pp. 147–180. [Google Scholar]
- Maddox WT, Ashby FG. Comparing decision bound and exemplar models of categorization. Perception & Psychophysics. 1993;53:49–70. doi: 10.3758/bf03211715. [DOI] [PubMed] [Google Scholar]
- Maddox WT, Ashby FG. Dissociating Explicit and Procedural-Learning Based Systems of Perceptual Category Learning. Behavioral Processes. 2004;66:309–332. doi: 10.1016/j.beproc.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Maddox WT, Ashby FG, Waldron EM. Multiple attention systems in perceptual categorization. Memory & Cognition. 2002;30:325–339. doi: 10.3758/bf03194934. [DOI] [PubMed] [Google Scholar]
- Maddox WT, Filoteo JV, Hejl KD, Ing AD. Category Number Impacts Rule-Based but not Information-Integration Category Learning: Further Evidence for Dissociable Category Learning Systems. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2004;30:227–235. doi: 10.1037/0278-7393.30.1.227. [DOI] [PubMed] [Google Scholar]
- Martin JH. Neuroanatomy: Text and atlas. 2nd ed. Stamford, CT: Appleton & Lange; 1996. [Google Scholar]
- Monchi O, Petrides M, Petre V, Worsley K, Dagher A. Wisconsin Card Sorting Revised: Distinct Neural Circuits Participating in Different Stages of the Task Identified by Event-Related Functional Magnetic Resonance Imaging. The Journal of Neuroscience. 2001;21:7733–7741. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustafa AA, Gluck MA. A Neurocomputational Model of Dopamine and Prefrontal-Striatal Interactions during Multicue Category Learning by Parkinson's Patients. Journal of Cognitive Neuroscience. doi: 10.1162/jocn.2010.21420. (in press) [DOI] [PubMed] [Google Scholar]
- Nelson HE. National adult reading test (NART) test manual. Windsor: NFER-Nelson; 1982. [Google Scholar]
- Owen AM, Roberts AC, Hodges JR, Summers BA, Polkey CE, Robbins TW. Contrasting mechanisms of impaired attentional set-shifting in patients with frontal lobe damage or Parkinsons's disease. Brain. 1993;116:1159–1175. doi: 10.1093/brain/116.5.1159. [DOI] [PubMed] [Google Scholar]
- Pasupathy A, Miller EK. Different time courses of learning-related activity in the prefrontal cortex and striatum. Nature. 2005;433:873–876. doi: 10.1038/nature03287. [DOI] [PubMed] [Google Scholar]
- Pickett ER, Kuniholm E, Protopapas A, Friedman J, Lieberman P. Selective speech motor, syntax and cognitive deficits associated with bilateral damage to the putamen and the head of the caudate nucleus: a case study. Neuropsychologia. 1998;36:173–188. doi: 10.1016/s0028-3932(97)00065-1. [DOI] [PubMed] [Google Scholar]
- Price A. Explicit category learning in Parkinson's disease: Deficits related to impaired rule generation and selection processes. Neuropsychology. 2006;20:249–257. doi: 10.1037/0894-4105.20.2.249. [DOI] [PubMed] [Google Scholar]
- Price A, Filoteo JV, Maddox WT. Rule-based category learning in patients with Parkinson's disease. Neuropsychologia. 2009;47:1213–1226. doi: 10.1016/j.neuropsychologia.2009.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SM, Bobholz JA, Hammeke TA, Rosen AC, Woodley SJ, Cunningham JM, Cox RW, Stein EA, Binder JR. Functional MRI evidence for subcortical participation in conceptual reasoning skills. Neuroreport. 1997;27:1987–1993. doi: 10.1097/00001756-199705260-00038. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behavioural Neurology. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Salatas H, Bourne LE. Learning Conceptual Rules III: Processes contributing to rule difficulty. Memory & Cognition. 1974;2:549–553. doi: 10.3758/BF03196919. [DOI] [PubMed] [Google Scholar]
- Schwarz G. Estimating the dimension of a model. The Annals of Statistics. 1978;6(2):461–464. [Google Scholar]
- Seger CA. How do the basal ganglia contribute to categorization? Their roles in generalization, response selection, and learning via feedback. Neuroscience and Biobehavioral Reviews. 2008;32:265–278. doi: 10.1016/j.neubiorev.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger CA, Cincotta CM. Dynamics of frontal, striatal, and hippocampal systems during rule learning. Cerebral Cortex. 2006;16:1546–1555. doi: 10.1093/cercor/bhj092. [DOI] [PubMed] [Google Scholar]
- Shepard RN, Hovland CI, Jenkins HM. Learning and memorization of classifications. Psychological Monographs. 1961;75 ((13, Whole No. 517)) [Google Scholar]
- Shohamy D, Myers CE, Geghman KD, Sage J, Gluck MA. L-Dopa impairs learning, but spares generalization, in Parkinson's disease. Neuropsychologia. 2006;44:774–784. doi: 10.1016/j.neuropsychologia.2005.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop RJ. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Swainson R, Robbins TW. Rule-abstraction deficits following a basal ganglia lesion. Neurocase. 2001;7:433–443. doi: 10.1076/neur.7.5.433.16248. [DOI] [PubMed] [Google Scholar]
- Teichmann M, Dupoux E, Kouider S, Brugieres P, Boisse MF, Baudic S, Cesaro P, Peschanski M, Bachoud-Levi AC. The role of the striatum in rule application: the model of Huntington's disease at early stage. Brain. 2005;128:1155–1167. doi: 10.1093/brain/awh472. [DOI] [PubMed] [Google Scholar]
- Ullman MT. Contributions of memory circuits to language: the declarative/procedural model. Cognition. 2004;92:231–270. doi: 10.1016/j.cognition.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3rd ed. San Antonio: Harcourt Assessment; 1997a. [Google Scholar]
- Wechsler D. Wechsler Memory Scale. 3rd ed. San Antonio: Harcourt Assessment; 1997b. [Google Scholar]
- Wickens TD. Models for behavior: Stochastic processes in psychology. San Francisco: W. H. Freeman; 1982. [Google Scholar]