Abstract
OBJECTIVE
Neointimal hyperplasia is an inflammatory and proliferative process that occurs as a result of injury to the vessel wall. We have shown that the homeostatic protein A20 prevents neointimal hyperplasia by affecting endothelial cell (EC) and smooth muscle cell (SMC) responses to injury. In this work, we questioned whether A20 impacts other pathogenic effectors of neointimal hyperplasia including homing of monocyte/macrophages and EC/SMC precursors to the site of vascular injury, vascular endothelial growth factor (VEGF) secretion, and adventitial neovascularization.
METHODS AND RESULTS
Carotid balloon angioplasty was performed on rat recipients of a bone marrow transplant from green fluorescent rats. Adenoviral delivery of A20 prevented neointimal hyperplasia and decreased macrophage infiltration. This was associated with decreased ICAM-1 and MCP-1 expression in vitro. Additionally, A20 reduced neovascularization in the adventitia of balloon injured carotid arteries, which correlated with fewer VEGF positive cells.
CONCLUSIONS
A20 down-regulates adhesion markers, chemokine production, and adventitial angiogenesis, all of which are required for macrophage trafficking to sites of vascular injury. This, in turn, diminishes the inflammatory milieu to prevent neointimal hyperplasia.
Keywords: Neointimal Hyperplasia, Vascular Endothelial Growth Factor, Neovascularization, Macrophage
1. Introduction
The hallmark of neointimal hyperplasia is the acquisition of an inflammatory and proliferative SMC phenotype that occurs as a result of injury to the vessel wall. Accordingly, signaling through the transcription factor NF-κB is a crucial early step in the development of neointimal hyperplasia.1, 2 In animal models, direct blockade of NF-κB with decoy oligodeoxynucleotides reduces neointimal hyperplasia through down regulating the inflammatory response.3–5 As demonstrated by our group and others, overexpression of the NF-κB dependent NF-κB inhibitory protein A20 prevents neointimal hyperplasia following rat carotid balloon injury, and reverts pre-existing lesions.6, 7
Vascular endothelial growth factor (VEGF) is a powerful angiogenic factor that has been associated with post-angioplasty restenosis and vein graft failure due to pathologic adventitial neovascularization.8–10 VEGF also has proinflammatory properties that promote infiltration and activation of monocytes, further exacerbating vascular disease.11, 12 Additionally, VEGF promotes homing of EC and SMC progenitors neointimal lesions via the Flt-1 receptor.13–15
Having previously demonstrated the ability of A20 to protect against neointimal hyperplasia, we hypothesized that A20 might also affect adventitial neovascularization and monocyte/macrophage infiltration, as well as homing of EC and SMC progenitors to the neointimal leison. To address this, we utilized a model of rat carotid balloon injury in which the rats had undergone bone marrow transplantation (BMT) from donor rats that ubiquitously expressed green fluorescent protein (GFP) so that we could track monocyte trafficking and bone marrow-derived EC and SMC contributing to neointimal lesions.
2. Materials and methods
2.1 Cell culture
Cells were grown at 37°C in 5% CO2. Human coronary artery SMC (Lonza, Walkersville, Maryland; Genlantis, San Diego, California) were grown in SMGM-2 (Lonza). Human coronary artery EC (Clonetics, San Diego, California) were grown in EGM MV-2 (Lonza). EC and SMC were used between passages 6 and 9. U937 and HEK293T cells were obtained from the American Type Culture Collection (Manassas, Virginia). Human recombinant VEGF was from R&D Systems (Minneapolis, Minnesota), as were IL1-β, TNFα, and IFNγ which were used at 100 U/ml, 400 U/ml, and 400 U/ml, respectively. Recombinant adenoviral vectors were generated in our laboratory as previously described, and as outlined in the supplemental methods.16 SMC were transduced at a multiplicity of infection (MOI) of 500 plaque forming units (pfu) per cell and EC were transduced at 100 pfu per cell, resulting in expression of the transgene in 95–100% of cells, without toxicity.
2.2 Bone Marrow Transplant
BMT was performed as described previously15 from green fluorescent protein (GFP) transgenic Sprague-Dawley rat (SLC, Shizuoka, Japan) donors to wild type Sprague-Dawley recipients (Harlan Industries, Indianapolis). Recipient rats were irradiated with 10 Gy (Gammacell 40 Exactor, Nordion International Inc., Kanata, Canada) 24 hours prior to injection with 1×108 unfractionated bone marrow cells. Engraftment was confirmed when >80% of peripheral blood cells were GFP–positive.
2.3 Balloon Angioplasty
Balloon injury was performed on the common carotid artery of adult male (300–400 g) GFP BMT recipient rats using a 2 Fr embolectomy catheter (Baxter Edwards, Irvine, California).17 The injured vessel was then filled with 30µl of PBS containing 5×108 pfu of recombinant adenovirus for 20 min.6
2.5 Histology, Immunohistochemistry, and Immunofluorescence
Morphometric analysis was performed on hematoxylin and eosin stained sections of carotid arteries (ImageJ 1.38×, National Institutes of Health, Bethesda, Maryland). Six to ten 5 µm serial sections spaced 50 µm apart were examined from each animal in a blinded fashion and intima to media (I/M) ratios were calculated based on cross-sectional area. For immunohistochemistry, sections were incubated with anti–ICAM–1 (PharMingen, San Diego, California) or anti–p65 (Santa Cruz Biotechnology, Santa Cruz, California) followed by biotinylated anti–mouse IgG (Vector, Burlingame, California) and developed with ImmPACT DAB (Vector). For immunofluorescence, sections were incubated with anti- ED1 (PharMingen), VEGF (Cell Signaling, Danvers, Massachusetts), or CD31 (Santa Cruz Biotechnology) primary antibodies followed AlexaFluor 594 conjugated secondary antibodies (Invitrogen, Carlsbad, California). Nuclei were imaged with Hoechst (Invitrogen). Representative images of IHC/IF sections were evaluated in a blinded fashion. VEGF, CD31, and ED1 IF was evaluated by ordered ranking of overall marker expression based on the number and intensity of positive cells per field; ICAM and p65 were evaluated by subjective grading using the following scale: 1 = no staining, 2 = faint, scattered staining, 3 = faint diffuse or focal intense staining, 4 = intense, diffuse staining.
2.6 Migration Assays
Migration assays were performed by the modified Boyden method.18 4×105 serum starved HCAEC were seeded in a BD BioCoat Fibronectin Cell Culture Insert (San Jose, California) with conditioned supernatant from SMC that had been transduced with rAd.A20 or rAd.βgal and stimulated with IL-1β, TNFα, and IFNγ for 24 hours. After overnight incubation HCAEC that had migrated across the membrane were quantified by light microscopy.
2.7 Adhesions Assays
U973 monocytes were labeled with 2’,7’–bis–(2–carboxyethyl)–5–(and–6)–carboxyfluorescein acetoxymethyl ester (Molecular Probes, Eugene, Oregon) as described19 and incubated in a ratio of 10:1 with SMC monolayers that had been transduced with rAd.A20 or rAd.βgal 72 hours prior and stimulated with IL–1β, TNFα and IFNγ 24 hours prior. After 1 hour of incubation, non–adherent U937 cells were removed by washing and attached cells were lysed. Fluorescence was measured at wavelengths of 485 and 530 nm (Wallac 1430, Perkin Elmer). To control for between assay variability, adherence of U937 macrophages is standardized to that of U937 cells that adherent to non-stimulated, non-transduced SMC, and expressed as relative fluorescence.
2.8 Statistics
Unpaired student’s t–tests with Welch’s correction and ANOVA with Tukey’s test were used to compare groups. The Mann-Whitney and Kruskal-Wallis test with Dunn’s Correction for multiple comparisons were used for non-parametric data, including analysis of IF/IHC grading/ranking. (InStat 3 & Prism, GraphPad Software, La Jolla, California).
3. Results
3.1 A20 prevents post-angioplasty neointimal hyperplasia
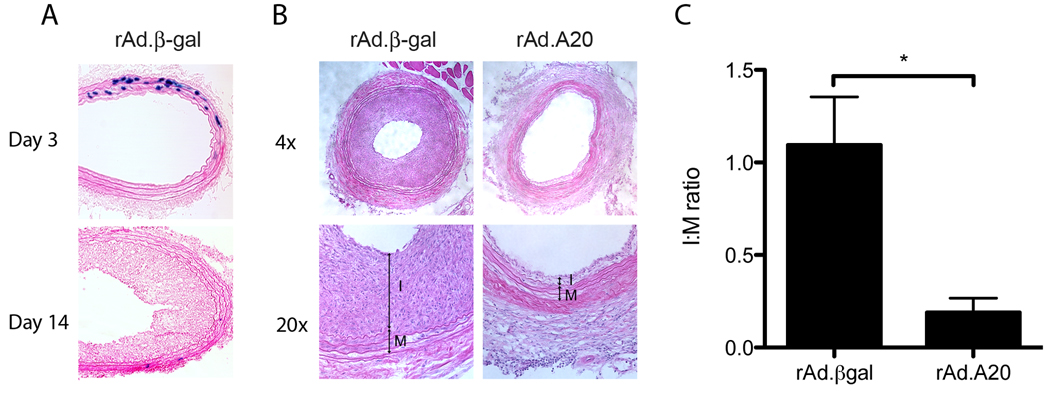
Carotid artery balloon injury followed by adenoviral treatment resulted in transgene expression in 30–40% of medial SMC by day 3 and lasted for up to 2 weeks (Figure. 1A). At 21 days post injury rats treated with rAd.βgal developed significant neointimal hyperplasia (Figure 1B&C), as previously reported,20 demonstrating that the myeloabaltive BMT protocol did not affect the response to balloon injury. In contrast, rats treated with rAd.A20 had negligible neointimal hyperplasia, with a significantly lower I/M ratio (n=5 per group; p=0.03). IF microscopy for α-actin demonstrated no co-localization with GFP, indicating that the SMC of the neointima were not of bone-marrow derived origin.
Figure 1.
A) Representative section showing rAd.βgal transduction. B) H&E sections demonstrating neointimal and medial layers. C) Quantitative morphometric analysis of I:M ratios. (p<0.05; n=5 per group)
3.2 A20 prevents neointimal hyperplasia through down-regulating inflammation
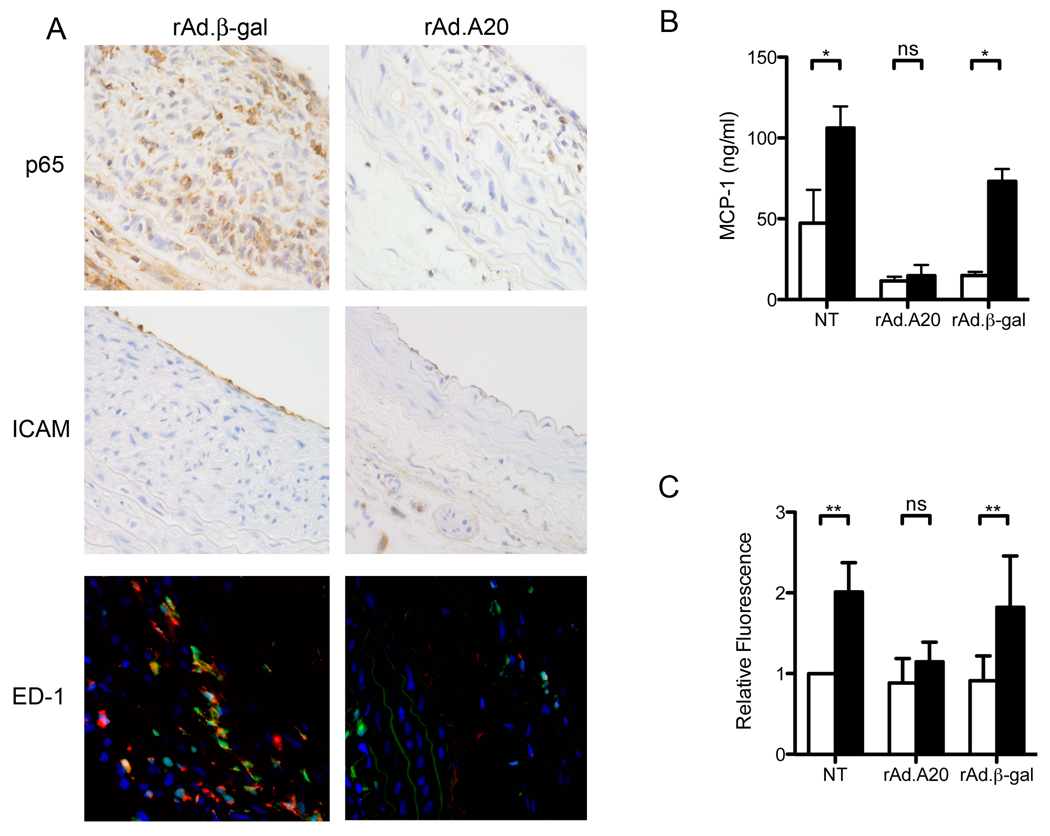
Because A20 is known to be a negative regulator of NF-κB, balloon injured carotid arteries were examined for inflammatory markers (Figure 2A). rAd.A20 treated vessels had significantly less macrophage infiltration than rAd.β-gal controls (n=5 per group, P=0.01). There was also a trend towards less nuclear p65 immunostaining in the neointima, and less ICAM-1 on the luminal endothelium, in rAd.A20 treated vessels; this, however, failed to reach statistical significance. This demonstrates that overexpression of A20 lowers the levels of inflammation and decreases macrophage infiltration following balloon injury.
Figure 2.
A) Representative immunofluorescence and immunhistorchemistry micrographs of injured rat carotids treated with rAd.βgal (n=5) or rAd.A20 (n=5) at 21 days. ED-1 positive macrophages appear orange due to the overlay of GFP and ED-1 (red). B) MCP-1 ELISA of cell culture supernatant taken at 48 h from NT, rAd.A20, and rAd.βgal transduced SMC cultured with (■) or without (□) inflammatory cytokines. Error bars represent SEM (n=3). C) Adhesion assays performed with SMC cultured with (■) or without (□) inflammatory cytokines. Error bars represent SEM (n=8). * P< 0.05, ** P<0.01
Macrophage infiltration depends on cellular recruitment to the site of injury and adhesion to the vessel wall. To test how A20 affects these processes, SMC were assayed for their ability to secrete monocyte chemoatractant protein 1 (MCP-1) after stimulation with inflammatory cytokines. Cytokine stimulation significantly increased MCP-1 mRNA (supplemental Figure 1) and protein levels (Figure 2B) in control, non-transduced (NT) or rAd.βgal transduced SMC (n=3;p<0.01), whereas A20 over-expression prevented cytokine induced increases in MCP-1 secretion (n=3). A20 had a similar effect in abrogating cytokine-mediated upregulation of ICAM-1 and VCAM-1 in SMC in culture (supplemental Figure 2).
In vitro monocyte adhesion assays were performed to determine if A20 could block monocyte adherence to SMC. Stimulation of NT or rAd.βgal transduced SMC with pro-inflammatory cytokines for 24 hours promoted a 2-fold increase in monocyte adherence (Figure 2C) (n=8; p<0.001). Over-expression of A20 abrogated this, yielding no significant increase in fluorescence following cytokine stimulation (n=8;p>0.05).
3.3 A20 prevents adventitial neovascularization
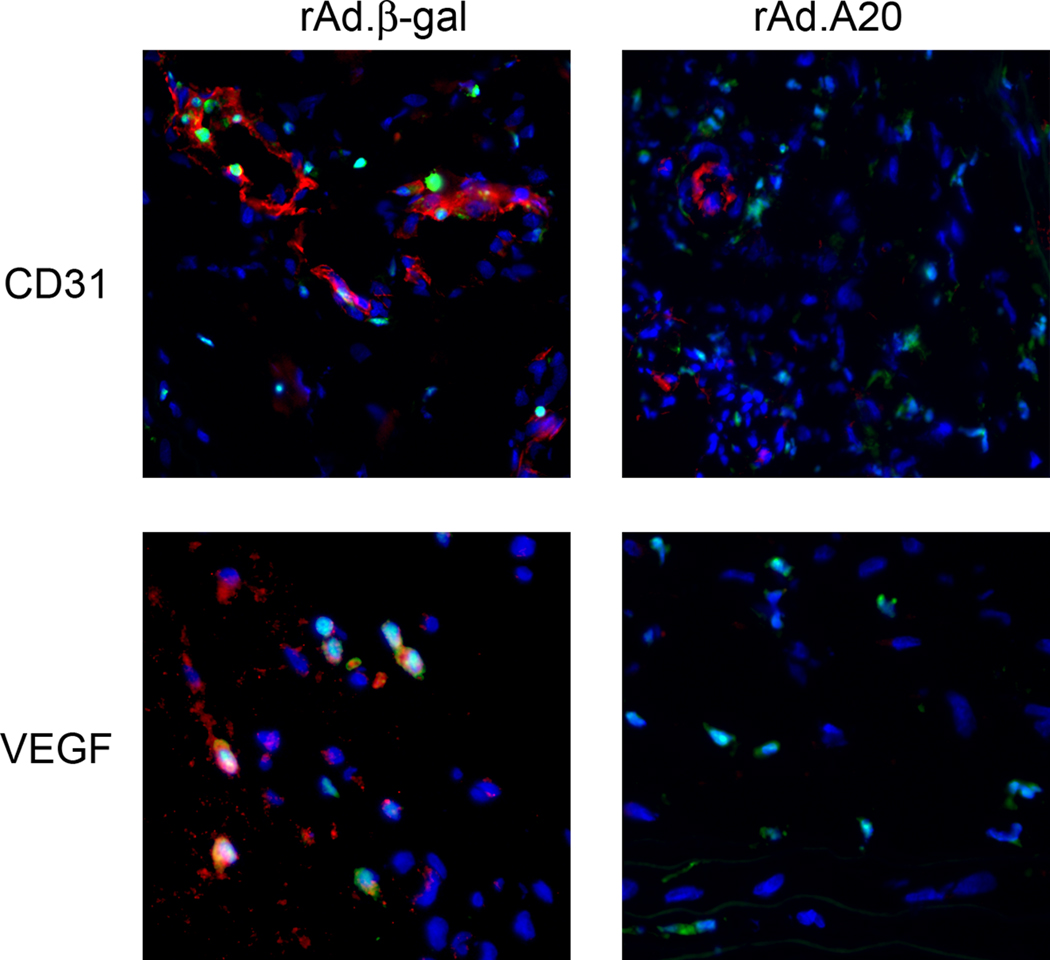
To determine the role of A20 in regulating neovascularization, balloon injured carotid arteries were probed for the EC specific marker CD31 by IF microscopy (Figure 3). rAd.A20 treated vessels had significantly less adventitial neovascularization than rAd.β-gal treated controls (n=5 per group, P=0.01). Some of the CD31 positive cells were also positive for GFP, indicating that they likely originated from bone marrow-derived EC progenitors. Correlating with the reduced neovascularization, there were significantly fewer VEGF expressing cells in the adventitia of rAd.A20 treated vessels as compared to rAd.βgal treated controls (n=5 per group, P=0.008). A20 overexpression did not affect VEGF mRNA or protein expression in SMC cultures (supplemental Figure 3), suggesting that A20’s effect on VEGF secretion is mediated indirectly. Interestingly, some of the VEGF positive cells were also GFP positive, indicating that they were of bone marrow origin, quite possibly monocytes that had trafficked to the injured vessel.
Figure 3.
Representative immunofluorescent micrographs staining for CD31 and VEGF (red) superimposed with GFP (green) staining of hematopoietic lineage cells (n=5 per group).
3.4 Re-endothelialization proceeds from vascular derived and not bone marrow derived CD31 progenitors
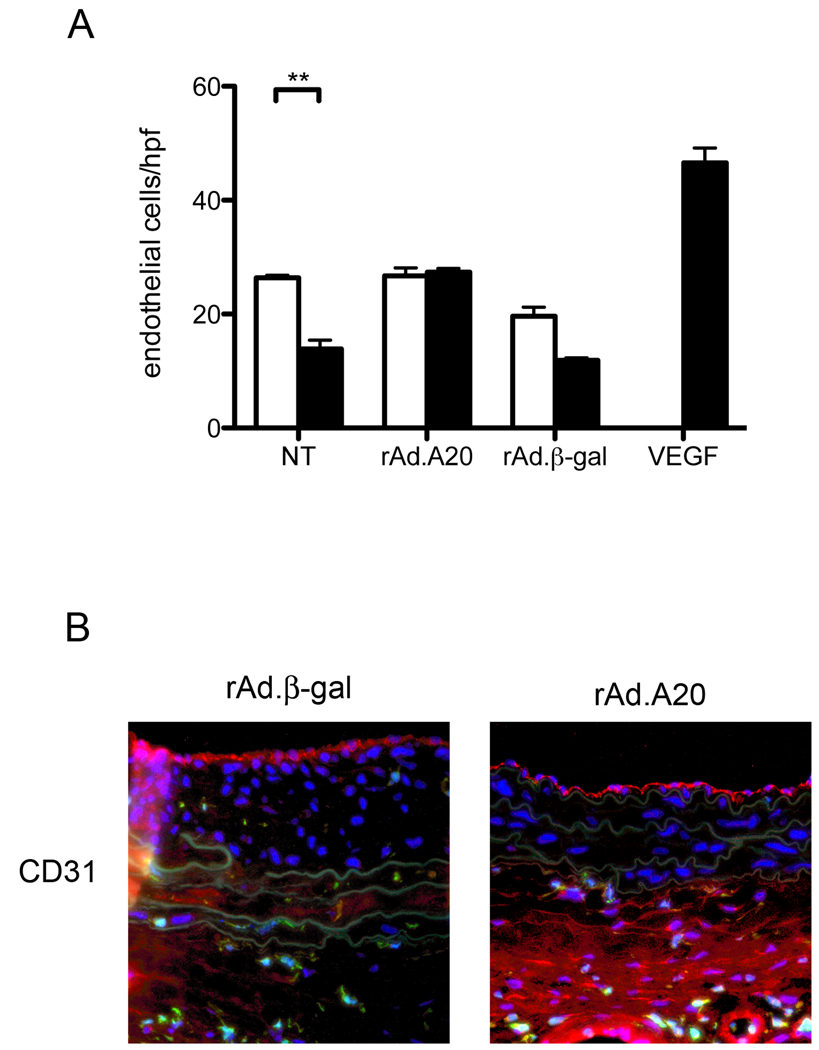
We have previously reported that A20 overexpression in injured carotid arteries increases the rate of re-endothelialization, with new CD31 positive cells appearing on the vessel lumen in rAd.A20 treated arteries at 7 days post injury.20. In order to demonstrate that this accelerated re-endothelialization was the result of A20 over-expression in medial SMC, we performed in vitro EC migration assays towards SMC conditioned media. EC migration was significantly decreased when using media from cytokine stimulated non-transduced SMC, as opposed to media from non-stimulated non-transduced SMC (n=3; p<0.01; Figure 4A). A similar, although not quite significant trend was seen with rAd.βgal transduced SMC. In contrast, medium from cytokine stimulated rAd.A20 transduced SMC had no effect on EC migration when compared to medium from non-stimulated rAd.A20 transduced SMC (n=3;p>0.05). These results indicate that although A20 overexpression reduces the migration of inflammatory cells (Figure 2) and of CD31+ EC precursors that contribute to pathological angiogenesis (Figure 3, bottom panels), it enhances the migration of EC that aid in luminal re-endotheliaization.
Figure 4.
A) EC migration assays using conditioned media from SMC cultured with (■) or without (□) inflammatory cytokines. Error bars represent SEM. (n=−3) * P<0.01, $ P<0.01. B) Representative (n=5 per group) immunofluorescence staining for CD31 (red), GFP (green), and Hoechst (blue). *** P<0.001
To determine whether in vivo re-endothelialization proceeds from bone marrow-derived progenitors, injured carotid segments were examined for the co-expression of CD31 and GFP on the luminal surface of the vessel. CD31-positive endothelial cells lining the vessel lumen were GFP negative in both rAd.A20 and rAd.βgal treated vessels (Figure 4B), indicating that they did not originate from the bone marrow.
4. Discussion
We have previously reported that over-expression of A20 by adenoviral mediated gene transfer to rat carotid arteries reduced post-angioplasty neointimal hyperplasia and reverted existing neointimal hyperplasia by decreasing smooth muscle cell inflammation and proliferation, and by promoting neointimal SMC apoptosis.6 In this work, we extend our understanding of the protective effects of A20 by examining downstream inflammatory events. We show that overexpression of A20 in rat carotid arteries results in decreased macrophage infiltration, reduced number of VEGF-producing cells, and less adventitial neovascularization.
Monocyte recruitment is an early component of the vascular response to injury.21, 22 Following de-endothelialization, chemokine secretion by SMC triggers the infiltration of bone marrow derived macrophages, which serve to entrench the inflammatory conditions and fuel neointimal development. Our demonstration that over-expression of A20 in the vessel blocks macrophage recruitment unravels an important mechanism involved in how A20 prevents neointimal hyperplasia. This effect of A20 results from its impact on multiple steps in the pathway leading to monocyte/macrophage accumulation. A20 inhibits SMC secretion of MCP-1, a chemokine necessary for attraction and transmigration of macrophages and reduces the expression of ICAM-1, a cell surface adhesion protein necessary for macrophage arrest. Our observation that these anti-inflammatory effects of A20 contribute to decreasing neointimal hyperplasia is supported by evidence from animal studies that show that direct blockade of ICAM-1 or MCP-1 diminishes in-stent and vein-bypass stenosis.23–25
In addition to its direct effects on ICAM-1 and MCP-1, A20 expression in the vessel wall may also affect macrophage trafficking through its effects on VEGF expression. VEGF is known to be present at high levels in lesions of neointimal hyperplasia26–28 and is thought to play a role in macrophage recruitment and activation through the Flt-1 receptor,11 as well as through NF-κB dependent upregulation of ICAM-1, VCAM-1, E-selectin, and MCP-1.12, 29 In our model, overexpression of A20 reduced the number of adventitial cells expressing VEGF. Some of the VEGF positive cells were of bone marrow origin, and likely represent VEGF producing macrophages. Ongoing studies in our laboratory are aimed at identifying this cell population.
VEGF secretion in the injured vessel also promotes the development of pathologic adventitial neovessels. Accordingly, the lower VEGF levels in rAd.A20 treated carotid arteries was associated with decreased numbers of adventitial neovessels. Given that the presence of these vessels has been associated with lesion progression,8, 10 this reveals another possible layer of protection afforded by A20 overexpression. Importantly the inhibitory effect of A20 upon neovascularization and attraction of EC progenitors to the site of vascular injury was limited to the adventitia and did not affect luminal re-endothelialization, a process that also requires endothelial cell migration. Rather, we have shown that luminal re-endothelialization is accelerated in rAd.20 transduced vessels.6 We confirmed this by demonstrating that expression of A20 in SMC helps maintain adequate EC migration despite an inflammatory milieu, indicating that expression of A20 in SMC inhibits the secretion of factors that impair vascular healing. This is in agreement with reports that stents coated with the anti-VEGF antibody bevacizumab inhibit neointimal hyperplasia without affecting endothelialization.30 Current experiments are underway in our laboratory to elucidate the mechanisms behind A20s paradoxical effects on EC migration.
VEGF has also been postulated to be involved in the mobilization and migration of bone marrow derived SMC to the neointima.15 We were unable to detect any bone marrow derived SMC in the neointima of our control rats, making it impossible to demonstrate the effect of A20 on SMC progenitor migration.
Overall, our data shed new light onto the mechanisms by which overexpression of A20 can inhibit the formation of neointimal hyperplasia through modulating downstream inflammatory events to decrease macrophage trafficking and adventitial neovascularization and to improve luminal re-endothelialization and healing. From a clinical standpoint, these novel data provide yet further evidence for the suitability of A20-based gene therapy to prevent post-angioplasty restenosis.
Supplementary Material
Acknowledgements
This research was supported by NIH grant RO1 HL080130 and in part by NIH grants RO1 DK063275, RO1HL57791, Juvenile Diabetes Research Foundation grant 1-2007-567 to CF. SMD, MDF, JJS, and VIP were supported by NIH T32 HL07734.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bourcier T, Sukhova G, Libby P. The nuclear factor kappa-B signaling pathway participates in dysregulation of vascular smooth muscle cells in vitro and in human atherosclerosis. J Biol Chem. 1997;272:15817–15824. doi: 10.1074/jbc.272.25.15817. [DOI] [PubMed] [Google Scholar]
- 2.Zuckerbraun BS, McCloskey CA, Mahidhara RS, Kim PKM, Taylor BS, Tzeng E. Overexpression of mutated I[kappa]B[alpha] inhibits vascular smooth muscle cell proliferation and intimal hyperplasia formation. Journal of Vascular Surgery. 2003;38:812–819. doi: 10.1016/s0741-5214(03)00427-0. [DOI] [PubMed] [Google Scholar]
- 3.Shintani T, Sawa Y, Takahashi T, Matsumiya G, Matsuura N, Miyamoto Y, Matsuda H. Intraoperative transfection of vein grafts with the NFkappaB decoy in a canine aortocoronary bypass model: a strategy to attenuate intimal hyperplasia. Ann Thorac Surg. 2002;74:1132–1137. doi: 10.1016/s0003-4975(02)03921-8. discussion 1137–1138. [DOI] [PubMed] [Google Scholar]
- 4.Yoshimura S, Morishita R, Hayashi K, Yamamoto K, Nakagami H, Kaneda Y, Sakai N, Ogihara T. Inhibition of intimal hyperplasia after balloon injury in rat carotid artery model using cis-element 'decoy' of nuclear factor-kappaB binding site as a novel molecular strategy. Gene Ther. 2001;8:1635–1642. doi: 10.1038/sj.gt.3301566. [DOI] [PubMed] [Google Scholar]
- 5.Squadrito F, Deodato B, Bova A, Marini H, Saporito F, Calo M, Giacca M, Minutoli L, Venuti FS, Caputi AP, Altavilla D. Crucial role of nuclear factor-kappaB in neointimal hyperplasia of the mouse carotid artery after interruption of blood flow. Atherosclerosis. 2003;166:233–242. doi: 10.1016/s0021-9150(02)00336-2. [DOI] [PubMed] [Google Scholar]
- 6.Patel VI, Daniel S, Longo CR, Shrikhande GV, Scali ST, Czismadia E, Groft CM, Shukri T, Motley-Dore C, Ramsey HE, Fisher MD, Grey ST, Arvelo MB, Ferran C. A20, a modulator of smooth muscle cell proliferation and apoptosis, prevents and induces regression of neointimal hyperplasia. FASEB J. 2006;20:1418–1430. doi: 10.1096/fj.05-4981com. [DOI] [PubMed] [Google Scholar]
- 7.Wang AB, Li HL, Zhang R, She ZG, Chen HZ, Huang Y, Liu DP, Liang CC. A20 attenuates vascular smooth muscle cell proliferation and migration through blocking PI3k/Akt singling in vitro and in vivo. J Biomed Sci. 2007;14:357–371. doi: 10.1007/s11373-007-9150-x. [DOI] [PubMed] [Google Scholar]
- 8.Kwon HM, Sangiorgi G, Ritman EL, Lerman A, McKenna C, Virmani R, Edwards WD, Holmes DR, Schwartz RS. Adventitial vasa vasorum in balloon-injured coronary arteries: visualization and quantitation by a microscopic three-dimensional computed tomography technique. J Am Coll Cardiol. 1998;32:2072–2079. doi: 10.1016/s0735-1097(98)00482-3. [DOI] [PubMed] [Google Scholar]
- 9.Kwon HM, Sangiorgi G, Ritman EL, McKenna C, Holmes DR, Jr, Schwartz RS, Lerman A. Enhanced coronary vasa vasorum neovascularization in experimental hypercholesterolemia. J Clin Invest. 1998;101:1551–1556. doi: 10.1172/JCI1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westerband A, Gentile AT, Hunter GC, Gooden MA, Aguirre ML, Berman SS, Mills JL. Intimal growth and neovascularization in human stenotic vein grafts. Journal of the American College of Surgeons. 2000;191:264–271. doi: 10.1016/s1072-7515(00)00320-3. [DOI] [PubMed] [Google Scholar]
- 11.Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marme D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;87:3336–3343. [PubMed] [Google Scholar]
- 12.Kim I, Moon SO, Kim SH, Kim HJ, Koh YS, Koh GY. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin through nuclear factor-kappa B activation in endothelial cells. J Biol Chem. 2001;276:7614–7620. doi: 10.1074/jbc.M009705200. [DOI] [PubMed] [Google Scholar]
- 13.Grosskreutz CL, Anand-Apte B, Duplaa C, Quinn TP, Terman BI, Zetter B, D'Amore PA. Vascular endothelial growth factor-induced migration of vascular smooth muscle cells in vitro. Microvasc Res. 1999;58:128–136. doi: 10.1006/mvre.1999.2171. [DOI] [PubMed] [Google Scholar]
- 14.Moore MA, Hattori K, Heissig B, Shieh JH, Dias S, Crystal RG, Rafii S. Mobilization of endothelial and hematopoietic stem and progenitor cells by adenovector-mediated elevation of serum levels of SDF-1, VEGF, and angiopoietin-1. Ann N Y Acad Sci. 2001;938:36–45. doi: 10.1111/j.1749-6632.2001.tb03572.x. discussion 45-37. [DOI] [PubMed] [Google Scholar]
- 15.Sata M, Saiura A, Kunisato A, Tojo A, Okada S, Tokuhisa T, Hirai H, Makuuchi M, Hirata Y, Nagai R. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med. 2002;8:403–409. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- 16.Ferran C, Stroka DM, Badrichani AZ, Cooper JT, Wrighton CJ, Soares M, Grey ST, Bach FH. A20 inhibits NF-kappaB activation in endothelial cells without sensitizing to tumor necrosis factor-mediated apoptosis. Blood. 1998;91:2249–2258. [PubMed] [Google Scholar]
- 17.Clowes AW, Reidy MA, Clowes MM. Mechanisms of stenosis after arterial injury. Lab Invest. 1983;49:208–215. [PubMed] [Google Scholar]
- 18.Boyden S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med. 1962;115:453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koga T, Claycombe K, Meydani M. Homocysteine increases monocyte and T-cell adhesion to human aortic endothelial cells. Atherosclerosis. 2002;161:365–374. doi: 10.1016/s0021-9150(01)00670-0. [DOI] [PubMed] [Google Scholar]
- 20.Daniel S, Patel VI, Shrikhande GV, Scali ST, Ramsey HE, Csizmadia E, Benhaga N, Fisher MD, Arvelo MB, Ferran C. The universal NF-kappaB inhibitor a20 protects from transplant vasculopathy by differentially affecting apoptosis in endothelial and smooth muscle cells. Transplant Proc. 2006;38:3225–3227. doi: 10.1016/j.transproceed.2006.10.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelken NA, Coughlin SR, Gordon D, Wilcox JN. Monocyte chemoattractant protein-1 in human atheromatous plaques. J Clin Invest. 1991;88:1121–1127. doi: 10.1172/JCI115411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yla-Herttuala S, Lipton BA, Rosenfeld ME, Sarkioja T, Yoshimura T, Leonard EJ, Witztum JL, Steinberg D. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc Natl Acad Sci U S A. 1991;88:5252–5256. doi: 10.1073/pnas.88.12.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tatewaki H, Egashira K, Kimura S, Nishida T, Morita S, Tominaga R. Blockade of monocyte chemoattractant protein-1 by adenoviral gene transfer inhibits experimental vein graft neointimal formation. J Vasc Surg. 2007;45:1236–1243. doi: 10.1016/j.jvs.2007.01.066. [DOI] [PubMed] [Google Scholar]
- 24.Nakano K, Egashira K, Ohtani K, Zhao G, Funakoshi K, Ihara Y, Sunagawa K. Catheter-based adenovirus-mediated anti-monocyte chemoattractant gene therapy attenuates in-stent neointima formation in cynomolgus monkeys. Atherosclerosis. 2007;194:309–316. doi: 10.1016/j.atherosclerosis.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki J, Izawa A, Isobe M. Anti-vascular cell adhesion molecule-1 and anti-very late antigen-4 monoclonal antibodies inhibit neointimal hyperplasia in the murine model of arterial injury. Acta Cardiol. 2004;59:147–152. doi: 10.2143/AC.59.2.2005169. [DOI] [PubMed] [Google Scholar]
- 26.Inoue M, Itoh H, Ueda M, Naruko T, Kojima A, Komatsu R, Doi K, Ogawa Y, Tamura N, Takaya K, Igaki T, Yamashita J, Chun TH, Masatsugu K, Becker AE, Nakao K. Vascular endothelial growth factor (VEGF) expression in human coronary atherosclerotic lesions: possible pathophysiological significance of VEGF in progression of atherosclerosis. Circulation. 1998;98:2108–2116. doi: 10.1161/01.cir.98.20.2108. [DOI] [PubMed] [Google Scholar]
- 27.Shibata M, Suzuki H, Nakatani M, Koba S, Geshi E, Katagiri T, Takeyama Y. The involvement of vascular endothelial growth factor and flt-1 in the process of neointimal proliferation in pig coronary arteries following stent implantation. Histochemistry and Cell Biology. 2001;116:471–481. doi: 10.1007/s00418-001-0336-4. [DOI] [PubMed] [Google Scholar]
- 28.Ohtani K, Egashira K, Hiasa K, Zhao Q, Kitamoto S, Ishibashi M, Usui M, Inoue S, Yonemitsu Y, Sueishi K, Sata M, Shibuya M, Sunagawa K. Blockade of vascular endothelial growth factor suppresses experimental restenosis after intraluminal injury by inhibiting recruitment of monocyte lineage cells. Circulation. 2004;110:2444–2452. doi: 10.1161/01.CIR.0000145123.85083.66. [DOI] [PubMed] [Google Scholar]
- 29.Marumo T, Schini-Kerth VB, Busse R. Vascular endothelial growth factor activates nuclear factor-kappaB and induces monocyte chemoattractant protein-1 in bovine retinal endothelial cells. Diabetes. 1999;48:1131–1137. doi: 10.2337/diabetes.48.5.1131. [DOI] [PubMed] [Google Scholar]
- 30.Stefanadis C, Toutouzas K, Stefanadi E, Lazaris A, Patsouris E, Kipshidze N. Inhibition of plaque neovascularization and intimal hyperplasia by specific targeting vascular endothelial growth factor with bevacizumab-eluting stent: An experimental study. Atherosclerosis. 2007;195:269–276. doi: 10.1016/j.atherosclerosis.2006.12.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.