Abstract
It has been suggested that a cardinal symptom of mania is over-activity and exaggerated goal-directed behavior. Nevertheless, few attempts have been made to quantify this behavior objectively in a laboratory environment. Having a methodology to assess over-activity reliably might be useful in distinguishing manic bipolar disorder (BD) from schizophrenia (SCZ) during highly activated states. In the current study, quantifiable measures of object-interaction were assessed using a multivariate approach. Additionally, symptom correlates of over-activity were assessed. Patients admitted to an acute care psychiatric hospital for either BD with mania or SCZ (paranoid and non-paranoid subtypes) as well as non-patient comparison (NC) participants were assessed in an open field setting referred to as the human Behavioral Pattern Monitor (hBPM). Activity and interactions with novel and engaging objects were recorded for 15 minutes via a concealed video camera and rated for exploratory behavior. Both BD and SCZ patients spent more time near the objects and exhibited more overall walking compared to NC. In contrast, BD patients exhibited greater physical contact with objects (number of object interactions and time spent with objects) relative to SCZ patients or NC participants, as well as more perseverative and socially disinhibited behaviors, indicating a unique pattern of over-activity and goal-directed behavior. Further analyses revealed a distinction between SCZ patients according to their subtype. The current study extends our methodology for quantifying exploration and over-activity in a controlled laboratory setting and aids in assessing the overlap and distinguishing characteristics of BD and SCZ.
1. Introduction
Over-activity is a characteristic feature of Bipolar Disorder (BD) with mania that has received considerable attention in recent years. Over-activity refers to an increase in goal-oriented activities and is often coupled with a tendency to be impulsive and a propensity for involvement in activities that violate social norms and have an increased potential for negative consequences (e.g., hypersexuality, spending, travelling, or stealing) (Swann et al., 2005; Swann et al., 2009). Several studies have suggested that the presence of over-activity is as important as elevated and/or irritable mood for the diagnosis of mania and should be added as a core or stem criterion (must always be present) for manic states and be given priority over other symptoms (Akiskal et al., 2001; Angst et al., 2003; Benazzi, 2007).
Surprisingly, there has been little effort to study over-activity in a controlled laboratory setting. This absence of a quantifiable means of assessing over-activity limits the feasibility of studying this core symptom. To address this paucity of research we recently designed the human open field paradigm, referred to as the human BPM (hBPM) (Perry et al., 2009), a translational paradigm based upon our work with the rodent Behavioral Pattern Monitor (BPM), (Geyer et al., 1986). The hBPM enables us to characterize exploratory behavior in response to novel stimuli as well as to quantify the amount of motor activity and spatial patterns of activity in a defined area (Minassian et al., 2009; Perry et al., 2009; Young et al., 2007). In our original hBPM study (Perry et al., 2009) we reported that individuals with BD and schizophrenia have distinctive profiles of exploratory behavior. Specifically, patients with bipolar mania demonstrated a unique exploratory pattern, characterized by high motor activity and increased object interaction. In contrast, patients with schizophrenia exhibited normal object interaction (Perry et al., 2009). Given that total object interaction was useful in differentiating these two diagnostic groups, we sought to develop new measures of object exploration in hopes of further characterizing exploratory activity among BD patients.
In this paper, we expand on our original finding by employing a multivariate approach to comprehensively characterize over-activity among BD and SCZ patients in the hBPM, including: 1) simultaneous interaction with multiple objects; 2) duration of object interaction; 3) perseverative object interactions; 4) walking behavior; and 5) the extent of time spent in and transitions between areas of the room adjacent to objects. Furthermore, previous groups have suggested that activated BD patients are symptomatically indistinguishable from the non-paranoid subtype of SCZ patients (Lindenmayer et al., 1995; Marder et al., 1997). Therefore, a second objective of this study was to compare the exploratory activity of manic BD patients to subtypes of paranoid and non-paranoid SCZ patients in the hBPM. We also investigated whether these diagnostic groups could be differentiated by a specific set of object interactions that constitute socially disinhibited behavior (e.g., opening another person’s desk drawer or putting on and wearing a mask). Finally, we tested the hypothesis that higher ratings of mania symptoms will be related to increased exploration and interaction with objects.
2. Methods
2.1 Subjects
This study was approved by the UCSD Human Research Protections Program. Acutely hospitalized inpatients with either SCID (Structured Clinical Interview for DSM-IV) diagnosed DSM-IV Bipolar Disorder, Current Episode Manic (n = 17 females, 9 males), Schizophrenia-paranoid subtype (n = 11 females, 6 males), or Schizophrenia non-paranoid subtype (including disorganized or undifferentiated) (n = 7 females, 4 males) between the ages of 18 to 55 participated in this study. Non-patient comparison participants who had never met criteria for an Axis I Disorder as determined by the SCID were recruited from the community (n = 26 females, 12 males). Participants were excluded if they had abused or been dependent on alcohol or substances within the past month, had a positive result on a urine toxicology screen, had a neurological condition, or had a medical condition that impaired motor functioning. All BD and all but three SCZ patients were prescribed psychotropic medication during the time of testing; the BD patients were typically treated with a combination of mood-stabilizing and atypical antipsychotic medications while the SCZ patients were prescribed an antipsychotic medication alone. The most common antipsychotic medication prescribed was risperidone and the most common mood stabilizers prescribed were lithium and valproate. While a recently published (Perry et al., 2009) study presents other hBPM measures on a subset of subjects in the current study, this paper reports novel measures and analyses that were not contained in our previous manuscript.
2.2 Measures used
After participants provided informed consent, the Brief Psychiatric Rating Scale (BPRS) (Overall and Gorham, 1980) and the Young Mania Rating Scale (YMRS) (Young et al., 1978) were administered to both patient groups. Demographic and illness factors are presented in Table 1.
Table 1.
Demographic and illness factors.
| Nonpatient Comparisons (NC) | Bipolar Mania (BD) | Schizophrenia (all SCZ) | Paranoid SCZ (pSCZ) | Non-paranoid SCZ (npSCZ) | Difference | |
|---|---|---|---|---|---|---|
| Age | 28.8 (9.5) | 31.6 (13.6) | 36.4 (12.0) | 39.5 (10.8) | 31.7 (13.2) | ns |
| Gender | 12 M, 14 F | 9 M, 8 F | 10 M, 8 F | 6 M, 5 F | 4 M, 3 F | ns |
| Years of education | 14.9 (1.7) | 13.5 (2.4) | 13.2 (2.3) | 12.5 (1.5) | 14.3 (2.9) | NC > BD and pSCZ (p<.03) |
| Age of onset of Illness | -- | 23.6 (9.5) | 25.4 (8.7) | 27.5 (8.9) | 22.1 (7.8) | ns |
| Years of illness duration | -- | 8.1 (7.3) | 10.6 (8.0) | 11.7 (8.4) | 8.9 (7.5) | ns |
| Number of days in treatment | -- | 2.4 (1.8) | 5.6 (10.2) | 7.5 (12.9) | 2.7 (1.7) | ns |
| Illness subtype | -- | Current Episode Manic | 11 paranoid, 2 disorganized, 5 undifferentiated | 11 paranoid | 2 disorg., 5 undiff. | -- |
| YMRS total score | -- | 27.3 (9.8) | 18.4 (7.6) | 17.0 (7.7) | 20.7 (7.3) | BD > all SCZ and pSCZ (p<.01) |
| BPRS total score | -- | 38.1 (14.5) | 36.2 (7.8) | 37.5 (9.0) | 34.2 (5.5) | ns |
| Risperidone dose (mg) | -- | n = 8 3.3 (2.1) |
n = 11 4.0 (1.2) |
n = 6 3.2 (1.6) |
n = 5 4.9 (1.0) |
ns |
| Valproate dose (mg) | -- | n = 6 1833.3 (516.4) |
n = 2 2250.0 (353.6) |
n = 1 2500.0 |
n = 1 2000.0 |
ns |
| Lithium Dose (mg) | -- | n = 5 1180.0 (563.0) |
-- | -- |
Note: For all cases except gender and illness subtype, values in the first five columns represent means and standard deviations. YMRS= Young Mania Rating Scale, BPRS = Brief Psychiatric Rating Scale
2.3 Procedure
The human Behavioral Pattern Monitor (hBPM) is a 2.7 m × 4.3 m room furnished with a desk, two bookcases, a standard filing cabinet, a short filing cabinet, two corkboards mounted on the walls, and vertical blinds covering the window. No chairs were placed in the room to encourage exploration. Eleven engaging toys (chosen using the criteria that they are safe, colorful, tactile, can be manipulated, and likely to invite human exploration) were placed around the room (on the desk, filing cabinets, and shelves of the bookcases, and one hung from a corkboard). Also among these items were objects that participants could wear, i.e., a feather mask and a pair of sunglasses.
Each participant was fitted with a LifeShirt (Vivometrics, 2002) that had a data-collection device in a fanny-pack around the waist. The LifeShirt is a continuous recording device used to measure acceleration and heart rate variability; these data are reported elsewhere (Henry et al., 2009; Minassian et al., 2009; Perry et al., 2009). Participants were directed into the hBPM and asked to wait there while the experimenter set up other parts of the study. They were left for 15 minutes, but were not told in advance how long their wait would be, and no other instructions were given. Their activity was monitored by a digital video camera embedded in a ceiling vent.
There are a total of 14 novel stimuli that can be interacted with in the hBPM: 11 engaging toys, exploration of drawers in a cabinet, manipulation of blinds over the window, and the LifeShirt’s recording device housed in the fanny-pack around the subject’s waist. A novel object interaction was considered any occasion when the subject interacted with one of the above-listed stimuli. Wearing the mask and glasses and exploring drawers were measured in more detail, as they were thought to demonstrate a failure to exercise social appropriate boundaries (e.g., opening potentially private drawers and wearing an item of clothing that may belong to another person).
Video footage was scored by hand for a second-by-second breakdown for sitting, standing, walking, and lying down, and subcategorized as to whether these activities occurred with or without holding an object.
2.4 Analyses
For the purposes of this study, analysis was conducted on 1) total object interaction, 2) mean time spent with all objects, 3) multiple object interactions, 4) percent of perseverative interactions, 5) wear mask/glasses, 6) explore drawer, and 7) time spent walking.
Object interaction was defined as follows: When a participant makes any physical contact with the object. This category includes when the object is in the hand, on the foot, becomes a physical extension of the body, or is used to push, poke, prod, or otherwise make physical contact with any other object or item of furniture.
Mean time spent with objects was calculated by totaling the number of seconds a participant spent interacting with objects during the 15-minute period and dividing by the total object interactions.
Multiple object interaction (MOI) was defined as: Any instance a participant interacts with more than one object at the same time. Should a participant pick up a new object, an additional multiple object interaction is scored; however, if that same object (or one of the other objects the participant is interacting with) is put down, a new MOI is not scored.
Percent of perseverative interaction was defined as: The total number of objects a participant touched more than once, divided by the total number of objects in the room.
Wear mask/glasses was defined as: Participant places the mask or glasses on head, (or arm, waist, leg, etc.) and releases the object completely – but the object is still on his or her person.
Explore drawer was defined as: The instant a participant first opens a drawer to the instant he or she closes it and releases the drawer.
Time spent walking was defined as: When the participant takes one or more steps in any direction (forward, backward, sideways, or diagonal) or when the participant shifts weight from one foot to another.
Video raters received at least one week of training with definitions and practice coding of videotapes of both patient and non-patient participants. Reliability checks were conducted over the course of the study. The kappa reliability coefficients for the rater-coded measures ranged from 0.91 to 0.96. The raters were blind to the clinical status of the participants.
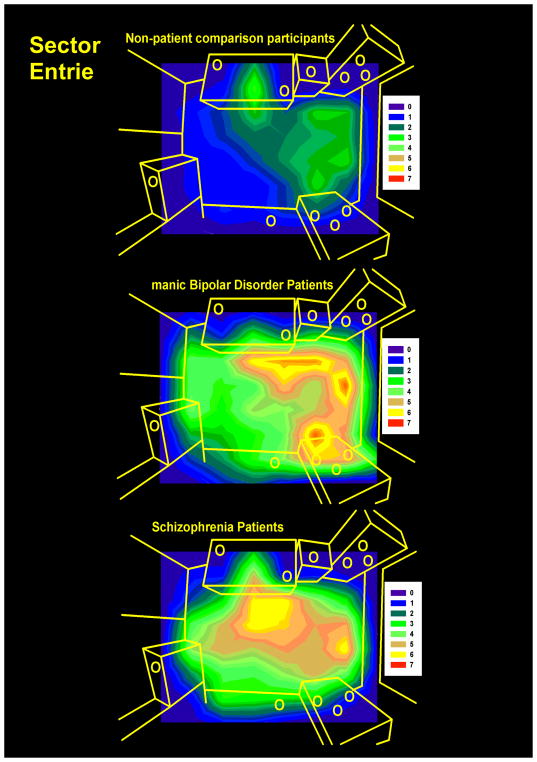
To assess participant movement in the hBPM, a grid of 64 sectors of equal sizes was generated for the open field paradigm. The x-y coordinate data were derived from digitized videos of the hBPM session as previously described (Perry et al., 2009), and time spent in each sector and sector entries were quantified. Average time and entries in 24 object–proximal sectors, defined as sectors that are immediately adjacent to the 14 objects in the room, were determined for each group. Figure 1 displays the contour map illustrating the distribution of average sector entries for the participant groups.
Figure 1. Contour maps of room exploration.
The distribution of average sector entries in each sector in seconds (over a grid of 64 equal size sectors) in the human Behavioral Pattern Monitor (hBPM) is illustrated for non-patient comparison participants (A), manic bipolar (B), and schizophrenia (C) patients. Intensity of activity is denoted by color. 11 objects placed around the room are shown as yellow circles.
2.5 Statistics
The effects of group on total number of object interactions, mean time spent with objects, multiple object interactions, percentage of object perseveration, time spent walking, and average time and entries in object-proximal sectors during the 15-minute hBPM session were analyzed using multivariate analysis of variance (MANOVA), followed by univariate ANOVAs for each parameter. This analysis was conducted for three groups comprised of NC, BD, and SCZ patients. A second MANOVA assessed group differences between BD and SCZ patients, with the SCZ patients being separated into paranoid and non-paranoid subtypes. Post-hoc analyses for significant effects were performed using the Least Significant Difference (LSD) post-hoc test, with α set at p<0.05. Pearson r correlations were conducted to determine the strength of the relationship between hBPM variables and YMRS and BPRS scores. Items from the BPRS were clustered into 5 factors as previously suggested (Shafer, 2005): 1) affect (anxiety, guilt, depressive mood, somatic concerns); 2) positive symptoms (unusual thought content, conceptual disorganization, hallucinations, grandiosity); 3) negative symptoms (blunted affect, emotional withdrawal, motor retardation); 4) resistance (hostility, uncooperativeness, suspiciousness); 5) activation (excitement, tension, mannerisms – posturing). Finally, differences between groups based upon whether a participant opened a drawer or wore the mask/glasses were analyzed using Pearson X2 analyses. Statistics were performed using SPSS 14.0 (Chicago, IL).
3. Results
Participant groups were matched for age and gender, but there was a significant difference in years of education (see Table 1). Subsequent investigation indicated there was no significant correlation between education level and any measure of object interaction (r-values from −0.202 to 0.057). To further determine any potential impact of education on exploration, subjects across all groups were divided into 4 education categories [pre-high school (n = 4); high school graduates (n = 18); some college (n = 18); college graduates (n = 21)] and the effect of education on object interaction variables was assessed using one-way ANOVAs. The results showed that education groups did not exhibit significant differences on any measure of object interaction (p values from 0.156 to 0.336). There were no significant differences between BD and SCZ patients on overall psychopathology (BPRS scores) or illness factors. The BD patients did exhibit higher overall YMRS scores compared to paranoid SCZ patients, but did not differ from the non-paranoid SCZ group. Finally, sector data were not obtained from 3 subjects (2 BD and 1 NC) due to malfunction of the software used to track participant location in the hBPM.
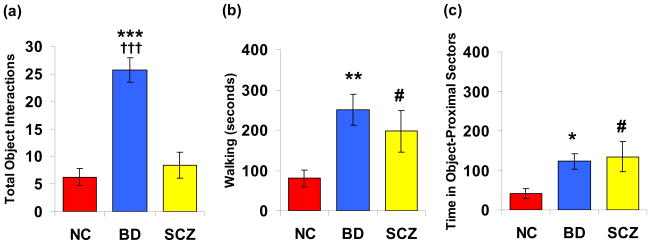
The MANOVA performed for NC, BD, and all SCZ subjects in the hBPM indicated a significant main effect of group (F(14,96)=4.4, p<0.001). Subsequent univariate ANOVAs revealed a main effect of group on total object interactions (F(2,53)=22.1, p<0.001), mean time spent with objects (F(2,53)=8.3, p<0.01), multiple object interactions (F(2,53)=8.4, p<0.01), percentage perseveration (F(2,53)=15.2, p<0.001), time spent walking (F(2,53)=4.5, p<0.05), average time in object proximal sectors (F(2,53)=4.2, p<0.05), and average sector entries (F(2,53)=7.1, p<0.01). Post hoc analyses revealed that BD patients exhibited significantly more total object interactions compared to NC (p<0.001) and SCZ patients (p<0.001), demonstrated more multiple object interactions compared to NC (p<0.001) and SCZ patients (p<0.01), and spent more time with objects relative to either group (NC, p < 0.01; SCZ, p < 0.01). In contrast, SCZ patients did not differ from NC subjects on any measure of physical contact with objects. (Table 2; Figure 2)
Table 2.
Multivariate assessment of hBPM object interaction for non-patient comparison participants (NC, n = 26), manic bipolar (BD, n = 17), and schizophrenia (SCZ, n = 18) patients.
| Measure | Bipolar Mania (BD) | Schizophrenia (SCZ) | Non-patient Comparisons (NC) | Group differences |
|---|---|---|---|---|
| Total Object Interactions | 25.7 (2.2) | 8.4 (2.3) | 6.3 (1.6) | BD > NC, SCZ |
| Mean Time Spent with Objects (seconds) | 573.2 (53.0) | 214.9 (58.5) | 206.9 (59.0) | BD > NC, SCZ |
| Multiple Object Interactions | 7.2 (2.3) | 1.2 (0.8) | 1.0 (0.5) | BD > NC, SCZ |
| Percent Object Perseveration | 28.4 (1.1) | 16.5 (3.5) | 8.5 (2.2) | BD > SCZ > NC |
| Time Spent Walking (seconds) | 250.3 (37.8) | 197.3 (51.0) | 80.5 (21.4) | BD, SCZ > NC |
| Time Spent in Object-Proximal Sectors (seconds) | 122.8(19.9) | 134.8 (37.9) | 41.2 (12.7) | BD, SCZ > NC |
| Object-Proximal Sector Entries | 34.9 (9.7) | 15.1 (4.2) | 6.4 (1.8) | BD > NC, SCZ |
Data are represented as means with S.E.M in parentheses.
Figure 2.
Object interaction and activity data for non-patient comparison participants (NC, n = 26), manic bipolar (BD, n = 17), and schizophrenia (SCZ, n = 18) patients. Measures include: (a) total object interactions, (b) time spent walking (seconds), and (c) mean time in object-proximal sectors (seconds). Data are represented as means ± S.E.M. Significant group differences are indicated as *(BD vs NC), † (BD vs SCZ); # (SCZ vs NC); * p < 0.05; ** p < 0.01; *** p < 0.001.
Both BD and SCZ patients exhibited significantly increased walking behavior in the hBPM compared to NC subjects and spent more time in object-proximal sectors (BD vs. NC, p < 0.05; SCZ vs. NC, p < 0.05), but the patient groups did not differ from one another on either time spent walking or time in object-proximal sectors. However, BD patients demonstrated significantly more object-proximal sector entries compared to both NC (p<0.001) and SCZ patients (p<0.05), suggesting that BD patients approached objects more frequently. Finally, all three groups displayed differences in perseverative object interaction. BD patients engaged in repeated object contact more than SCZ patients (p<0.01), who in turn, demonstrated more perseveration compared to NC subjects (p<0.05).
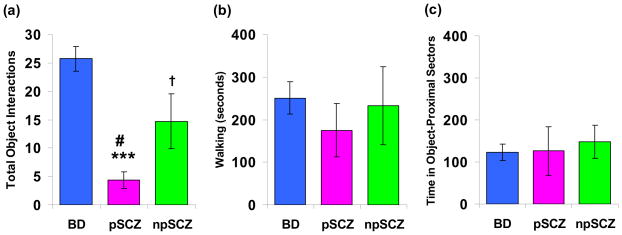
A second MANOVA comparing BD patients to the paranoid and non-paranoid SCZ sub-groups indicated a main effect of group (F(14,50)=2.2, p<0.05). Subsequent univariate ANOVAs revealed a main effect of group on total object interactions (F(2,30)=18.3, p<0.001), mean time spent with objects (F(2,30)=12.5, p<0.001), multiple object interactions (F(2,30)=4.4, p<0.05), and percentage perseveration (F(2,30)=4.5, p<0.05). No significant group differences were observed for time spent walking or object-proximal sector time or entries. Post hoc analyses revealed that BD patients exhibited significantly more total object interactions compared to both paranoid (p<0.001) and non-paranoid SCZ (p<0.05) patients; the BD group also demonstrated more object perseverations relative to both SCZ subtypes (paranoid, p<0.05; non-paranoid, p<0.05). While BD also spent more time with objects (p<0.001) and showed more multiple object interactions (p<0.01) compared to paranoid SCZ participants, they did not differ from the non-paranoid SCZ on these measures. (Table 3; Figure 3)
Table 3.
Multivariate assessment of hBPM object interaction for manic bipolar (BD, n = 17), paranoid schizophrenia (pSCZ, n = 11), and non-paranoid schizophrenia (npSCZ, n = 7) patients.
| Measure | Bipolar Mania (BD) | Schizophrenia (pSCZ) | Schizophrenia (npSCZ) | Group differences |
|---|---|---|---|---|
| Total Object Interactions | 25.7 (2.2) | 4.4 (1.5) | 14.7 (4.8) | BD > npSCZ > pSCZ |
| Mean Time Spent with Objects (seconds) | 573.2 (53.0) | 114.5 (58.3) | 372.6 (96.2) | BD, npSCZ > pSCZ |
| Multiple Object Interactions | 7.2 (2.3) | 0 (0) | 3.0 (1.9) | BD > pSCZ |
| Percent Object Perseveration | 28.4 (1.1) | 17.6 (5.2) | 14.6 (4.4) | BD > pSCZ, npSCZ |
| Time Spent Walking (seconds) | 250.3 (37.8) | 174.7 (62.5) | 232.7 (91.4) | ns |
| Time Spent in Object-Proximal Sectors (seconds) | 122.8 (19.9) | 126.4 (58.3) | 147.9 (38.9) | ns |
| Object-Proximal Sector Entries | 34.9 (9.7) | 10.4 (3.2) | 22.4 (9.2) | ns |
Data are represented as means with S.E.M in parentheses.
Figure 3.
Object interaction and activity data for manic bipolar (BD, n = 17), paranoid schizophrenia (pSCZ, n = 11), and non-paranoid schizophrenia (npSCZ, n = 7) patients. Measures include: (a) total object interactions, (b) time spent walking (seconds), and (c) mean time in object-proximal sectors (seconds). Data are represented as means ± S.E.M. Significant group differences are indicated as *(BD vs pSCZ), † (BD vs npSCZ); # (pSCZ vs npSCZ); †, # p < 0.05; *** p < 0.001.
Comparisons between the SCZ subtypes revealed that paranoid SCZ patients engaged in fewer total object interactions (p<0.05) and spent less time with objects (p<0.05) compared to the non-paranoid group, but both groups showed equivalent multiple object interactions and perseverative interaction.
While none of the NC participants or paranoid SCZ patients put on the mask or glasses, several non-paranoid SCZ (14%) and almost half of the BD subjects (47%) wore one of these items. A high percentage of BD subjects also investigated the drawer (53%), in contrast to relatively few NC (4%), paranoid SCZ (9%), or non-paranoid SCZ (14%) participants. Pearson X2 analyses revealed that the percentage of BD patients that wore the mask was significantly greater than NC (X2=15.0, p<0.001) and paranoid SCZ (X2=7.2, p<0.01). The percentage of BD patients opening the drawer was also significantly higher than NC participants (X2=13.9, p<0.001) and paranoid SCZ patients (X2=5.6, p<0.05). There were no differences in either measure between the NC and SCZ patient groups.
We observed a number of modest but significant correlations between YMRS scores and object interaction activity in the hBPM (Table 4). The number of total object interactions and time spent with objects were positively correlated with elevated mood, decreased sleep, and more severe language-thought disorder. Perseverative object interaction was also significantly correlated with higher ratings of motor activity, decreased sleep, and increased rate and quantity of speech. Finally, participant entries into object-proximal sectors were associated with more pronounced symptoms of elevated mood and greater motor activity.
Table 4.
Pearson r correlation coefficients between YMRS items and hBPM variables for all patients.
| Total Object Interactions | Time Spent with Objects | Multiple Object Interactions | Percent Object Perseveration | Time Spent Walking | Time in Object-Proximal Sectors | Object-Proximal Sector Entries | |
|---|---|---|---|---|---|---|---|
| YMRS ITEM 1: Elevated Mood | .55** | .51** | .30 | .20 | .20 | .01 | .41* |
| YMRS ITEM 2: Motor Activity | .07 | .17 | .05 | .63** | .49** | −.24 | .35* |
| YMRS ITEM 3: Sexual Interest | .36* | .18 | .20 | .25 | .08 | −.13 | .01 |
| YMRS ITEM 4: Sleep | .41* | .35* | .32 | .34* | −.07 | −.06 | .03 |
| YMRS ITEM 5: Irritability | .11 | .14 | .07 | .32 | .04 | .07 | .23 |
| YMRS ITEM 6: Speech | .05 | .02 | .10 | .49** | −.12 | −.28 | −.10 |
| YMRS ITEM 7: Language – Thought Disorder | .51** | .42* | .30 | .33 | −.07 | −.06 | .01 |
| YMRS ITEM 8: Content | −.28 | −.27 | −.05 | −.28 | .02 | .14 | −.18 |
| YMRS ITEM 9: Disruptive-Aggressive Behavior | .22 | .14 | .22 | .26 | −.02 | −.14 | .30 |
| YMRS ITEM 10: Appearance | .15 | .13 | .22 | .11 | −.04 | −.05 | −.19 |
| YMRS ITEM 11: Insight | −.26 | −.22 | −.01 | −.21 | −.33 | −.05 | −.39* |
| TOTAL YMRS SCORE | .26 | .20 | .29 | .35* | −.02 | −.11 | .06 |
Significant correlations are denoted by an asterisk (* p < 0.05; ** p < 0.01).
Although total BPRS scores were not related to hBPM activity, patients who spent more time with objects exhibited more severe positive symptoms (Table 5). In contrast, negative symptoms were significantly correlated with decreased time spent with objects and reduced object perseveration. Subjects with higher BPRS activation factor scores demonstrated more total object interactions and increased object-proximal sector entries. Notably, two of the individual BPRS items (Item 8, grandiosity and Item 16, blunted affect) were associated with several hBPM variables. Patients with higher ratings of grandiosity exhibited more total object interactions (r=0.38, p<0.05), greater time spent with objects (r = 0.53, p <0.01), increased object perseveration (r = 0.39, p <0.05), and engaged in more object-proximal sector entries (r = 0.42, p <0.05). In contrast, higher ratings of blunted affect were associated with fewer total object interactions (r = −0.42, p <0.05), less time with objects (r = −0.53, p <0.01), reduced object perseveration (r = −0.48, p <0.05), and decreased walking (r = −0.38, p < 0.05). (See Table 5)
Table 5.
Pearson r correlation coefficients between BPRS factors and hBPM variables for all patients.
| Total Object Interactions | Time Spent with Objects | Multiple Object Interactions | Percent Object Perseveration | Time Spent Walking | Time in Object- Proximal Sectors | Object- Proximal Sector Entries | |
|---|---|---|---|---|---|---|---|
| BPRS TOTAL SCORE | −.03 | .07 | −.15 | .01 | .03 | .28 | .15 |
| BPRS FACTOR 1: Affect | −.15 | −.11 | .01 | .06 | −.16 | .06 | −.15 |
| BPRS FACTOR 2: Positive Symptoms | .19 | .36* | .01 | .05 | .24 | .26 | .26 |
| BPRS FACTOR 3: Negative Symptoms | −.29 | −.42* | .03 | −.38* | −.32 | .18 | −.27 |
| BPRS FACTOR 4: Resistance | .21 | .14 | .16 | .26 | .11 | .08 | .44* |
| BPRS FACTOR 5: Activation | .36* | .19 | .21 | .28 | .05 | −.15 | .34* |
Significant correlations are denoted by an asterisk (* p < 0.05).
Several additional post-hoc analyses were conducted to examine potential effects of psychotropic medications on exploratory behavior. Pearson r correlations between dose of risperidone and the hBPM variables yielded no significant relationships with the exception of a weak positive relationship between time spent walking and risperidone dose (r=0.48, p=0.04). Patients were then divided based upon whether they were taking (n=19) or not taking (n=16) risperidone and t-tests were conducted using the hBPM variables; no significant differences were found. A similar analysis was conducted for subjects on (n=5) and off (n=30) lithium, and on (n=8) and off (n=27) valproate. Apart from a weak trend such that BD patients on lithium had slightly more perseverations than those off lithium (p=0.04), no significant medication effects were observed.
4. Discussion
4.1 Using a multivariate approach to assess exploratory behavior in BD mania and SCZ
The objective of this study was to apply a multivariate methodology to assess differences in exploratory behavior in BD and SCZ. Using this multivariate approach, the characteristics of over-activity in manic BD patients as compared to SCZ patients and a non-patient sample can be summarized as follows: Both manic BD and SCZ patients show equivalent levels of ambulation in the room, as illustrated by time spent walking and time in object-proximal sectors. Manic BD patients, however, can be distinguished based upon greater physical interaction with novel objects in a repeated fashion, interaction with multiple objects simultaneously, and interactions that may be reflective of social disinhibition such as engaging with objects that may belong to another person. The sector-entries map in Figure 2 illustrates the key differences in activity and exploration among the subject groups. While both SCZ and BD spent more time near the objects relative to NC, the sector entry data indicate that SCZ subjects were most active in the center of the room, apparently independent of object placement. In contrast, peak sector entries for BD patients occurred in areas containing the most objects, suggesting a different pattern of exploration characterized by greater repeated object interaction (percent perseveration) and interaction with multiple objects. These data are promising and yet should be viewed with caution. For example, while our analyses did not show prominent medication effects, we cannot discount that medications can have differentially impacted patients and may contribute to the differences between patient groups. Clearly, the best means of assessing medication effects would be to control for medication and/or to substantially increase the sample size of the study.
4.2 Differentiating schizophrenia subtypes based upon object interaction
Patients with schizophrenia (SCZ) have been observed to demonstrate mania-like symptoms (Lindenmayer et al., 1995) including over-activity and goal-directed behavior. These behaviors are particularly common during highly symptomatic states (Marder et al., 1997) and among people with the non-paranoid subtype of schizophrenia (undifferentiated and disorganized). Consequently, differential diagnosis between BD and SCZ is difficult due to “overlapping boundaries with regard to symptomatology” (Lindenmayer et al., 1995). Due to the difficulty in distinguishing these “mania-like” behaviors between BD and SCZ and the emergence of genetic data that support overlap in genetic susceptibility of the two disorders (Ivleva et al., 2008), some authors have suggested that BD and SCZ may represent a single disorder that falls along a genetic continuum (Crow, 1990). We assessed object interaction in the context of exploration at the level of diagnostic subtype. Non-paranoid SCZ patients (disorganized and undifferentiated) demonstrated significantly more total object interactions and spent more time with objects relative to paranoid SCZ patients. In addition, BD patients exhibited significantly greater total object interactions and more object perseveration compared to both SCZ groups; however they did not differ from non-paranoid SCZ on mean time spent with objects, multiple object interactions, or time and entries in object-proximal sectors. The data support the current thinking that during highly symptomatic and activated states non-paranoid SCZ and BD patients with mania have similarities in their behavioral presentation (Lindenmayer et al., 1995). However, the multivariate approach utilized here reveals that the total number of object interactions may be useful in discriminating manic BD patients from SCZ patients regardless of schizophrenia subtype. The current findings lend support to the contention that over-activity is a core feature of the mania of BD (Akiskal et al., 2001; Benazzi, 2007), but also suggest that non-paranoid SCZ patients may exhibit features of over-activity. Taken together, these data underscore the advantages of using a multivariate approach to determine signature characteristics of BD and SCZ. However it is important to keep in mind that distinctions in subtyping are based primarily on cross-section symptom assessment and other distinctions, such as disease course, genetic indices or brain functional measures, while beyond the scope of the present investigation, may offer a more relevant means of subtyping. For example, Turetsky and colleagues (Turetsky et al., 2002) reported clinical, neuroanatomical and neurophysiological correlates associated with schizophrenia subtypes that were based upon ‘memory’ profiles. Thus, future studies should consider analyzing different object interaction patterns based upon subtypes other than symptom clusters.
4.3 The relationship between manic symptoms and measures of object interaction
Our hypothesis that higher mania symptoms would be related to increased exploration and interaction with objects was not supported. There does not appear to be a relationship between our measures of exploration and either general symptoms of psychopathology or of mania. Review of the distribution of BD symptom ratings did not reveal problems with a restricted range; therefore this lack of a relationship appears reliable. These results are consistent with our previous findings that hBPM measures appear to capture diagnostic differences better than observer-rated measures of symptoms (Perry et al., 2009)
The current results suggest that more severe symptoms of psychopathology may be related to increased exploration and interaction with objects. While total BPRS scores were not correlated with any hBPM measure, more specific measures of mania and activation (such as the elevated mood item on the YMRS scale and the BPRS activation factor) are associated with more extensive exploration. In contrast, negative symptoms (such as blunted affect) are correlated with reduced activity. These findings are consistent with previous work reporting that motor activity in psychiatric patients (as quantified by an accelerometer) is positively correlated with elevated mood and inversely related to ratings of blunted affect (Minassian et al., 2009).
4.4 Increased object interaction may represent a form of behavioral disinhibition
Collectively, we interpret the increased goal-directed object interaction or over-activity in manic BD patients as representing a form of behavioral disinhibition. Several authors (Hirshfeld-Becker et al., 2006) have defined behavioral disinhibition as an extreme tendency to seek out novelty, approach unfamiliar stimuli, and to engage in socially undesirable actions (Iacono et al., 2008). Individuals who display behavioral disinhibition often do so in unfamiliar settings (Hirshfeld-Becker et al., 2006) and appear to do so as an automatic reaction to novelty (Cloninger, 1986). Consequently, BD patients appear to lack the capacity for self-regulation and violate typical social etiquette that is observed among healthy volunteers. This inability to regulate behavior can be understood as a failure to inhibit inappropriate responses or impulses, which is a major role of the prefrontal cortex (PFC) (Miller and Cohen, 2001). The PFC exerts control over a wide range of cognitive processes in the service of goal-directed behavior and in novel situations where “behavior must be guided by internal states or intentions” (Miller and Cohen, 2001). A large number of studies suggest that BD is associated with functional and structural PFC deficits, including decreases in grey matter density, reduced neuronal and glial density, decreased glucose metabolism, reduced cerebral blood flow, and diminished levels of PFC brain metabolites (Baxter et al., 1985; Buchsbaum et al., 1986; Drevets et al., 1997; Rajkowska et al., 2001). Consequently, the increases in exploration, object interaction, and the incidence of preservative interactions observed among the BD patients in the novel setting of the hBPM could be interpreted as a failure of top-down control processes. This proposition is consistent with reports of patients with frontal lesions appear who interact with objects within their reach in a perseverative fashion, irrespective of social appropriateness (Lhermitte, 1983).
4.5 The role of dopamine in over-activity
Several authors (Braver and Cohen, 2000; Derryberry and Rothbart, 1997) have suggested that dopamine (DA) dysregulation may play a critical role in top-down control processes. They hypothesized that DA can exert a neuromodulatory influence on the PFC and is critical in “gating” or modulating the influence particularly during novel situations. Cloninger (1987) has also suggested that high novelty-seeking behavior is rooted in DA dysregulation, specifically hyperdopaminergia. Accordingly, the increased exploration and engagement with novel objects observed in the present study may be a result of hyperdopaminergic tone among manic BD patients. While this hypothesis is speculative, our current findings are consistent with work from our group on the dopamine transporter (DAT) knockdown mouse (Ralph-Williams et al., 2003). DAT knockdown mice have approximately 90% loss of DAT expression resulting in a hyperdopaminergic tone and show increased holepoking behavior when placed in the rodent BPM. Holepoking behavior is considered the animal analogue of goal-oriented specific exploration behavior in humans in the hBPM (Paulus and Geyer, 1993). Furthermore, Ralph-Williams and colleagues (Ralph-Williams et al., 2003) found that the mood-stabilizer valproate significantly attenuated these behaviors in DAT knockout mice, while having no effect on their wildtype littermates. Additionally, acute administration of the DAT-specific inhibitor GBR12909 in mice appears to mimic the mania phenotype in the BPM (Young et al., 2010). Finally, variation in the dopamine transporter gene (DAT1, SLC6A3) is now considered a candidate gene in several neuropsychiatric disorders, including BD and the hyperactivity of ADHD (Gill et al., 1997; Greenwood et al., 2006).
4.6 Summary
We previously reported that BD subjects exhibit increases in the amount of locomotor activity when compared to SCZ patients (Perry et al., 2009). Furthermore, discriminant function analyses revealed that the hBPM measures in general and object interactions in particular correctly classified BD and SCZ patients better than did symptom domain scores (Perry et al., 2009). Whereas our previous work (Perry et al., 2009) reported only one summary measure of object interaction in a cross-species examination of exploration, in the current paper we extended our methodology for quantifying object interaction and determined distinguishing characteristics of BD and SCZ. While both BD and SCZ patients spent more time near the objects and exhibited more overall walking compared to NC, BD patients exhibited greater physical contact with objects (number of object interactions and time spent with objects) relative to SCZ patients or NC participants, as well as more perseverative and socially disinhibited behaviors. This unique pattern of over-activity and goal-directed behavior may represent a signature of BD with mania.
Kraepelin (1921) divided the major psychotic disorders into a bipolar type and a schizophrenia type and distinguished them by symptom profile and prognosis. More recently, this characterization has been questioned. The present findings suggest that during highly symptomatic states BD and SCZ patients demonstrate differences in activity levels when in a novel environment. It has been suggested that BD and SCZ share many symptoms particularly during highly symptomatic states. Still, there are cardinal features that help to differentiate these two disorders, namely the increased goal-directed behavior of patients with BD. Surprisingly, although well recognized, this distinction has been understudied. The data from our current study support the position that the two disorders share many symptoms, as indicated in the similar BPRS scores, but also have distinct characteristics, namely increased exploration.
In conclusion, the current study introduces a new multivariate methodology for objectively characterizing over-activity. This methodology offers promise as a cross-species translational paradigm because human and animal behavior can be studied in a parallel way using the human and animal BPMs. Consequently, this unique approach to studying over-activity in the context of exploration in BD may provide an opportunity to identify critical phenotypes that may reveal putative neurobiology underlying the mania of BD and facilitate the development and testing of novel antimanic agents.
Acknowledgments
This work was supported by a grant from the National Institute of Mental Health (NIMH) (R01-MH071916) and by the Veteran’s Administration VISN 22 Mental Illness Research, Education, and Clinical Center. The authors gratefully acknowledge Dr. Martin Paulus as well as Virginia Masten, Richard Sharp, Christin Seed, and Eliza Ferguson for their contributions to this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiskal HS, Hantouche EG, Bourgeois ML, Azorin JM, Sechter D, Allilaire JF, Chatenet-Duchene L, Lancrenon S. Toward a refined phenomenology of mania: combining clinician-assessment and self-report in the French EPIMAN study. Journal of Affective Disorders. 2001;67:89–96. doi: 10.1016/s0165-0327(01)00441-4. [DOI] [PubMed] [Google Scholar]
- Angst J, Gamma A, Benazzi F, Ajdacic V, Eich D, Rossler W. Toward a redefinition of subthreshold bipolarity: epidemiology and proposed criteria for bipolar-II, minor bipolar disorders and hypomania. Journal of Affective Disorders. 2003;73:133–146. doi: 10.1016/s0165-0327(02)00322-1. [DOI] [PubMed] [Google Scholar]
- Baxter LR, Jr, Phelps ME, Mazziotta JC, Schwartz JM, Gerner RH, Selin CE, Sumida RM. Cerebral metabolic rates for glucose in mood disorders. Studies with positron emission tomography and fluorodeoxyglucose F 18. Archives of General Psychiatry. 1985;42:441–447. doi: 10.1001/archpsyc.1985.01790280019002. [DOI] [PubMed] [Google Scholar]
- Benazzi F. Is overactivity the core feature of hypomania in bipolar II disorder? Psychopathology. 2007;40:54–60. doi: 10.1159/000096513. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD. On the control of control: The role of dopamine in regulating prefrontal function and working memory. In: Monsell S, Driver J, editors. Control of Cognitive Processes: Attention and Performance XVIII. MIT Press; Cambridge, MA: 2000. pp. 713–737. [Google Scholar]
- Buchsbaum MS, Wu J, DeLisi LE, Holcomb H, Kessler R, Johnson J, King AC, Hazlett E, Langston K, Post RM. Frontal cortex and basal ganglia metabolic rates assessed by positron emission tomography with [18F]2-deoxyglucose in affective illness. Journal of Affective Disorders. 1986;10:137–152. doi: 10.1016/0165-0327(86)90036-4. [DOI] [PubMed] [Google Scholar]
- Cloninger CR. A unified biosocial theory of personality and its role in the development of anxiety states. Psychiatric Developments. 1986;4:167–226. [PubMed] [Google Scholar]
- Cloninger CR. A systematic method for clinical description and classification of personality variants. A proposal. Archives of general psychiatry. 1987;44:573–588. doi: 10.1001/archpsyc.1987.01800180093014. [DOI] [PubMed] [Google Scholar]
- Crow TJ. The continuum of psychosis and its genetic origins. The sixty-fifth Maudsley lecture. British Journal of Psychiatry. 1990;156:788–797. doi: 10.1192/bjp.156.6.788. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Rothbart MK. Reactive and effortful processes in the organization of temperament. Development and Psychopathology. 1997;9:633–652. doi: 10.1017/s0954579497001375. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Russo PV, Masten VL. Multivariate assessment of locomotor behavior: pharmacological and behavioral analyses. Pharmacology, Biochemistry, and Behavior. 1986;25:277–288. doi: 10.1016/0091-3057(86)90266-2. [DOI] [PubMed] [Google Scholar]
- Gill M, Daly G, Heron S, Hawi Z, Fitzgerald M. Confirmation of association between attention deficit hyperactivity disorder and a dopamine transporter polymorphism. Molecular Psychiatry. 1997;2:311–313. doi: 10.1038/sj.mp.4000290. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Schork NJ, Eskin E, Kelsoe JR. Identification of additional variants within the human dopamine transporter gene provides further evidence for an association with bipolar disorder in two independent samples. Molecular Psychiatry. 2006;11:125–133. 115. doi: 10.1038/sj.mp.4001764. [DOI] [PubMed] [Google Scholar]
- Henry BL, Minassian A, Paulus MP, Geyer MA, Perry W. Heart rate variability in bipolar mania and schizophrenia. Journal of Psychiatric Research. 2009;44:168–176. doi: 10.1016/j.jpsychires.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfeld-Becker DR, Biederman J, Henin A, Faraone SV, Cayton GA, Rosenbaum JF. Laboratory-observed behavioral disinhibition in the young offspring of parents with bipolar disorder: a high-risk pilot study. The American Journal of Psychiatry. 2006;163:265–271. doi: 10.1176/appi.ajp.163.2.265. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: common and specific influences. Annual Review of Clinical Psychology. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- Ivleva E, Thaker G, Tamminga CA. Comparing genes and phenomenology in the major psychoses: schizophrenia and bipolar 1 disorder. Schizophrenia Bulletin. 2008;34:734–742. doi: 10.1093/schbul/sbn051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraepelin E. Manic Depressive Insanity and Paranoia. Edinburgh: Livingstone; 1921. [Google Scholar]
- Lhermitte F. ‘Utilization behaviour’ and its relation to lesions of the frontal lobes. Brain. 1983;106 (Pt 2):237–255. doi: 10.1093/brain/106.2.237. [DOI] [PubMed] [Google Scholar]
- Lindenmayer JP, Grochowski S, Hyman RB. Five factor model of schizophrenia: replication across samples. Schizophrenia Research. 1995;14:229–234. doi: 10.1016/0920-9964(94)00041-6. [DOI] [PubMed] [Google Scholar]
- Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. The Journal of Clinical Psychiatry. 1997;58:538–546. doi: 10.4088/jcp.v58n1205. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Minassian A, Henry BL, Geyer MA, Paulus MP, Young JW, Perry W. The quantitative assessment of motor activity in mania and schizophrenia. Journal of Affective Disorders. 2009;120:200–206. doi: 10.1016/j.jad.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall JR, Gorham DR. The brief psychiatric rating scale. J Oper Psychiatry. 1980;11:48–64. [Google Scholar]
- Paulus MP, Geyer MA. Three independent factors characterize spontaneous rat motor activity. Behavioural Brain Research. 1993;53:11–20. doi: 10.1016/s0166-4328(05)80262-1. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Paulus MP, Young JW, Kincaid MJ, Ferguson EJ, Henry BL, Zhuang X, Masten VL, Sharp RF, Geyer MA. A reverse-translational study of dysfunctional exploration in psychiatric disorders: from mice to men. Archives of General Psychiatry. 2009;66:1072–1080. doi: 10.1001/archgenpsychiatry.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Halaris A, Selemon LD. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biological Psychiatry. 2001;49:741–752. doi: 10.1016/s0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- Ralph-Williams RJ, Paulus MP, Zhuang X, Hen R, Geyer MA. Valproate attenuates hyperactive and perseverative behaviors in mutant mice with a dysregulated dopamine system. Biological Psychiatry. 2003;53:352–359. doi: 10.1016/s0006-3223(02)01489-0. [DOI] [PubMed] [Google Scholar]
- Shafer A. Meta-analysis of the brief psychiatric rating scale factor structure. Psychological Assessment. 2005;17:324–335. doi: 10.1037/1040-3590.17.3.324. [DOI] [PubMed] [Google Scholar]
- Swann AC, Dougherty DM, Pazzaglia PJ, Pham M, Steinberg JL, Moeller FG. Increased impulsivity associated with severity of suicide attempt history in patients with bipolar disorder. The American Journal of Psychiatry. 2005;162:1680–1687. doi: 10.1176/appi.ajp.162.9.1680. [DOI] [PubMed] [Google Scholar]
- Swann AC, Lijffijt M, Lane SD, Steinberg JL, Moeller FG. Severity of bipolar disorder is associated with impairment of response inhibition. Journal of Affective Disorders. 2009;116:30–36. doi: 10.1016/j.jad.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Moberg PJ, Mozley LH, Moelter ST, Agrin RN, Gur RC, Gur RE. Memory-delineated subtypes of schizophrenia: relationship to clinical, neuroanatomical, and neurophysiological measures. Neuropsychology. 2002;16:481–490. [PubMed] [Google Scholar]
- Vivometrics. The LifeShirt System™. Ventura, CA: 2002. [Google Scholar]
- Young JW, Goey AK, Minassian A, Perry W, Paulus MP, Geyer MA. GBR 12909 administration as a mouse model of bipolar disorder mania: mimicking quantitative assessment of manic behavior. Psychopharmacology. 2010;208:443–454. doi: 10.1007/s00213-009-1744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Minassian A, Paulus MP, Geyer MA, Perry W. A reverse-translational approach to bipolar disorder: rodent and human studies in the Behavioral Pattern Monitor. Neuroscience and Biobehavioral Reviews. 2007;31:882–896. doi: 10.1016/j.neubiorev.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]