Abstract
The survival of poliovirus 1 (LSc) and echovirus 1 (Farouk) in estuarine water and sediment was studied in Galveston Bay, Texas. Viruses were suspended in estuarine water and sediment both in dialysis tubing and in chambers constructed with polycarbonate membrane walls. Virus inactivation rates in seawater were similar in both types of chambers. Virus adsorption to sediment greatly increased survival time. The time required to inactivate 99% (T-99) of poliovirus increased from 1.4 days in seawater alone to 6.0 days for virus adsorbed to sediment at a relatively nonpolluted site. At a more polluted site, poliovirus T-99 was increased from approximately 1 h to 4925 days by virus adsorption to sediment. This study demonstrates that under field conditions virus association with estuarine sediment acts to prolong its survival in the marine environment.
Full text
PDF
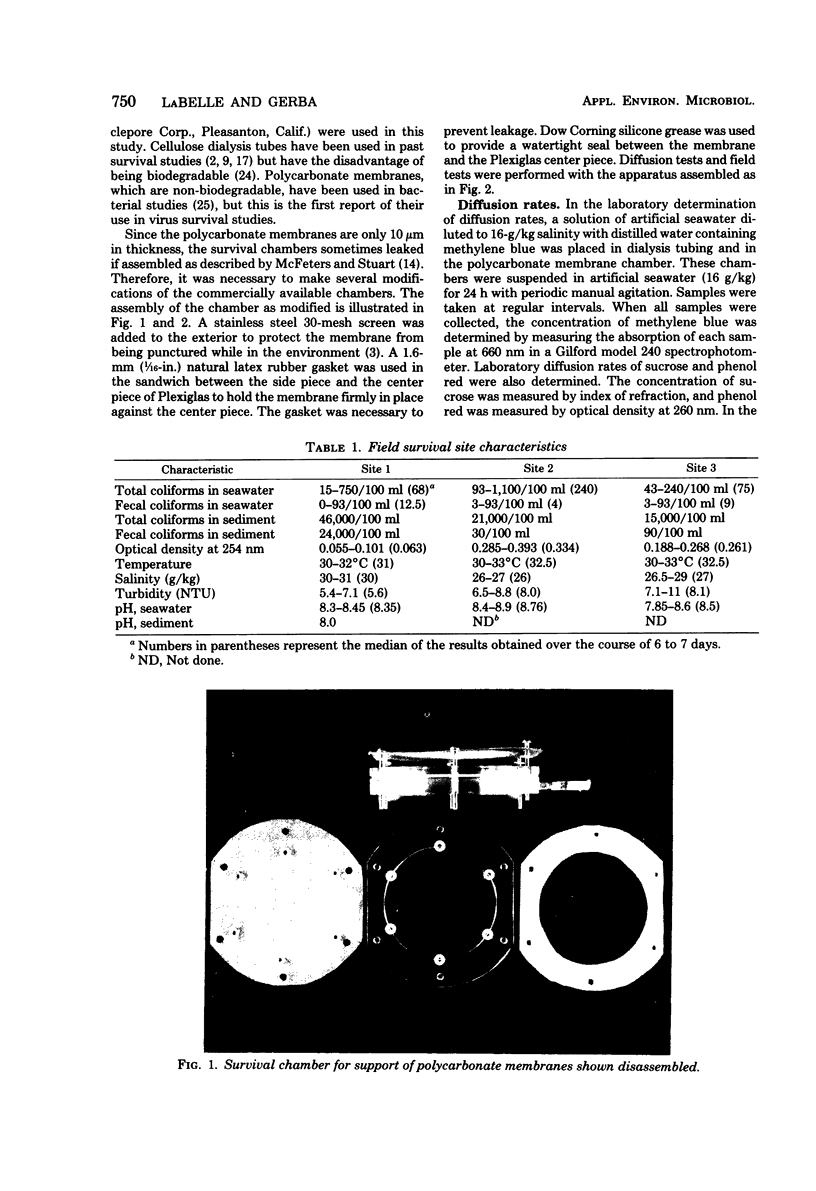

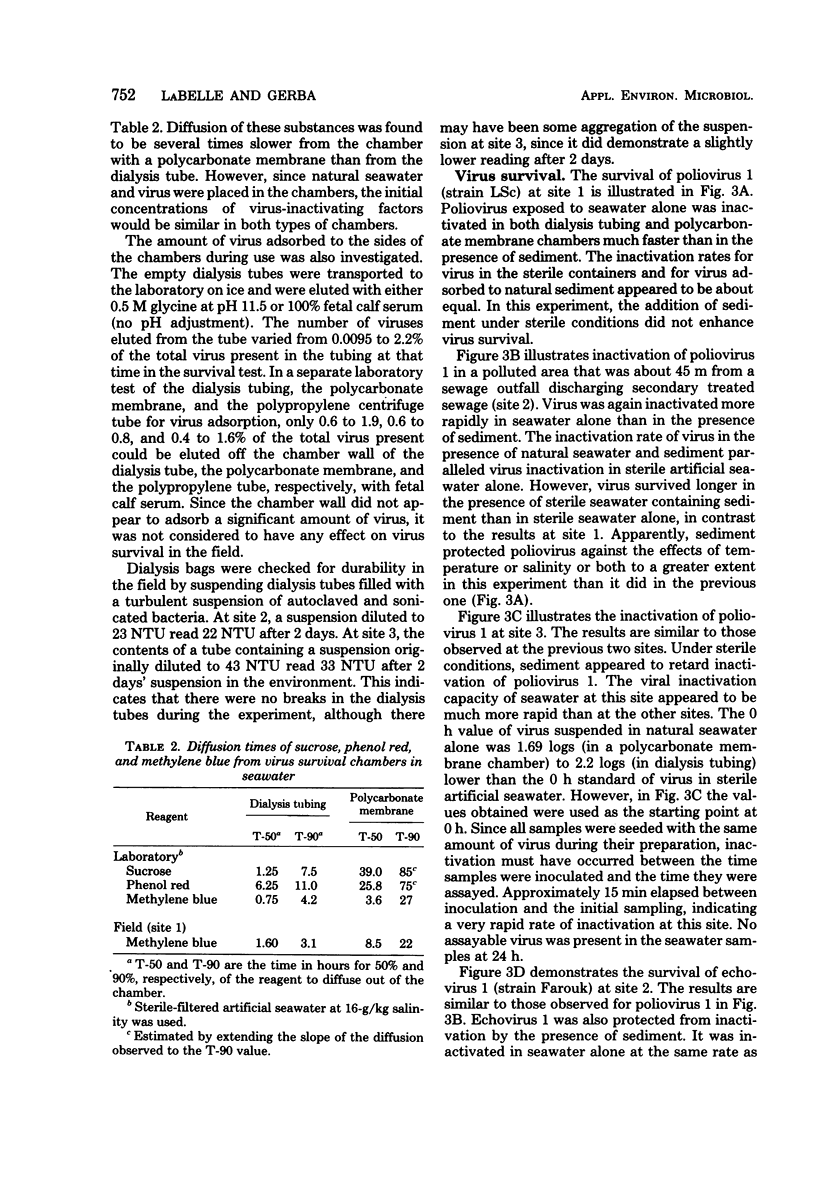
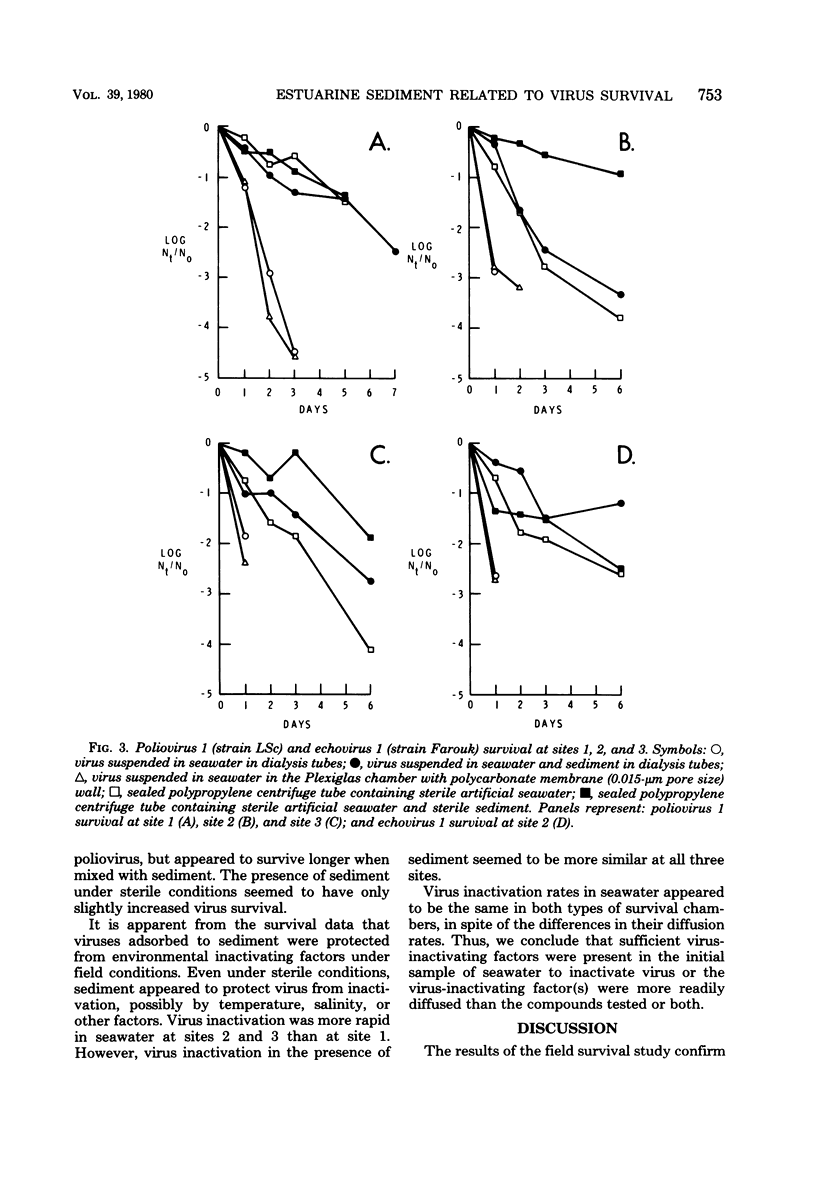


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fliermans C. B., Gorden R. W. Modification of membrane diffusion chambers for deep-water studies. Appl Environ Microbiol. 1977 Jan;33(1):207–210. doi: 10.1128/aem.33.1.207-210.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba C. P., Farrah S. R., Goyal S. M., Wallis C., Melnick J. L. Concentration of enteroviruses from large volumes of tap water, treated sewage, and seawater. Appl Environ Microbiol. 1978 Mar;35(3):540–548. doi: 10.1128/aem.35.3.540-548.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal S. M., Gerba C. P., Melnick J. L. Occurrence and distribution of bacterial indicators and pathogens in canal communities along the Texas coast. Appl Environ Microbiol. 1977 Aug;34(2):139–149. doi: 10.1128/aem.34.2.139-149.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson M., Wallis C., Melnick J. L. Concentration and purification of enteroviruses by membrane chromatography. Appl Environ Microbiol. 1976 Nov;32(5):689–693. doi: 10.1128/aem.32.5.689-693.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann J. E., Kostenbader K. D., Jr, CLIVER D. O. Persistence of enteroviruses in lake water. Appl Microbiol. 1974 Nov;28(5):895–896. doi: 10.1128/am.28.5.895-896.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill W. F., Jr, Jakubowski W., Akin E. W., Clarke N. A. Detection of virus in water: sensitivity of the tentative standard method for drinking water. Appl Environ Microbiol. 1976 Feb;31(2):254–261. doi: 10.1128/aem.31.2.254-261.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBelle R. L., Gerba C. P., Goyal S. M., Melnick J. L., Cech I., Bogdan G. F. Relationships between environmental factors, bacterial indicators, and the occurrence of enteric viruses in estuarine sediments. Appl Environ Microbiol. 1980 Mar;39(3):588–596. doi: 10.1128/aem.39.3.588-596.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBelle R. L., Gerba C. P. Influence of pH, salinity, and organic matter on the adsorption of enteric viruses to estuarine sediment. Appl Environ Microbiol. 1979 Jul;38(1):93–101. doi: 10.1128/aem.38.1.93-101.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matossian A. M., Garabedian G. A. Virucidal action of sea water. Am J Epidemiol. 1967 Jan;85(1):1–8. doi: 10.1093/oxfordjournals.aje.a120666. [DOI] [PubMed] [Google Scholar]
- McFeters G. A., Stuart D. G. Survival of coliform bacteria in natural waters: field and laboratory studies with membrane-filter chambers. Appl Microbiol. 1972 Nov;24(5):805–811. doi: 10.1128/am.24.5.805-811.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub S. A., Sagik B. P. Association of enteroviruses with natural and artificially introduced colloidal solids in water and infectivity of solids-associated virions. Appl Microbiol. 1975 Aug;30(2):212–222. doi: 10.1128/am.30.2.212-222.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. M., Gerba C. P., Melnick J. L. Role of sediment in the persistence of enteroviruses in the estuarine environment. Appl Environ Microbiol. 1978 Apr;35(4):685–689. doi: 10.1128/aem.35.4.685-689.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobsey M. D., Gerba C. P., Wallis C., Melnick J. L. Concentration of enteroviruses from large volumes of turbid estuary water. Can J Microbiol. 1977 Jun;23(6):770–778. doi: 10.1139/m77-114. [DOI] [PubMed] [Google Scholar]
- Vasconcelos G. J., Swartz R. G. Survival of bacteria in seawater using a diffusion chamber apparatus in situ. Appl Environ Microbiol. 1976 Jun;31(6):913–920. doi: 10.1128/aem.31.6.913-920.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]




