Abstract
Conditioned responses to drug-related environmental cues (such as craving) play a critical role in relapse to drug use. Animal models demonstrate that repeated exposure to drug-associated cues in the absence of drug administration leads to the extinction of conditioned responses, but the few existing clinical trials focused on extinction of conditioned responses to drug-related cues in drug-dependent individuals show equivocal results. The current study examined drug-related cue reactivity and response extinction in a laboratory setting in methamphetamine-dependent individuals. Methamphetamine cue-elicited craving was extinguished during two sessions of repeated (3) within-session exposures to multi-modal (picture, video, and in-vivo) cues, with no evidence of spontaneous recovery between sessions. A trend was noted for a greater attenuation of response in participants with longer (4-7 day) inter-session intervals. These results indicate that extinction of drug-cue conditioned responding occurs in methamphetamine-dependent individuals, offering promise for the development of extinction- based treatment strategies.
Keywords: methamphetamine addiction, drug cues, cue exposure, cue exposure therapy
INTRODUCTION
Consistent with a Pavlovian conditioning theory, cues associated with drug using (e.g. paraphernalia, locations where drug is used) acquire the capacity to elicit conditioned responses, such as craving, as a consequence of repeated pairings between the cues and the central nervous system effects of the drug (i.e. activation of reward pathways in the brain; Pavlov, 1927). This conditioned association has been systematically examined in human laboratory settings using paradigms in which participants are exposed to cues related to the use of nicotine (LaRowe, Saladin, Carpenter, & Upadhyaya, 2007; McClernon et al., 2007; Tiffany, Cox, & Elash, 2000), alcohol (Glautier & Drummond, 1994; Monti et al., 1993; Szegedi et al., 2000), heroin (Moring & Strang, 1989; Sell et al., 2000; Yu et al., 2007), and cocaine (Avants, Margolin, Kosten, & Cooney, 1995; Robbins, Ehrman, Childress, & O’Brien, 1999; Saladin, Brady, Graap, & Rothbaum, 2006), and subjective, behavioral, and physiological responses are recorded. Several studies have shown that craving is related to relapse to drug-taking behavior (Back, Brady, Sonne, & Verduin, 2006; Brady et al., 2006; Cooney, Litt, Morse, Bauer, & Gaupp, 1997; Drummond & Glautier, 1994; Killen & Fortmann, 1997; Rohsenow et al., 1994), and conditioned responses to drug cues play a critical role in relapse during abstinence, when craving is likely to be elevated (Childress, McLellan, Ehrman, & O’Brien, 1988; O’Brien, Childress, Ehrman, & Robbins, 1998; Sinha, Fuse, Aubin, & O’Malley, 2000).
While craving and reactivity to cocaine-associated cues is reliable and robust (Coffey et al., 2002; Robbins et al., 1999; Saladin et al., 2006; Sinha et al., 2000), relatively little attention has been given to methamphetamine (METH), a related psychostimulant with high abuse and dependence liability. Emerging evidence suggests that craving to METH cues can be reliably measured in METH-dependent individuals (Bruehl, Lende, Schwartz, Sterk, & Elifson, 2006; Newton et al., 2006; Tolliver et al., 2010) and cue-elicited craving is a strong predictor of subsequent METH use (Hartz, Frederick-Osborne, & Galloway, 2001). Accordingly, cue-elicited METH craving should be viewed as a clinically important phenomenon.
Animal models demonstrate that repeated exposure to drug-associated cues in the absence of drug administration leads to the extinction of conditioned responses (Barr et al., 1983; Neisewander, O’Dell, Tran-Nguyen, Castaneda, & Fuchs, 1996; See, 2002). Early work applying these principles to drug-dependent clinical populations showed promise in reducing reactivity to drug-associated cues and improving drug-use related outcomes (e.g. Childress, McLellan, & O’Brien, 1986; O’Brien, Childress, McLellan, & Ehrman, 1990). Nevertheless, the limited number of studies employing such techniques in clinical settings have had mixed results (e.g. Cooney et al., 1997; Drummond & Glautier, 1994; Monti et al., 1993; Rohsenow et al., 2001). Recent studies of experimentally-controlled acquisition, extinction, and renewal of conditioned appetitive responses have elucidated nuances of extinction and renewal, including the importance of context and expectation (Thewissen, Snijders, Havermans, van den Hout, & Jansen, 2006; Van Gucht, Vansteenwegen, Beckers, & Van der Bergh, 2008); however, if and how to integrate these subtleties into treatment-oriented extinction paradigms is not yet clear. A recent review of the use of cue extinction paradigms in drug-dependent clinical populations (Conklin & Tiffany, 2002) discussed the disconnect between the theoretical promise of this type of intervention and the use of extinction training in clinical practice. The authors cited the lack of clear optimal parameters for conducting cue extinction studies as one possible contribution to the inconsistent results. In addition, the time course and parameters influencing extinction may differ across substances, as use patterns tend to vary across classes of drugs of abuse. Thus, further research to characterize drug cue extinction is warranted. The purpose of this study is to explore extinction of craving response to METH-related cues following repeated exposure in METH-dependent individuals.
METHODS
Subjects
Men and women aged 18-50 who met DSM-IV criteria for METH dependence within the past six months were eligible to participate. The study was approved by the Institutional Review Board of the Medical University of South Carolina. All participants provided written informed consent after being fully informed of potential risks of participation before any study assessments/procedures were undertaken. Both treatment-seeking and nontreatment-seeking participants were recruited through referrals from local substance abuse treatment clinics or advertisements in the community and were compensated with vouchers for their participation. All subjects were required to maintain abstinence from METH, alcohol, and all other drugs of abuse except nicotine as confirmed by breathalyzer and urine drug screening on the day of test assessments. Exclusion criteria included a history of or current psychotic disorder, bipolar affective disorder, or major depressive disorder requiring antidepressant pharmacotherapy or presenting with significant suicidal risk. Subjects with current severe anxiety disorders including panic disorder, posttraumatic disorder, or generalized anxiety disorder were excluded due to potential interference with the measurement of cue reactivity. Current treatment with benzodiazepines, ß-blockers, anti-arrhythmic agents, psychostimulants or any other agents known to interfere with heart rate and skin conductance monitoring was exclusionary. Subjects with significant hematologic, endocrine (including diabetes mellitus), cardiovascular, pulmonary, renal, gastrointestinal, or neurological disease were also excluded.
Study Design
All study procedures were conducted at the research clinic of Behavioral Health Services in Pickens, South Carolina, a NIDA Clinical Trials Network site. After giving informed consent, potential participants were screened using the MINI International Neuropsychiatric Interview (Sheehan et al., 2003), a structured interview based on the DSM-IV for assessment of psychiatric and substance use symptoms. Quantitative METH and other substance use data for the past 90 days were assessed using the Time-Line Follow-Back (TLFB), a calendar-based instrument used to assess daily self-reported substance use (Sobell & Sobell, 1992) and breathalyzer and urine drug screening was conducted to assess recent substance use. Once all inclusion/exclusion criteria were satisfied, subjects were eligible to begin cue exposure sessions.
Cue Reactivity Procedures
Participants were administered three 20-minute sequences of multi-modal METH cue exposure over each of two one-hour sessions, resulting in exposure to a total of six cue exposure sequences. Multi-modal METH cues were counterbalanced for order of presentation, and consisted of photographs and video of individuals procuring and using METH and “in vivo” paraphernalia and simulated METH. Baseline craving ratings and physiologic measures were collected 20 minutes and 5 minutes prior to initial cue exposure for each session and subsequently during each cue sequence. The intervals between the two cue exposure sessions varied from 1 day to 7 days. Subjects were required to provide a negative urine drug screen prior to each cue exposure session. Self-reported baseline and cue-induced craving were assessed using a modification of the Within-Session Rating Scale (Childress et al., 1986), a visual analog scale (0-10) assessment of subjective desire to use METH. Physiologic data [heart rate (BPM) and skin conductance (micro-Seimans)] were collected using Ag/AgCl electrodes interfaced to a Biopac MP-100 data acquisition system and analyzed using AcKnowledge software (Biopac, Goleta, CA). Two electrodes were placed on the second phalanx of the first and third fingers of the non-dominant hand for the measurement of skin conductance, and additional electrodes were placed on the anterior chest and left abdomen to record heart rate; participants were instructed not to move during recordings to limit movement artifact.
Statistical Analysis
Standard descriptive statistics were used to summarize the general demographic and clinical data. Descriptive statistics are denoted as Mean ± Standard Error of the Mean (SEM) for continuous variables and percentages for categorical variables. Demographic and clinical characteristics were tabulated for all subjects and were also compared between individuals who reported METH craving (> 0) during the initial baseline assessment and those who did not. The comparisons were done using the Wilcoxon 2-Sample Rank Sum Test Statistic for continuous variables and the Pearson Chi-Square test for categorical characteristics.
In order to establish that the selected cues were effective in eliciting a conditioned craving response, the Wilcoxon Signed Rank test was used to analyze the difference between craving scores at baseline and those reported during the first cue sequence. The first and second session baseline values were also compared to assess whether non-cue-elicited craving was reduced across sessions.
Extinction of craving response was assessed via covariance pattern models, which account for the correlation across repeated measures as well as data that are missing at random. A type III sums of squares F-test for the sequence effect was used to determine whether significant decreases in conditioned craving occurred over the course of the six cue sequences. To assess whether baseline craving level impacted extinction, secondary analyses included a between-group comparison between those participants who did and those who did not report craving at baseline (i.e., prior to cue presentations in session 1). These comparisons were made by fitting repeated measures ANOVA with craving Group assignment, Sequence, and the interaction between Group and Sequence as the factors of interest. Secondarily, to track the possible extinction of elevated heart rate and skin conductance in response to the cues, similar models as described above were fit with the within-sequence mean heart rate and skin conductance data.
The number of days varied between Sessions 1 and 2 among study participants. Similar repeated measures ANOVAs were used to determine whether the amount of time between cue exposure sessions affected the process of extinction. Baseline group comparisons were done using the Wilcoxon 2-Sample Rank Sum Test Statistic for continuous variables and the Pearson Chi-Square test for categorical characteristics.
All statistical analyses were performed using the SAS System version 9.2. A type I error rate was controlled at 0.05 for all analyses; reported p-values were not corrected for multiple comparisons.
RESULTS
Twenty-eight subjects were enrolled in the study; four subjects dropped out and multiple cue-sequence extinction data was collected for 24 of subjects. The descriptive and clinical data for the participants are listed in Table 1. The participants had a mean age of 32.1 (±7.4) years and were mostly female (79.2%). The majority of the participants smoked cigarettes (83.3%) and were currently in drug treatment (79.2%); less than 1/3 of the subjects were currently employed full-time (29.2%). Baseline craving values represent the mean of the two craving ratings taken 20 & 5 minutes prior to cue presentation. The two baseline craving ratings for session 1 were not statistically different from one another (20 min: 1.75 ± 2.40, 5 min: 1.96 ± 2.69; p = 0.375).
Table 1.
Demographic and clinical characteristics for all subjects and dichotomized by METH craving status at study baseline.
| Demographics and Characteristics† |
All Subjects | Baseline Non Cravers |
Baseline Cravers | P Value§* |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | Std Err |
n | Mean | Std Err |
n | Mean | Std Err |
||
| Age (yrs) | 24 | 32.1 | 1.5 | 14 | 31.4 | 1.7 | 10 | 33.2 | 2.8 | 0.58 |
| HR at Baseline (BPM) | 22 | 79.2 | 2.7 | 14 | 80.4 | 3.8 | 8 | 77.2 | 3.6 | 0.87 |
| Skin Cond at Baseline(μS) | 24 | 2.5 | 0.3 | 14 | 2.6 | 0.3 | 10 | 2.4 | 0.6 | 0.34 |
| Craving at Baseline | 24 | 1.9 | 0.5 | 14 | 0 | 0 | 10 | 4.5 | 0.6 | <0.01 |
| % Male | 24 | 20.8 | 14 | 7.1 | 10 | 40.0 | 0.12 | |||
| % Smoker | 24 | 83.3 | 14 | 78.6 | 10 | 90.0 | 0.62 | |||
| % Currently Employed | 24 | 29.2 | 14 | 21.4 | 10 | 40.0 | 0.39 | |||
| % High School Edu | 24 | 37.5 | 14 | 21.4 | 10 | 60.0 | 0.07 | |||
| % in Treatment | 24 | 79.2 | 14 | 85.7 | 10 | 70.0 | 0.62 | |||
| % Craving At Baseline | 24 | 41.7 | NA | NA | ||||||
P Value for Difference between baseline cravers and non cravers.
Continuous Variables were compared by use of the Wilcoxon 2-Sample Test Statistic and Categorical Variables were compared by use of the 2-Sided Fisher Exact Test Statistic.
Continuous Variables are listed as mean and standard deviation while categorical variables are listed as percentages.
To establish that the selected METH-related cues activated craving, the change in craving scores before and after exposure to the initial cue sequence was evaluated. The participants reported a mean craving score at baseline of 1.85 ± 0.51, and following cue sequence 1 reported a craving level of 4.03 ± 0.65 (p < 0.001). The mean craving (and standard deviations) for the two session 2 baselines were 0.67 ± 2.04 and 0.54 ± 1.89, which are not significantly different p = 0.75. The averaged session 2 baseline craving score was 0.60 (± 0.39), which was significantly lower than the session 1 baseline (p =0.031).
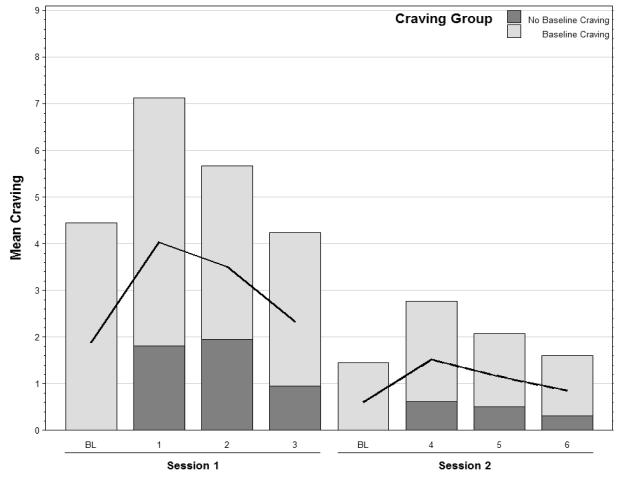
To assess extinction of cue-elicited responses after repeated exposure, the within-subject effect of cue sequence (6 sequences over 2 sessions) was assessed. The main effect of sequence was significant (F(5,21) = 7.82, p < 0.001), indicating a decrease in craving response to the cues at an average rate of 0.65 per sequence (Figure 1). There was a decrease from 4.03 ± 0.65 following sequence 1 to 0.85 ± 0.35 following sequence 6. Twenty of the 24 participants reported craving for METH following the initial cue sequence (response > 0). Of these 20 participants, the mean % change in craving score through the end of the last cue sequence was −84.4% and 11 (of the 20) participants reported no craving at the end of sequence 6.
Figure 1.
Mean craving score by sequence across session 1 and session 2 for all subjects and dichotomized by METH craving status at study baseline. The solid line represents the overall mean (n=24).
Similar analyses were run on the physiologic data (heart rate and skin conductance) and no significant changes were identified. Neither the heart rate nor the skin conductance patterns mirrored those seen for craving ratings.
Baseline Cravers versus Non Cravers
Following the primary full-cohort analysis, the participants were grouped according to their initial baseline craving. The groupings consisted of 14 participants who had no baseline craving for METH and 10 participants who reported craving at baseline (4.45 ± 0.58). The two groups did not differ with respect to age (non cravers 31.4 ± 1.7 vs. cravers 33.2 ± 2.8; p = 0.58), baseline heart rate (80.4 ± 3.8 vs. 77.2 ± 3.6; p = 0.87), baseline skin conductance (2.6 ± 0.3 vs. 2.4 ± 0.6; p = 0.34), percent current smokers (78.6 % vs. 90.0 %; p = 0.62), or percent currently in treatment (85.7% vs. 70.0%; p = 0.62).
The participants that failed to crave at baseline (0 ± 0) reported a minimal response to the initial cue (1.8 ± 0.44; p = 0.002) while those that did crave METH at baseline rated their craving at 7.1 ± 0.62 following cue sequence 1, an increase of 2.6 ± 0.73 (p = 0.012). Of the ten participants who reported craving at session 1 baseline, only 3 reported craving at the session 2 baseline. Of the 14 participants who reported no craving at session 1 baseline, all continued to report a lack of craving at the session 2 baseline.
The effect of baseline craving groups, sequences, and the interaction of the two were examined for statistical significance. The interaction of sequence and craving group was not significant (F(5,21) = 1.65; p = 0.190). The overall effect of sequence in the model remained significant (F(5,21) = 10.34; p < 0.001) as did the effect of the grouping (F(1,21) = 15.19; p = <0.001), which was to be expected due to the dichotomization on the craving at baseline. Extinction of the conditioned craving response to METH cues was then analyzed independently for those individuals who did and did not report baseline craving. There was a significant effect of sequence (F(5,8) = 11.34; p = 0.001) in the group of baseline cravers, in which craving response to the cues decreased at an average rate of 1.12 per cue exposure sequence (decreasing from 7.13 ± 0.62 to 1.60 ± 0.75). Similarly, those who did not crave at baseline showed a significant decrease in cue-elicited craving over the course of the six cue exposure sequences (F(5,65) = 2.69; p = 0.028) with an average decrease of 0.32 per sequence (decreasing from 1.81 ± 0.44 to 0.31 ± 0.21; see Figure 1).
Effect of Inter-session Interval
Each subject was assigned an inter-session interval (ISI) between 1 and 7 days (median = 2 days). Extinction rates of the individuals with ≤ 3 days between cue exposure sessions (n=17) were compared with those that had ≥ 4 days between sessions (n=7). Mean baseline craving values did not differ between the ISI Groups (1.79 ± 0.62 for ≤ 3 days vs. 2.00 ± 1.00 for ≥ 4 days; p = 0.86), nor did their rate of response extinction (F(5,21) = 1.95; p = 0.13). There was also no significant difference in craving response between the two ISI groups (F(1,21) = 0.07; p = 0.796). However, in an unadjusted nonparametric (Wilcoxon Rank Sums) analysis of the total decline in craving from sequence 1 to sequence 6, a trend toward significance was found (p = 0.06), showing a slightly greater average decrease in conditioned craving in the group with > 4 days between session 1 and session 2 (See Table 2).
Table 2.
Inter-session Interval Group craving scores (mean ± SE) during session 1 baseline, session 1 and 2 by sequence, as well as the absolute change from baseline to the last cue sequence.
| Group | Change 1 From BL |
Baseline | Session 1 | Session 2 | ||||
|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S1 | S2 | S3 | |||
| ≤ 3 Days | 2.43 ± 0.67 | 1.79 ± 0.62 | 3.55 ± 0.78 | 3.25 ± 0.71 | 2.31 ± 0.61 | 1.73 ± 0.62 | 1.49 ± 0.54 | 1.12 ± 0.48 |
| ≥ 4 Days | 5.00 ± 1.12 | 2.00 ± 1.00 | 5.19 ± 1.18 | 4.10 ± 1.47 | 2.33 ± 1.20 | 1.00 ± 0.74 | 0.33 ± 0.22 | 0.19 ± 0.12 |
| P-Value * | 0.06 | 0.86 | 0.23 | 0.64 | 0.99 | 0.30 | 0.13 | 0.16 |
2 Sample Wilcoxon Rank Sums test p value.
DISCUSSION
In this laboratory study of cue-elicited response extinction in METH users, we have shown that multi-modal METH cues elicit a robust craving response that can be extinguished with multiple presentations of the cues in the absence of METH administration. Several study parameters and subject characteristics were examined to determine their impact on extinction of responses to METH-associated cues. To our knowledge, these are the first data to be published concerning extinction of cue-induced craving in this population of drug-dependent individuals.
The multi-modal cue presentation utilized in this study involved pictures and video of the procurement and use of drug, as well as the ‘in vivo’ cues of simulated drug and paraphernalia associated with METH use. Similar paradigms have been shown to be effective in eliciting robust craving responses in previously published studies of cocaine-dependent individuals (Childress, McLellan, Ehrman, & O’Brien, 1987; Childress et al., 1986; Robbins et al., 1999; Waldrop et al., in press). Characterization of factors contributing to the elicitation of subjective and physiological responses to different cue modalities in METH users have been detailed and published elsewhere (Tolliver et al., 2010). In an analysis of the clinical and demographic factors contributing to initial METH cue reactivity in the entire study cohort, the strongest predictor of METH cue reactivity was baseline craving (Tolliver et al., 2010).
Of those participants that completed the extinction procedures, 58% did not report any baseline craving while 42% reported some level of craving for METH before exposure to the cues. Most (83%), but not all, participants had a positive craving response to the METH cues, which is consistent with (or even higher than) reports of cue-induced craving among individuals dependent on a number of abused substances (Avants et al., 1995; Coffey et al., 2002; Monti et al., 1993). Because baseline craving was strongly predictive of initial cue reactivity in the larger cohort, we anticipated that this factor might also influence the extinction of response to METH cues. However, both individuals who reported baseline craving and those who did not exhibited significant increases in craving after the initial cue presentation compared to baseline craving levels, as well as a significant decreases of craving response to cues across six sequences of cue exposures (see Figure 1).
A well-established phenomenon associated with extinction of a conditioned response in animal studies is ‘spontaneous recovery,’ whereby a previously extinguished response is emitted when cues are reintroduced (cf., Pavlov, 1927; Rescorla, 2004a). This has been demonstrated in a number of animal models of extinction of conditioned response to drug-associated stimuli (Di Ciano & Everitt, 2002; Meil & See, 1996), and may play a role in relapse to drug-taking behavior in long-abstinent drug-dependent individuals (Childress et al., 1988; Cooney et al., 1997; Kosten et al., 2006; Sinha & Li, 2007). In this study, no spontaneous recovery of the extinguished response was evident. As the majority of study participants were in drug treatment and had not used METH in the month prior to initiation of extinction, this is consistent with evidence that spontaneous recovery is less likely when the interval between conditioning and extinction training is long (Rescorla, 2004b). There were no increases in cue-elicited responses during the second session, which was conducted a variable number of days after session one, indicating that the METH cues were no longer salient enough to elicit significant craving for the drug. In addition, the level of craving exhibited during the baseline measure of session 2 was significantly lower than the baseline craving rating in session 1, indicating that no increase in ambient craving had occurred between the cue sessions. The lack of spontaneous recovery suggests that the extinction procedures during the first session had sustained effects. Future studies focused on the demonstration of spontaneous recovery in human laboratory paradigms might be of interest as this might guide the timing of exposure session to maximize extinction.
Since the time between cue exposure sessions has been shown to be relevant in general models of extinction of conditioned responses (Bouton, 1993; Rescorla, 2004b), we varied the length of time between sessions to determine whether this might impact the rate or level of extinction to METH cues; it is important to note that none of our subjects relapsed to METH use between sessions 1 and 2. While the rate of extinction did not vary between those that had shorter (≤ 3 days) versus longer (≥ 4 days) inter-session intervals, there was a trend for those that had the longer inter-session interval to exhibit a greater degree of extinction of the craving response (see Table 2). This finding is generally consistent with recent well-controlled animal studies of extinction (Li & Westbrook, 2008). This may prove to be an important variable to consider in extinction of responses to cues associated with different classes of drugs, as use patterns may govern whether spaced versus massed exposure trials are more effective (Wagner, Siegel, & Fein, 1967).
While this experiment has established that elicitation and extinction of METH cue craving response can be established in a laboratory setting, there are limitations to be acknowledged. Without a control group, in which there was no cue exposure and craving data was collected at identical time-points, it is impossible to conclude definitively that the reduction in craving ratings seen in the cue-exposed participants was not merely the result of repeated questioning over time, nor whether the trend in inter-session interval impact on extinction was due to time itself, an effect of time on memory consolidation, or something else. To enable more definitive conclusions, future studies investigating extinction would benefit by including such control conditions.
The lack of relationship between the subjective and physiological responses is troublesome, but not necessarily surprising. A number of drug cue exposure studies have reported a similar lack of correlation between subjective and physiologic response to drug-related cues (for review, see Tiffany & Conklin, 2000), including those that examined extinction of response to drug cues (Childress et al., 1986; Robbins et al., 1999). A number of hypotheses have been proposed for this phenomenon, including discrepancies in whether drug-conditioned cues elicit drug-like (Robinson & Berridge, 1993) or drug-withdrawal (Ludwig & Wikler, 1974) effects, or that cue-elicited responses represent something altogether different, such as a form of cognitive dissonance (Tiffany & Conklin, 2000).
While the current findings are preliminary in nature, they offer promise for the treatment of METH-dependent individuals by demonstrating an intact ability to extinguish conditioned responses in a population in which drug-associated cognitive deficits are commonly reported (for review, see Cruickshank & Dyer, 2009). Recently, the cognitive processes of extinction have become better understood; for example, instead of ‘re-learning’ associations, extinction is now believed to involve the development of new associations (Santini, Muller, & Quirk, 2001; for review, see Lovibond, 2009), a finding that may guide the development of more effective extinction procedures. Cue exposure therapy for anxiety disorders is based on the same behavioral tenets as those used to explain the extinction of conditioned responses to drug cues. However, exposure therapy in the treatment of anxiety disorders has been much more extensively studied and has been used clinically with a great deal of success (Krijn, Emmelkamp, Olafsson, & Biemond, 2004; Rothbaum & Schwartz, 2002; Zinbarg, 1993). In contrast, extinction training is not widely used in the clinical treatment of addictions. Despite the opposite motivational valence of fear and drug cue conditioning, recent evidence suggests that the brain circuits for extinction of fear and drug addiction may overlap (Peters, Kalivas, & Quirk, 2009). Therefore, repeated non-reinforced exposure to METH-related cues, perhaps in adjunct with more active therapeutic modalities (e.g. cognitive behavioral therapy), may result in a newly-learned response which would enable METH-dependent individuals to better resist the use formerly associated with drug-related cues outside of the laboratory setting.
In conclusion, this study demonstrates robust extinction of conditioned craving response to drug-related cues in METH-dependent individuals over the course of two extinction sessions. These findings have relevant clinical implications that warrant further exploration to establish the effectiveness of extinction training as a component of treatment in METH-dependent populations.
ACKNOWLEDGEMENTS
This study was supported by grant # 5P20 DA022658-03 awarded to the Translational Research in Addiction Center at MUSC (Dr. Ron See, PI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Avants SK, Margolin A, Kosten TR, Cooney NL. Differences between responders and nonresponders to cocaine cues in the laboratory. Addict Behav. 1995;20(2):215–224. doi: 10.1016/0306-4603(94)00066-2. [DOI] [PubMed] [Google Scholar]
- Back SE, Brady KT, Sonne SC, Verduin ML. Symptom improvement in co-occurring PTSD and alcohol dependence. J Nerv Ment Dis. 2006;194(9):690–696. doi: 10.1097/01.nmd.0000235794.12794.8a. [DOI] [PubMed] [Google Scholar]
- Barr GA, Sharpless NS, Cooper S, Schiff SR, Paredes W, Bridger H. Classical conditioning, decay and extinction of cocaine-induced hyperactivity and stereotypy. Life Sci. 1983;33(14):1341–1351. doi: 10.1016/0024-3205(83)90817-2. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol Bull. 1993;114(1):80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Brady KT, Back SE, Waldrop AE, McRae AL, Anton RF, Upadhyaya HP, et al. Cold pressor task reactivity: predictors of alcohol use among alcohol-dependent individuals with and without comorbid posttraumatic stress disorder. Alcohol Clin Exp Res. 2006;30(6):938–946. doi: 10.1111/j.1530-0277.2006.00097.x. [DOI] [PubMed] [Google Scholar]
- Bruehl AM, Lende DH, Schwartz M, Sterk CE, Elifson K. Craving and control: methamphetamine users’ narratives. J Psychoactive Drugs, Suppl. 2006;3:385–392. doi: 10.1080/02791072.2006.10400602. [DOI] [PubMed] [Google Scholar]
- Childress AR, McLellan AT, Ehrman R, O’Brien CP. Classically conditioned responses in opioid and cocaine dependence: a role in relapse? NIDA Res Monogr. 1988;84:25–43. [PubMed] [Google Scholar]
- Childress AR, McLellan AT, Ehrman RN, O’Brien CP. Extinction of conditioned responses in abstinent cocaine or opioid users. NIDA Res Monogr. 1987;76:189–195. [PubMed] [Google Scholar]
- Childress AR, McLellan AT, O’Brien CP. Abstinent opiate abusers exhibit conditioned craving, conditioned withdrawal and reductions in both through extinction. Br J Addict. 1986;81(5):655–660. doi: 10.1111/j.1360-0443.1986.tb00385.x. [DOI] [PubMed] [Google Scholar]
- Coffey SF, Saladin ME, Drobes DJ, Brady KT, Dansky BS, Kilpatrick DG. Trauma and substance cue reactivity in individuals with comorbid posttraumatic stress disorder and cocaine or alcohol dependence. Drug Alcohol Depend. 2002;65(2):115–127. doi: 10.1016/s0376-8716(01)00157-0. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97(2):155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Morse PA, Bauer LO, Gaupp L. Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. J Abnorm Psychol. 1997;106(2):243–250. doi: 10.1037//0021-843x.106.2.243. [DOI] [PubMed] [Google Scholar]
- Cruickshank CC, Dyer KR. A review of the clinical pharmacology of methamphetamine. Addiction. 2009;104(7):1085–1099. doi: 10.1111/j.1360-0443.2009.02564.x. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Reinstatement and spontaneous recovery of cocaine-seeking following extinction and different durations of withdrawal. Behav Pharmacol. 2002;13(5-6):397–405. doi: 10.1097/00008877-200209000-00013. [DOI] [PubMed] [Google Scholar]
- Drummond DC, Glautier S. A controlled trial of cue exposure treatment in alcohol dependence. J Consult Clin Psychol. 1994;62(4):809–817. doi: 10.1037//0022-006x.62.4.809. [DOI] [PubMed] [Google Scholar]
- Glautier S, Drummond DC. Alcohol dependence and cue reactivity. J Stud Alcohol. 1994;55(2):224–229. doi: 10.15288/jsa.1994.55.224. [DOI] [PubMed] [Google Scholar]
- Hartz DT, Frederick-Osborne SL, Galloway GP. Craving predicts use during treatment for methamphetamine dependence: a prospective, repeated-measures, within-subject analysis. Drug Alcohol Depend. 2001;63(3):269–276. doi: 10.1016/s0376-8716(00)00217-9. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP. Craving is associated with smoking relapse: findings from three prospective studies. Exp Clin Psychopharmacol. 1997;5(2):137–142. doi: 10.1037//1064-1297.5.2.137. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, et al. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;31(3):644–650. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- Krijn M, Emmelkamp PM, Olafsson RP, Biemond R. Virtual reality exposure therapy of anxiety disorders: a review. Clin Psychol Rev. 2004;24(3):259–281. doi: 10.1016/j.cpr.2004.04.001. [DOI] [PubMed] [Google Scholar]
- LaRowe SD, Saladin ME, Carpenter MJ, Upadhyaya HP. Reactivity to nicotine cues over repeated cue reactivity sessions. Addict Behav. 2007;32(12):2888–2899. doi: 10.1016/j.addbeh.2007.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SH, Westbrook RF. Massed extinction trials produce better short-term but worse long-term loss of context conditioned fear responses than spaced trials. J Exp Psychol Anim Behav Process. 2008;34(3):336–351. doi: 10.1037/0097-7403.34.3.336. [DOI] [PubMed] [Google Scholar]
- Lovibond PF. Cognitive Processes in Extinction. Learn Mem. 2004;11(12):495–5000. doi: 10.1101/lm.79604. [DOI] [PubMed] [Google Scholar]
- Ludwig AM, Wikler A. “Craving” and relapse to drink. Q J Stud Alcohol. 1974;35(1 pt A):108–130. [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Liu J, Salley AN, Behm FM, Rose JE. Selectively reduced responses to smoking cues in amygdala following extinction-based smoking cessation: results of a preliminary functional magnetic resonance imaging study. Addict Biol. 2007;12(3-4):503–512. doi: 10.1111/j.1369-1600.2007.00075.x. [DOI] [PubMed] [Google Scholar]
- Meil WM, See RE. Conditioned cued recovery of responding following prolonged withdrawal from self-administered cocaine in rats: an animal model of relapse. Behav Pharmacol. 1996;7(8):754–763. [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Rubonis AV, Niaura RS, Sirota AD, Colby SM, et al. Alcohol cue reactivity: effects of detoxification and extended exposure. J Stud Alcohol. 1993;54(2):235–245. doi: 10.15288/jsa.1993.54.235. [DOI] [PubMed] [Google Scholar]
- Moring J, Strang J. Cue exposure as an assessment technique in the management of a heroin addict: case report. Drug Alcohol Depend. 1989;24(2):161–167. doi: 10.1016/0376-8716(89)90080-x. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, O’Dell LE, Tran-Nguyen LT, Castaneda E, Fuchs RA. Dopamine overflow in the nucleus accumbens during extinction and reinstatement of cocaine self-administration behavior. Neuropsychopharmacology. 1996;15(5):506–514. doi: 10.1016/S0893-133X(96)00097-8. [DOI] [PubMed] [Google Scholar]
- Newton TF, Roache JD, De La Garza R, 2nd, Fong T, Wallace CL, Li SH, et al. Bupropion reduces methamphetamine-induced subjective effects and cue-induced craving. Neuropsychopharmacology. 2006;31(7):1537–1544. doi: 10.1038/sj.npp.1300979. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12(1):15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, McLellan T, Ehrman R. Integrating systemic cue exposure with standard treatment in recovering drug dependent patients. Addict Behav. 1990;15(4):355–365. doi: 10.1016/0306-4603(90)90045-y. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes. Dover Publications; New York: 1927. [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16(5):279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. Spontaneous recovery. Learn Mem. 2004a;11(5):501–509. doi: 10.1101/lm.77504. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Spontaneous recovery varies inversely with the training-extinction interval. Learn Behav. 2004b;32(4):401–408. doi: 10.3758/bf03196037. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O’Brien CP. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend. 1999;53(3):223–230. doi: 10.1016/s0376-8716(98)00135-5. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Rubonis AV, Gulliver SB, Colby SM, Binkoff JA, et al. Cue exposure with coping skills training and communication skills training for alcohol dependence: 6- and 12-month outcomes. Addiction. 2001;96(8):1161–1174. doi: 10.1046/j.1360-0443.2001.96811619.x. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Rubonis AV, Sirota AD, Niaura RS, Colby SM, et al. Cue reactivity as a predictor of drinking among male alcoholics. J Consult Clin Psychol. 1994;62(3):620–626. doi: 10.1037//0022-006x.62.3.620. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Schwartz AC. Exposure therapy for posttraumatic stress disorder. Am J Psychother. 2002;56(1):59–75. doi: 10.1176/appi.psychotherapy.2002.56.1.59. [DOI] [PubMed] [Google Scholar]
- Saladin ME, Brady KT, Graap K, Rothbaum BO. A preliminary report on the use of virtual reality technology to elicit craving and cue reactivity in cocaine dependent individuals. Addictive Behaviors. 2006;31(10):1881–1894. doi: 10.1016/j.addbeh.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Santini E, Muller RU, Quirk GJ. Consolidation of extinction learning involves transfer from NMDA-independent to NMDA-dependent memory. J Neurosci. 2001;21(22):9009–9017. doi: 10.1523/JNEUROSCI.21-22-09009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav. 2002;71(3):517–529. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- Sell LA, Morris JS, Bearn J, Frackowiak RS, Friston KJ, Dolan RJ. Neural responses associated with cue evoked emotional states and heroin in opiate addicts. Drug Alcohol Depend. 2000;60(2):207–216. doi: 10.1016/s0376-8716(99)00158-1. [DOI] [PubMed] [Google Scholar]
- Sheehan D, Janavs J, Baker R, Harnett-Sheehan K, Knapp E, Sheehan M. MINI: Mini International Neuropsychiatric Interview. Florida and Paris: 2003. [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O’Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology (Berl) 2000;152(2):140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26(1):25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Sobell L, Sobell MB. Timeline follow-back: A technique for assessing self-reported ethanol assumption. In: Allen J, Litten RZ, editors. Measuring alcohol consumption: Psychological and biological methods. Humana Press; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- Szegedi A, Lorch B, Scheurich A, Ruppe A, Hautzinger M, Wetzel H. Cue exposure in alcohol dependent patients: preliminary evidence for different types of cue reactivity. J Neural Transm. 2000;107(6):721–730. doi: 10.1007/s007020070073. [DOI] [PubMed] [Google Scholar]
- Thewissen R, Snijders SJ, Havermans RC, van den Hout M, Jansen A. Renewal of cue-elicited urge to smoke: Implications for cue exposure treatment. Behav Res Ther. 2006;44(10):1441–1449. doi: 10.1016/j.brat.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Conklin CA. A cognitive processing model of alcohol craving and compulsive alcohol use. Addiction. 2000;95(Suppl 2):S145–153. doi: 10.1080/09652140050111717. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Cox LS, Elash CA. Effects of transdermal nicotine patches on abstinence-induced and cue-elicited craving in cigarette smokers. J Consult Clin Psychol. 2000;68(2):233–240. doi: 10.1037//0022-006x.68.2.233. [DOI] [PubMed] [Google Scholar]
- Tolliver BK, McRae-Clark AL, Saladin M, Price KL, Simpson AN, DeSantis SM, et al. Determinants of cue-elicited craving and physiologic reactivity in methamphetamine-dependent subjects in the laboratory. Am J Drug Alcohol Ab. 2010;36:106–13. doi: 10.3109/00952991003686402. [DOI] [PubMed] [Google Scholar]
- Van Gucht D, Vansteenwegen D, Beckers T, Van den Bergh O. Return of experimentally induced chocolate craving after extinction in a different context: Divergence between craving for and expecting to eat chocolate. Behav Res Ther. 2008;46(3):375–391. doi: 10.1016/j.brat.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Wagner AR, Siegel LS, Fein GG. Extinction of conditioned fear as a function of percentage of reinforcement. J Comp Physiol Psychol. 1967;63(1):160–164. doi: 10.1037/h0024172. [DOI] [PubMed] [Google Scholar]
- Waldrop AE, Price KL, DeSantis SM, Simpson AE, Back SE, McRae AL, et al. Community-dwelling cocaine-dependent men and women respond differently to social stressors versus cocaine cues. Psychoneuroendocrinology. doi: 10.1016/j.psyneuen.2009.11.005. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Zhang S, Epstein DH, Fang Y, Shi J, Qin H, et al. Gender and stimulus difference in cue-induced responses in abstinent heroin users. Pharmacol Biochem Behav. 2007;86(3):485–492. doi: 10.1016/j.pbb.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Zinbarg RE. Information processing and classical conditioning: implications for exposure therapy and the integration of cognitive therapy and behavior therapy. J Behav Ther Exp Psychiatry. 1993;24(2):129–139. doi: 10.1016/0005-7916(93)90041-t. [DOI] [PubMed] [Google Scholar]