Abstract
The most intractable feature of drug addiction is the high rate of relapse, even following extended periods of abstinence from drug-taking. Evidence suggests that allowing rats extended access to cocaine self-administration leads to behavioral characteristics in these animals that are consistent with the development of addiction in humans. In the current study, rats were allowed to self-administer cocaine over a total of 22 daily sessions, the final 7 of which were long-access (LgA) sessions of 6 hours duration. Assessments of reinstatement of drug-seeking behavior were made following reintroduction to the drug-taking environment and noncontingent priming with either CS or cocaine in both extinguished and abstinent subject groups. Three separate groups of rats were treated with either saline or D-serine (100mg/kg i.p.) administered 2 hrs prior to, or immediately following, each extinction training session. Saline-treated LgA rats were resistant to the effects of extinction training to reduce noncontingent priming of reinstatement of drug-seeking behavior with either CS or cocaine. In contrast, treatment with D-serine either before or immediately following the sessions resulted in a significant enhancement in the ability of extinction training to reduce cocaine-primed reinstatement of drug-seeking behavior. These results suggest that D-serine can act to enhance the consolidation of extinction learning in LgA rats, and is therefore a promising adjunctive agent along with behavioral therapy for the treatment of cocaine addiction.
The development of an effective treatment regimen for the state of addiction to psychostimulants such as cocaine and amphetamines has proved to be problematic, and currently there is no FDA approved treatment for this condition. A prominent feature of addiction is the propensity for relapse, and craving is thought to be a contributing factor for this component of the disease (Jaffe et al., 1989; Ehrman et al., 1992). One therapeutic approach showing efficacy involves behavioral therapy, including the attempt to restructure past experiences and learn new behaviors that would decrease the frequency and/or severity of relapse in individuals attempting to remain abstinent (Dutra et al., 2008; Waldron and Turner, 2008). Enhancing the effectiveness of such psychosocial treatments through the use of adjunctive pharmacotherapy such as cognitive enhancement is therefore a reasonable strategy to pursue, as evidenced by the successful use of D-cycloserine in the treatment of phobia (Ressler et al., 2004).
Relapse to drug-taking is commonly investigated in preclinical studies using rodent drug self-administration and extinction/reinstatement protocols (deWit and Stewart, 1981; Epstein et al., 2006). In the current report, the cognitive enhancer D-serine was administered in concert with extinction training in an attempt to reduce the reinstatement of drug-seeking behavior in rats allowed to self-administer cocaine. Previously, using a short access (ShA) self-administration protocol, we determined that the NMDAR coagonist D-serine could significantly enhance the effect of extinction in reducing cocaine-primed reinstatement (Kelamangalath et al., 2009), and an earlier investigation found that an NMDAR antagonist blocked the effect of extinction to reduce cocaine-primed reinstatement (Kelamangalath et al., 2007). Together, these results demonstrated that the activity of NMDA receptors either during or immediately following an extinction learning experience can modulate drug-primed reinstatement of drug-seeking behavior. In order to optimize the relevance of our rodent model, a long-access (LgA) self-administration protocol was utilized, as extended access to cocaine has been argued to provide an improved rat correlate for the human state of addiction (Ahmed and Koob, 1998; Deroche-Gamonet et al., 2004; Vanderschuren and Everitt, 2004).
The reinstatement of drug-seeking behavior following cocaine priming is enhanced in LgA rats (Mantsch et al. 2004), an effect that is not due to either increased cocaine intake or escalation of drug-taking per se (Knackstedt and Kalivas 2007), but is instead dependent upon the level of operant training during cocaine self-administration (Kippin et al. 2006). Determining the conditions that reduce drug-primed reinstatement of drug-seeking behavior in these LgA rats is an important step towards the development of an effective treatment for cocaine addiction in humans. Considering that the treatment of psychostimulant addiction will likely require elements of both pharmacological and psychotherapy, the LgA self-administration/extinction/reinstatement protocol provides the opportunity to assess a combined pharmaco/behavioral approach in rats voluntarily ingesting cocaine. In this report we have tested the efficacy of systemically administered D-serine as an adjunctive pharmacotherapy combined with extinction training in rats allowed extended access to cocaine. The findings suggest that this is a promising strategy for reducing drug-induced relapse in cocaine dependent individuals.
Methods
Animals
Male Sprague-Dawley rats (Harlan) weighed approximately 300 g at the beginning of the experiment and were housed individually in a temperature and humidity controlled vivarium having a 12 hour light/dark cycle (lights off at 7:00 P.M.). They were given access to food and water ad libitum and were handled daily for 5 days prior to the surgery in order to diminish stress associated with handling. The housing and experimental procedures followed the Guide for the Care and Use of Laboratory Animals and were approved by the local IACUC at the University of Georgia.
Jugular catheterization protocol
The animals were anesthetized using a combination of ketamine (70 mg/kg), xylazine (10 mg/kg) and acepromazine (1 mg/kg) administered i.p. Depth of anaesthesia was assessed by monitoring respiration rate and palpebral and pedal withdrawal reflexes. Under anesthesia, the right jugular vein was isolated. The catheter was exteriorized by passing it subcutaneously to the base of the skull, where it was connected to a modified 22 gauge cannula. A silastic catheter (Dow Corning) was then inserted into the vein (4–5 cm) and secured in position with silk sutures (6/0). The animal was placed in a stereotaxic frame (Stoelting), where the right-angled cannula (Plastics One) was mounted to the top of the skull using dental cement and 4 screws. Immediately after surgery, and once daily for 5 days, the animals were treated with gentamicin at a dose of 5 mg/kg i.v. The catheters were flushed every day with saline prior to each self-administration session and with heparin (10 USP/ml) after the session to maintain the patency of the catheter. Catheter patency was verified daily by drawing blood from the catheter.
Self-administration environment
The operant chambers (Med Associates) were equipped with 2 levers, one “active” and another “inactive” with lights positioned above each lever. The chambers had a rod grid floor, a house light, a speaker/tone generator (2.9 kHz, 10 dB above ambient) and were housed inside enclosures equipped with ventilation fans. A syringe pump was located outside the enclosure. The method for delivering a cocaine infusion was as follows: The modified 22 gauge cannula mounted on the rat’s skull was connected to a liquid swivel with PE-50 tubing protected by a metal spring. The swivel was connected with tygon tubing to the syringe mounted in the infusion pump. Infusion volumes were calculated according to the animal’s weight. For cocaine animals, the syringes mounted in the infusion pump contained cocaine hydrochloride (NIDA, RTI) dissolved in normal saline at 4 mg cocaine/ml of solution. Each infusion delivered an infusion volume of 0.125 ml/kg body weight; hence the dose of cocaine self-administered was 0.5 mg/kg/infusion. The MED-PC software program recorded the number of active lever presses, inactive lever presses and the number of infusions.
Self-administration protocol (days 1–22)
The animals having patent indwelling catheters were subjected to self-administration training for a period of 22 days with one session each day. The first 15 days the self-administration training sessions were 90 minutes in duration referred to as the short access (ShA) protocol. The next 7 days the self-administration was extended to 6 hours and is referred to as the long access (LgA) protocol. Upon entry into the self-administration environment, the house light and the ventilation fan were on. In addition to triggering an infusion, active lever presses had the following programmed consequences: the house light was turned off, and the active lever light/tone (i.e. the CS) was turned on for a period of 30 seconds. Additional responses on the active lever during this 30 second period had no programmed consequences, although the program continued to count the number of active/inactive lever presses and infusions. After this 30 second period the lever light and tone were terminated and the house light came back on. Rats were initially trained for 10 days on an FR-1 (fixed ratio schedule-1) schedule in which each active lever press outside the timeout period triggered the programmed consequences. For the next 12 days of self-administration training, an FR-3 schedule was imposed where 3 active lever presses outside the time out period were required to trigger an infusion and the CS. Each rat was placed in the same operant conditioning chamber throughout the course of the experiment.
Extinction protocol (days 23–27)
After the 22 days of self-administration training, the animals were divided into 4 groups (balanced for cocaine intake): 1) extinguished (saline), 2) extinguished (D-serine pre-extinction), 3) extinguished (D-serine post-extinction) and 4) abstinent (saline). All the groups except D-serine post extinction group received i.p. injections of their respective treatments in their home cage environment approximately 2 hours before the extinction training session. The D-serine post group was administered D-serine immediately after the extinction session and placed back in the home cage after the injection. Both the extinguished group 1 and the abstinent group 4 received injections of saline (1ml/kg). Groups 2 and 3 received an i.p. injection of D-serine (100 mg/kg). During the extinction training sessions, the animals in the operant chambers were attached to the drug tether but otherwise exposed only to the diffuse, contextual cues of the operant environment. Responses on the active lever had no programmed consequences during the extinction training phase. For protocol days 23–27, responses on both active and inactive levers, as well as the equivalent “number of infusions” were counted by the software (although as stated above, syringe pumps were not activated during this phase of training). Extinction proceeded for a period of 5 days; with one 90 minute daily session during which the animals in the extinction training groups 1, 2 and 3 were taken to the operant chambers. Under these conditions, the animals extinguished their lever-pressing behavior to less than 20% of their former activity during self-administration. Group 4 abstinent animals remained in their home cages throughout days 23–27.
Reinstatement tests (protocol days 28–30)
On days 28–30, all the animals (including the home cage abstinent animals), were placed back in the operant chambers for reinstatement tests. The reinstatement test session conditions were similar to an extinction session in that the animals were exposed only to the contextual cues of the operant chamber environment and the active lever responding were not reinforced by the contingent availability of either CS or US.
Reinstatement following reintroduction to the drug-taking environment (extinction responding)
On test day 28, response to the diffuse contextual stimuli was assessed from active lever-pressing response during the initial 10 minutes on reintroduction to the operant chamber environment in which the animals were exposed only to the contextual cues of the operant chamber environment.
Reinstatement following presentation of CS cues
Later during the same test session on day 28, lever presses evoked in response to a single, noncontingent presentation of the CS were then assessed. The CS (light/tone) was delivered at the 40th minute of the 120 minute test session. Thus the initial 40 minutes of the 120 minute session served as an extinction period to allow lever presses initiated by exposure to contextual stimuli to subside. As the CS was expected to evoke an immediate response from animals, response to this non-contingent CS was quantified as the number of lever presses during the subsequent 10 minutes following the priming event (t=40–50 min).
Reinstatement following drug-priming
Responses to drug prime were assessed on days 28–30. The reinstatement of drug-seeking behavior was tested using 3 different doses (0.25 mg/kg, 0.5 mg/kg and 1 mg/kg) of cocaine on the three consecutive days (day 28, 29 and 30), respectively. In order to minimize the number of test days, the 0.25 mg/kg cocaine priming dose was tested in the latter half of the 120 minute session on day 28. A single, noncontingent drug prime was programmed to be infused intravenously by the syringe pump at the 80th minute of the 120 minute session on the first reinstatement test day. Thereafter on day 29 and 30 ,the drug prime sessions were of 90 minutes duration and a single non-contingent infusion was delivered at time=40 minutes of the session for the 0.5 and 1 mg/kg cocaine doses. Again, the initial 40 minutes of these 90 minute sessions served as an extinction period which allowed lever presses initiated by exposure to contextual stimuli to subside before the drug-prime reinstatement test. Drug-seeking behavior elicited by the different doses of cocaine was quantified from the number of responses on the active lever following the drug prime for 30 minutes immediately after the priming event (i.e. t= 80–110 min on day 28 and t=40–70 min on days 29 and 30).
Drugs
Cocaine hydrochloride was obtained from NIDA (RTI). The NMDA coagonist D-serine was obtained from Sigma (St. Louis).
Statistics
The number of active lever presses, infusions and inactive lever presses were recorded for each session. These data were used to calculate the responses during each experimental session. A one-way repeated measures ANOVA was applied for figure 1A and a two-way RM ANOVA was applied for figure 2A. One-way or two-way ANOVAs were used for the other data analysis. The two factors taken into consideration for the two-way ANOVA were: 1) either trial (pre vs. post priming responses) or days and 2) either condition (extinction vs. abstinence) or treatment, as the case may be. A value of p<.05 was taken as significant, being determined from the Holm-Sidak post-hoc test method. All the statistics was performed using SigmaStat (SigmaStat 3.1) software.
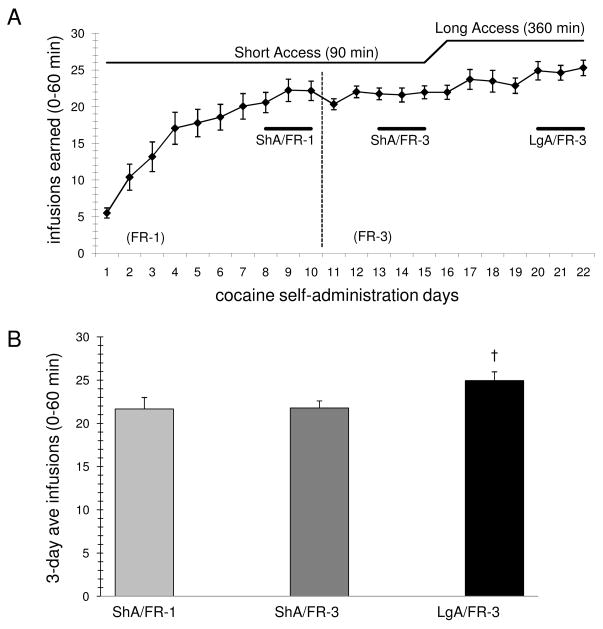
Figure 1.
Cocaine self-administration and escalation of drug intake. (A) Cocaine self-administration training was carried out over three phases: 1st starting from day1 with an FR-1 schedule during short-access (ShA, 90 min) daily sessions, 2nd starting from day 11 with an Fr-3 schedule, 3rd starting from day 16 with long-access (LgA, 360 min) daily sessions. The results are the mean ± SEM (n=33) of daily infusions. (B) Three day average earned infusions during the first 60 min for each of these phases are illustrated from the days indicated by the horizontal bars in panel A (ShA/FR-1, ShA/FR-3, LgA/FR-3) and show a significant increase in earned infusions during the LgA phase compared with either ShA group (†, p<0.05, one-way ANOVA/Holm-Sidak).
Figure 2.
Extinction of drug-seeking behavior. (A) Data are the mean ± SEM of active lever presses during the 90 min extinction sessions for the indicated saline (n=8) and D-serine pre (n=8) and post (n=9) treatment groups. Active lever responding diminished over the five daily sessions. (B) Active lever presses from the fifth extinction session on protocol day 27. Data are the mean ± SEM of active lever presses during consecutive 10 min bins of the session.
Results
Cocaine self-administration and extinction of the drug-seeking behavior
Animals having indwelling jugular catheters were trained to self-administer cocaine in an operant chamber environment for 22 consecutive days (Fig. 1A). Rats were initially trained on an FR-1 schedule during short access (ShA) 90 minute sessions for first 10 days, and then they were switched to an FR-3 schedule for the next 12 days of self-administration training. The last 7 days of self-administration (day 16–22) the animals were switched to a long access (LgA) protocol in which the session duration was extended to 6 hours. Animals typically achieved stable self-administration by day 10, and imposing the FR-3 schedule did not significantly alter the number of earned infusions per session (days 8–10: 21.7 ± 1.3 vs. days 13–15: 21.8 ± 0.8). A significant increase in the average number of infusions earned during the first hour of self-administration occurred after the animals were switched from ShA to LgA. Analysis by one-way RM ANOVA showed a significant effect of ShA vs. LgA, F(3,96)= 7.13, p<.001 for the 33 rats completing the study. A planned comparison (Fig. 1B) between the average of the first hour of infusions during the last 3 days of ShA with the first hour of infusions during the last 3 days of LgA (25.0 ± 1.0; days 20–22) showed a significant increase in earned infusions (†, one-way ANOVA/Holm-Sidak, p<0.05). There were no significant differences in the average number of infusions earned per animal among the four different groups of rats subsequently utilized for the reinstatement studies that followed (data not shown).
After 22 days of SA, three groups of rats were subjected to extinction training in the same operant chamber environment for 90 minute extinction sessions in the absence of both CS and US (i.e. the active lever had no programmed consequences) for 5 days (protocol days 23–27) and a fourth group was kept abstinent in their home cage environment (abstinent group) during this time. Active and inactive lever presses were monitored during the extinction sessions and it was found that the animals rapidly extinguish their drug-seeking behavior under these conditions (Fig. 2A). In two of the extinction groups, the effect of facilitation of NMDARs on the extinction learning process was evaluated following the systemic administration of D-serine (a full agonist of NMDAR at the glycine modulatory site). In one of these groups, D-serine was administered (100 mg/kg i.p.) 2–3 hours prior to each extinction session (D-serine pre) and in the other group D-serine was administered (100 mg/kg i.p.) immediately following the extinction session (D-serine post).
A comparison of drug-seeking behavior between extinction sessions demonstrated that lever-pressing activity observed on the second thru the fifth day of extinction was significantly decreased as compared to the first day of extinction (p<.01, two-way RM ANOVA/Holm-Sidak) for all groups. A two-way RM ANOVA indicated that there was no significant difference between the saline extinguished and the D-serine treated groups during the five days of training, and by protocol day 27 all of the extinction groups exhibited similar levels of drug-seeking behavior during the 90 minute sessions.
Evaluation of cocaine-seeking behavior within an extinction session illustrates that the majority of lever-pressing activity occurs during the initial ten minutes in the operant chamber environment, suggesting that the diffuse contextual cues of the drug-taking environment are priming this response. After this initial activity, active lever-pressing decreases rapidly. As illustrated during the fifth extinction session on protocol day 27 (Fig. 2B), active lever responses became minimal (< 2) by 20–30 minutes and remained low for the remainder of the 90 minute session. This within session response pattern was observed during all extinction sessions (data not shown). Therefore, during the reinstatement experiments involving the noncontingent presentation of either CS or US stimuli, the priming event was delivered at time that allowed a temporal distinction to be made between the initial drug-seeking activity induced by reintroduction to the operant chamber environment (i.e. active lever responses during the first ten minutes) versus the subsequent activity induced via noncontingent presentation of priming events delivered later within the same test session.
The effects of D-serine administration during extinction training on the reinstatement of drug-seeking behavior
Once the animals underwent either extinction training or enforced abstinence for a period of 5 days (protocol days 23–27), the resumption of lever-pressing activity was induced using three forms of priming stimuli: diffuse contextual cues, discrete conditioned cues, and drug infusion. In the saline abstinent group of rats, context induced lever-pressing activity was assessed under extinction conditions during the first ten minutes of the session conducted on protocol day 28 (Fig. 3A). For comparison, responding from the first ten minutes of the session conducted on protocol day 23 of the saline extinction group (i.e. one-day abstinent, A1 group) is also shown with the six-day saline abstinent (A6) group. The extinction response of the A6 group was significantly higher than that of the saline extinguished group (†, p<.05, one-way ANOVA/Holm-Sidak), a result consistent with other reports of incubation of this response (e.g. (Lu et al., 2004). Among the three groups of rats extinguished for five days, the resumption of lever-pressing activity induced by diffuse contextual cues of the operant chamber environmental is illustrated in Figure 3B. The level of responding on the active lever in both saline and D-serine treated rats was similar. Thus the facilitation of NMDAR activity during/following extinction training did not have a significant impact on the reinstatement induced in LgA rats by reintroduction to the drug-taking environment following a period of five days of extinction. This observation is in keeping with our previous results using the same protocol in ShA animals which concluded that the accumulated decrease in extinction responding did not involve an NMDAR-dependent mechanism (Kelamangalath et al., 2007). As expected, the active lever responding of the A6 group was significantly higher compared to the response in the saline treated and the D-serine treated extinguished groups (##, p<.01, one-way ANOVA/Holm-Sidak), confirming the effectiveness of extinction training in reducing the context-induced reinstatement of responding as compared to enforced abstinence in these LgA rats.
Figure 3.
Incubation and extinction responding. Data are the mean ± SEM of active lever presses (left bar of pair) during the first 10 min of the extinction session for the indicated groups. The right bar of each pair is the inactive lever responding. (A) Active lever responding six days after cocaine self-administration (A6 group, n= 8) was significantly increased compared to one day (A1 group, n= 8) after cocaine self-administration (†, p<0.05, one-way ANOVA/Holm-Sidak). (B) Active lever responding for the indicated saline, D-serine pre, and D-serine post extinguished groups (n= 8,9,8, respectively) did not differ significantly. Active lever responding in the A6 group in panel A. was significantly increased compared with each of the extinguished groups in panel B. (##, p<.01, one-way ANOVA/Holm-Sidak).
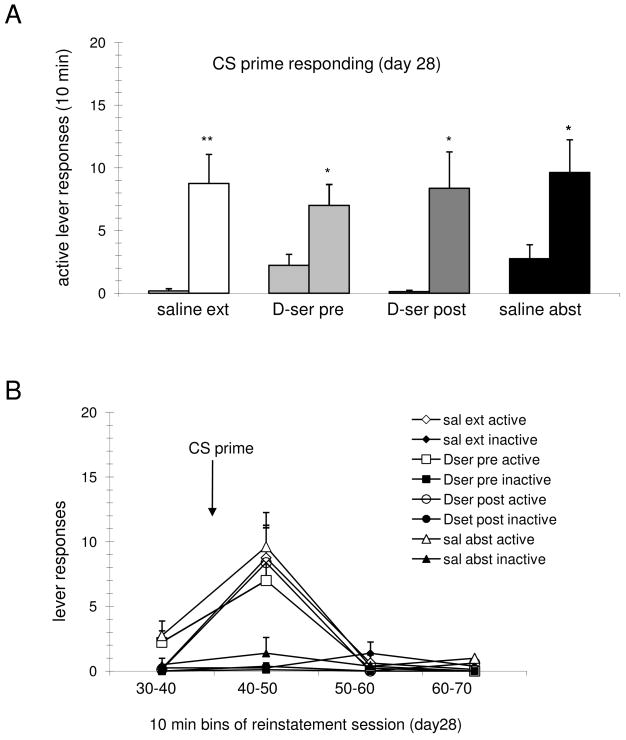
In order to investigate the effects of facilitation of NMDAR activity during extinction on the discrete CS-induced reinstatement, a single presentation of lever light/tone was delivered at t=40 min of the day 28 reinstatement session. Active lever-pressing was measured during a “pre-prime” ten minute period of time as well as a “post-prime” ten minute period (Fig. 4A). Analysis by paired t-test demonstrated that the post-priming activity following the CS prime was significantly greater than the pre-priming activity for the saline extinguished, the D-serine pre extinguished, the D-serine post extinguished, and the saline abstinent groups (**, p<.01; *, p<.05). Planned comparisons among the post-prime responses of the D-serine treated extinguished groups were not significantly different compared to either the saline extinguished group or the abstinent group, indicating that the facilitation of NMDAR activity during or following extinction was not effective in reducing the CS-induced reinstatement. Once again, this observation is in keeping with our previous results in ShA rats which concluded that CS-primed responding did not involve an NMDAR-dependent mechanism (Kelamangalath et al., 2007). Additionally, and in contrast to those previous ShA findings, the 5-day extinction protocol itself was not effective in these LgA animals in reducing their reinstatement response to the discrete CS, as the primed response of the saline extinguished animals was not significantly reduced compared to the saline abstinent group (p>.8, planned comparison, unpaired t-test).
Figure 4.
CS primed reinstatement of drug-seeking behavior. (A) Data are the mean ± SEM of active lever presses for the indicated treatment groups (n= 8,9,8,8, respectively) during 10 min periods before (left bar) and after (right bar) noncontingent CS priming. Following a single presentation of the CS (lever light/tone), active lever responding was significantly increased (**, p<0.01; *, p<0.05; paired t-tests). (B) Inactive lever presses remained low during the 10 min following delivery of the CS, and all responding was minimal 10–30 min post priming.
Finally, cocaine-induced reinstatement was tested on 3 consecutive days (protocol days 28–30) at 3 different priming doses (0.25mg/kg, 0.5mg/kg, 1mg/kg) with a single, noncontingent intravenous infusion of cocaine (Fig. 5). The lowest priming dose of 0.25 mg/kg was tested on protocol day 28 (forty minutes following the CS reinstatement test), at time=80 min of the 120 min session (Fig. 5A). Subsequent doses of cocaine were tested within a 90 min reinstatement test session on protocol days 29 and 30, the priming infusion was delivered at time=40 min of these sessions. The active lever responding during the next 30 minutes was measured as an indication of the reinstatement of drug-seeking behavior evoked by the US (Fig. 5A–C). The dose-response results are plotted to illustrate the shift in the cocaine-induced drug-seeking activity (Fig. 5D). Over these three days of reinstatement testing, rats experienced 50 minutes of extinction conditions following each drug prime and 40 minutes of extinction prior to the next primed reinstatement test the following day. On day 31, the saline abstinent group was tested with a saline infusion and no significant active lever-pressing occurred post-prime (range 0–3 responses, data not shown). Thus the dose-dependent increase in responding observed for days 29 and 30 was not merely due to a conditioned response to infusion itself during the reinstatement test sessions.
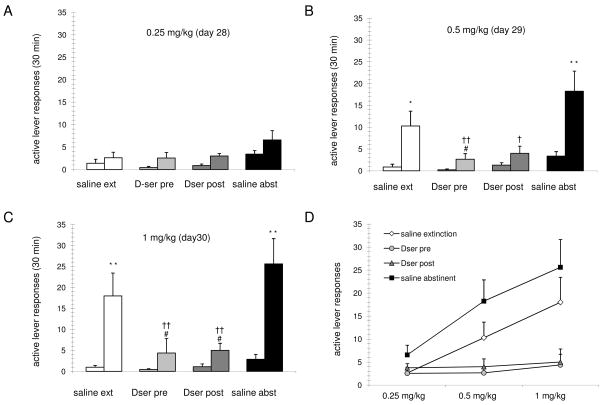
Figure 5.
Cocaine primed reinstatement of drug-seeking behavior. (A-C) Data are the mean ± SEM of active lever presses for the indicated treatment groups (n= 8,9,8,8, respectively) during 30 min periods before (left bar) and after (right bar) noncontingent i.v. drug priming at the indicated doses. (A) Following a single 0.25mg/kg infusion, active lever responding did not differ significantly among all groups. (B) Following a single 0.5mg/kg infusion, active lever responding was significantly increased in both the saline extinguished and saline abstinent groups (**, p<0.01; *, p<0.05; two-way ANOVA/Holm-Sidak), and responding of the D-serine treatment groups was significantly decreased as compared with the saline abstinent group (††, p<0.01; †, p<0.05; two-way ANOVA/Holm-Sidak). Also, the D-serine pretreatment group was significantly decreased as compared with the saline extinction group (#, p<0.05; two-way ANOVA/Holm-Sidak). (C) Following a single 1mg/kg infusion, active lever responding was significantly increased in both the saline extinguished and saline abstinent groups (**, p<0.01; two-way ANOVA/Holm-Sidak), and responding of the D-serine treatment groups was significantly decreased as compared with either the saline abstinent group (††, p<0.01; two-way ANOVA/Holm-Sidak) or the saline extinction group (#, p<0.05; two-way ANOVA/Holm-Sidak). (D) Summary dose-response of the active lever presses for the indicated treatment groups and drug priming doses. Note that 0.5mg/kg was used during cocaine self-administration.
At the 0.5mg/kg priming dose of cocaine, two-way ANOVA confirmed an effect of trial, Ftrial (1,54)=21.5 and an effect of treatment, Ftreatment (3,54)=7.3 and an effect of interaction between these two factors, Finteraction (3,54)= 3.7 (Fig. 5B). The post-hoc analysis of this data showed that the 30 minute post priming response was significantly greater than the pre-priming response for the saline extinguished (t=2.8, *, p<.01) and the saline abstinent (t=4.8, **, p<.01) groups. Among the D-serine extinguished groups, the post-prime response of the D-serine pre extinguished group was significantly lower than the post-prime response of the saline extinguished group (t=2.4, #, p<.05) indicating an enhancement in the effectiveness of extinction by facilitation of NMDAR activity. The post-prime responses of both the D-serine pre extinguished group (t=5.2, ††, p<.01), and the D-serine post extinguished group (t=4.5, †, p<.05) were significantly less than that of the saline abstinent group. Although reduced, the reinstatement response observed in the saline extinction group was not significantly different when compared to saline abstinent group (p>.2), indicating that extinction alone was ineffective in these LgA animals to significantly reduce the drug-induced reinstatement at this priming dose.
An analysis at the priming dose of 1mg/kg showed an effect of trial, Ftrial (1,54)=28.6 and an effect of treatment, Ftreatment (3,54)=6.6 and an effect of interaction between these two factors Finteraction(3,54)=4.7 (Fig. 5C). The post-priming responses were significantly greater than the pre-priming responses for both the saline extinguished (t=3.6, **, p<.01) and the saline abstinent (t=5.2, **, p<.01) groups. Planned comparisons between the post-prime responses of the different treatments showed that both the D-serine pre (t=3, #, p<.05) and D-serine post (t=2.9, #, p<.05) extinguished groups showed significantly lower responses to the 1mg/kg drug prime as compared to the saline extinguished group. The post-priming response of both the D-serine pre (t=4.7, ††, p<.01) or D-serine post (t=4.9, ††, p<.01) extinguished groups were also significantly less than that of the saline abstinent group. Once again, the reinstatement response observed in the saline extinguished group was not significantly different when compared to saline abstinent group (p>.3), indicating that extinction by itself was not effective in reducing the drug-induced reinstatement at this priming dose. Thus extinction training is effective in significantly reducing the response to cocaine-priming only when combined with D-serine treatment in LgA rats. These results demonstrate that the ability of extinction to reduce drug-primed reinstatement is enhanced by the facilitation of NMDAR activity during/following extinction training in rats previously allowed one week of extended access to cocaine self-administration.
Discussion
In this report we have described the efficacy of treatment with D-serine to significantly reduce cocaine-primed reinstatement in rats having extended access to cocaine self-administration. This current finding, obtained with a NMDA receptor coagonist, is consistent with and extends with our previous results demonstrating that treatment with an NMDA receptor antagonist during extinction blocked the effects of that training to reduce drug-primed reinstatement (Kelamangalath et al., 2007). Thus although the effectiveness of extinction training to reduce responding by the end of the five day protocol is not affected by the NMDAR coagonist (Fig. 2), the benefit of such training for suppressing cocaine-induced reinstatement of drug-seeking behavior assessed 2–3 days later is significant when D-serine is also administered (Fig. 5). As argued below, since D-serine givern either before or immediately following each of the five daily extinction sessions was equally effective, the results are consistent with an enhancement of the consolidation process for extinction learning.
In the ongoing efforts to improve animal self-administration models as they pertain to human addiction, it has been found that increased access to drug during daily sessions (≥6hrs vs. <3hrs) leads to an increase in the initial rate of drug intake (Ahmed and Koob, 1998), and that this extended access can be correlated with other behaviors consistent with drug dependence in humans, such as increased willingness to work for the drug and an increased capacity to withstand negative stimuli associated with drug-taking (Deroche-Gamonet et al., 2004; Vanderschuren and Everitt, 2004). In this study, we have tested the efficacy of D-serine to facilitate the effects of five days of extinction training in rats subjected to the long-access (LgA) self-administration model. In addition to monitoring responses during the five-day extinction training period, three other assessments of reinstatement of drug-seeking behavior were subsequently measured. First, in terms of both the rate of extinction and the final extent of reduced active lever-pressing achieved over the five-day protocol, there was a lack of consistent effects of D-serine on this process when evaluated either between or within sessions (Fig. 2). Thus extinction learning itself does not appear to be significantly altered by modulating the activity of NMDARs with D-serine, a result in keeping with our previous report with ShA rats (Kelamangalath et al., 2007) and another study testing D-cycloserine in both rats and monkeys (Nic Dhonnchadha et al., 2010). Even though evidence for incubation of extinction responding (Grimm et al., 2001) was obtained in another group of LgA rats held abstinent over the same period (Fig. 3A), the five-day extinction protocol was very effective in reducing the active lever response initiated by reintroduction to the drug-taking environment. Therefore, it is not unexpected that D-serine treatment conferred no additional benefit (Fig. 3B). Our recent report (Kelamangalath et al., 2009) using a suboptimal extinction protocol in ShA rats also found that D-serine had no effect, indicating that NMDA receptor-mediated mechanisms are not involved in this form of environmental context-induced resumption of drug-seeking.
Second, it is interesting to note that extinction of lever pressing in the absence of cues was not effective in significantly reducing the reinstatement response to a single, noncontingent presentation of the CS (Fig. 4). Extinction in the absence of cues is often employed in self-administration studies of reinstatement of drug-seeking behavior (Yahyavi-Firouz-Abadi and See, 2009), and we have previously observed a reduction of CS-induced reinstatement in ShA rats (Kelamangalath et al., 2007). Our current result suggests that the LgA animals are resistant to the effects of extinction under these conditions with respect to the ability of such training to reduce CS primed reinstatement. Once again, treatment with D-serine had no facilitatory effect; however it remains untested whether facilitation of NMDA receptors would have had any effect using an extinction protocol in which the CS itself was extinguished.
Finally, cocaine-primed reinstatement of drug-seeking behavior was measured across 3 consecutive daily sessions during which a single, noncontingent dose of cocaine was delivered (Fig. 5). Although a trend towards decreased reinstatement was evident, it is noteworthy that the cocaine-primed responses of the saline group were not significantly decreased following the five-day extinction training experience-again suggesting a resistance in these LgA rats that is in contrast to our prior experience with ShA rats (c.f. Fig 3B of Kelamangalath et al., 2007). In spite of this resistance, treatment with D-serine either before or immediately following each of the five extinction sessions dramatically decreased cocaine-primed reinstatement to minimal response levels during the test sessions performed 48–72 hours following the final administration of D-serine. Previous results from our own studies and those of other investigators have demonstrated that either D-serine or D-cycloserine treatment alone, in the absence of extinction training, does not affect either drug-primed reinstatement (Kelamangalath et al., 2009) or reacquisition of cocaine self-administration (Nic Dhonnchadha et al., 2010). In light of these reports, as well as other evidence indicating that D-serine can enhance learning (Duffy et al., 2008), we conclude that D-serine likely acts to facilitate the effects of extinction training to significantly reduce cocaine-primed reinstatement of drug-seeking behavior. Alternatively, it remains a possibility that D-serine is acting independently of extinction to reduce reinstatement under these conditions.
Evidence has recently been provided that reintroduction to the self-administration environment promotes habitual drug-seeking behavior, whereas exposure to a discriminative cue evokes goal-directed drug-seeking behavior (Root et al., 2009). Another report posits a compulsive relationship between cocaine-priming exposure and drug-seeking behavior such that “rats do not press the lever for cocaine, they press the lever because of cocaine” (Norman and Tsibulsky, 2006). These examples support the concept that various forms of environmental context, cue- or drug-priming exposures are likely to engage distinct neural mechanisms that underlie the reinstatement of drug-seeking and drug-taking behavior. Thus it is not unexpected that our results demonstrate a differential effect of D-serine on cocaine-primed reinstatement as compared with other means of initiating drug-seeking behavior, as NMDAR-dependent mechanisms may be selectively involved in some but not all cases. With respect to its potential clinical significance, given that cocaine itself is a potent inducer of cocaine wanting/craving (Jaffe et al., 1989), the efficacy of the combination of extinction training and D-serine treatment to reduce drug-primed reinstatement of cocaine-seeking behavior in these LgA rats is particularly striking.
The dose of D-serine employed in these studies with LgA rats (100mg/kg) was selected based upon our prior experience in the reinstatement of cocaine-seeking behavior in ShA rats (Kelamangalath et al., 2007 & 2009), reports of D-serine effects on other forms of learning and memory (Stouffer EM et al., 2004), the pharmacokinetic parameters of D-serine (Hashimoto and Chiba, 2004; Smith et al., 2009), and the known sensitivity of rats to D-serine induced nephrotoxicity (Williams et al., 2003). Although it is often administered at much higher levels (600–800mg/kg) to demonstrate effects on learning/memory performance (Duffy et al., 2008; Karasawaa et al., 2008), we have not tested such larger doses and the assessment of lower doses of D-serine on the reinstatement of drug-seeking is a future topic of study. Our choice of D-serine was motivated by the discovery of this compound as an endogenous ligand (Schell MJ et al., 1995), its ability to facilitate NMDAR-dependent synaptic plasticity (Yang et al., 2003) and its characterization as a full agonist acting at the glycine modulatory site of the NMDA receptor (Furukawa and Gouaux, 2003). Importantly, several studies in schizophrenia patients have reported symptom improvement with D-serine adjunctive pharmacotherapy (Tsai et al., 1998; Tsai et al., 1999; Heresco-Levy et al., 2005), results that indicate the potential safety and efficacy of D-serine in humans.
With respect to the underlying actions of D-serine to facilitate the effects of extinction training to reduce cocaine-primed reinstatement of drug-seeking behavior, we propose that an enhanced consolidation of extinction learning is the most parsimonious mechanism consistent with our reported findings. The observation that D-serine was also effective when administered immediately following the extinction sessions, suggests that an interaction with the acquisition of learning during the session was not required and therefore a facilitation of the consolidation of extinction learning is likely. As the reinstatement of drug-seeking behavior is subsequently tested in the absence of D-serine, an effect on the retrieval of extinction memory is also not indicated. Similar conclusions have been reached using D-cycloserine in cocaine-induced conditioned place preference studies (Botreau et al., 2006) and in cocaine self-administering ShA rats (Nic Dhonnchadha et al., 2010). It is important to distinguish among the effects of extinction training to reduce 1) the initial extinction responding upon reintroduction to the operant chamber environment vs. 2) the effects to reduce responding to a noncontingent presentation of the CS vs. 3) the effects to reduce responding to a noncontingent priming with drug, as we have previously reported that only the latter form of evoked reinstatement of drug-seeking behavior is modulated by NMDAR activity (Kelamangalath et al., 2007). This highlights the heterogeneous nature of the neural mechanisms that contribute to the complex interplay between priming stimuli and drug-seeking in animal models as well as the potential relationship between various methods of eliciting craving and relapse in human addicts. These findings suggest that multiple approaches, pharmacological and behavioral, are likely needed to comprise an effective regimen for the treatment of drug addiction. Our current results using the rat LgA model of cocaine self-administration demonstrate that D-serine is an effective enhancer of behavioral training known to decrease drug-seeking behavior, and is therefore worthy of further consideration and study as adjunctive pharmacotherapy in cocaine dependent patients.
Acknowledgments
This work was supported by the National Institutes of Health National Institute on Drug Abuse [DA016302]; and the University of Georgia Interdisciplinary Toxicology Program [graduate stipend award].
Abbreviations
- ShA
short access
- LgA
long access
- FR
fixed ratio
- CS
conditioned stimulus
- US
unconditioned stimulus
- NMDAR
n-methyl, d-aspartate receptor
- ANOVA
analysis of variance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: Change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Botreau F, Paolone G, Stewart J. D-Cycloserine facilitates extinction of a cocaine-induced conditioned place preference. Behav Brain Res. 2006;172:173–178. doi: 10.1016/j.bbr.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- deWit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology. 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Duffy S, Labri V, Roder JC. D-Serine augments NMDA-NR2B receptor-dependent hippocampal long-term depression and spatial reversal learning. Neuropsychopharmacology. 2008;33:1004–1018. doi: 10.1038/sj.npp.1301486. [DOI] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165:179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, O’ Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology (Berl) 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: An assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa H, Gouaux E. Mechanisms of activation, inhibition and specificity: crystal structures of the NMDA receptor NR1 ligand-binding core. EMBO J. 2003;22:2873–2885. doi: 10.1093/emboj/cdg303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation: Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto A, Chiba Y. Effect of systemic administration of -serine on the levels of L- and D-serine in several brain areas and periphery of rat. Eur J Pharmacol. 2004;495:153–158. doi: 10.1016/j.ejphar.2004.05.036. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Daniel CJ, Richard E, Agnes V, Pesach L, Gali B, Sara C, Marina E. D-serine efficacy as add-on pharmacotherapy to risperidone and olanzapine for treatment-refractory schizophrenia. Biol Psychiatry. 2005;57:577–585. doi: 10.1016/j.biopsych.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Jaffe JH, Cascell NG, Kumor KM, Sherer MA. Cocaine-induced cocaine craving. Psychopharmacology (Berl) 1989;97:59–64. doi: 10.1007/BF00443414. [DOI] [PubMed] [Google Scholar]
- Karasawa J, Hashimoto K, Chaki S. D-Serine and a glycine transporter inhibitor improve MK-801-induced cognitive deficits in a novel object recognition test in rats. Behav Brain Res. 2008;186:78–83. doi: 10.1016/j.bbr.2007.07.033. [DOI] [PubMed] [Google Scholar]
- Kelamangalath L, Seymour CM, Wagner JJ. D-serine facilitates the effects of extinction to reduce cocaine-primed reinstatement of drug-seeking behavior. Neurobiol Learn Mem. 2009;92:544–551. doi: 10.1016/j.nlm.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelamangalath L, Swant J, Stramiello M, Wagner JJ. The effects of extinction training in reducing the reinstatement of drug-seeking behavior: Involvement of NMDA receptors. Behav Brain Res. 2007;185:119–128. doi: 10.1016/j.bbr.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, See RE. Contributions of prolonged contingent and noncontingent cocaine exposure to enhanced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2006;187:60–7. doi: 10.1007/s00213-006-0386-3. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW. Extended access to cocaine self-administration enhances drug-primed reinstatement but not behavioral sensitization. J Pharmacol Exp Ther. 2007;322:1103–9. doi: 10.1124/jpet.107.122861. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47:214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology (Berl) 2004;175:26–36. doi: 10.1007/s00213-004-1778-x. [DOI] [PubMed] [Google Scholar]
- Nic Dhonnchadha BA, Szalay JJ, Achat-Mendes C, Platt DM, Otto MW, Spealman RD, Kantak KM. D-cycloserine deters reacquisition of cocaine self-administration by augmenting extinction learning. Neuropsychopharmacology. 2010;35:357–367. doi: 10.1038/npp.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AB, Tsibulsky VL. The compulsion zone: A pharmacological theory of acquired cocaine self-administration. Brain Res. 2006;1116:143–152. doi: 10.1016/j.brainres.2006.07.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cognitive enhancers as adjuncts to psychotherapy: Use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- Root DH, Fabbricatore AT, Barker DJ, Ma S, Pawlak AP, West MO. Evidence for habitual and goal-directed behavior following devaluation of cocaine: a multifaceted interpretation of relapse. PLoS One. 2009;4:e7170. doi: 10.1371/journal.pone.0007170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell MJ, Molliver ME, Snyder S. D-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci U S A. 1995;92:3948–3952. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Uslaner JM, Yao L, Mullins CM, Surles NO, Huszar SL, McNaughton CH, Pascarella DM, Kandebo M, Hinchliffe RM, Sparey T, Brandon NJ, Jones B, Venkatraman S, Young MB, Sachs N, Jacobson MA, Hutson PH. The behavioral and neurochemical effects of a novel D-amino acid oxidase inhibitor compound 8 [4H-thieno [3,2-b]pyrrole-5-carboxylic Acid] and D-serine. J Pharmacol Exp Ther. 2009;328:921–930. doi: 10.1124/jpet.108.147884. [DOI] [PubMed] [Google Scholar]
- Stouffer EM, Petri HL, Devan B. Effect of D-serine on a delayed match-to-place task for the water maze. Behav Brain Res. 2004;152:447–452. doi: 10.1016/j.bbr.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Tsai GE, Li-Chen C, Nicholas L, Joseph TC. D-serine added to antipsychotics for the treatment of schizophrenia. Biol Psychiatry. 1998;44:1081–1089. doi: 10.1016/s0006-3223(98)00279-0. [DOI] [PubMed] [Google Scholar]
- Tsai GE, Yang P, Chung L-C, Tsai I-C, Tsai C-W, Coyle JT. D-Serine added to clozapine for the treatment of schizophrenia. Am J Psychiatry. 1999;156:1822–1825. doi: 10.1176/ajp.156.11.1822. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Waldron HB, Turner CW. Evidence-based psychosocial treatments for adolescent substance abuse. J Clin Child Adol Psych. 2008;37:238–261. doi: 10.1080/15374410701820133. [DOI] [PubMed] [Google Scholar]
- Williams RE, Jacobsen M, Lock EA. 1H NMR Pattern recognition and 31P NMR studies with D-Serine in rat urine and kidney, time- and dose-related metabolic effects. Chemical Research in Toxicology. 2003;16:1207–1216. doi: 10.1021/tx030019q. [DOI] [PubMed] [Google Scholar]
- Yahyavi-Firouz-Abadi N, See RE. Anti-relapse medications: preclinical models for drug addiction treatment. Pharmacol Ther. 2009;124:235–247. doi: 10.1016/j.pharmthera.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ge W, Chen Y, Zhang Z, Shen W, Wu C, Poo M, Duan S. Contribution of astrocytes to hippocampal long-term potentiation through release of D-serine. Proc Natl Acad Sci U S A. 2003;100:15194–15199. doi: 10.1073/pnas.2431073100. [DOI] [PMC free article] [PubMed] [Google Scholar]