Abstract
Pramipexole is a hydrophilic, weakly basic drug, but exhibits high oral bioavailability in humans (> 90%). In rats, rOct1 and rOct2 contribute towards pramipexole excretion into urine. The objective of this study was to assess whether pramipexole is a substrate for human OCT1-3. In vitro uptake studies were performed using hOCT1-MDCK monolayers, hOCT2-HEK cells and hOCT3-HEK cells. hOCT2 transported pramipexole in a high affinity manner (Kt = 15.4 ± 4.1 μM, Jmax = 0.476 ± 0.028 pmol/sec/cm2). hOCT3 transported pramipexole in a low affinity manner (Kt = 138 ± 31 μM, Jmax = 1.10 ± 0.08 pmol/sec/cm2). Although previously reported to be translocated by rOct1, pramipexole was not a substrate for hOCT1. The human intestinal absorption of pramipexole may involve transport by OCT3 and possibly OCT2. OCT2- and OCT3-mediated transport of pramipexole have implications in the drug’s elimination from the kidney and distribution in the brain, respectively.
Keywords: Pramipexole, organic cation transporter (OCT), uptake, drug transport, drug disposition
INTRODUCTION
Cationic drugs represent approximate 40% of FDA approved drugs on the market.1 They are frequently hydrophilic at physiologic pH, which can confer low membrane permeability. Pramipexole is a weakly basic drug with pKa of 9.60 2 and a dopamine D2 receptor full agonist approved for treatment of Parkinson’s disease and restless legs syndrome. Pramipexole is hydrophilic (logD of −0.77 at pH 7) and possesses a very high water solubility [> 20 mg/ml].3, 4 Nevertheless, pramipexole exhibits an oral human bioavailability of more than 90%.5 Pramipexole is largely eliminated by the kidney in humans, which involves tubular secretion.6 In rats, organic cation transporter 1 and 2 (rOct1 and rOct2) contribute towards excretion of pramipexole into urine.7
OCTs belong to the solute carrier family SLC 22A. Three distinct OCT isoforms, namely OCT1-3, have been identified and mediate the entry of organic cations into cells. Both OCT1 and OCT2 are detected in many organs.8 Human OCT1 (hOCT1) and hOCT2 are most strongly expressed in the liver and kidney, respectively, playing important roles in the removal of toxic drugs and their metabolites.8 OCT3 shows widespread tissue distribution, including in skeletal muscle, liver, placenta, heart and brain.8 Like OCT1 and OCT2, OCT3 is expressed in epithelial cells and neurons. In addition, it is also expressed in glial cells and muscle cells.8 OCT1-3 mRNA expression has been detected in human small intestine and Caco-2 cells.8, 9 Immunostaining analyses of human jejunum revealed that hOCT3 is localized to the brush border membrane.10 In this study, stably transfected cell lines, including human Embryonic Kidney 293 (HEK) cells for hOCT2 and hOCT3, and Madin-Darby canine kidney (MDCK) cells for hOCT1, were used to assess whether pramipexole is a substrate for human OCT1-3, which may have implications for its absorption, distribution and elimination.
EXPERIMENTAL SECTION
Materials
Pramipexole dihydrochloride was provided by Cadila Health Care Ltd. Fetal bovine serum, trypsin, and Dulbecco’s modified Eagle medium (DMEM) were purchased from Invitrogen Corporation (Carlsbad, CA). Stably transfected hOCT1-MDCK and mock cells were described previously.11 For stably transfected hOCT2-HEK and mock cells, HEK293 cells were transfected with pcDNA5/FRT vector (Invitrogen; Carlsbad, CA) containing the hOCT2 cDNA inserts or empty vector using LipofectamineTM 2000 (Invitrogen; Carlsbad, CA) and selected following the manufacturer’s protocols for pcDNA5/FRT. Stably transfected hOCT3-HEK and mock cells were kindly provided by Dr. Kathleen M. Giacomini from the University of San Francisco.12
Cell culture
Stably transfected cells and mock cells were cultured at 37 °C, 90% relative humidity, and 5% CO2 atmosphere and fed every 2 days. Media was composed of DMEM supplemented with 10% FBS, 50 units/mL penicillin, 50 μg/mL streptomycin, and 75 μg/mL hygromycin B (for HEK 293 cells). Cells were passaged after reaching 80% confluence.
OCT1-3 uptake studies
hOCT1-MDCK and mock cells were seeded in 12-well plates (Corning; Corning, NY). hOCT2-HEK and hOCT3-HEK cells, as well as mock cells, were seeded in Biocoat™ poly-D-Lysine 24-well plates (BD Biosciences; Franklin Lakes, NJ). Seeding density was 52,000 cells/cm3. On day four after seeding, uptake studies were performed at pramipexole concentrations over 0 to 200 μM for hOCT1-MDCK cells, 0 to 500 μM for hOCT2-HEK cells, and 0 to 1000 μM for hOCT3-HEK cells, in HBSS. Non-transporter-mediated passive uptake was assessed by measuring pramipexole uptake in the mock cells.
Uptake was determined to be linear over 10 min. At the end of the assay (10 min), cells were washed thrice with chilled HBSS buffer. Acetonitrile was then applied and, after its evaporation, cells were incubated with a mixture of acetonitrile and water (1:1, containing internal standard compound) for 30 min on a plate shaker at 50 oscillations per min. Pramipexole was quantified by LC/MS/MS. Tetraethylammonium (TEA) was quantified by scintillation counting.
Uptake data from mock cells was fitted to a modified passive transport model 13 (eqn 1):
| (1) |
where J is uptake, Pp is the passive permeability, and PABL is the aqueous boundary layer (ABL) permeability, and S is pramipexole concentration. PABL was 150 × 10−6 cm/s for uptake studies.
Uptake data from stably transfected cells was fitted to the Michaelis-Menten model with parallel passive permeability (eqn 2):
| (2) |
where Jmax and Kt are the Michaelis-Menten coefficients. Equations 1 and 2 were applied sequentially to mock and transporter-expressing cell data to estimate Pp, Kt, and Jmax. The Pp estimate from mock cells was applied to eqn 2. Nonlinear curve fitting was performed using WinNonlin 4.1 (Pharsight, Mountain View, CA).
Additionally, apparent permeability of pramipexole uptake was calculated from eqn 3:
| (3) |
where Papp is apparent pramipexole permeability, J is pramipexole uptake, and S is pramipexole concentration. The surface area was 3.80 cm2 for the 12 well plates that were used for hOCT1-MDCK (and mock cells) and was 2.00 cm2 for the 24 well plates that were used for hOCT2-HEK (and mock cells) and for hOCT3-HEK (and mock cells).
Analytical methods
Pramipexole samples were quantified by LC/MS/MS. The analytical system consisted of Finnigan Surveyor® Plus Autosampler, Finnigan Surveyor® LC Pump Plus and Finnigan TSQ® Quantum Discovery MAX™ mass spectrometer with an electrospray ionization source and triple-quadrupole mass analyzer. The column used was Synergi™ Polar-RP column (4 μm, 50 × 2.00 mm; Phenomenex, Torrance, CA). Selective reaction monitoring (SRM) monitors precursor/product ion pair of pramipexole at m/z 212.1/152.1. An acetonitrile with 0.1% formic acid/50 mM ammonia formate (pH 3.2) was used as the mobile phase. The gradient was 50% acetonitrile over 1 min, a linear increase to 90% acetonitrile over 1 min, a platform of 90% acetonitrile for 1 min, a linear decrease to 50% acetonitrile for 1 min and a platform of 50% acetonitrile for 1 min, with a flow rate of 0.4 mL/min. The assay was linear (r2>0.999) over 5–10000 ng/ml.
Data analysis
Uptake and inhibition data are expressed as mean ± SEM derived from three independent studies. Statistical significance was evaluated using Graphpad Prism (Graphpad Software, Inc.; La Jolla, CA).
RESULTS
Uptake of pramipexole by hOCT1 in MDCK cells
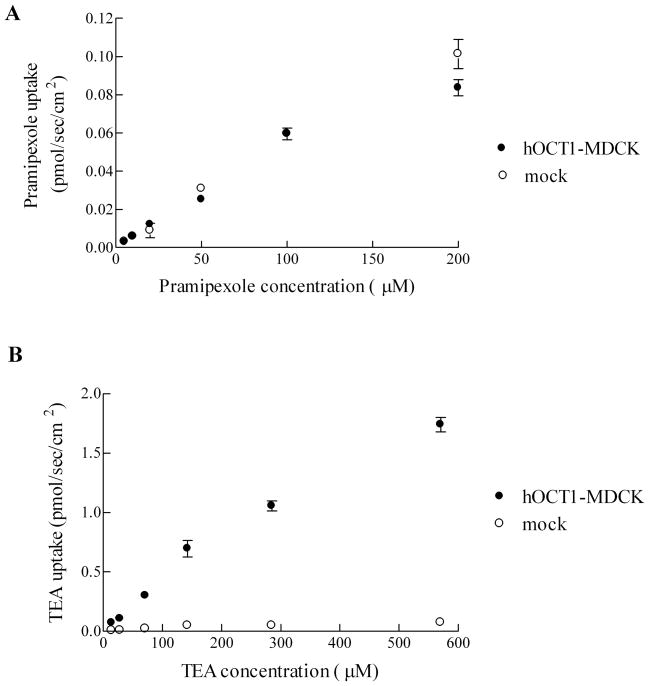
The uptake of pramipexole by hOCT1 was evaluated using stably transfected hOCT1-MDCK and mock cells. Pramipexole uptake into hOCT1-MDCK and mock cells did not differ from 0–200 μM (Figure 1A). The uptake of pramipexole by either hOCT1-MDCK or mock cells was low and passive. TEA served as positive control and was taken up by hOCT1-MDCK cells from 14.3–570 μM (Figure 1B). Our data suggest that pramipexole is not a substrate for hOCT1.
Figure 1.
Uptake of pramipexole and TEA into hOCT1-MDCK and mock cells. A, Pramipexole uptake did not differ between hOCT1-MDCK cells and mock cells over a 5–200 μM pramipexole concentration range. Pramipexole permeability into mock cell monolayers was a low 1.21 (±0.06) ×10−7 cm/sec. B, TEA uptake into hOCT1-MDCK cells was higher than uptake into mock cells over a 14.3–570 μM TEA concentration range. Error bars denote SEM.
Uptake of pramipexole by hOCT2 in HEK cells
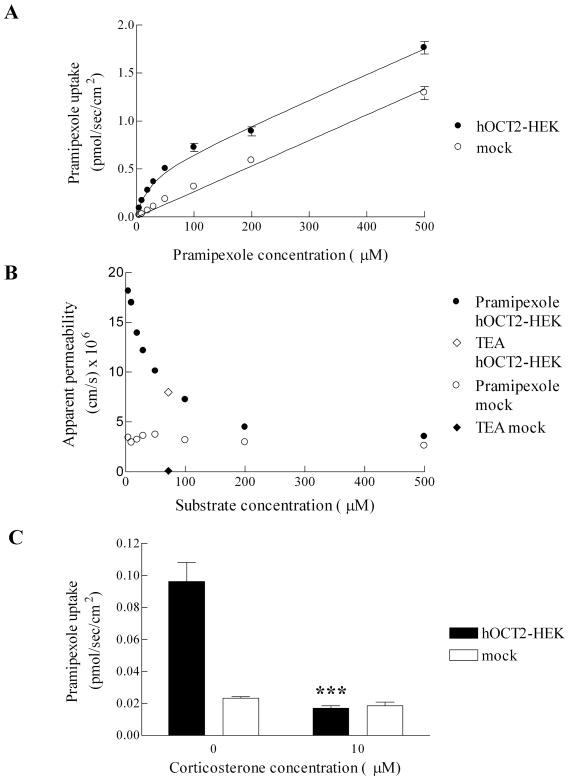
The uptake of pramipexole by hOCT2 was evaluated using stably transfected hOCT2-HEK and mock cells. In Figure 2A, the uptake of pramipexole by mock cells demonstrated linear kinetics over 0–500 μM, while the uptake of pramipexole by hOCT2-HEK cells demonstrated a saturable kinetic. Fitted Kt and Jmax for hOCT2-mediated pramipexole uptake were 15.4 (±4.1) μM and 0.476 (±0.028) pmol/sec/cm2, respectively. The uptake of pramipexole into mock cells was low and exhibited a passive permeability of 2.71 (±0.06) ×10−6 cm/sec.
Figure 2.
Uptake of pramipexole into hOCT2-HEK and mock cells. A, Pramipexole uptake was greater into hOCT1-MDCK cells than into mock cells. B, Apparent permeability of pramipexole versus concentration is plotted for pramipexole uptake into hOCT2-HEK cells and mock cells. Also plotted is the apparent permeability of TEA at 71.3 μM into hOCT2-HEK cells and mock cells. C, Inhibition of pramipexole uptake (5 μM) into hOCT2-HEK and mock cells by 10 μM corticosterone. ***, p < 0.001, compared with hOCT2-HEK cells not treated with corticosterone. Error bars denote SEM.
The apparent permeability of pramipexole is plotted at each experimental concentration in Figure 2B. Also plotted, for comparison, is the apparent permeability of TEA at 71.3 μM. Apparent permeabilities of pramipexole into mock cells were similar across the concentrations, reflecting low passive uptake when OCT2 is not present. However, the apparent permeability of pramipexole into hOCT2-HEK cells was high at low concentrations and decreased at high concentrations, approaching its apparent permeability in mock cells. This profile reflects the role of OCT2 in pramipexole uptake. The uptake of TEA by OCT2 fell on the pramipexole profile, suggesting pramipexole as a strong substrate for OCT2.
To further confirm that the transport into hOCT2-HEK cells was mediated by OCT2, uptake experiments were carried out using the OCT inhibitor corticosterone (Figure 2C). Corticosterone (10 μM) significantly inhibited the uptake of pramipexole (5 μM) into hOCT2-HEK cells (p < 0.001), while had minimal effect on the uptake of pramipexole into mock cells (p > 0.05). After corticosterone inhibition, the uptake of pramipexole into hOCT2-HEK cells was comparable to mock cells, indicating that OCT2-mediated uptake was abolished.
Uptake of pramipexole by hOCT3 in HEK cells
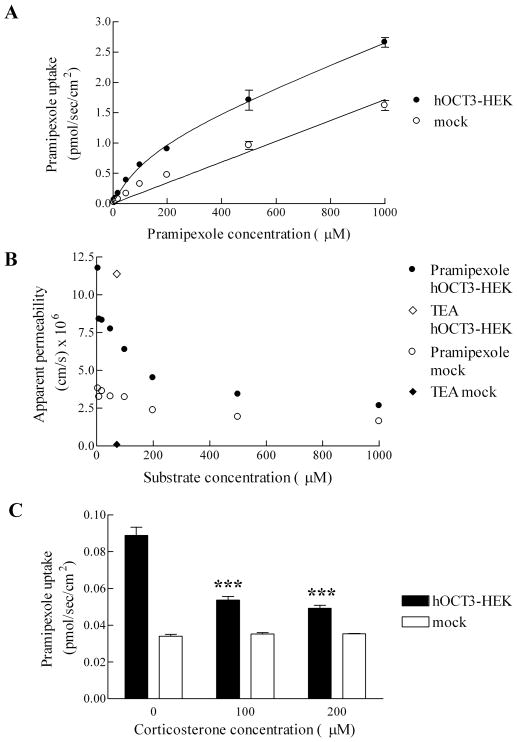
The uptake of pramipexole by hOCT3 was evaluated using stably transfected hOCT3-HEK and mock cells. In Figure 3A, the uptake of pramipexole by mock cells demonstrated linear kinetics over 0–1000 μM, while the uptake of pramipexole by hOCT3-HEK cells demonstrated a saturable kinetics. Fitted Kt and Jmax for hOCT3-mediated pramipexole uptake were 138(±31) μM and 1.10 (±0.08) pmol/sec/cm2, respectively. In contrast to the uptake of pramipexole by hOCT2 by a high affinity manner, the uptake of pramipexole by hOCT3 is a low affinity process, consistent with the nature of the OCT3 for certain substrates.14 The uptake of pramipexole into mock cells was low and exhibited a passive permeability of 1.74 (±0.06) ×10−6 cm/sec.
Figure 3.
Uptake of pramipexole into hOCT3-HEK and mock cells. A, Pramipexole uptake was greater into hOCT3-HEK cells than into mock cells. B, Apparent permeability of pramipexole versus concentration is plotted for pramipexole uptake into hOCT3-HEK cells and mock cells. Also plotted is the apparent permeability of TEA at 71.3 μM into hOCT3-HEK cells and mock cells. C, Inhibition of pramipexole uptake (10 μM) into hOCT3-HEK and mock cells by 100 or 200 μM corticosterone. ***, p < 0.001, compared with hOCT3-HEK cells not treated with corticosterone. Error bars denote SEM.
The apparent permeability of pramipexole is plotted at each experimental concentration in Figure 3B. Apparent permeabilities of pramipexole into mock cells were similar across the concentrations, reflecting low passive uptake. However, the apparent permeability of pramipexole into hOCT3-HEK cells was high at low concentrations and decreased at high concentrations, approaching the passive permeability in mock cells.
To further confirm that the transport into hOCT3-HEK cells was mediated by OCT3, uptake experiments were carried out using the OCT inhibitor corticosterone (Figure 3C). Corticosterone at high concentrations (100 and 200 μM) significantly inhibited the uptake of pramipexole (10 μM) into hOCT3-HEK cells (p < 0.001), while had minimal effect on the uptake of pramipexole into mock cells (p > 0.05). After corticosterone inhibition, the uptake of pramipexole into hOCT3-HEK cells was reduced by about 75%. Given corticosterone is a hOCT3 inhibitor with a Ki value in the sub-μM range, we do not know why inhibition was not complete.
DISCUSSION
This study demonstrated that pramipexole was a substrate for hOCT2 and hOCT3. hOCT2 and hOCT3 transported pramipexole in a high and low affinity manner, respectively. This study shows for the first time that pramipexole is a substrate for OCT3 and not a substrate for hOCT1.
Although a previous report indicated that pramipexole was transported by rOct1 7, our results show that pramipexole was not a substrate for hOCT1. The cell model in the present study was the stably transfected hOCT1-MDCK cells. The usefulness of the cell model to examine hOCT1 function was supported by the results of apically applying OCT substrate TEA. The uptake of TEA was much higher in hOCT1-MDCK cells than in mock cells across a 14.3–570 μM concentration range (Figure 1B). Although OCT1 is physiologically expressed on the basolateral side of kidney tubular cells, the over-expression system might cause the transporter to be expressed on the apical membrane as well. Additionally, cells were likely not fully differentiated and polarized when the uptake studies were performed. The difference between rOct1 and hOCT1 pramipexole uptake results appears to reflect a species difference. Substantial differences in substrate kinetics between rOct1 and hOCT1 have been observed previously.15 For example, the smallest n-tetraalkylammonium (nTAA), tetramethylammonium (TMA) was transported by rOCT1, but to a much lesser extent by hOCT1.15 hOCT1 shares 80% identity with rOct1.16 Amino acid residue changes may account for a functional difference between species.
Implication for pramipexole absorption
Pramipexole is a polar, weakly basic drug, but exhibits high oral bioavailability. OCTN2, a Na+-L-carnitine cotransporter as well as Na+-independent cation transporter, is the most abundantly expressed cation transporter in small intestine. 17, 18 In mouse enterocytes, OCTN2 is localized on the apical membrane.19 However, pramipexole (500 μM) did not inhibit L-carnitine (2.5 μM) uptake by hOCTN2-MDCK cells.20 Furthermore, L-carnitine (5 mM) did not inhibit [14C] pramipexole (3.9 μM) uptake by rat brain capillary endothelial cells, which highly expresses OCTN2.21 Therefore, these studies suggest that OCTN2 is not involved in the intestinal absorption of pramipexole, assuming OCTN2 has only one substrate binding site.
The human intestinal absorption of pramipexole appears to involve transport by OCT3 and possibly OCT2. OCT2 and OCT3 mRNA expression have been detected in human small intestine and Caco-2 cells.8, 9 OCT3 protein is localized on the brush border membrane of human jejunum.10 It should be noted that other intestinal transporters that may contribute were not examined in this study, including plasma membrane monoamine transporter (PMAT).22
Other implications of OCT-mediated uptake
Our results have implications for OCT2- and OCT3-mediated disposition of pramipexole in other tissues, such as kidney and brain. The kidney is the major organ for pramipexole elimination in humans and rats. Urinary excretion is the major route of pramipexole clearance in humans, with 80% of pramipexole dose recovered in the urine, mainly (about 90%) as unchanged drug.6 Renal clearance of pramipexole in humans is 400 ml/min, indicating active tubular secretion.6 In rodents, both Oct1 and Oct2 are expressed in the kidney.8 Pramipexole was found to be substrates for both rOct1 and rOct2.7 Kinetic analysis using kidney slices indicated that both rOct1 and rOct2 are involved in the renal uptake of pramipexole across the basolaterol membrane of the proximal tubular epithelial cells.7 Meanwhile, pramipexole was not the substrate of hOCT1 here, but a high affinity substrate for hOCT2. Coincidently, OCT2, but not OCT1, is strongly expressed on the basolateral membrane of kidney tubular cells in humans. Results here may suggest that OCT2 contributes to the renal elimination of pramipexole in humans.
Highly expressed on nondopaminergic cells, such as astrocytes adjacent to both the soma and terminals of midbrain dopaminergic neurons, OCT3 was proposed to modulate dopaminergic damage by bidirectionally regulating the local extracellular bioavailability of exogenous and endogenous neurotoxins in the brain.23 The present finding of pramipexole being an OCT3 substrate implicates a potential reservoir gate role of OCT3 in the disposition of the dopamine receptor agonist pramipexole in the brain. OCT3 may take up and hence inactivate pramipexole, so as to prevent uncontrolled activity of pramipexole when pramipexole is in excess outside the dopaminergic neurons. Alternatively, when pramipexole is below the therapeutic effective concentration outside the dopaminergic neurons, OCT3 may release and replenish pramipexole. Also, rat OCT3 is highly expressed at choroid plexus epithelial cells forming the blood-CSF barrier, which could also affect the brain distribution of pramxipexole.24, 25
A consideration for the potential relevance of hOCT3 for pramipexole disposition in the brain is pramipexole concentration. The steady state maximum plasma concentration of pramipxole at a common dose to treat Parkinson’s disease is about 4.9 ng/ml, or 0.016 μM.26 This concentration is low compared to our measured pramipexole Kt of 138 μM for hOCT3. However, it may be possible that OCT3 plays a role in local brain distribution of pramipexole, since OCT3 is considered a high capacity transporter. OCT3 is known as the “uptake-2 high capacity low affinity system” for removal of extracellular monoamine neurotransmitters14, such as domapine, whose concentration in brain is below 1 μM.27
In summary, this is the first study to characterize the in vitro permeability of pramipexole and examine the interaction of pramipexole with human OCTs. Its high human intestinal absorption may be promoted by OCT3 and possibly OCT2. Results indicate possible OCT2-mediated elimination from the kidney and OCT3-mediated distribution in the brain.
Acknowledgments
We gratefully acknowledge Dr. Kathleen M. Giacomini (University of California San Francisco) for providing hOCT3-HEK and mock cells. This work was supported in part by National Institutes of Health grant DK67530.
ABBREVIATIONS
- OCT
Organic Cation Transporter
- MDCK
Madin-Darby canine kidney
- HEK
Human Embryonic Kidney 293
- TEA
tetraethylammonium
- CSF
cerebrospinal fluid
Reference List
- 1.Neuhoff S, Ungell AL, Zamora I, Artursson P. pH-Dependent Bidirectional Transport of Weakly Basic Drugs Across Caco-2 Monolayers: Implications for Drug–drug Interactions. Pharm Res. 2003;20:1141–1148. doi: 10.1023/a:1025032511040. [DOI] [PubMed] [Google Scholar]
- 2.Moffat AC, Osselton DM, Widdop B. Clarke’s analysis of drugs and poisons in pharmaceuticals, body fluids and postmortem material. Pharmaceutical Press; London: 2004. [Google Scholar]
- 3.Friedl T, Eisenreich W. Extended release tablet formulation containing pramipexole or a pharmaceutically acceptable salt thereof. 20060051417. United States Patent Application 2006 Mar 9;
- 4.Block J, Beale JM. Wilson & Gisvold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry. Lippincott Williams & Wilkins; 2009. [Google Scholar]
- 5.Boehringer Ingelheim Pharmaceuticals, I. Mirapex ® (pramipexole dihydrochloride) prescribing information. 2009 [Google Scholar]
- 6.Wright CE, Sisson TL, Ichhpurani AK, Peters GR. Steady-state pharmacokinetic properties of pramipexole in healthy volunteers. J Clin Pharmacol. 1997;37:520–525. doi: 10.1002/j.1552-4604.1997.tb04330.x. [DOI] [PubMed] [Google Scholar]
- 7.Ishiguro N, Saito A, Yokoyama K, Morikawa M, Igarashi T, Tamai I. Transportof the dopamine D2 agonist pramipexole by rat organic cation transporters Oct1 and Oct2 in kidney. Drug Metab Dispos. 2005;33:495–499. doi: 10.1124/dmd.104.002519. [DOI] [PubMed] [Google Scholar]
- 8.Koepsell H, Lips K, Volk C. Polyspecific Organic Cation Transporters: Structure, Function, Physiological Roles, and Biopharmaceutical Implications. Pharm Res. 2007;24:1227–1251. doi: 10.1007/s11095-007-9254-z. [DOI] [PubMed] [Google Scholar]
- 9.Hayer-Zillgen M, Bruss M, Bonisch H. Expression and pharmacological profile of the human organic cation transporters hOCT1, hOCT2 and hOCT3. Br J Pharmacol. 2002;136:829–836. doi: 10.1038/sj.bjp.0704785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muller J, Lips KS, Metzner L, Neubert RHH, Koepsell H, Brandsch M. Drug specificity and intestinal membrane localization of human organic cation transporters (OCT) Biochem Pharmacol. 2005;70:1851–1860. doi: 10.1016/j.bcp.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Shu Y, Leabman MK, Feng B, Mangravite LM, Huang CC, Stryke D, Kawamoto M, Johns SJ, DeYoung J, Carlson E, Ferrin TE, Herskowitz I, Giacomini KM. Evolutionary conservation predicts function of variants of the human organic cation transporter, OCT1. Proc Natl Acad Sci U S A. 2003;100:5902–5907. doi: 10.1073/pnas.0730858100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang S, Lovejoy KS, Shima JE, Lagpacan LL, Shu Y, Lapuk A, Chen Y, Komori T, Gray JW, Chen X, Lippard SJ, Giacomini KM. Organic Cation Transporters Are Determinants of Oxaliplatin Cytotoxicity. Cancer Res. 2006;66:8847–8857. doi: 10.1158/0008-5472.CAN-06-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balakrishnan A, Hussainzada N, Gonzalez P, Bermejo M, Swaan PW, Polli JE. Bias in Estimation of Transporter Kinetic Parameters from Overexpression Systems: Interplay of Transporter Expression Level and Substrate Affinity. J Pharmacol Exp Ther. 2007;320:133–144. doi: 10.1124/jpet.106.107433. [DOI] [PubMed] [Google Scholar]
- 14.Grundemann D, Schechinger B, Rappold GA, Schomig E. Molecular identification of the corticosterone-sensitive extraneuronal catecholamine transporter. Nat Neurosci. 1998;1:349–351. doi: 10.1038/1557. [DOI] [PubMed] [Google Scholar]
- 15.Dresser MJ, Gray AT, Giacomini KM. Kinetic and Selectivity Differences between Rodent, Rabbit, and Human Organic Cation Transporters (OCT1) J Pharmacol Exp Ther. 2000;292:1146–1152. [PubMed] [Google Scholar]
- 16.Ciarimboli G. Organic cation transporters. Xenobiotica. 2008;38:936–971. doi: 10.1080/00498250701882482. [DOI] [PubMed] [Google Scholar]
- 17.Terada T, Shimada Y, Pan X, Kishimoto K, Sakurai T, Doi R, Onodera H, Katsura T, Imamura M, Inui KI. Expression profiles of various transporters for oligopeptides, amino acids and organic ions along the human digestive tract. Biochem Pharmacol. 2005;70:1756–1763. doi: 10.1016/j.bcp.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 18.Hilgendorf C, Ahlin G, Seithel A, Artursson P, Ungell AL, Karlsson J. Expression of Thirty-six Drug Transporter Genes in Human Intestine, Liver, Kidney, and Organotypic Cell Lines. Drug Metab Dispos. 2007;35:1333–1340. doi: 10.1124/dmd.107.014902. [DOI] [PubMed] [Google Scholar]
- 19.Kato Y, Sugiura M, Sugiura T, Wakayama T, Kubo Y, Kobayashi D, Sai Y, Tamai I, Iseki S, Tsuji A. Organic Cation/Carnitine Transporter OCTN2 (Slc22a5) Is Responsible for Carnitine Transport across Apical Membranes of Small Intestinal Epithelial Cells in Mouse. Mol Pharmacol. 2006;70:829–837. doi: 10.1124/mol.106.024158. [DOI] [PubMed] [Google Scholar]
- 20.Diao L, Ekins S, Polli J. Novel Inhibitors of Human Organic Cation/Carnitine Transporter (hOCTN2) via Computational Modeling and In Vitro Testing. Pharm Res. 2009;26:1890–1900. doi: 10.1007/s11095-009-9905-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okura T, Ito R, Ishiguro N, Tamai I, Deguchi Y. Blood-brain barrier transport of pramipexole, a dopamine D2 agonist. Life Sci. 2007;80:1564–1571. doi: 10.1016/j.lfs.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 22.Zhou M, Xia L, Wang J. Metformin Transport by a Newly Cloned Proton-Stimulated Organic Cation Transporter (Plasma Membrane Monoamine Transporter) Expressed in Human Intestine. Drug Metab Dispos. 2007;35:1956–1962. doi: 10.1124/dmd.107.015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui M, Aras R, Christian WV, Rappold PM, Hatwar M, Panza J, Jackson-Lewis V, Javitch JA, Ballatori N, Przedborski S, Tieu K. The organic cation transporter-3 is a pivotal modulator of neurodegeneration in the nigrostriatal dopaminergic pathway. Proc Natl Acad Sci U S A. 2009;106:8043–8048. doi: 10.1073/pnas.0900358106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haag C, Berkels R, Grundemann D, Lazar A, Taubert D, Schomig E. The localisation of the extraneuronal monoamine transporter (EMT) in rat brain. J Neurochem. 2004;88:291–297. doi: 10.1111/j.1471-4159.2004.02180.x. [DOI] [PubMed] [Google Scholar]
- 25.Vialou V, Amphoux A, Zwart R, Giros B, Gautron S. Organic Cation Transporter 3 (Slc22a3) Is Implicated in Salt-Intake Regulation. J Neurosci. 2004;24:2846–2851. doi: 10.1523/JNEUROSCI.5147-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jenner P, Konen-Bergmann M, Schepers C, Haertter S. Pharmacokinetics of a Once-Daily extended-release formulation of pramipexole in healthy male volunteers: Three studies. Clin Ther. 2009;31:2698–2711. doi: 10.1016/j.clinthera.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 27.Iniouchine M, Sibarov D, Volnova A, Jimenez-Rivera C, Nozdrachev A. Blockers of monoamine transporters influence high dopamine concentration uptake in rat brain slices. Dokl Biol Sci. 2008;419:80–82. doi: 10.1134/s0012496608020038. [DOI] [PubMed] [Google Scholar]