Abstract
Brain cell loss has been reported in subjects with alcoholism. However, the molecular mechanisms are unclear. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and monoamine oxidase B (MAO B) reportedly play a role in cellular dysfunction with regards to ethanol exposure. We have recently reported that GAPDH protein expression was increased in the brains of rats fed with ethanol. Furthermore, GAPDH interacts with the transcriptional activator, transforming growth factor-beta-inducible early gene 2 (TIEG2), to augment TIEG2-mediated MAO B activation, resulting in neuronal cell damage due to ethanol exposure. The current study investigates whether the TIEG2–MAO B cascade is also active in the brains of rats fed with ethanol. Ten ethanol-preferring rats were fed with a liquid diet containing ethanol, with increasing amounts of ethanol up to a final concentration of 6.4% representing a final diet containing 36% of calories for 28 days. Ten control rats were fed the liquid diet without ethanol. The expression of TIEG2 protein, MAO B mRNA levels, MAO B catalytic activity, and the levels of anti-apoptotic protein Bcl 2 and apoptotic protein caspase 3 were determined in the prefrontal cortex of the rats. Ethanol significantly increased protein levels of TIEG2, active caspase 3, MAO B mRNA and enzyme activity, but significantly decreased Bcl 2 protein expression compared to control rats. In summary, ethanol increases the TIEG2–MAO B brain cell death cascade in rat brains, suggesting that the TIEG2–MAO B pathway is a novel pathway for brain cell damage resulting from ethanol exposure, and may contribute to chronic alcohol-induced brain damage.
Keywords: Alcoholism, Ethanol-preferring rats, Transforming growth factor-beta-inducible early gene 2, Monoamine oxidase B, Apoptosis, Bcl 2, Active caspase 3, Cell death pathway
Introduction
Alcoholism is a major psychiatric condition as long term heavy ethanol consumption results in neuropsychological difficulties that affect physical health and memory along with social, family, and job responsibilities (Rodgers et al. 2000; Caldwell et al. 2002). Reduced volume of brain tissue, increased brain damage accompanied by cognitive deficits, and low densities of neuronal and glial cells have been reported in brains from human subjects with alcohol dependence (Kril and Halliday 1999; Brooks 2000; Miguel-Hidalgo et al. 2002; Prendergast 2004; Miguel-Hidalgo et al. 2006; Prendergast and Little 2007). Ethanol also induces neuronal cell death and cell cycle delay in cell model systems in vitro (Luo et al. 1999; Chen et al. 2006) and causes neurotoxicity and brain lesions in animal models (Pfefferbaum et al. 2007; Choi et al. 2008). However, the molecular mechanism underlying the ethanol-related brain damage is not yet clear.
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is a multifunctional protein that catalyzes and breaks down glucose with the release of energy and carbon. Apart from this traditionally described role, GAPDH has been recently implicated in several non-metabolic processes including transcriptional activation and initiation of apoptosis. GAPDH is known to translocate into the nucleus and mediate stress signaling, resulting in cellular dysfunction and death (Ishitani et al. 1996; Chen et al. 1999; Chuang et al. 2005; Hara et al. 2005; Bae et al. 2006; Sen et al. 2008). Furthermore, the protein level of GAPDH was not only increased in the prefrontal cortex (PFC) of human brains from alcoholics (Alexander-Kaufman et al. 2006), but also elevated in rat brains that were exposed to ethanol (Ou et al. 2009b). However, the molecular mechanism for the observed GAPDH-mediated damage in alcoholism remains unclear. Recently, we identified a possible mechanism by which GAPDH may interact with the transcriptional activator, transforming growth factor-beta-inducible early gene 2 (TIEG2) to augment TIEG2-mediated mono-amine oxidase B (MAO B) activation, resulting in neuronal cell injury as a result of exposure to ethanol (Ou et al. 2009a, b). Therefore, TIEG2 appears to be the target of the stress sensor, GAPDH (Ou et al. 2009b).
MAO B has also been implicated in alcoholism (Carlsson et al. 1980). This enzyme degrades a number of biogenic amines (such as phenylethylamine, tyramine, tryptamine, and dopamine) and generates inert hydrogen peroxide (H2O2), which can interact with iron, initiating Fenton’s reaction to produce reactive hydroxyl radicals that cause cellular dysfunction and death (Youdim et al. 2004; Gerlach et al. 2006). An MAO B transcriptional activator, TIEG2, induces MAO B expression by interacting with the core promoter region of the enzyme (Ou et al. 2004). TIEG2 reportedly inhibits cell growth (Cook et al. 1998) and induces apoptosis in murine oligodendroglial cells (Wang et al. 2007). We have recently reported that GAP-DH interacts with TIEG2 and augments TIEG2-mediated MAO B activation and subsequent cell damage in neuronal cells exposed to ethanol (Ou et al. 2009a). Thus, the GAPDH–TIEG2–MAO B cascade may play a prominent role in cell dysfunction and damage. However, whether ethanol could increase the levels of both TIEG2 and MAO B simultaneously in the brain has not yet been studied.
In this study, we investigated whether chronic ethanol exposure increased levels of both TIEG2 and MAO B in the PFC of ethanol-preferring rats. This ethanol-preferring line of rats is a long-standing, reputable choice as an excellent animal model for investigating the effects of alcoholism (Li et al. 1979; Waller et al. 1984).
Materials and Methods
Materials
Male ethanol-preferring Wistar rats [weighing 180–220 g, from the Indiana University Alcohol Research Center (Li et al. 1979)] were fed with an ethanol diet or control diet (Dyets, Bethlehem, PA). The Lieber–DeCarli ethanol diet was purchased from Dyets (Bethlehem, PA). Antibodies were obtained from the following sources: mouse anti-TIEG2 (BD, Franklin Lakes, New Jersey); mouse anti-Bcl 2 (Santa Cruz Technology, Santa Cruz, CA); and rabbit anti-active caspase 3 (ABCAM, Cambridge, MA). Restore PLUS Western Blot Stripping Buffer was purchased from Fisher. The SYBR supermix kit for quantitative real-time RT-PCR was purchased from Bio-Rad Laboratories (Hercules, CA).
Animal Care, Group Size and Feeding
All protocols for the animal experiments described in this study were carried out according to the Ethical Guidelines on Animal Experimentation and were approved by the Animal Usage Committee at the University of Mississippi Medical Center. Ethanol-preferring Wistar rats were housed in individual cages in a temperature- and humidity-controlled room with a 12:12-h light–dark cycle. The standard group size in our studies (control group and treatment group) was 10 per group. Rats were randomly assigned to each experimental group. The number of rats (10/group) was based on our preliminary data and is considered to be a sufficient quantity to power the studies to obtain significant results.
Rats were acclimatized for 3 days after arrival and provided free access to Purina rat chow and water. Rats were then allowed free access to the liquid diet without ethanol for 3 days and then randomly assigned to the ethanol-fed or control groups. The liquid ethanol diet contained increasing amounts of ethanol until a final diet containing 36% of calories from ethanol (6.4% EtOH) was achieved as follows: no EtOH for 3 days, followed by 2.5% for 3 days, then 5.0% for 5 days, and finally 6.4% for 17 days. This final diet containing 36% of calories from ethanol (~14 g EtOH/day/kg rat) achieved a blood ethanol concentration of less than 50 mM (Kalev-Zylinska and During 2007). The liquid ethanol diet (#710260) was purchased from Dyets Inc. (Bethlehem, PA). In the ethanol diets, glucose was isocalorically substituted with ethanol according to the Lieber–DeCarli diet formulae following the manufacturer’s instructions. The control rats were fed a glucose liquid diet (without ethanol; #710027, Dyets) that contained the same amount of calories that their ethanol-fed pair had consumed (Ou et al. 2009b).
The ethanol concentrations used in this study (less than 50 mM) are within the range that results in the physiological effects observed in alcoholics (Henriksen et al. 1997; Yao et al. 2001).
Sacrifice
All rats, in the ethanol-fed and control groups, were killed by decapitation on day 29, and the PFC was immediately removed and stored at −80°C until used. The PFC was chosen in our studies because alcohol-use disorders have been shown to reduce PFC volume compared to healthy controls (Paul et al. 2008) interfering with executive function. The protein levels of TIEG2, Bcl 2, active caspase 3 and MAO B mRNA, and catalytic activity were determined as described below.
Tissue Preparation
The PFC from each animal was homogenized on ice with a 0.5 ml solution containing 1 mM EDTA, 10 mM Tris–HCL (pH 7) and fresh protease inhibitors (1× Protease Inhibitor Cocktail, Sigma). The resulting homogenate was briefly stored on dry ice for ~10 min, thawed and centrifuged at 4°C (12,500 rpm) for 5 min in a microcentrifuge. The supernatant was then stored at −80°C until used. Protein concentrations of the homogenized samples were calculated using the BCA Protein Assay Kit (PIERCE).
Western Blotting
Thirty micrograms (or 40 mg for active caspase 3) of total protein for each rat were separated on 10.5% SDS–poly-acrylamide gels by electrophoresis and transferred to PVDF membranes in a BioRad mini tank apparatus. After transfer, the membranes were blocked at room temperature for 1 h with 5% nonfat dry milk. Following blocking, membranes were incubated with the respective primary antibody, mouse anti-TIEG2 (1:500) or anti-Bcl 2 (1:500) or rabbit anti-active caspase 3 (1:250), overnight at 4°C. After incubation with the appropriate secondary antibody (Santa Cruz) for 2 h, the bands were visualized by horse-radish peroxidase reaction using SuperSignal Chemiluminescent Substrate (PIERCE). As a control for sample loading, the same blot, after having been immunostained with TIEG2, Bcl 2 or active caspase 3, was stripped for 20 min at room temperature in Restore Western Blot Stripping Buffer (Fisher) and then re-probed with mouse anti-actin primary antibody at 1:2500 overnight at 4°C.
The relative intensity (relative optical density × pixel area) of the autoradiographic bands from western blotting was determined using a gel analysis software and computer-assisted image analysis system (Ou et al. 2001). Pairs of rat subjects were immunoblotted on the same gel with duplicates on separate gels. Linear regression was used to plot a standard curve for each gel, from which relative optical density (ROD) values of the samples were converted to cortical standard protein units for each experimental sample for each gel (Ou et al. 2009b). The level of TIEG2, Bcl 2 or active caspase 3 protein was defined as the ratio of the optical density value of the TIEG2, Bcl 2 or active caspase 3 band to the optical density value of each prospective actin band expressed as a ratio of [protein of interest]/[actin]. Duplicate optical density ratios were obtained for each sample and averaged. The anti-TIEG2 antibody recognizes a protein band of 72 kDa, the anti-Bcl 2 antibody recognizes a band of 26 kDa and the anti-active caspase 3 antibody recognizes a band of 20 kDa.
Quantitative Real-Time RT-PCR
Total RNA was isolated from the rat PFC using TRIzol reagent (Invitrogen; ~50 mg tissue/1 ml TRIzol) following manufacturer’s instructions. The mRNA was then reverse-transcribed into cDNA using SuperScript® III Reverse Transcriptase (Invitrogen). MAO B mRNA levels for the ethanol-treated group and untreated control group were analyzed by quantitative real-time RT-PCR using a Bio-Rad iCycler system. Specific primers used for the rat MAO B are as follows: sense, 5′-GGGGGCGGCATCT-CAGGT-3′ and antisense, 5′-TCAGCCGCTCAACTT-CATTCACTT-3′. Real-time RT-PCR was performed with a SYBR supermix kit, and 18S Ribosomal RNA primer was used as an internal control in every plate to avoid sample variations. The values were calculated as described previously (Ou et al. 2004).
MAO B Catalytic Activity
One hundred microliters of total protein (~100 μg of protein) were incubated with 10 μM 14C-labeled phenyl-ethylamine (Amersham) in 900 μl of assay buffer (50 mM sodium phosphate buffer, pH 7.4) and incubated at 37°C for 20 min; the reaction was stopped by the addition of 100 μl of 6 N HCl. The reaction products were then extracted with toluene and centrifuged at 2500 rpm (800×g) for 7 min. The organic phase containing the reaction product was extracted and quantified by liquid scintillation spectroscopy (Ou et al. 2004).
Statistical Analysis
Student’s t-test was used for experiments with the two groups, and a value of P < 0.05 was considered statistically significant.
Results
Increased Expression of TIEG2 in Rats Treated with Physiologically High Levels of Ethanol
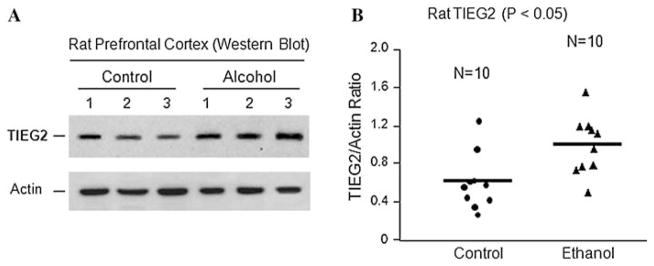
Because alcohol-use disorders have been shown to reduce PFC volume as compared to healthy controls (Paul et al. 2008), to examine the role of TIEG2, the target of the stress sensor GAPDH (Ou et al. 2009b), in the brain’s response to ethanol, we fed rats an ethanol liquid diet or control diet for 28 days, and examined the protein level of TIEG2 in the PFC. Results from western blot analysis showed significant induction of TIEG2 in the PFC of rats exposed to ethanol (Fig. 1). Ethanol increased the average protein level of TIEG2 by ~1.6-fold compared to control rats (Fig. 1b, P < 0.05). Actin was used as loading control. As shown in Fig. 1a, actin levels were not affected by ethanol.
Fig. 1.
The effect of ethanol on the expression of TIEG2 protein. Rats were fed with an ethanol diet or control diet for 28 days, and the protein levels of TIEG2 in the prefrontal cortex were examined by Western blotting. a Representative western blots showing the immunolabelling of TIEG2 in the prefrontal cortex of 3 untreated controls and 3 ethanol-treated rats. The anti-actin antibody was used as the loading controls. b Quantitative analysis of western blot results. Each TIEG2 protein band was evaluated by the relative intensity (relative optical density × pixel area) of its autoradiographic band and normalized to the density of actin. The graph of the average optical density of TIEG2/actin for the individual subjects (circles or triangles) and mean values (horizontal lines) are shown with 10 rats (n = 10) for each the control group (circles) and the ethanol-fed group (triangles). Expression of TIEG2 is significantly increased in the ethanol-treated group as compared to the untreated control group (P < 0.05)
The increases in TIEG2 by ethanol in rat brains suggest that TIEG2 may play an important role in alcohol-related brain damage.
Ethanol Increased MAO B mRNA Level and Catalytic Activity in Rats with Chronic Ethanol Exposure
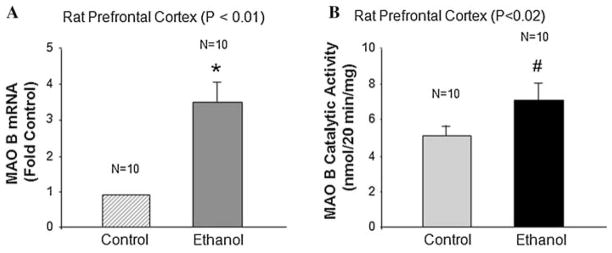
TIEG2 protein is significantly increased in rats fed with physiological concentrations of ethanol and because TIEG2 is a transcription factor that activates MAO B expression by interacting with its core promoter region (Ou et al. 2004), we, therefore, hypothesized that rat brain MAO B levels would be increased as well. MAO B mRNA levels (Fig. 2a) and catalytic activity (Fig. 2b) were determined in the rat PFC by quantitative real-time RT-PCR and enzymatic activity assay, respectively. As shown in Fig. 2a, the level of MAO B mRNA was increased by 3.5-fold in the ethanol-treated group compared to the untreated control group (P < 0.01). We also observed a significant increase (by ~1.37-fold) in MAO B enzymatic activity in the PFC of rats treated with ethanol as compared to controls (Fig. 2b; P < 0.02).
Fig. 2.
The effect of ethanol on MAO B mRNA level and catalytic activity. Rats were fed with an ethanol diet or control diet for 28 days, and a the rat MAO B mRNA levels were determined by quantitative real-time RT-PCR, and b the rat MAO B catalytic activity was determined by enzymatic activity assay using the prefrontal cortex. Data represent the mean ± S.D. of three independent experiments with 10 rats (n = 10) in each group. Controls were the untreated group, which were taken as 1 in a. The MAO B mRNA level and catalytic activity are significantly increased in the ethanol-treated group as compared to the untreated control group (* P < 0.01 and # P < 0.02)
Reduced Expression of Anti-Apoptotic Bcl 2, but Increased Expression of Apoptotic Protein Active Caspase 3, in Rats Treated with Physiologically High Levels of Ethanol
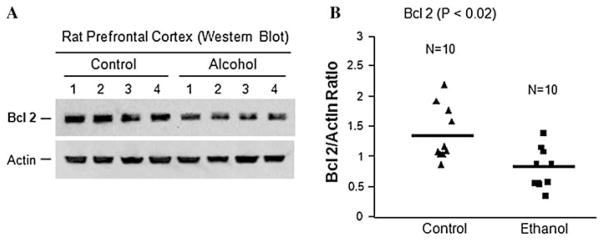
To test whether ethanol could induce apoptosis in rat brains, we determined the expression of both the anti-apoptotic protein, Bcl 2 and the apoptotic protein, active caspase 3. Results from western blot analysis showed significant reduction of Bcl 2 in the PFC of rats exposed to ethanol (Fig. 3). Ethanol decreased the average protein level of Bcl 2 by ~41% compared to control rats (Fig. 3b, P < 0.02). Similarly, as shown in Fig. 3a, actin levels were not affected by ethanol.
Fig. 3.
The effect of ethanol on the expression of Bcl 2 anti-apoptotic protein. Rats were fed with ethanol diet or control diet for 28 days, and the protein levels of Bcl 2 in the prefrontal cortex were examined by western blotting. a Representative western blots showing the immunolabelling of Bcl 2 in the prefrontal cortex of 4 untreated controls and 4 ethanol-treated rats. The anti-actin antibody was used as the loading controls. b Quantitative analysis of western blot results. Each Bcl 2 protein band was evaluated by the relative intensity (relative optical density × pixel area) of its autoradiographic band and normalized to the density of actin. The graph of the average optical density of Bcl 2/actin for the individual subjects (triangles or squares) and mean values (horizontal lines) are shown with 10 rats (n = 10) for each the control group (triangles) and the ethanol-fed group (squares). Expression of Bcl 2 is significantly decreased in the ethanol-treated group as compared to the untreated control group (P < 0.02)
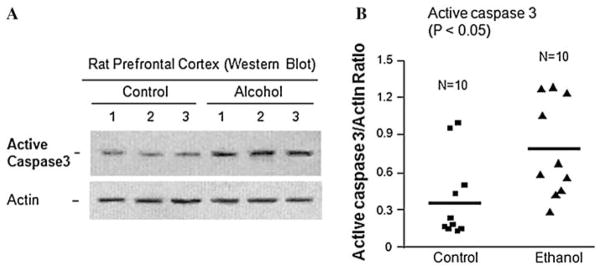
Furthermore, the western blot analysis showed significant increase in active caspase 3 in the PFC of rats exposed to ethanol (Fig. 4). Ethanol increased the average protein level of active caspase 3 by ~1.8-fold compared to control rats (Fig. 4b, P < 0.05).
Fig. 4.
The effect of ethanol on the expression of caspase 3 apoptotic protein. Rats were fed with ethanol diet or control diet for 28 days, and the protein levels of active caspase 3 in the prefrontal cortex were examined by western blotting. a Representative western blots showing the immunolabelling of active caspase 3 in the prefrontal cortex of 3 untreated controls and 3 ethanol-treated rats. The anti-actin antibody was used as the loading controls. B Quantitative analysis of western blot results. Each active caspase 3 protein band was evaluated by the relative intensity (relative optical density × pixel area) of its autoradiographic band and normalized to the density of actin. The graph of the average optical density of active caspase 3/actin for the individual subjects (squares or triangles) and mean values (horizontal lines) are shown with 10 rats (n = 10) for each the control group (squares) and the ethanol-fed group (triangles). Expression of active caspase 3 is significantly increased in the ethanol-treated group as compared to the untreated control group (P < 0.05)
The decrease in anti-apoptotic Bcl 2 and increase in apoptotic caspase 3 in rat brains upon ethanol exposure suggest that ethanol induces apoptosis that may be mediated by an increase in the TIEG2–MAO B cell death cascade in the PFC of rats exposed to ethanol.
Discussion
In the present study, we demonstrate that our newly proposed mechanism of the ethanol-elicited TIEG2–MAO B cascade is significantly increased in the rat model of alcoholism. This suggests that the TIEG2–MAO B cascade may be involved in the ethanol-induced brain cell death observed in this study. Thus, this study demonstrates the suitability of using chronic ethanol feeding of rats (ethanol-preferring rats) as a model to study the molecular mechanism of ethanol-induced brain tissue damage.
The transcription factor, TIEG2, is an activator of MAO B (Ou et al. 2004) which promotes apoptotic cell death (Wang et al. 2007). We have previously reported that exposing neuronal cells (in vitro) to ethanol (75 mM) increased the expression of TIEG2 and MAO B catalytic activity, while ethanol in the presence of an MAO B inhibitor (deprenyl) reduced the expression of both TIEG2 and MAO B and increased cell viability (Lu et al. 2008). Recently, we found that ethanol also increased the levels of nuclear GAPDH and MAO B activity in brain derived cell lines (human glioblastoma and neuroblastoma). We have also revealed that GAPDH physically interacted with TIEG2, and this interaction was enhanced in the nucleus by ethanol but reduced by MAO B inhibitors in human brain-derived cell lines (Ou et al. 2009a). Thus, this newly identified GAPDH–TIEG2–MAO B-mediated brain cell death pathway may provide the new insights into drug design for the treatment of neurobiological diseases including alcohol-use disorders.
It is interesting to note that the GAPDH–TIEG2–MAO B cascade is up-regulated in the rat PFC following chronic alcohol treatment, because we have recently shown that in ethanol-preferring rats, both GAPDH and MAO B proteins are significantly increased by approximately twofold (P < 0.02) for GAPDH and by ~1.7-fold (P < 0.05) for MAO B compared to normal controls as determined by western blot analysis (Ou et al. 2009b). Our current study used the same rat model and experiment, and we found that TIEG2 protein expression is also significantly increased. More recently, we found that both GAPDH and MAO B proteins were significantly elevated in postmortem studies of PFC tissue of alcohol-dependent subjects (Ou et al. 2009b). Because of the elevated levels of GAPDH and MAO B, it is possible that TIEG2 expression may be equally elevated in human subjects with alcoholism and needs to be investigated in the future. To our knowledge, this is the first report in animals to show increased TIEG2 protein in the PFC of rats fed with physiologically relevant amounts of ethanol.
In addition, we found that ethanol significantly increased MAO B catalytic activity by ~1.37-fold (P < 0.02; Fig. 2b) as compared to untreated rats in this study, suggesting that ethanol-induced cell death is partially through MAO B-mediated oxidative stress because MAO B is the major H2O2 generating enzyme (Ou et al. 2009a; Youdim and Lavie 1994). The increase in H2O2 induces cellular (oxidative) stress that can result in cyto-toxicity (neurotoxicity) and apoptotic cell death.
Consistent with the increase in activation of the TIEG2–MAO B cell death pathway, we have found that the anti-apoptotic protein Bcl-2 was reduced while the apoptotic protein caspase 3 was increased in ethanol-treated rats. Interestingly, these results are similar to another transforming growth factor inducible gene, TIEG1. TIEG1 has been reported to promote apoptosis (triggered by homoharringtonine or velcade) through the down-regulation of the Bcl-2 gene and activation of caspase 3 (Jin et al. 2007). Furthermore, TIEG1-induced apoptosis involved the release of cytochrome c from mitochondria into the cytosol and disruption of the mitochondrial membrane potential (Jin et al. 2007). However, whether TIEG2-mediated cell death (triggered by ethanol) also involve the release of cytochrome c and disruption of the mitochondrial membrane potential as seen in TIEG1-mediated cell death is unknown yet and needs to be investigated. In addition, a TIEG2-knockout mouse has been generated (Song et al. 2005) and can be a powerful tool to further characterize the TIEG2-mediated cell death mechanism induced by ethanol. Additionally, future studies should also examine the specificity of cell types regarding susceptibility to alcohol exposure.
In summary, we show that chronic ethanol consumption can significantly induce the expression of TIEG2 protein in the rat brain, and this increase is accompanied by corresponding increases in MAO B mRNA level and enzymatic activity. In addition, the anti-apoptotic protein Bcl-2 was reduced while the apoptotic protein caspase 3 was increased in ethanol-treated rats, which indicated that the initiation of cell death has occurred. Taken together, it suggests that the rat TIEG2–MAO B cell death pathway may contribute to chronic alcohol-induced brain tissue injury. The current chronic ethanol feeding paradigm in rats may also provide a suitable model to investigate the molecular pathophysiology of ethanol-induced brain damage in humans.
Acknowledgments
This study was supported by Public Health Service Grants P20 RR 017701, a NARSAD Young Investigator Award, an Intramural Research Support grant from The University of Mississippi Medical Center and an R24 Alcohol Research Resource Award Grant (R24 AA015512-02) from NIAAA.
References
- Alexander-Kaufman K, James G, Sheedy D, Harper C, Matsumoto I. Differential protein expression in the prefrontal white matter of human alcoholics: a proteomics study. Mol Psychiatry. 2006;11:56–65. doi: 10.1038/sj.mp.4001741. [DOI] [PubMed] [Google Scholar]
- Bae BI, Hara MR, Cascio MB, Wellington CL, Hayden MR, Ross CA, Ha HC, Li XJ, Snyder SH, Sawa A. Mutant huntingtin: nuclear translocation and cytotoxicity mediated by GAPDH. Proc Natl Acad Sci USA. 2006;103:3405–3409. doi: 10.1073/pnas.0511316103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks PJ. Brain atrophy and neuronal loss in alcoholism: a role for DNA damage? Neurochem Int. 2000;37:403–412. doi: 10.1016/s0197-0186(00)00051-6. [DOI] [PubMed] [Google Scholar]
- Caldwell TM, Rodgers B, Jorm AF, Christensen H, Jacomb PA, Korten AE, Lynskey MT. Patterns of association between alcohol consumption and symptoms of depression and anxiety in young adults. Addiction. 2002;97:583–594. doi: 10.1046/j.1360-0443.2002.00092.x. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Adolfsson R, Aquilonius SM, Gottfries CG, Oreland L, Svennerholm L, Winblad B. Biogenic amines in human brain in normal aging, senile dementia, and chronic alcoholism. Adv Biochem Psychopharmacol. 1980;23:295–304. [PubMed] [Google Scholar]
- Chen RW, Saunders PA, Wei H, Li Z, Seth P, Chuang DM. Involvement of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and p53 in neuronal apoptosis: evidence that GAPDH is upregulated by p53. J Neurosci. 1999;19:9654–9662. doi: 10.1523/JNEUROSCI.19-21-09654.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CP, Kuhn P, Chaturvedi K, Boyadjieva N, Sarkar DK. Ethanol induces apoptotic death of developing beta-endorphin neurons via suppression of cyclic adenosine monophosphate production and activation of transforming growth factor-beta1-linked apoptotic signaling. Mol Pharmacol. 2006;69:706–717. doi: 10.1124/mol.105.017004. [DOI] [PubMed] [Google Scholar]
- Choi DS, Wei W, Deitchman JK, Kharazia VN, Lesscher HM, McMahon T, Wang D, Qi ZH, Sieghart W, Zhang C, Shokat KM, Mody I, Messing RO. Protein kinase Cdelta regulates ethanol intoxication and enhancement of GABA-stimulated tonic current. J Neurosci. 2008;28:11890–11899. doi: 10.1523/JNEUROSCI.3156-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang DM, Hough C, Senatorov VV. Glyceraldehyde-3-phosphate dehydrogenase, apoptosis, and neurodegenerative diseases. Annu Rev Pharmacol Toxicol. 2005;45:269–290. doi: 10.1146/annurev.pharmtox.45.120403.095902. [DOI] [PubMed] [Google Scholar]
- Cook T, Gebelein B, Mesa K, Mladek A, Urrutia R. Molecular cloning and characterization of TIEG2 reveals a new subfamily of transforming growth factor-beta-inducible Sp1-like zinc finger-encoding genes involved in the regulation of cell growth. J Biol Chem. 1998;273:25929–25936. doi: 10.1074/jbc.273.40.25929. [DOI] [PubMed] [Google Scholar]
- Gerlach M, Double KL, Youdim MB, Riederer P. Potential sources of increased iron in the substantia nigra of parkinsonian patients. J Neural Transm Suppl. 2006;70:133–142. doi: 10.1007/978-3-211-45295-0_21. [DOI] [PubMed] [Google Scholar]
- Hara MR, Agrawal N, Kim SF, Cascio MB, Fujimuro M, Ozeki Y, Takahashi M, Cheah JH, Tankou SK, Hester LD, Ferris CD, Hayward SD, Snyder SH, Sawa A. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat Cell Biol. 2005;7:665–674. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- Henriksen JH, Gronbaek M, Moller S, Bendtsen F, Becker U. Carbohydrate deficient transferrin (CDT) in alcoholic cirrhosis: a kinetic study. J Hepatol. 1997;26:287–292. doi: 10.1016/s0168-8278(97)80043-8. [DOI] [PubMed] [Google Scholar]
- Ishitani R, Kimura M, Sunaga K, Katsube N, Tanaka M, Chuang DM. An antisense oligodeoxynucleotide to glyceraldehyde-3-phosphate dehydrogenase blocks age-induced apoptosis of mature cerebrocortical neurons in culture. J Pharmacol Exp Ther. 1996;278:447–454. [PubMed] [Google Scholar]
- Jin W, Di G, Li J, Chen Y, Li W, Wu J, Cheng T, Yao M, Shao Z. TIEG1 induces apoptosis through mitochondrial apoptotic pathway and promotes apoptosis induced by homoharringtonine and velcade. FEBS Lett. 2007;581:3826–3832. doi: 10.1016/j.febslet.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Kalev-Zylinska ML, During MJ. Paradoxical facilitatory effect of low-dose alcohol consumption on memory mediated by NMDA receptors. J Neurosci. 2007;27:10456–10467. doi: 10.1523/JNEUROSCI.2789-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kril JJ, Halliday GM. Brain shrinkage in alcoholics: a decade on and what have we learned? Prog Neurobiol. 1999;58:381–387. doi: 10.1016/s0301-0082(98)00091-4. [DOI] [PubMed] [Google Scholar]
- Li TK, Lumeng L, McBride WJ, Waller MB. Progress toward a voluntary oral consumption model of alcoholism. Drug Alcohol Depend. 1979;4:45–60. doi: 10.1016/0376-8716(79)90040-1. [DOI] [PubMed] [Google Scholar]
- Lu D, Johnson C, Johnson S, Tazik S, Ou XM. The neuroprotective effect of antidepressant drug via inhibition of TIEG2-MAO B mediated cell death. Drug Discov Ther. 2008;2:289–295. [PMC free article] [PubMed] [Google Scholar]
- Luo J, West JR, Cook RT, Pantazis NJ. Ethanol induces cell death and cell cycle delay in cultures of pheochromocytoma PC12 cells. Alcohol Clin Exp Res. 1999;23:644–656. [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Wei J, Andrew M, Overholser JC, Jurjus G, Stockmeier CA, Rajkowska G. Glia pathology in the prefrontal cortex in alcohol dependence with and without depressive symptoms. Biol Psychiatry. 2002;52:1121–1133. doi: 10.1016/s0006-3223(02)01439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Overholser JC, Meltzer HY, Stockmeier CA, Rajkowska G. Reduced glial and neuronal packing density in the orbitofrontal cortex in alcohol dependence and its relationship with suicide and duration of alcohol dependence. Alcohol Clin Exp Res. 2006;30:1845–1855. doi: 10.1111/j.1530-0277.2006.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou XM, Storring JM, Kushwaha N, Albert PR. Heterodimerization of mineralocorticoid and glucocorticoid receptors at a novel negative response element of the 5-HT1A receptor gene. J Biol Chem. 2001;276:14299–14307. doi: 10.1074/jbc.M005363200. [DOI] [PubMed] [Google Scholar]
- Ou XM, Chen K, Shih JC. Dual functions of transcription factors, transforming growth factor-beta-inducible early gene (TIEG)2 and Sp3, are mediated by CACCC element and Sp1 sites of human monoamine oxidase (MAO) B gene. J Biol Chem. 2004;279:21021–21028. doi: 10.1074/jbc.M312638200. [DOI] [PubMed] [Google Scholar]
- Ou XM, Lu D, Johnson C, Chen K, Youdim MB, Rajkowska G, Shih JC. Glyceraldehyde-3-phosphate dehydrogenase-mono-amine oxidase B-mediated cell death-induced by ethanol is prevented by rasagiline and 1-R-aminoindan. Neurotox Res. 2009a;16:148–159. doi: 10.1007/s12640-009-9064-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou XM, Stockmeier CA, Meltzer HY, Overholser JC, Jurjus GJ, Dieter L, Chen K, Lu D, Johnson C, Youdim MB, Austin MC, Luo J, Sawa A, May W, Shih JC. A novel role for glyceraldehyde-3-phosphate dehydrogenase and monoamine oxidase B cascade in ethanol-induced cellular damage. Biol Psychiatry. 2009b Dec 18; doi: 10.1016/j.biopsych.2009.10.032. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul CA, Au R, Fredman L, Massaro JM, Seshadri S, Decarli C, Wolf PA. Association of alcohol consumption with brain volume in the Framingham study. Arch Neurol. 2008;65:1363–1367. doi: 10.1001/archneur.65.10.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Bell RL, Sullivan EV. Development and resolution of brain lesions caused by pyrithiamine- and dietary-induced thiamine deficiency and alcohol exposure in the alcohol-preferring rat: a longitudinal magnetic resonance imaging and spectroscopy study. Neuropsychopharmacology. 2007;32:1159–1177. doi: 10.1038/sj.npp.1301107. [DOI] [PubMed] [Google Scholar]
- Prendergast MA. Do women possess a unique susceptibility to the neurotoxic effects of alcohol? J Am Med Womens Assoc. 2004;59:225–227. [PubMed] [Google Scholar]
- Prendergast MA, Little HJ. Adolescence, glucocorticoids and alcohol. Pharmacol Biochem Behav. 2007;86:234–245. doi: 10.1016/j.pbb.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Rodgers B, Korten AE, Jorm AF, Christensen H, Henderson S, Jacomb PA. Risk factors for depression and anxiety in abstainers, moderate drinkers and heavy drinkers. Addiction. 2000;95:1833–1845. doi: 10.1046/j.1360-0443.2000.9512183312.x. [DOI] [PubMed] [Google Scholar]
- Sen N, Hara MR, Kornberg MD, Cascio MB, Bae BI, Shahani N, Thomas B, Dawson TM, Dawson VL, Snyder SH, Sawa A. Nitric oxide-induced nuclear GAPDH activates p300/CBP and mediates apoptosis. Nat Cell Biol. 2008;10:866–873. doi: 10.1038/ncb1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song CZ, Gavriilidis G, Asano H, Stamatoyannopoulos G. Functional study of transcription factor KLF11 by targeted gene inactivation. Blood Cells Mol Dis. 2005;34:53–59. doi: 10.1016/j.bcmd.2004.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller MB, McBride WJ, Gatto GJ, Lumeng L, Li TK. Intragastric self-infusion of ethanol by ethanol-preferring and -nonpreferring lines of rats. Science. 1984;225:78–80. doi: 10.1126/science.6539502. [DOI] [PubMed] [Google Scholar]
- Wang Z, Spittau B, Behrendt M, Peters B, Krieglstein K. Human TIEG2/KLF11 induces oligodendroglial cell death by downregulation of Bcl-X(L) expression. J Neural Transm. 2007;114:867–875. doi: 10.1007/s00702-007-0635-6. [DOI] [PubMed] [Google Scholar]
- Yao Z, Zhang J, Dai J, Keller ET. Ethanol activates NFkappaB DNA binding and p56lck protein tyrosine kinase in human osteoblast-like cells. Bone. 2001;28:167–173. doi: 10.1016/s8756-3282(00)00425-7. [DOI] [PubMed] [Google Scholar]
- Youdim MB, Lavie L. Selective MAO-A and B inhibitors, radical scavengers and nitric oxide synthase inhibitors in Parkinson’s disease. Life Sci. 1994;55:2077–2082. doi: 10.1016/0024-3205(94)00388-2. [DOI] [PubMed] [Google Scholar]
- Youdim MB, Fridkin M, Zheng H. Novel bifunctional drugs targeting monoamine oxidase inhibition and iron chelation as an approach to neuroprotection in Parkinson’s disease and other neurodegenerative diseases. J Neural Transm. 2004;111:1455–1471. doi: 10.1007/s00702-004-0143-x. [DOI] [PubMed] [Google Scholar]