Abstract
The neural crest is a transient population of migratory cells that differentiates to form a variety of cell types in the vertebrate embryo, including melanocytes, the craniofacial skeleton, and portions of the peripheral nervous system. These cells initially exist as adherent epithelial cells in the dorsal aspect of the neural tube and only later become migratory after an epithelial-to-mesenchymal transition (EMT). Snail2 plays a critical role in mediating chick neural crest cell EMT and migration due to its expression by both premigratory and migratory cranial neural crest cells and its ability to down-regulate intercellular junctions components. In an attempt to delineate the role of cellular junction components in the neural crest, we have identified the adherens junction molecule neural alpha-catenin (αN-catenin) as a Snail2 target gene whose repression is critical for chick neural crest cell migration. Knock-down and overexpression of αN-catenin enhances and inhibits neural crest cell migration, respectively. Furthermore, our results reveal that αN-catenin regulates the appropriate movement of neural crest cells away from the neural tube into the embryo. Collectively, our data point to a novel function of an adherens junction protein in facilitating the proper migration of neural crest cells during the development of the vertebrate embryo.
Keywords: Neural crest, αN-catenin, EMT, delamination, migration, adherens junctions, chick embryo
Introduction
The neural crest, a population of migratory cells derived from the future central nervous system, gives rise to a diverse range of cell types in the developing vertebrate embryo (Le Douarin and Kalcheim, 1999). Initially existing as adherent epithelial cells in the dorsal neural tube (the premigratory neural crest), these cells undergo an epithelial-to-mesenchymal transition (EMT), characterized by the loss of intercellular contacts and the generation of motile, mesenchymal neural crest cells (Hay, 1995). The acquisition of motility by premigratory neural crest cells is crucial to permit these cells to leave the neural tube and migrate to their final destinations, allowing for the formation of appropriate structures and, ultimately, a functioning vertebrate embryo.
EMTs have been documented to characterize a myriad of normal (gastrulation, neural crest emigration) and aberrant (cancer metastasis) processes (Leptin et al., 1992; Thiery, 2002; Hemavathy et al., 2004). The molecular mechanisms underlying EMTs have been elucidated, in part, both in the developing embryo and during tumorigenesis (Hemavathy et al., 2000; Nieto, 2002; Barrallo-Gimeno and Nieto, 2005; De Craene et al., 2005; Perez-Mancera et al., 2005; Taneyhill et al., 2007; Wu and McClay, 2007; Sauka-Spengler and Bronner-Fraser, 2008; Yang and Weinberg, 2008; Ahlstrom and Erickson, 2009; Acloque et al., 2009), and involve the rearrangement of cell adhesion factors and cytoskeletal elements, including the regulation of Cadherin family members, in order to facilitate cell motility. These studies have shown that repression of cadherins, such as the epithelial cadherin (E-cadherin) and cadherin6B, is critical for EMTs. Cadherins are the transmembrane structural component of the apically localized adherens junctions that are found in polarized epithelial cells, including premigratory neural crest cells, and these molecules function to mediate calcium-dependent adhesive interactions among neighboring cells (Hyafil et al., 1981; Volk and Geiger, 1984; Shirayoshi et al., 1986; Gumbiner, 2005; Taneyhill, 2008). Down-regulation of cadherins occurs through the direct interaction of Snail proteins with Snail binding sites (E boxes) (Nieto, 2002) in a palindromic position in the promoter region of E-cadherin and within the cadherin6B regulatory region (Giroldi et al., 1997; Cano et al., 2000; Bolos et al., 2003; Come et al., 2004; Taneyhill et al., 2007). Snail proteins comprise a large family of transcriptional repressors known to play key roles during EMTs to promote the formation of motile cells (Nieto, 2002; Barrallo-Gimeno and Nieto, 2005). With respect to chick neural crest cell development, Snail2 is expressed by premigratory and migratory cranial neural crest cells, and its function appears to be necessary for the emigration of neural crest cells (Nieto et al., 1994).
Given the importance of down-regulating cell-cell junctions in order to generate various migratory cell types, we investigated whether additional adherens junctions components might play an important role in regulating neural crest cell migration. To this end, we have identified neural α-catenin (αN-catenin) as a critical player during chick neural crest cell migration. α-catenins are cytoplasmic proteins that function by interacting with the actin cytoskeleton and with β-catenin at adherens junctions (Gumbiner, 2005; Pokutta et al., 2008). In addition, loss of αN-catenin has been shown to have deleterious effects on embryonic development in several model systems (Kofron et al., 1997; Torres et al., 1997; Oda et al., 1998), including a disruption in the motility of various cell types in the mouse (Magie et al., 2002; Togashi et al., 2002; Uemura and Takeichi, 2006). Perturbation of αN-catenin in the chick reveals a previously undocumented role for this gene in the neural crest, particularly with respect to the appropriate migration of neural crest cells. Furthermore, we demonstrate that αN-catenin is a Snail2 target gene whose repression occurs through the interaction of Snail2 with an E box in the αN-catenin regulatory region. Collectively, our studies indicate that the down-regulation of αN-catenin, mediated in part by Snail2 repression, is important for the migration of neural crest cells in the developing vertebrate embryo.
Materials and Methods
Chicken embryo culture
Fertilized chicken eggs were obtained from Hy-Line North America, L.L.C. (Elizabethtown, PA) and incubated at 38°C in humidified incubators (EggCartons.com, Manchaug, MA). Embryos were staged according to the number of pairs of somites (somite stage (ss)).
Design and electroporation of αN-catenin antisense morpholino
A 3′ lissamine-labeled antisense αN-catenin morpholino (MO), 5′-CGTTGCAGAAGTCATACTCCCTCA-3′, was designed to target the αN-catenin mRNA according to the manufacturer's criteria (GeneTools, L.L.C.). A 5 base pair mismatch lissamine-labeled antisense αN-catenin control MO 5′-CcTTcCAGAAcTCATAgTCCgTCA-3′ (mutated bases are in lower case; GeneTools, L.L.C.) was used that does not target αN-catenin mRNA. The sequences for the Snail2 and Snail2 control MOs are available in (Taneyhill et al., 2007). MOs were introduced into the developing chick embryo using a modified version of the electroporation technique (Itasaki et al., 1999). Briefly, MOs were injected at a final total concentration of 500 μM (Taneyhill et al., 2007) into the neural tube lumen at the desired axial level and 2, 25 volt, 30 mSec pulses were applied across the embryo.
Overexpression of αN-catenin in vivo
The full-length αN-catenin cDNA was directionally cloned into the pCIG chick expression construct by PCR using a chick cDNA library (7-12ss) as the template in order to produce pCIG-αN-catenin, and sequenced to confirm accuracy. The control (pCIG) or pCIG-αN-catenin expression construct was introduced into the embryo at a concentration of 3 μg/μl, as described above for the MO electroporations.
Whole-mount in situ hybridization
Whole-mount in situ hybridization was performed as described previously in (Wilkinson, 1992; Taneyhill et al., 2007; Coles et al., 2007). Stained embryos were imaged in 70% glycerol using a camera mounted on a Zeiss SteREO Discovery.V8 microscope. Transverse-sections were obtained by cryostat-sectioning gelatin-embedded embryos at 10 or 14 μm. Images were captured using a Zeiss AxioObserver.Z1 microscope and processed using Adobe Photoshop 9.0 (Adobe Systems).
Chick embryo and explant culture
The developing neural crest cell population was electroporated with the appropriate MO or overexpression construct, and electroporated embryos were re-incubated for 45 minutes or 4 hours, respectively, prior to dissection of premigratory neural crest tissue, explantation, incubation for 2.5 hours, and processing as described previously in (Taneyhill et al., 2007; Coles et al., 2007).
Immunohistochemistry
Immunohistochemical detection of αN-catenin (Developmental Studies Hybridoma Bank (DSHB), clone NCAT2; 1:200) was performed in whole-mount or on transverse sections following methanol fixation of embryos and cryostat-sectioning (modified from (Nakagawa and Takeichi, 1998; Coles et al., 2007)). Immunohistochemical detection of Cadherin6B (DSHB, clone CCD6B-1, 1:100) and Cadherin7 (DSHB, clone CCD7-1, 1:100) was performed as described previously (Nakagawa and Takeichi, 1998; Coles et al., 2007). Immunohistochemical detection of phospho-histone H3 (Millipore Ser10; 1:500) and HNK-1 (1:100) were performed as in (Coles et al., 2007). Imaging of embryos and sections, and quantification of apoptosis in sections, was carried out as described in (Coles et al., 2007). Sections were stained with 4′,6-diamidino-2-phenylindole (DAPI) to mark cell nuclei. Samples were analyzed with a Zeiss AxioObserver.Z1 microscope.
Cell counts
Cell counts of Sox10- or Snail2-expressing cells in sections were performed as described previously (Coles et al., 2007). Briefly, embryos electroporated with control MO, αN-catenin MO, pCIG or pCIG-αN-catenin and hybridized with Sox10 or Snail2 antisense riboprobes were imaged and subsequently cryostat-sectioned at 14 μm. Sections were stained with DAPI to enable the identification of individual nuclei and mounted for imaging. In midbrain regions where neural crest cell migration had commenced, 7-10 serial images were captured for at least 3 embryos that had MO or GFP localized to the dorsal neural tube. Every DAPI positive nuclei that was surrounded by cytoplasmic Sox10 or Snail2 staining in the migratory streams on both the electroporated and non-electroporated side was counted and recorded. The fold differences were averaged over the number of sections in which cells were counted, and the standard error of the mean was calculated and compared for embryos electroporated with either control MO, αN-catenin MO, pCIG or pCIG-αN-catenin. Significance of results was established using the unpaired Student's t test. In vitro explants were fixed and double-stained with DAPI and phalloidin according to the manufacturer's instructions (Molecular Probes), or DAPI-stained and immunostained with an anti-vimentin antibody (DSHB, clone 40E-C; 1:50) (Coles et al., 2007). All cell counts and statistics were conducted as described previously in (Taneyhill et al., 2007; Coles et al., 2007). Briefly, emigrating/migrating neural crest cells that were double-positive for phalloidin (or vimentin) and MO (or GFP) were counted and divided by the total number of emigrating/migrating neural crest cells that were phalloidin- (or vimentin-) positive. Results are presented as the standard error of the mean, with statistical significance established using the unpaired Student's t test.
RNA and cDNA preparation
Total RNA was isolated using the RNAqueous Total RNA Isolation Kit (Ambion), and cDNA was synthesized using random hexamers and the Superscript II RT-PCR System (Invitrogen) according to the manufacturer's instructions.
Quantitative Polymerase Chain Reaction
Quantitative polymerase chain reaction (QPCR) was performed using a SYBR Green assay (Applied Biosystems) as described in (Taneyhill and Bronner-Fraser, 2005; Taneyhill et al., 2007). Data analysis was carried out as described in (Taneyhill et al., 2007). Primer sequences for E boxes used in chromatin immunoprecipitation reactions are listed below:
E box 1_Forward: 5′-TCTGTTAATGCACAACTGAGGAA-3′; E box 1_Reverse: 5′-TGCTGATGGGTGTTTCAGAT-3′; E box 2_Forward: 5′-TTGGTGGGAACATTCAGAAG-3′; E box 2_Reverse: 5′-CTCAAACAAAGCCCTGTGG-3′; E box 3_Forward: 5′-TGGCTGTAGATAAGGAAAAGCA-3′; E box 3_Reverse: 5′-TGAGAGAGAAGAGCTATTCCACAA-3′; E box 4_Forward: 5′-TCTGAAGTGGCCTGGTATGA-3′; E box 4_Reverse: 5′-TGTGGCTCCGTGTTGACTAT-3′; E box 5_Forward: 5′-TGCAACATTCAAAACAACTTGA-3′; E box 5_Reverse: 5′-AAAAGTGACAGCGTTTCACAA-3′; E box 6_Forward: 5′-AGTCGCAGCACAGAAATGAG-3′; E box 6_Reverse: 5′-ATTGTTTTCCTGCACAATGG-3′; E box 7_ Forward: 5′-CTTTCTCCAGCTAAGCAATCC-3′; E box 7_Reverse: 5′-AAGTTACCTGCACAACTGCAA-3′; E box 8_Forward: 5′-GCTCTTGATTTTTGTTCACTTAAG -3′; E box 8_Reverse: 5′-CTGAAAAATCTGATAAAAATAAATGG-3′; 3′UTR control region_Forward_2846: 5′-GCGTTCTCTTCCAATGTTCA-3′; 3′UTR control region_Reverse_3112: 5′-GCAAATTCTTTGGCCAATTT-3′.
Chicken αN-catenin sequence identification and isolation
The chick αN-catenin genomic locus was identified using Ensembl (www.ensembl.org) by searching with the complete chick αN-catenin cDNA sequence (accession number NM_205136). Sequence reads were assembled and analyzed using DNAStar™. Genomic sequence was confirmed by PCR amplification and sequencing of regions subcloned from two independent BAC clones, CH261-28H21 and CH261-30L22, using Taq DNA polymerase (Roche) and appropriate primers (IDT). Specific E box regions analyzed by chromatin immunoprecipitation are delineated in Figure 6B. Some regions contained multiple E boxes (with the exception of E box region 1 and 2), and QPCR primers were designed accordingly to amplify the entire region in these cases in order to detect potential association of Snail2 to them.
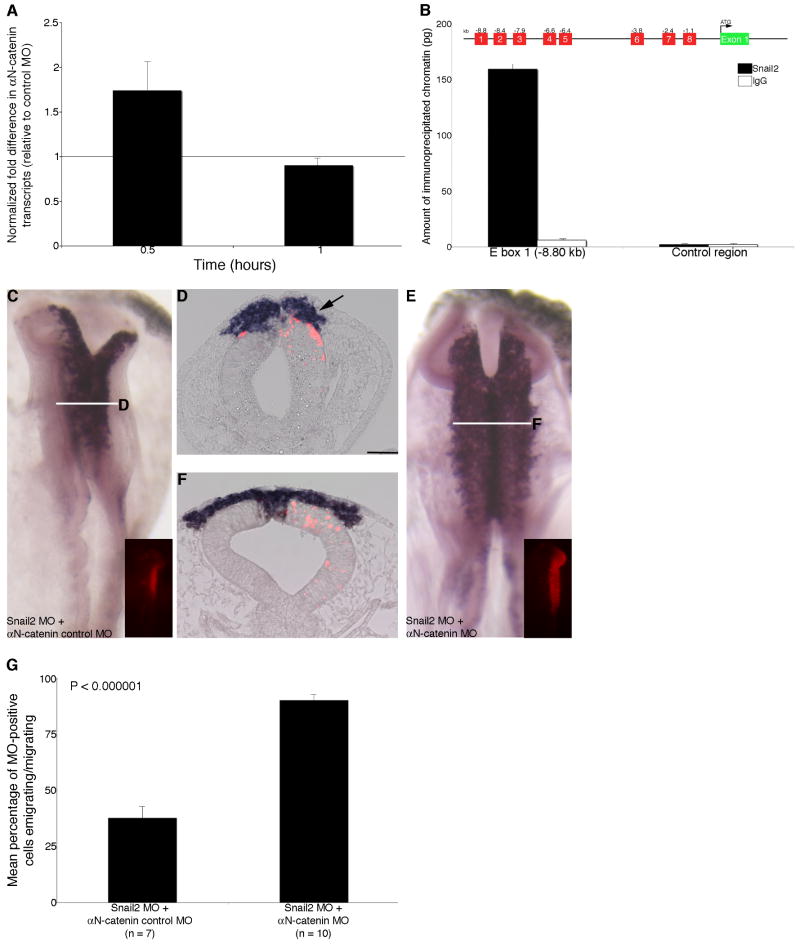
Figure 6. αN-catenin expression is regulated in the chick midbrain premigratory neural crest by Snail2.
(A) Up-regulation of αN-catenin following Snail2 depletion in a QPCR time course experiment. (B) ChIP experiments identify the association of Snail2, but not an IgG control antibody, with E box 1 in the αN-catenin regulatory region. No association of Snail2 or IgG to distal sequences lacking E box motifs in the αN-catenin 3′UTR (control) is observed, and no significant association to other E box motifs was detected for either antibody. (C,E) Whole-mount in situ hybridization for Sox10 followed by indicated transverse sections (D,F) after 8 hour treatment with Snail2 MO and αN-catenin control MO (C,D) or Snail2 MO and αN-catenin MO (E,F). Arrow in (D) points to a decrease in the size of the Sox10-positive migratory neural crest cell domain, which is restored to “wild-type” levels upon loss of both Snail2 and αN-catenin (E,F). (G) Quantification of data obtained from explanted premigratory neural crest tissue taken from embryos electroporated with Snail2 MO and αN-catenin control MO or Snail2 MO and αN-catenin MO following fixation and staining with phalloidin. Rescue of neural crest cell migration occurs upon depletion of both Snail2 and αN-catenin. Results are reported as the standard error of the mean, with statistical significance established using an unpaired Student's t test (p < 0.000001). Scale bar in (D) is 50 μm and applicable to all whole-mount and section images. MO, red; kb, kilobases; UTR, untranslated region.
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) of Snail2 protein associated with the αN-catenin regulatory region was carried out as described in (Taneyhill et al., 2007; Taneyhill and Adams, 2008). Briefly, premigratory neural crest/dorsal neural fold tissue was isolated from the midbrain region of chick embryos using tungsten needles. Tissue was fixed for 10 minutes in 4% paraformaldehyde, lysed, and then sonicated to achieve a chromatin size of 200-1000 base pairs. Equivalent amounts of chromatin were used in two different immunoprecipitations: an anti-mouse IgG control antibody (5 μg; Santa Cruz Biotechnology) and an anti-Snail2 antibody (8 μg; clone 62.1E6 concentrate, DSHB). Antibody:protein:chromatin complexes were then captured using protein A-sepharose beads. After cross-link reversal and proteinase K treatment, bound chromatin was precipitated and used for QPCR to detect association of Snail2 to E boxes and to the αN-catenin 3′UTR as a negative control. Experiments were repeated at least three times with triplicate replicates in each. Because the amount of chromatin obtained from tissue prior to immunoprecipitation differs between experiments, the results are presented as a representative ChIP experiment, with the reported standard deviations, as in (Taneyhill et al., 2007).
Results
αN-catenin is distributed throughout the apical region of the neural tube but is absent from newly migrating neural crest cells
Given the critical role of Cadherin6B in modulating neural crest cell migration (Coles et al., 2007), we chose to examine other adherens junctions components for a possible function in the neural crest. To this end, we determined the spatio-temporal expression pattern of αN-catenin in the chick midbrain through a series of whole-mount in situ hybridization and immunostaining experiments for mRNA and protein, respectively. Whole-mount in situ hybridization for αN-catenin reveals transcripts distributed throughout the neural tube of the embryo prior to neural crest cell migration (Fig. 1A,C,E,G), commencing from at least the 4 somite stage (ss). This observation was corroborated by examining transverse sections of embryos after whole-mount in situ hybridization (Fig. 1B,D,F,H,I). During neural crest cell migration at the 7ss, αN-catenin transcripts are absent, however, from newly migratory neural crest cells (Fig. 1H,I, arrowheads). αN-catenin protein is observed apically in a discrete pattern along the lumenal side of the neural tube (Fig. 1J,L,M,O,P,R,S,U), a distribution pattern indicative of a protein associated with adherens junctions (Niessen and Gottardi, 2008), with somewhat diminished protein levels dorsally at the 7ss (Fig. 1S, arrow). In keeping with our in situ hybridization results, αN-catenin protein is absent from newly migratory neural crest cells (Fig. 1S,V, arrowheads). αN-catenin antibody staining is noted in the ectoderm in the absence of any primary (αN-catenin) antibody (Fig. 1Y), indicating that this is non-specific or background staining. These data suggest that αN-catenin possesses the proper spatio-temporal distribution to play a potential role in the developing neural crest cell population.
Figure 1. αN-catenin transcripts and protein distribution in the chick embryo midbrain.
(A,C,E,G) Whole-mount in situ hybridization for αN-catenin at various stages of development, with indicated transverse sections (B,D,F,H) for whole-mounts, respectively. (I) Higher magnification image of the dorsal neural tube region found in (H). In (H) and (I), neural tube cells express αN-catenin but newly migratory neural crest cells lack αN-catenin (arrowheads). (J,M,P,S) Immunohistochemistry for αN-catenin protein (green) on transverse sections taken through the chick embryo midbrain, with representative sections shown for specific developmental stages. (K,N,Q,T) DAPI staining of (J,M,P,S) to mark cell nuclei. (L,O,R,U) Merge αN-catenin and DAPI images. (V-X) Magnified field of dorsal neural tube region found at 7ss in (S-U). Arrow (S) and arrowheads (S,V) indicate diminished αN-catenin protein in the apico-dorsal region of the neural tube and the absence of αN-catenin protein on newly migratory neural crest cells, respectively. (Y,Z) Negative control immunohistochemistry experiments for αN-catenin in the absence of primary (αN-catenin) antibody (Y) and secondary antibody (Z). Scale bar in (A) is 20 μm and is applicable to all whole-mount images, while individual scale bars (50 μm) are shown for each section. Scale bar in (J) is 50 μm and applicable to images (J-U,Y,Z). Scale bar for (V-X) is 50 μm and is shown in (V). ss, somite stage.
Depletion of αN-catenin enhances neural crest cell migration in vivo
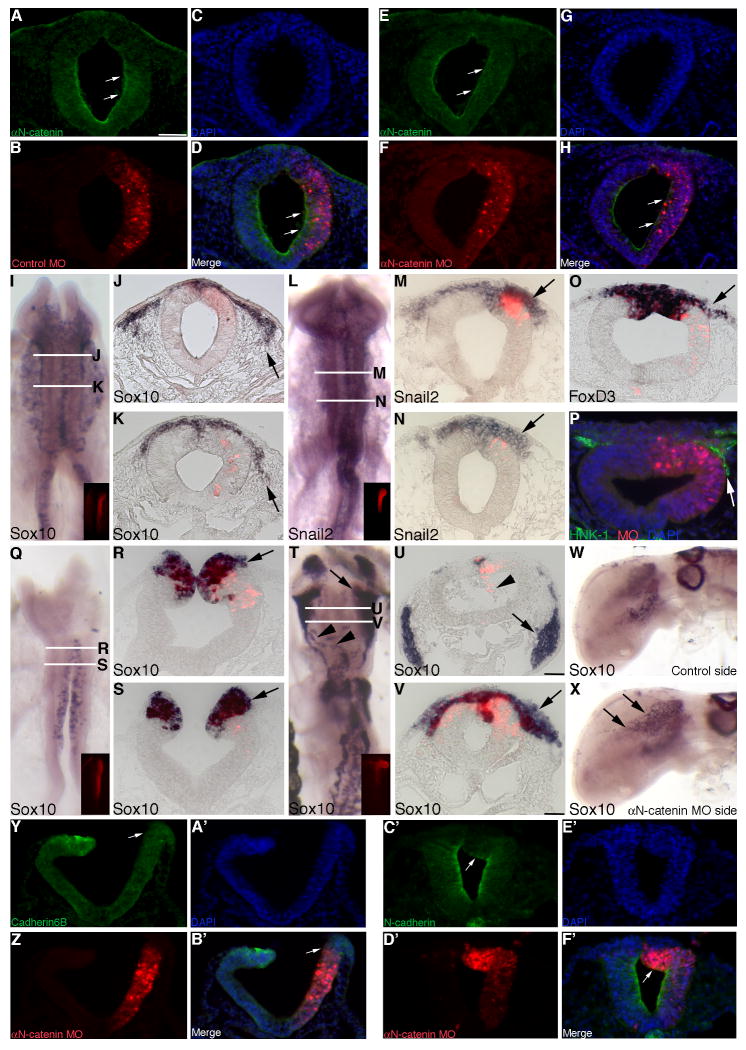
In order to better characterize a potential role for αN-catenin in the neural crest, we designed a morpholino antisense oligonucleotide (MO) to target and knockdown αN-catenin mRNA translation, thus depleting protein levels in the chick premigratory neural crest cell population. Transfection of midbrain neural tube cells using the in ovo electroporation method (Itasaki et al., 1999; Taneyhill et al., 2007; Coles et al., 2007) with a 5 base pair (bp) mismatch αN-catenin control MO (hereafter referred to as control MO) has no effect on αN-catenin protein levels (Fig. 2A,D, arrows), while introduction of αN-catenin MO results in a loss of αN-catenin protein in the apical region of the neural tube of the transfected area after an 8 hour incubation with the MO (Fig. 2E,H, arrows; 9/10 embryos).
Figure 2. Morpholino-mediated depletion of αN-catenin from the developing neural crest cell population of the chick midbrain enhances neural crest cell migration in vivo.
(A-C) Individual channel and (D) merge images of a representative transverse section taken through an embryo electroporated with a 5 bp mismatch αN-catenin control MO (control MO, red) after 8 hours of MO incubation and processing by whole-mount immunohistochemistry for αN-catenin protein (A,D, green). Note the presence of αN-catenin protein throughout the apical region of the neural tube (A,D, arrows). (E-G) Individual channel and (H) merge images of a representative transverse section taken through an embryo electroporated with αN-catenin MO (red) after 8 hours of MO incubation and processing by whole-mount immunohistochemistry for αN-catenin protein (E,H, green). Note the loss of αN-catenin protein throughout the transfected side of the neural tube (E,H, arrows). (I,L) Whole-mount in situ hybridization followed by indicated rostral and caudal transverse sections for Sox10 (J, K) and Snail2 (M,N), respectively, after 8 hour incubation following treatment with αN-catenin MO. Arrows indicate the presence of more Sox10- and Snail2-positive migratory neural crest cells (J,K and M,N, respectively), on the electroporated side of the embryo. (O,P) Representative transverse sections taken from embryos treated with αN-catenin MO for 8 hours followed by FoxD3 whole-mount in situ hybridization (O) or immunohistochemistry for HNK-1 (P, green), respectively. Arrows reveal more migratory neural crest cells on the electroporated side of the embryo. (Q) Whole-mount in situ hybridization followed by indicated rostral and caudal transverse sections (R,S) for Sox10 after 3 hour incubation following treatment with αN-catenin MO. Arrows in (R,S) indicate the presence of more Sox10-positive migratory neural crest cells on the electroporated side of the embryo. (T) Whole-mount in situ hybridization followed by indicated rostral and caudal transverse sections (U,V) for Sox10 after 20 hour incubation following treatment with αN-catenin MO. Arrows in (T-V) and arrowheads in (T) point to an increase in the number of Sox10-positive migratory neural crest cells and to aberrant pathways being traversed by Sox10-positive cells, respectively. Arrowhead in (U) shows αN-catenin MO-positive cells in the neural tube lumen. (W,X) Side views of an embryo processed for whole-mount in situ hybridization for Sox10 after 20 hour incubation following treatment with αN-catenin MO. Arrows in (X) reveal more Sox10-positive cells entering the head region on the electroporated side of the embryo that are not observed on the contralateral control side (W). (Y-A′) Individual channel and (B′) merge images of a representative transverse section taken through an embryo electroporated with αN-catenin MO (red) after 4 hours of MO incubation and processing by whole-mount immunohistochemistry for Cadherin6B protein (Y,B′, green). Note the loss of Cadherin6B protein from the dorsal region of the neural tube on the transfected side (Y,B′, arrows). (C′-E′) Individual channel and (F′) merge images of a representative transverse section taken through an embryo electroporated with αN-catenin MO (red) after 4 hours of MO incubation and processing by whole-mount immunohistochemistry for N-cadherin protein (C′,F′, green). Note the decrease in N-cadherin protein in the dorsal region of the neural tube on the transfected side (C′,F′, arrows). In all experiments, the right side of the embryo is electroporated, as indicated by the lissamine (red) fluorescence of the MO in the transverse sections and/or in the inset images of each whole-mount. Scale bar in (A) is 50 μm and applicable to all whole-mount and section images except for that shown in (U) which is also applicable to (V) (scale bar is 50 μm).
Upon electroporation of either αN-catenin or control MO, transfected embryos were re-incubated for 8 hours and then processed for whole-mount in situ hybridization for Sox10, Snail2, and FoxD3, or immunostained using an antibody to HNK-1, to assess for changes in the premigratory or migratory neural crest cell populations. Treatment with control MO has no effect on Sox10 (Supp.Fig. 1A,B; 8/8 embryos), Snail2 (Supp.Fig. 1C,D; 10/11 embryos), FoxD3 (Supp.Fig. 1E; 4/4 embryos), and HNK-1 immunostaining (Supp.Fig. 1F; 5/5 embryos). Depletion of αN-catenin from the premigratory neural crest, however, enhances neural crest cell migration, as determined by the presence of more Sox10-positive migratory neural crest cells on the transfected side (right) of the embryo, compared to the contralateral control side (and to control embryos), throughout multiple axial levels examined (Fig. 2I-K, arrows; 11/11 embryos). In addition, we determined effects of αN-catenin depletion on the expression of Snail2, a molecular marker of premigratory and migratory cranial neural crest cells. We observe an increase in the Snail2-positive migratory neural crest cell domain on the transfected side (Fig. 2L-N, arrows; 12/14 embryos) compared to the contralateral control side, at all noted axial levels. This same observation is made for the premigratory and migratory neural crest cell marker FoxD3 (Fig. 2O, arrow; 6/7 embryos), as well as the migratory neural crest cell marker HNK-1 (Fig. 2P, arrow; 5/5 embryos).
In order to quantify effects on neural crest cell migration, we counted the number of Sox10- and Snail2-positive migratory neural crest cells on both the electroporated and contralateral control sides in at least 7 serial sections obtained from a minimum of 3 embryos treated with αN-catenin or control MO. Our data indicate that depletion of αN-catenin results in a statistically significant 1.5- and 1.4-fold increase, respectively, in the number of Sox10- and Snail2-positive cells compared to the contralateral control side (Sox10-positive cells: control side = 84 +/- 6, αN-catenin MO side = 129 +/- 9; Snail2-positive cells: control side = 90 +/- 6, αN-catenin MO side = 123 +/- 9), with a Student's t test of p < 0.000001 for Sox10 and p < 0.00001 for Snail2. No statistically significant difference is observed in the presence of control MO compared to the contralateral control side (Sox10-positive cells: control side = 48 +/- 3; control MO side = 48 +/- 4, fold difference of 1.0; Snail2-positive cells: control side = 111 +/- 6, control MO side = 108 +/- 7, fold difference of 0.98). We eliminated the possibility that αN-catenin depletion was altering neural crest cell migration through changes in the premigratory neural crest cell population by noting no apparent difference in Snail2 and FoxD3 expression in the dorsal neural tube of embryos treated with αN-catenin or control MO (Fig. 2M-O). Taken together, these data suggest that reduced levels of αN-catenin augment neural crest cell migration.
In order to ensure that the increase in migratory neural crest cells observed upon αN-catenin depletion was not due to changes in cell proliferation in the neural tube or migratory neural crest cell population, we performed phospho-histone H3 (PH3) immunostaining on transverse sections taken from embryos treated with αN-catenin or control MO for 8 hours. We observe no striking difference in cell proliferation in the presence of either MO, compared to each other and the contralateral control side of the embryo (Supp. Fig. 2A,B, arrowheads). In addition, no difference in the amount of cell death is noted by TUNEL assay upon treatment with either MO (Supp.Fig. 2C,D, arrowheads). Furthermore, examination of the Sox10 and Snail2 in situ hybridization brightfield images and immunohistochemical images for HNK-1 (and Cadherin7, data not shown) at a higher magnification reveals no changes in neural crest cell size and/or architecture. As such, our results indicate that αN-catenin depletion enhances neural crest cell migration in vivo in the absence of any alterations in the premigratory neural crest population, cell proliferation, cell death or cell size/architecture.
We also assessed effects of αN-catenin depletion on neural crest cell migration at both early (3 hours) and later (20 hours) time points post-electroporation. Depletion of αN-catenin at both of these time points significantly reduces the amount of αN-catenin protein in the neural tube (Supp.Fig.3A,D (3 hrs), 9/10 embryos; Fig. 3E,H (20 hrs), 9/10 embryos). MO-mediated knock-down of αN-catenin for 3 hours results in the presence of increased numbers of Sox10-positive migratory neural crest cells at stages of embryonic development during which neural crest emigration is just initiating (Fig. 2Q-S, arrows). Furthermore, long-term depletion of αN-catenin results in the presence of more Sox10-positive migratory neural crest cells in the head region of the embryo, an observation not noted on the contralateral control side or in control embryos (Fig. 2T-V,X, arrows). This is not due to any changes in cell proliferation, as assessed by PH3 immunostaining, or in neural crest cell size/architecture (data not shown). Later in development, we often observe neural crest cells aberrantly migrating to ectopic locations in the embryo (Fig. 2T, arrowheads) and MO-positive cells in the neural tube lumen (Fig. 2U, arrowhead). These phenotypes are not found in control MO-treated embryos (data not shown). Moreover, this increase in neural crest cell migration may be due to the loss of Cadherin6B- and/or N-cadherin-type adherens junctions within the neural tube, as depletion of αN-catenin results in diminished Cadherin6B (Fig. 2Y,B′, arrows) and N-cadherin (Fig. 2C′,F′, arrows) levels in dorsal neural tube cells in 7/9 embryos and 8/12 embryos examined, respectively. These phenotypes are not observed on the contralateral control side of the embryo (Fig. 2Y,B′,C′,F′) nor in control-treated embryos (data not shown). Thus, depletion of αN-catenin promotes an increase in the number of migratory neural crest cells and, later in development, the inappropriate migration of neural crest cells that may be due, in part, to the loss of adherens junctions proteins in the premigratory neural crest cell population of the developing embryo.
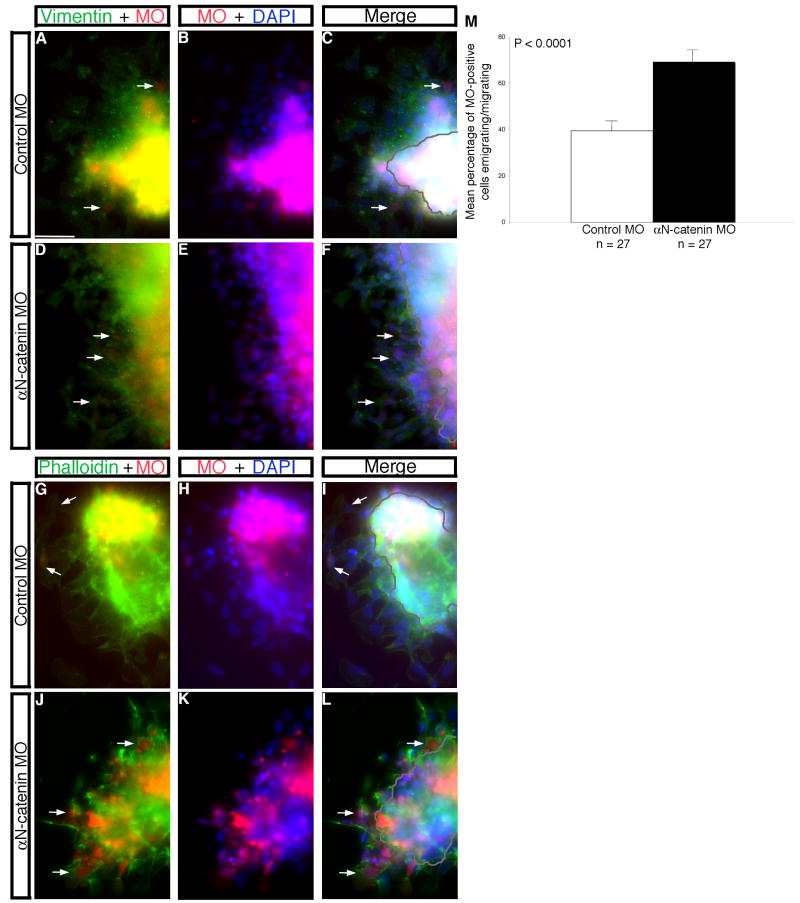
Figure 3. Morpholino-mediated depletion of αN-catenin from the developing neural crest cell population of the chick midbrain enhances neural crest cell migration in vitro.
(A,B; D,E; G,H; J,K) Combined channel and (C,F,I,L) merge images of explanted premigratory neural crest tissue taken from embryos electroporated with control (A-C, G-I) or αN-catenin (D-F,J-L) MO. Explants were fixed and processed for vimentin immunostaining (A-F, green) or stained with phalloidin (G-L, green). Arrows in (D,F,J,L) reveal more vimentin- or phalloidin-positive migratory neural crest cells, respectively, upon knock-down of αN-catenin compared to control MO-treated explants (A,C,G,I). (M) Quantification of explant data demonstrates a 1.7-fold increase in the number of emigrating/migrating neural crest cells in the presence of αN-catenin MO compared to the control. Results are reported as the standard error of the mean, with statistical significance established using an unpaired Student's t test (p < 0.0001). Scale bar in (A) is 20 μm and applicable to all explant images. MO, morpholino (red); DAPI, blue.
Depletion of αN-catenin enhances neural crest cell migration in vitro
To quantitatively demonstrate effects of αN-catenin depletion on neural crest cell migration, we used an in vitro explantation assay (Shoval et al., 2007; Taneyhill et al., 2007; Coles et al., 2007). Effects on EMT were assessed with either an antibody to vimentin to label intermediate filaments (Fig. 3A,C,D,F) or phalloidin stain to mark the actin cytoskeleton (Fig. 3G,I,J,L), as well as by counting the number of emigrating/migrating neural crest cells (Fig. 3C,F,I,L, arrows; Fig. 3M). Our results demonstrate a statistically significant approximate 2-fold increase in the number of neural crest cells emigrating/migrating upon αN-catenin knockdown compared to control (Fig. 3M). Furthermore, we quantified the distance traveled by migratory neural crest cells in these explantation experiments, and we find no significant change in the distance migrated upon depletion of αN-catenin compared to control (data not shown). As such, neural crest cells appear to be migrating normally in this in vitro assay, in the presence or absence of αN-catenin, with no subsequent change in cell migration rates observed. Taken together with our in vivo data, these in vitro results implicate αN-catenin in regulating chick neural crest cell migration at the level of the numbers of migratory neural crest cells generated in the embryo.
Overexpression of αN-catenin disrupts neural crest cell migration in vivo
To corroborate our knock-down data, we cloned the full-length αN-catenin cDNA into a chick expression construct (pCIG) containing a bi-cistronic GFP cassette to mark transfected cells, and introduced the construct into the premigratory neural crest cell population of the chick midbrain by electroporation. Overexpression of αN-catenin in this region increases the amount of αN-catenin protein in the neural tube after 8 hours of incubation with the overexpression construct, as indicated by the presence of αN-catenin protein laterally in neural tube cells outside of the apical region where αN-catenin is normally observed (Fig. 4E,H, arrows). Furthermore, αN-catenin overexpression results in the migration of cells into the neural tube lumen (Fig. 4E,H, arrowheads and asterisks). These phenotypes are observed in 10/12 embryos, are similar to that observed upon overexpression of other Cadherins (Nakagawa and Takeichi, 1998; Coles et al., 2007), and are not observed with the control pCIG construct (Fig. 4A,D).
Figure 4. Overexpression of αN-catenin in the developing neural crest cell population of the chick midbrain disrupts neural crest cell migration in vivo.
(A-C) Individual channel and (D) merge images of a representative transverse section taken through an embryo electroporated with the pCIG control construct (B, green) after 8 hours of incubation and subsequent processing by whole-mount immunohistochemistry for αN-catenin protein (red). Note the presence of αN-catenin protein throughout the apical region of the neural tube (A,D, arrows). (E-G) Individual channel and (H) merge images of a representative transverse section taken through an embryo electroporated with the αN-catenin overexpression construct (pCIG-αN-catenin; F, green) after 8 hours of incubation and subsequent processing by whole-mount immunohistochemistry for αN-catenin protein (red). Note the increase in αN-catenin protein observed on the transfected side of the embryonic neural tube (E,H, arrows), as well as the presence of multiple αN-catenin-positive (H, arrowheads) and –negative (asterisks) cells in the neural tube lumen. (I,K) Whole-mount in situ hybridization followed by indicated transverse sections (J,L) for Sox10 and Snail2, respectively, after 8 hour incubation following treatment with pCIG-αN-catenin. Arrows in (J,L) reveal Sox10- and Snail2-positive migratory neural crest cells on the electroporated side of the embryo that have aggregated adjacent to the neural tube lumen just below the ectoderm. Arrowhead in (J) indicates Sox10-positive cells in the neural tube lumen. (M,N) Representative transverse sections taken from embryos treated with pCIG-αN-catenin for 8 hours followed by FoxD3 whole-mount in situ hybridization (M) or immunohistochemistry for HNK-1 (N, red), respectively. Arrows reveal fewer migratory neural crest cells on the electroporated side of the embryo, many of which have clustered near the neural tube. Arrowheads in (N) point to cells double-positive (yellow) for HNK-1 (red) and GFP (green) in the neural tube lumen, while asterisks indicate cells positive only for HNK-1. (O) Whole-mount in situ hybridization followed by indicated transverse section (P) for Sox10 after 20 hour incubation following treatment with pCIG-αN-catenin. Arrowheads in (O,P) point to aggregates of Sox10-positive migratory neural crest cells in the neural tube lumen. Arrows in (P) indicate Sox10-positive migratory neural crest cells on the electroporated side of the embryo that appear to be “lagging behind” their counterparts on the contralateral control side with respect to their migration. (Q-S) Individual channel and (T) merge images of a representative transverse section taken through an embryo electroporated with pCIG-αN-catenin (green) after 8 hours of incubation and processing by whole-mount immunohistochemistry for Cadherin6B protein (Q,T, red). Note the increase in Cadherin6B protein throughout the transfected side of the neural tube (Q,T, arrows), on cells in the neural tube lumen (Q,T, arrowheads), and in some migratory neural crest cells (Q,T, asterisks). (U-W) Individual channel and (X) merge images of a representative transverse section taken through an embryo electroporated with pCIG-αN-catenin (green) after 8 hours of incubation and processing by whole-mount immunohistochemistry for N-cadherin protein (U,X, red). Note the reduction in N-cadherin protein in the dorsal region of the neural tube (U,X, arrows). In all experiments, the right side of the embryo is electroporated, as indicated by the GFP (green) fluorescence in (B,F), in the inset images in (I,K,O), and in sections (N,R,T,V,X). Scale bar in (A) is 50 μm and applicable to all whole-mount and section images except for (P) (scale bar for P is also 50 μm). BF, brightfield.
To assess effects on neural crest cell migration, we electroporated embryos with either the control pCIG or pCIG-αN-catenin construct, re-incubated them for 8 hours, and then processed embryos as described previously for the MO electroporations. Treatment with the pCIG control construct has no effect on Sox10 (Supp.Fig. 4A,B; 12/12 embryos), Snail2 (Supp.Fig. 4C,D; 11/12 embryos), FoxD3 (Supp.Fig. 4E; 4/4 embryos), and HNK-1 staining (Supp.Fig. 4F; 5/5 embryos). In contrast, overexpression of αN-catenin disrupts neural crest cell migration, as evidenced by the aggregation of Sox10- (13/15 embryos) and Snail2-positive (14/15 embryos) migratory neural crest cells on the transfected side (right), compared to the contralateral control side (and to control embryos) (Fig. 4I,J for Sox10, Fig. 4K,L for Snail2; arrows). This altered migratory phenotype is consistently observed upon αN-catenin overexpression, as evidenced by FoxD3 expression (Fig. 4M, arrow; 6/7 embryos) and HNK-1 immunostaining (Fig. 4N, arrow; 5/5 embryos). In addition, we often see cells that stain positive for neural crest molecular markers, such as Sox10 or HNK-1 (Fig. 4J,N, arrowheads), and, in some instances, GFP-negative, HNK-1-positive neural crest cells (Fig. 4N, asterisks), within the neural tube lumen. As with our knock-down experiments, overexpression of αN-catenin leads to no significant differences in 1) Snail2 and FoxD3 expression in the premigratory neural crest (Fig. 4L,M), 2) the amount of cell proliferation (Supp.Fig. 5A,B, arrowheads) or cell death (Supp.Fig. 5C,D, arrowheads) in the neural tube or migratory neural crest cell population, and 3) migratory neural crest cell size and/or architecture (data not shown). It is important to note, however, that some cells observed in the neural tube lumen upon overexpression of αN-catenin eventually become apoptotic, as indicated by TUNEL-positive staining (Supp.Fig. 5D, arrows). Collectively, these results point to a disruption of neural crest cell migration upon overexpression of αN-catenin that is not due to any alterations in the premigratory neural crest cell population, cell death, cell proliferation or cell size/architecture, in keeping with the enhanced migration phenotype observed upon depletion of αN-catenin.
In order to quantify effects on neural crest cell migration, we counted the number of Sox10- and Snail2-positive migratory neural crest cells on both the electroporated and contralateral control sides in at least 7 serial sections obtained from a minimum of 3 embryos treated with pCIG or pCIG-αN-catenin. Our data indicate that overexpression of αN-catenin results in a statistically significant 1.4- and 1.7-fold decrease, respectively, in the number of Sox10- and Snail2-positive migratory neural crest cells compared to the contralateral control side (Sox10-positive cells: control side = 86 +/- 3, pCIG-αN-catenin side = 59 +/- 4; Snail2-positive cells: control side = 144 +/- 8, pCIG-αN-catenin side = 87 +/- 6), with a Student's t test of p < 0.000001 for Sox10 and p < 0.0000001 for Snail2. No statistically significant difference is observed in the presence of pCIG compared to the contralateral control side (Sox10-positive cells: control side = 78+/- 4; pCIG control side = 81 +/- 5, fold difference of 1.0; Snail2-positive cells: control side = 97 +/- 4, pCIG control side = 96 +/- 5, fold difference of 1.0). These data are in good agreement with those obtained in our MO knock-down experiments.
We also assessed effects of αN-catenin overexpression on neural crest cell migration at later time points (20 hours) post-electroporation. Overexpression of αN-catenin leads to the presence of Sox10-positive migratory neural crest cells within the neural tube lumen (Fig. 4O,P, arrowheads). In addition, neural crest cell migration is perturbed in these embryos (Fig. 4P, arrows), as Sox10-positive cells cluster below the ectoderm in a more dorsal location compared to the contralateral control side of the embryo (and to control-treated embryos; data not shown). This disruption in neural crest cell migration may be due to the retention of Cadherin6B-containing adherens junctions within the neural tube proper, as αN-catenin overexpression results in increased Cadherin6B protein in neural tube cells and on those cells entering the neural tube lumen (Fig. 4Q,T, arrows and arrowheads, respectively; 10/12 embryos), as well as in some migratory neural crest cells, where Cad6B is normally not observed (Fig. 4Q,T, asterisks). Conversely, N-cadherin protein levels in the neural tube are reduced dorsally (Fig. 4U,X, arrows; 7/7 embryos). These phenotypes are not observed on the contralateral control side of the embryo (Fig. 2Q,T,U,X) nor in control-treated embryos (data not shown). Thus, overexpression of αN-catenin permits neural crest cell delamination but impairs normal migration of neural crest cells away from the chick neural tube in a manner that may be partially dependent upon the retention of Cadherin6B-containing adherens junctions.
Overexpression of αN-catenin impedes neural crest cell migration in vitro
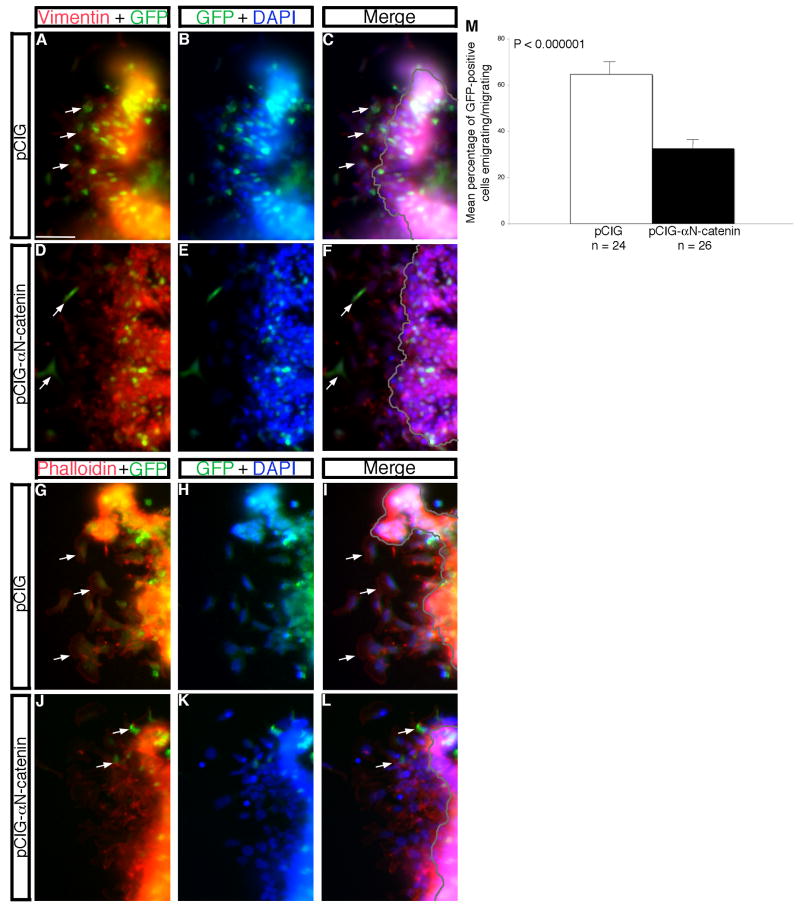
As in our knock-down experiments, we overexpressed αN-catenin (or the control pCIG construct) in the chick midbrain premigratory neural crest cell population and performed in vitro explantation assays to more easily quantify possible effects on neural crest cell migration. Both vimentin and phalloidin staining (Fig. 5) demonstrate a statistically significant two-fold decrease in the number of neural crest cells emigrating/migrating upon overexpression of αN-catenin (Fig. 5D,F,J,L) compared to control, as noted by the presence of more GFP- and vimentin- or phalloidin-positive migratory neural crest cells in control explants (Fig. 5A,C,G,I, arrows, respectively; Fig. 5M). As with our MO knock-down experiments, we quantified the distance traveled by migratory neural crest cells in these explantation experiments and found no significant difference compared to control (data not shown). As such, neural crest cell migratory behavior appears to be unchanged upon overexpression of αN-catenin in this in vitro assay. Thus, our in vivo and in vitro data point to a unique role for αN-catenin in regulating neural crest cell migration in the chick embryo.
Figure 5. Overexpression of αN-catenin in the developing neural crest cell population of the chick midbrain disrupts neural crest cell migration in vitro.
(A,B; D,E; G,H; J,K) Combined channel and (C,F,I,L) merge images of explanted premigratory neural crest tissue taken from embryos electroporated with control pCIG (A-C, G-I) or pCIG-αN-catenin (D-F,J-L). Explants were fixed and processed for vimentin immunostaining (A-F, red) or stained with phalloidin (G-L, red). Arrows in (A,C,G,I) reveal greater numbers of vimentin- or phalloidin-positive migratory neural crest cells, respectively, in pCIG-treated explants compared to that observed in the presence of pCIG-αN-catenin (D,F,J,L). (M) Quantification of explant data demonstrates a 2-fold decrease in the number of emigrating/migrating neural crest cells in the presence of pCIG-αN-catenin compared to pCIG. Results are reported as the standard error of the mean, with statistical significance established using an unpaired Student's t test (p < 0.000001). Scale bar in (A) is 20 μm and applicable to all explant images. GFP, green; DAPI, blue.
Depletion of Snail2 from the chick midbrain premigratory neural crest cell population results in αN-catenin up-regulation
The reported pattern of Snail2 protein distribution in the midbrain region of the chick indicates that Snail2 protein and αN-catenin transcripts possess expression patterns somewhat analogous to that observed for Snail2 and its direct target cadherin6B (Taneyhill et al., 2007). Therefore, we tested if αN-catenin was indeed a target of Snail2 regulation in vivo. To this end, we depleted Snail2 from the chick midbrain premigratory neural crest cell population by electroporation of a Snail2 MO that is capable of knocking-down Snail2 protein levels by 82% (see immunohistochemistry data in (Taneyhill et al, 2007); also our current observations). After electroporation of the Snail2 MO or a 5 bp mismatch Snail2 control MO (from Taneyhill et al., 2007), we dissected out the premigratory neural crest tissue, prepared RNA and cDNA, and assessed αN-catenin transcript levels by quantitative PCR (QPCR) (Fig. 6A). In a time course experiment, αN-catenin is up-regulated as early as 0.5 hour after Snail2 MO treatment, with αN-catenin transcripts returning to control levels after 1 hour (Fig. 6A), a kinetic profile similar to that observed for cadherin6B. This return to control levels after 1 hour is not due to an up-regulation of Snail2 protein levels, as Snail2 remains depleted in our assay (Taneyhill et al., 2007; data not shown). Furthermore, an increase in αN-catenin transcripts is observed upon Snail2 depletion in the premigratory neural crest forming region of the chick trunk (data not shown), suggesting that this regulatory mechanism may be conserved in premigratory neural crest cells found along the anterior-posterior axis of the chick. Thus, our data indicate that αN-catenin may be a bona fide target of Snail2 regulation in the chick embryo.
Snail2 associates with the αN-catenin regulatory region in vivo
Based on our QPCR data and the observed effects on neural crest cell migration upon αN-catenin perturbation, we examined whether Snail2 associates with the αN-catenin regulatory region in vivo. By scanning 10 kilobases (kb) upstream of the αN-catenin start site of translation, we identified 8 regions containing putative Snail2 binding sites (E boxes) (Nieto, 2002) (Fig. 6B). Chromatin immunoprecipitation (ChIP) was performed using chick midbrain premigratory neural crest tissue and an antibody to Snail2, coupled with QPCR to amplify these E box-containing regions (Taneyhill et al., 2007; Taneyhill and Adams, 2008). Our ChIP-QPCR results demonstrate that Snail2 preferentially associates with an E box of the sequence ‘CAACTG’, located in the αN-catenin regulatory region at -8.80 kb upstream of the translational start site (E box 1), with little to no association detected to this region with the mouse IgG negative control antibody (Fig. 6B). No statistically significant interactions with the other remaining E box regions and a region in the αN-catenin 3′UTR (control) that lacks any putative E boxes were observed for either antibody (Fig. 6B). To further test this regulatory relationship, we simultaneously depleted both Snail2 and αN-catenin from the premigratory neural crest cell population of the chick midbrain (Fig. 6C-F). Nieto et al. (1994) originally reported a defect in neural crest cell migration upon loss of Snail2 (Nieto et al., 1994), and we observe a similar phenotype in embryos treated with Snail2 MO (Fig. 6C,D, arrow, 16/20 embryos). This defect can be rescued, however, upon loss of αN-catenin in a Snail2-depleted background (Fig. 6E,F; 14/16 embryos). No phenotypic alterations are observed upon electroporation of αN-catenin control MO with a 5 bp mismatch control Snail2 MO (6/6 embryos; representative wild-type phenotype shown in Supp.Fig. 1A,B), or upon electroporation of the control Snail2 MO with αN-catenin MO (9/10 embryos) (data not shown). In addition, in vitro explant experiments using tissue taken from embryos electroporated with the various combinations of MOs used in the in vivo rescue experiment described above reveal that simultaneous knockdown of both Snail2 and αN-catenin rescues the migration defects associated with knock-down of Snail2 alone, with an approximate 2.5-fold increase in the number of neural crest cells migrating upon depletion of both Snail2 and αN-catenin (Fig. 6G, P < 0.00001). Taken together, our combined results are suggestive of a role for Snail2 in binding and repressing αN-catenin transcription in the chick midbrain premigratory neural crest in order to promote neural crest cell migration.
Discussion
αN-catenin localizes to apical adherens junctions in the neural tube and is absent from newly migratory neural crest cells
Existing in the apical region of cells, adherens junctions are comprised of several proteins, including nectin/afadin and cadherin/catenin complexes, that function to link the cell surface to the cortical actin cytoskeleton (for review, see (Niessen and Gottardi, 2008)). Cadherins interact in trans on neighboring cells in a calcium-dependent manner, and the cytoplasmic tail of cadherins is bound by two catenin proteins, p120-catenin and β-catenin (Gumbiner, 2005). Originally discovered as an E-cadherin-associated protein bridged by β-catenin, α-catenin possesses sequence similarity throughout its protein domain structure to the actin binding protein vinculin (Herrenknecht et al., 1991; Nagafuchi et al., 1991; Gumbiner, 2005) and has been shown to have a half-life of 7 hours (Suzuki et al., 2008).
A novel subtype of α-catenin, neural α-catenin or αN-catenin, was identified in 10-day chick embryonic brain cells and found to confer cadherin-mediated cell-cell adhesion upon transfection into non-adherent PC9 lung carcinoma cells (Hirano et al., 1992). Studies in 2- to 8-day chick embryos, in cultured monolayers of 10-day chick embryonic brain cells, and in mouse revealed αN-catenin protein distribution in the somites and apical region of the neural tube, with later localization observed in the central nervous system (CNS) (Hirano et al., 1992; Hirano and Takeichi, 1994; Uchida et al., 1994). More recent experiments demonstrated binding of monomeric α-catenin to the N-terminal region of β-catenin, whereas α-catenin dimers interact with actin filaments (Pokutta et al., 2008). Furthermore, a report by Cavey et al. describes a two-tiered model by which cell surface E-cadherin complex stabilization in Drosophila is achieved through α-catenin-mediated tethering of E-cadherin complexes to an underlying F-actin network, thus limiting E-cadherin complex mobility and stabilizing the overall epithelium (Cavey et al., 2008).
Our experimental results demonstrate the presence of αN-catenin transcripts and protein in the chick neural tube as early as the 4ss. During stages of neural crest cell migration in the chick midbrain, αN-catenin RNA and protein are maintained in the neural tube but are absent from newly migratory neural crest cells. Prior studies in Xenopus documenting the distribution of α-catenin protein during embryogenesis also noted the presence and absence of α-catenin (possibly αN-catenin due to its localization in neural tissue) in the apical region of the neural tube and in migratory neural crest cells, respectively (Schneider et al., 1993). Collectively, these results suggest that αN-catenin is expressed in the correct spatio-temporal pattern to play a key role in the neural crest.
αN-catenin regulates the appropriate migration of multiple cell types, including the neural crest
To investigate the functional role of αN-catenin in chick neural crest cell development, we employed MO-mediated knock-down of αN-catenin mRNA translation in the midbrain premigratory neural crest cell population. Depletion of αN-catenin augments neural crest cell migration both in vivo and in vitro, as evidenced by a statistically significant increase in the number of migratory neural crest cells. Earlier emigration and migration of neural crest cells are consistently observed at multiple time points after αN-catenin knock-down. Moreover, we observe no alterations in neural crest cell size and/or architecture, nor any increase in the proliferation and migration rates of the migratory neural crest cell population upon perturbation of αN-catenin. These data are consistent with the prior finding that neural crest cells do not divide considerably during their migration (LeDouarin and Kalcheim, 1999). In addition, we observe no change in the premigratory neural crest cell domain, which suggests that the ventral to dorsal movement of neural tube cells that underlies chick trunk neural crest cell emigration (Krispin et al., 2010) is also occurring in the midbrain region and is not altered upon depletion of αN-catenin. Thus, the enhanced migration phenotype we observe is most likely due to increased emigration of neural crest cells. Given the inherent mosaic nature of transfection resulting from the electroporation technique, this observed effect is truly compelling, as not all cells will receive the MO and, consequently, inhibit αN-catenin mRNA translation. Our data demonstrate, however, significant knock-down of αN-catenin protein levels at all time points (3, 8, 20 hours) in approximately 90% of embryos examined, resulting in augmented neural crest cell migration. One possible explanation for this increase in the number of migratory neural crest cells may be the loss of Cadherin6B- and/or N-cadherin-containing adherens junctions within premigratory neural crest cells of the dorsal neural tube, thus facilitating their detachment from the neuroepithelium and subsequent migration.
Although little research to date has examined the specific functional role of αN-catenin in embryogenesis, previous studies have examined α-catenin in the embryonic development of Xenopus and Drosophila, through both depletion experiments and targeted deletions, and in cultured cells. Maternal depletion of α-catenin in Xenopus using antisense α-catenin oligonucleotides abrogates adhesion at the blastula stage (Kofron et al., 1997). In Drosophila, the EMT underlying mesoderm formation requires a loss of α-catenin from the cell surface (Oda et al., 1998), and RNAi-mediated knock-down of α-catenin leads to disrupted cell movements during gastrulation (Magie et al., 2002). Moreover, research conducted in mouse and rat hippocampal cultures has investigated functions of αN-catenin in the CNS, with the resulting phenotypes further indicating a role for αN-catenin in cell migration. Using two independent lines of mice with deficiencies in αN-catenin (Park et al., 2000; Park et al., 2002a; Park et al., 2002b; Togashi et al., 2002), researchers noted that loss of αN-catenin results in abnormal migration of cerebellar Purkinje cell precursors. Furthermore, αN-catenin deficiency causes the aberrant migration of anterior commissure axons to ectopic locations in the mouse brain (Uemura and Takeichi, 2006). Collectively, these results are interesting in light of our αN-catenin depletion data showing 1) decreased adhesion and increased numbers of migratory neural crest cells at all time points examined, and 2) aberrant neural crest cell migration observed upon prolonged depletion of αN-catenin (20 hours), with neural crest cells moving in a different pattern than that observed normally in the embryo (such as on the non-transfected side of the embryo). Finally, loss of α-catenin promotes a mesenchymal morphology and leads to increased tumorigenicity in cultured cells (Buillons et al., 1997). Taken together, these findings strongly indicate a general function for αN-catenin in regulating the appropriate migration of a variety of cell populations. As such, the depletion of αN-catenin from premigratory neural crest cells most likely leads to a premature decrease in local cell-cell adhesion as evidenced by our data, the detachment of neural crest cells from the neuroepithelium, and the promotion of neural crest cell migration.
We further validated our αN-catenin knock-down phenotype by conducting in vivo and in vitro overexpression studies. αN-catenin overexpression perturbs neural crest cell migration in vivo at both early and later time points without having a considerable effect on the delamination process, as neural crest cells are able to successfully detach from the basal lamina but at times migrate inappropriately into the neural tube lumen. Together with our MO knock-down data, these results suggest that αN-catenin functions after the neural crest cell delamination process and plays an important role in regulating neural crest cell migration. In our overexpression experiments, we often see αN-catenin (GFP)-negative cells in the neural tube lumen, and this phenotype may be due to the initial expression then loss of αN-catenin/GFP in these cells, as our TUNEL data show that these lumenal cells eventually undergo apoptosis. An alternative explanation, however, is that overexpression of αN-catenin has a non-cell autonomous effect on other cells within the neural tube, resulting in the inappropriate movement of non-transfected cells into the neural tube lumen. Other reports have documented the interaction of αN-catenin with the transcriptional repressor ZASC1 in the nuclei of cultured cells (Bogaerts et al., 2005), the association of αN-catenin dimers with actin filaments (Gumbiner, 2005; Pokutta et al., 2008), and a reduction in N-cadherin protein levels upon αN-catenin overexpression (our work, see below). As such, the overexpression of αN-catenin in some neural tube cells could be imparting them with a different transcriptional program due to its interaction with ZASC1, the result of which is a signaling event that affects neighboring non-transfected neural tube cells and causes them to move into the neural tube lumen. Another possibility is that the enhanced association of αN-catenin with actin filaments may result in the rearrangement of the cellular architecture of the neural tube, effectively “squeezing out” non-transfected cells that eventually end up spilling into the neural tube lumen. Finally, the reduction in N-cadherin protein levels that we observe upon overexpression of αN-catenin would provide an additional mechanism by which other neural tube cells (not premigratory neural crest cells) could end up in the lumen due to diminished cell-cell adhesion within the neural tube proper, resulting in cell movement into the lumen. It is important to note that these explanations for negative cells may not be mutually exclusive in our embryos.
The localization of cells in the neural tube lumen upon αN-catenin overexpression is reminiscent of the phenotype noted upon overexpression of other Cadherins (Nakagawa and Takeichi, 1998; Coles et al., 2007). These latter reports suggest that the regulated expression of Cadherins may be required for establishing apicobasal polarity in neural tube cells, and that this polarity is critical in directing neural crest cell migration away from the neural tube into the embryo proper. As such, the mis-expression of Cadherins throughout the cell surface (and thus no longer strictly in the apical region) would disrupt apicobasal polarity in the neural tube, and, consequently, lead to the inappropriate movement of neural crest cells into the neural tube lumen. Our data lend further credence to the importance of neural tube/neuroepithelium apicobasal polarity established by adherens junctions and the effect of apicobasal polarity on neural crest cell migration. Interestingly, αN-catenin overexpression increases Cadherin6B protein levels in the neural tube and, to some extent, in migratory neural crest cells, arguing in favor of the hypothesis that the observed reduction in neural crest cell migration could be related to increased adhesion generated by Cadherin6B-based adherens junctions. Moreover, the loss of N-cadherin-based adherens junctions upon overexpression of αN-catenin may provide an explanation for the observed disruption in neural tube structure and the localization of non-neural crest cells in the neural tube lumen. Thus, the regulation of one adherens junction component, αN-catenin, is important to maintain proper epithelial architecture within the embryonic neural tube, as well as to permit the appropriate migration of neural crest cells away from the neural tube.
Neural crest cell EMT is requisite for subsequent neural crest cell migration and relies partly upon transcriptional repression of αN-catenin by Snail2
Emigration of neural crest cells from the embryonic dorsal neural tube is contingent upon EMT in order to confer adherent, premigratory neural crest cells with the ability to migrate. The Snail superfamily of transcriptional repressors plays important roles in developmental EMTs that control the morphogenetic movements required for the formation of the mesoderm (Grau et al., 1984; Boulay et al., 1987; Carver et al., 2001), skeletogenic mesenchyme (Wu and McClay, 2007), and neural crest (Nieto et al., 1994; Taneyhill et al., 2007), as well as those EMTs that underscore the metastasis observed in a wide range of human cancers (Nieto, 2002; Barrallo-Gimeno and Nieto, 2005; Yang and Weinberg, 2008). These seemingly different processes are linked by their dependence on EMT in which genes required to maintain the “epithelial” state of the cell are down-regulated in order to disrupt cell-cell interactions, apicobasal polarity, and foster the breakdown of the basement membrane (Hay, 1995; Nakaya et al., 2008), followed by the up-regulation of mesenchymal molecules that facilitate the remodeling of the cytoskeleton to permit cell shape changes and motility.
Here we demonstrate that αN-catenin is up-regulated in the chick premigratory neural crest cell population as early as 30 minutes after Snail2 depletion. The kinetics of up-regulation at both 30 minutes and at 1 hour are remarkably similar to that observed for cadherin6B (Taneyhill et al., 2007), and thus support the hypothesis that αN-catenin is a direct target of Snail2 repression. In vivo ChIP experiments confirm this hypothesis and reveal that Snail2 associates with E box 1 (‘CAACTG’) in the αN-catenin regulatory region at -8.80 kb upstream of the translational start site, an E box sequence that differs slightly from that reported for cadherin6B (‘CAGGTA’). These data are interesting because the repression of both cadherin6B and αN-catenin by Snail2 is occurring simultaneously in premigratory neural crest cells during neural crest cell EMT. In addition, MO-mediated knock-down of αN-catenin, in a Snail2-depleted background, rescues the neural crest cell migration defects associated with the loss of Snail2 both in vivo and in vitro, implicating αN-catenin as a key target of Snail2 regulation. These combined results suggest that Snail2 represses αN-catenin expression through association with an E box in the αN-catenin regulatory region, and that loss of regulation by Snail2 upon perturbation of αN-catenin further encumbers neural crest cell migration.
We have identified an additional target of Snail2 repression within chick premigratory neural crest cell adherens junctions, αN-catenin, that regulates neural crest cell migration. Our data suggest that modulation of αN-catenin by Snail2 may provide one of the pivotal control points during chick neural crest cell EMT. Moreover, our work bolsters the hypothesis that the apicobasal polarity provided by neural tube adherens junctions may regulate the appropriate migration of neural crest cells into the embryo periphery. As in other organisms and cell culture systems, we find that αN-catenin directly impinges upon cell motility, with neural crest cells migrating inappropriately upon perturbation of αN-catenin. Taken together, our studies define a novel regulatory relationship between Snail2 and αN-catenin that plays an important role in controlling proper neural crest cell migration in the chick embryo.
Supplementary Material
Acknowledgments
We thank Ms. Vineeta Singh for assistance with literature searches and Dr. Edward Coles for critically reading the manuscript. The Snail2 and Snail2 control MOs were generous gifts from Dr. Marianne Bronner-Fraser. The αN-catenin, N-cadherin, Cadherin6B, and Cadherin7 antibodies were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242. This work was supported by a grant from the NIH-NICHD (R00 HD055034 to L.A.T.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438–49. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlstrom JD, Erickson CA. The neural crest epithelial-mesenchymal transition in 4D: a ‘tail’ of multiple non-obligatory cellular mechanisms. Development. 2009;136:1801–1812. doi: 10.1242/dev.034785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–3161. doi: 10.1242/dev.01907. [DOI] [PubMed] [Google Scholar]
- Bolos V, Peinado H, Perez-Moreno M, Fraga MF, Esteller M, Cano A. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J Cell Sci. 2003;116:499–511. doi: 10.1242/jcs.00224. [DOI] [PubMed] [Google Scholar]
- Bogaerts S, Vanlandschoot A, van Hengel J, van Roy F. Nuclear translocation of alphaN-catenin by the novel zinc finger transcriptional repressor ZASC1. Exp Cell Res. 2005;311:1–13. doi: 10.1016/j.yexcr.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Boulay JL, Dennefeld C, Alberga A. The Drosophila developmental gene snail encodes a protein with nucleic acid binding fingers. Nature. 1987;330:395–8. doi: 10.1038/330395a0. [DOI] [PubMed] [Google Scholar]
- Bullions LC, Notterman DA, Chung LS, Levine AJ. Expression of wild-type alpha-catenin protein in cells with a mutant alpha-catenin gene restores both growth regulation and tumor suppressor activities. Mol Cell Biol. 1997;17:4501–8. doi: 10.1128/mcb.17.8.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno M, Rodrigo I, Locascio A, Blanco M, del Barrio MG, Portillo F, Nieto MA. The transcription factor Snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Carver EA, Jiang R, Lan Y, Oram KF, Gridley T. The mouse snail gene encodes a key regulator of the epithelial-mesenchymal transition. Mol Cell Biol. 2001;21:8184–8. doi: 10.1128/MCB.21.23.8184-8188.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavey M, Rauzi M, Lenne PF, Lecuit T. A two-tiered mechanism for stabilization and immobilization of E-cadherin. Nature. 2008;453:751–6. doi: 10.1038/nature06953. [DOI] [PubMed] [Google Scholar]
- Coles EG, Taneyhill LA, Bronner-Fraser M. A critical role for Cadherin6B in regulating avian neural crest emigration. Dev Biol. 2007;312:533–44. doi: 10.1016/j.ydbio.2007.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Come C, Arnoux V, Bibeau F, Savagner P. Roles of the transcription factors snail and slug during mammary morphogenesis and breast carcinoma progression. J Mammary Gland Biol Neoplasia. 2004;9:183–93. doi: 10.1023/B:JOMG.0000037161.91969.de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Craene B, van Roy F, Berx G. Unraveling signalling cascades for the Snail family of transcription factors. Cellular Signaling. 2005;17:535–547. doi: 10.1016/j.cellsig.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Giroldi LA, Bringuier PP, de Weijert M, Jansen C, van Bokhoven A, Schalken JA. Role of E boxes in the repression of E-cadherin expression. Biochem Biophys Res Commun. 1997;241:453–8. doi: 10.1006/bbrc.1997.7831. [DOI] [PubMed] [Google Scholar]
- Grau Y, Carteret C, Simpson P. Mutations and Chromosomal Rearrangements Affecting the Expression of Snail, a Gene Involved in Embryonic Patterning in DROSOPHILA MELANOGASTER. Genetics. 1984;108:347–360. doi: 10.1093/genetics/108.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–34. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- Hemavathy K, Guru SC, Harris J, Chen JD, Ip YT. Human Slug is a repressor that localizes to sites of active transcription. Mol Cell Biol. 2000;26:5087–5095. doi: 10.1128/mcb.20.14.5087-5095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemavathy K, Hu X, Ashraf SI, Small SJ, Ip YT. The repressor function of snail is required for Drosophila gastrulation and is not replaceable by Escargot or Worniu. Dev Biol. 2004;269:411–20. doi: 10.1016/j.ydbio.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Herrenknecht K, Ozawa M, Eckerskorn C, Lottspeich F, Lenter M, Kemler R. The uvomorulin-anchorage protein alpha catenin is a vinculin homologue. Proc Natl Acad Sci U S A. 1991;88:9156–60. doi: 10.1073/pnas.88.20.9156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S, Kimoto N, Shimoyama Y, Hirohashi S, Takeichi M. Identification of a neural alpha-catenin as a key regulator of cadherin function and multicellular organization. Cell. 1992;70:293–301. doi: 10.1016/0092-8674(92)90103-j. [DOI] [PubMed] [Google Scholar]
- Hirano S, Takeichi M. Differential expression of alpha N-catenin and N-cadherin during early development of chicken embryos. Int J Dev Biol. 1994;38:379–84. [PubMed] [Google Scholar]
- Hyafil F, Babinet C, Jacob F. Cell-cell interactions in early embryogenesis: a molecular approach to the role of calcium. Cell. 1981;26:447–54. doi: 10.1016/0092-8674(81)90214-2. [DOI] [PubMed] [Google Scholar]
- Itasaki N, Bel-Vialar S, Krumlauf R. ‘Shocking’ developments in chick embryology: electroporation and in ovo gene expression. Nat Cell Biol. 1999;1:E203–7. doi: 10.1038/70231. [DOI] [PubMed] [Google Scholar]
- Kofron M, Spagnuolo A, Klymkowsky M, Wylie C, Heasman J. The roles of maternal alpha-catenin and plakoglobin in the early Xenopus embryo. Development. 1997;124:1553–60. doi: 10.1242/dev.124.8.1553. [DOI] [PubMed] [Google Scholar]
- Krispin S, Nitzan E, Kassem Y, Kalcheim C. Evidence for a dynamic spatiotemporal fate map and early fate restrictions of premigratory avian neural crest. Development. 2010;137:585–95. doi: 10.1242/dev.041509. [DOI] [PubMed] [Google Scholar]
- Le Douarin N, Kalcheim C. The neural crest. New York: Cambridge University Press; 1999. [Google Scholar]
- Leptin M, Casal J, Grunewald B, Reuter R. Mechanisms of early Drosophila mesoderm formation. Dev Suppl. 1992:23–31. [PubMed] [Google Scholar]
- Magie CR, Pinto-Santini D, Parkhurst SM. Rho1 interacts with p120ctn and alpha-catenin, and regulates cadherin-based adherens junction components in Drosophila. Development. 2002;129:3771–82. doi: 10.1242/dev.129.16.3771. [DOI] [PubMed] [Google Scholar]
- Nagafuchi A, Takeichi M, Tsukita S. The 102 kd cadherin-associated protein: similarity to vinculin and posttranscriptional regulation of expression. Cell. 1991;65:849–57. doi: 10.1016/0092-8674(91)90392-c. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Takeichi M. Neural crest emigration from the neural tube depends on regulated cadherin expression. Development. 1998;125:2963–2971. doi: 10.1242/dev.125.15.2963. [DOI] [PubMed] [Google Scholar]
- Nakaya Y, Sukowati EW, Wu Y, Sheng G. RhoA and microtubule dynamics control cell-basement membrane interaction in EMT during gastrulation. Nat Cell Biol. 2008;10:765–75. doi: 10.1038/ncb1739. [DOI] [PubMed] [Google Scholar]
- Niessen CM, Gottardi CJ. Molecular components of the adherens junction. Biochim Biophys Acta. 2008;1778:562–71. doi: 10.1016/j.bbamem.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto MA, Sargent MG, Wilkinson DG, Cooke J. Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science. 1994;264:835–839. doi: 10.1126/science.7513443. [DOI] [PubMed] [Google Scholar]
- Nieto MA. The Snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–166. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- Oda H, Tsukita S, Takeichi M. Dynamic behavior of the cadherin-based cell-cell adhesion system during Drosophila gastrulation. Dev Biol. 1998;203:435–50. doi: 10.1006/dbio.1998.9047. [DOI] [PubMed] [Google Scholar]
- Park C, Longo CM, Ackerman SL. Genetic and physical mapping of the cerebellar deficient folia (cdf) locus on mouse chromosome 6. Genomics. 2000;69:135–8. doi: 10.1006/geno.2000.6322. [DOI] [PubMed] [Google Scholar]
- Park C, Falls W, Finger JH, Longo-Guess CM, Ackerman SL. Deletion in Catna2, encoding alpha N-catenin, causes cerebellar and hippocampal lamination defects and impaired startle modulation. Nat Genet. 2002a;31:279–84. doi: 10.1038/ng908. [DOI] [PubMed] [Google Scholar]
- Park C, Finger JH, Cooper JA, Ackerman SL. The cerebellar deficient folia (cdf) gene acts intrinsically in Purkinje cell migrations. Genesis. 2002b;32:32–41. doi: 10.1002/gene.10024. [DOI] [PubMed] [Google Scholar]
- Perez-Mancera PA, Gonzalez-Herrero I, Perez-Caro M, Gutierrez-Cianca N, Flores T, Gutierrez-Adan A, Pintado B, Snachez-Martin M, Sanchez-Garcia I. SLUG in cancer development. Oncogene. 2005;24:3073–3082. doi: 10.1038/sj.onc.1208505. [DOI] [PubMed] [Google Scholar]
- Pokutta S, Drees F, Yamada S, Nelson WJ, Weis WI. Biochemical and structural analysis of alpha-catenin in cell-cell contacts. Biochem Soc Trans. 2008;36:141–7. doi: 10.1042/BST0360141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauka-Spengler T, Bronner-Fraser M. A gene regulatory network orchestrates neural crest formation. Nat Rev Mol Cell Biol. 2008;9:557–68. doi: 10.1038/nrm2428. [DOI] [PubMed] [Google Scholar]
- Schneider S, Herrenknecht K, Butz S, Kemler R, Hausen P. Catenins in Xenopus embryogenesis and their relation to the cadherin-mediated cell-cell adhesion system. Development. 1993;118:629–40. doi: 10.1242/dev.118.2.629. [DOI] [PubMed] [Google Scholar]
- Shirayoshi Y, Hatta K, Hosoda M, Tsunasawa S, Sakiyama F, Takeichi M. Cadherin cell adhesion molecules with distinct binding specificities share a common structure. EMBO J. 1986;5:2485–8. doi: 10.1002/j.1460-2075.1986.tb04525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoval I, Ludwig A, Kalcheim C. Antagonistic roles of full-length N-cadherin and its soluble BMP cleavage product in neural crest delamination. Development. 2007;134:491–501. doi: 10.1242/dev.02742. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Ueda A, Kobayashi N, Yang J, Tomaru K, Yamamoto M, Takeno M, Ishigatsubo Y. Proteasome-dependent degradation of α-catenin is regulated by interaction with ARMc8α. Biochem J. 2008;411:581–91. doi: 10.1042/BJ20071312. [DOI] [PubMed] [Google Scholar]
- Taneyhill LA. To adhere or not to adhere: the role of Cadherins in neural crest development. Cell Adh Migr. 2008;2:223–30. doi: 10.4161/cam.2.4.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneyhill LA, Adams MS. Investigating regulatory factors and their DNA binding affinities through real time quantitative PCR (RT-QPCR) and chromatin immunoprecipitation (ChIP) assays. Methods Cell Biol. 2008;87:367–89. doi: 10.1016/S0091-679X(08)00219-7. [DOI] [PubMed] [Google Scholar]
- Taneyhill LA, Bronner-Fraser M. Dynamic alterations in gene expression after Wnt-mediated induction of avian neural crest. Mol Biol Cell. 2005;16:5283–93. doi: 10.1091/mbc.E05-03-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneyhill LA, Coles EG, Bronner-Fraser M. Snail2 directly represses cadherin6B during epithelial-to-mesenchymal transitions of the neural crest. Development. 2007;134:1481–90. doi: 10.1242/dev.02834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- Togashi H, Abe K, Mizoguchi A, Takaoka K, Chisaka O, Takeichi M. Cadherin regulates dendritic spine morphogenesis. Neuron. 2002;35:77–89. doi: 10.1016/s0896-6273(02)00748-1. [DOI] [PubMed] [Google Scholar]
- Uchida N, Shimamura K, Miyatani S, Copeland NG, Gilbert DJ, Jenkins NA, Takeichi M. Mouse alpha N-catenin: two isoforms, specific expression in the nervous system, and chromosomal localization of the gene. Dev Biol. 1994;163:75–85. doi: 10.1006/dbio.1994.1124. [DOI] [PubMed] [Google Scholar]
- Uemura M, Takeichi M. Alpha N-catenin deficiency causes defects in axon migration and nuclear organization in restricted regions of the mouse brain. Dev Dyn. 2006;235:2559–66. doi: 10.1002/dvdy.20841. [DOI] [PubMed] [Google Scholar]
- Volk T, Geiger B. A 135-kd membrane protein of intercellular adherens junctions. EMBO J. 1984;3:2249–60. doi: 10.1002/j.1460-2075.1984.tb02123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DG. Whole mount in situ hybridization of vertebrate embryos. In: Wilkinson DG, editor. In Situ Hybridization. Oxford: Oxford University Press; 1992. pp. 75–83. [Google Scholar]
- Wu SY, McClay DR. The Snail repressor is required for PMC ingression in the sea urchin embryo. Development. 2007;134:1061–70. doi: 10.1242/dev.02805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–29. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.