Abstract
Glioblastoma patients have a poor prognosis, even after surgery, radiotherapy, and chemotherapy with temozolomide or 1,3-bis(2-chloroethy)-1-nitrosourea. We developed an in vitro recovery model using neurosphere cultures to analyze the efficacy of chemotherapy treatments, and tested whether glioblastoma neurosphere initiating cells are resistant. Concentrations of chemotherapy drugs that inhibit neurosphere formation are similar to clinically relevant doses. Some lines underwent a transient cell cycle arrest and a robust recovery of neurosphere formation. These results indicate that glioblastoma neurospheres can regrow after treatment with chemotherapy drugs. This neurosphere recovery assay will facilitate studies of chemo-resistant subpopulations and methods to enhance glioblastoma therapy.
Keywords: chemotherapy, cancer stem cells, DNA damage, temozolomide, BCNU, glioblastoma, neurosphere
1. Introduction
Temozolomide (TMZ) is the most common chemotherapy drug used to treat glioblastomas [1]. 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU, carmustine) is an older drug that surgeons now deposit in the tumor bed as dissolvable wafers [2]. Both of these drugs alkylate DNA at multiple sites, including the O6 position of guanine, which can result in futile cycles of DNA repair and, ultimately, cell death. [3].
Even after aggressive treatment that eliminates most of the tumor load, these tumors recur in an average of 6.9 months after initial treatment [4]. Tumor regrowth implies that glioblastomas include a population of cells that are resistant to therapy and maintain the ability to proliferate. Recently, a population of cells with stem cell-like properties was identified in brain tumors [5], and several laboratories have reported that these cells are relatively resistant to chemotherapy and radiation [6; 7].
Neurosphere cultures are heterogeneous, and only a fraction of cells are capable of sphere formation. These cells are classified as neurosphere initiating cells (NICs) [8; 9; 10]. To quantify the NICs, normal or transformed cells are plated at low density in defined medium and after 1–2 weeks, the numbers of neurospheres are scored [11; 12]. For normal neural cells, this assay was developed to assess stem cells [8], but is now thought to also detect early progenitor cells [13; 14; 15]. It is not known whether there is a similar ambiguity for tumor NICs. Spheroid-based drug screens for other tumor types are under development [16].
The glioblastoma cells used for this assay are grown as neurospheres in defined medium to prevent differentiation. Unlike serum-supplemented cultures, glioblastoma neurosphere cells form invasive brain tumors in immunodeficient mice [17]. In addition, based on expression profiling, neurosphere cultures resemble glioblastoma tumors from patients more closely than serum-supplemented cultures [17].
Using the neurosphere-formation assay, we found that clinically relevant doses of BCNU or TMZ inhibit neurosphere formation. For four of five cell cultures, neurosphere formation resumes following a recovery period; dissociation of these initial spheres allows robust formation of secondary spheres. BCNU and TMZ induce S and/or G2/M cell cycle arrest, which partially reverses by seven days post-treatment. Collectively, these results indicate that neurosphere formation is highly sensitive to chemotherapy drugs, and in some cases, the NICs may enter a reversible cell cycle arrest. This reversible cell cycle arrest may protect the NICs from chemotoxicity [18; 19; 20; 21], allowing regrowth of some cultures after chemotherapy. In addition, an ex vivo TMZ-treated culture that resumes sphere formation is also capable of tumor initiation as subcutaneous xenografts. This model for the survival and recovery of cultured glioblastoma neurosphere cells may provide insights for tumor recurrence in vivo.
2. Materials and Methods
2.1 Cell culture
In a previous study, PTEN −/− neural precursor cells [22] were infected with a retrovirus bearing the human mutant receptor EGFRvIII, and we refer to these as EGFRvIII PTEN −/− cells [23]. These transformed cells formed glioblastoma-like tumors in immunodeficient mice, and we established the aggressive PET2 line from one of these tumors. As a control, PTEN +/+ neural precursor cells were infected with an empty GFP MSCV-XZ066 virus [24]. We refer to these as GFP PTEN +/+ cells. A major advantage of these mouse cultures is the easy comparison of normal and transformed cells.
The human adherent glioblastoma cell line U373MG (obtained from Dr. Larry Recht, Stanford University) was converted to a floating neurosphere culture, U373NS, by growing cells in 20% FBS DMEM/F12 until confluent and then in defined serum-free DMEM/F12 medium supplemented with B27 (GIBCO Carlsbad, CA), 20 ng/ml bFGF (Invitrogen Carlsbad, CA) and 20 ng/ml EGF (Invitrogen). These cells formed neurospheres after they were in serum-free media for approximately one month. U373NS neurospheres could be maintained for >30 passages by mechanical trituration. Primary human glioblastoma cultures (GS7-2 and GS9-6) were established from resected tumor tissue with Institutional Review Board approval. The tumors were cut into small sections and dissociated with trypsin. Dissociated tumor cells were grown as neurosphere cultures in DMEM/F12 medium supplemented with B27, 20 ng/ml bFGF and 20 ng/ml EGF. The primary glioblastoma cultures were passaged using basic solution to dissociate the spheres [25]. The characterization of GS7-2 was reported by Dr. Cochran and co-workers [26]. All experiments using primary human GBM cultures were carried out with cells from passages 10–20.
All of the glioblastoma cultures, but not GFP PTEN +/+, formed subcutaneous tumors in immunodeficient mice (data not shown). As reported for other glioblastoma neurosphere cultures [27], fetal bovine serum induced astroglial differentiation of GFP PTEN +/+, PET2, U373NS, GS7-2, and GS9-6 (data not shown). All cultures used in this paper are O6-methylguanine DNA methyltransferase (MGMT) negative (data not shown).
As part of the basic characterization of these lines, we determined if the tumor suppressor p53 was mutated. The mRNA was extracted using a QuickPrep micro mRNA purification kit (GE Healthcare, Piscataway, NJ). One hundred ng of mRNA was incubated for 2 hrs at 37°C with 1 µl of Oligo(dT)12–18 (500 µg/ml), 4 µl of 5X First strand cDNA buffer, 2 µl of 0.1 mM DTT and 1 µl of 10 mM dNTP Mix (final volume 20 µl). Then 1 µl of SSII Reverse Transcriptase (200 U) was added and incubated for 50 min at 42°C, followed by heat inactivation for 15 min at 70°C. The p53 cDNA (2 µl) was amplified with the Platinum Taq DNA High Fidelity Polymerase (Invitrogen Carlsbad, CA). The primers for amplification of the human p53 cDNA were 5’-ATGGAGGAGCCGCAGTCAGAT-3’ and 5’-TGCGCCGGTCTCTCCCAGGAC-3’; 5’-AAGGAAATTTGCGTGTGGAGT-3’ and 5’-CAGTCGGAGTCAGGCCCTTCT-3’. The primers for amplifying the mouse p53 cDNA were 5’-TTGGGACCATCCTGGCTGTAG-3’ and 5’-ATAAGGTACCACCACGCTGTG-3’ for exon1–5, 5’-ATGGTGATGGCCTGGCTCCTC-3’ and 5’-CAGTTCAGGGCAAAGGACTTC-3’ for exon 6–8, 5’-AAGTCCTTTGCCCTGAACTGC-3’ and 5’-GACCGGGAGGATTGTGTCTCA-3’ for exon 9. The PCR products were analyzed by automated DNA sequencing in both directions (GENEWIZ, South Plainfield, NJ).
PET2 showed only a C132W mutation, which is not active as judged by a WAF1 reporter construct (data not shown). GS9-6 had the wild type p53 sequence. The GS7-2 showed only a mutated F113V sequence. Amino acid 113 was mutated in four tumors (Sanger Cosmic Database), including one F113V mutation in a glioblastoma. We did not test U373NS cells because the parent line, U373MG, is already known to lack functional p53 [28].
2.2 Primary and secondary neurosphere formation
Neurospheres were mechanically triturated into single cell suspensions or disassociated with basic solution [25] and plated at 3,000 cell/ml of defined medium. To measure the effect of chemotoxicity, BCNU (Sigma-Aldrich St. Louis, MO) or TMZ (a gift from Dr. Michael Glantz) were added to these cultures at a range of concentrations. Neurospheres with at least 25 cells/sphere were counted after 7 or 10 days after addition of drug. Then, to measure recovery from drug treatment, cultures were supplemented with fresh defined media, and neurospheres were counted a second time, 14 or 20 days after drug treatment. The primary glioblastoma cultures (GS7-2 and GS9-6) formed spheres slightly slower than the other cultures. As a result, we allowed about 10 days for sphere formation by the primary human cultures and 7 days for the U373NS line and mouse cultures. For the secondary neurosphere assay, treated cells were collected after recovery on day 14 or 20, and dissociated into single cells. Cells from each well were replated into 6-well plates in 2 ml of defined medium. Secondary neurosphere formation was quantified by counting the number of neurospheres (>25 cells) after 7 or 10 days.
2.3 Proliferation assay
Single-cell suspensions were plated at 5,000 cells/well in a 96 well plate with or without BCNU or TMZ. After one week, viable cells were measured using colorimetric assays following the manufacturer’s instructions (Promega Corp, Madison, WI). In initial experiments with the mouse cells, we used 3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide (MTT). In later experiments with the human cell cultures, we used 3-[4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H- tetrazolium (MTS) because of its greater solubility. In both cases, mitochondria in viable cells reduce these compounds to a formazan, which is detected by absorbance in a Microplate reader.
2.4 Subcutaneous xenografts with ex vivo TMZ treatment
U373NS neurospheres were dissociated and plated at 2.5 × 104 cells/ml in defined media and treated with DMSO or 200 µM TMZ. On day 7, the cells were dissociated and 3 × 106 live cells were re-suspended in 100 µl PBS. Cells were subcutaneously injected into the right flank of female athymic nude mice. The mice were observed for tumor formation for 100 days post-injection and were sacrificed when the tumor reached a volume of 1.5 cm3. These procedures were approved by the University of Massachusetts Medical School IACUC.
2.5 Comet assay
DNA damage in neurosphere cells with and without TMZ or BCNU treatment was measured by a comet assay, using protocols and reagents provided by Trevigen Inc. (Gaithersburg, MD). Briefly, single cells were embedded in agarose, lysed and treated with an alkaline solution to unwind and denature the DNA. The agarose-embedded cells were then subjected to electrophoresis. Cleaved DNA fragments caused by single or double stranded breaks migrate out of the nuclei and are detected using SYBR Green. For each experiment, at least 75 cells were examined with a Zeiss Axiovert S100 microscope, and DNA was quantified with OpenLab 4.0 software (Improvision Inc., Waltham, MA). The comet tail moment is calculated as the product of the tail length and the relative pixel intensity of the comet tail compared with the pixel intensity and the area of the nucleus. Comet tail moments are proportional to the extent of DNA damage in the cell [29]. Comet tail moments of treated cells were normalized to the comet tail moments of untreated cells.
2.6 Flow cytometry
Flow cytometry was used to measure the percentages of CD133+, CD15+ and A2B5+ cells following treatment with chemotherapy drugs. Cells were suspended in PBS containing 5% fetal calf serum, and stained with antibodies against CD133, CD15, and A2B5. Mouse cells were stained with monoclonal anti-mouse CD133 antibody (1:100; Clone 13A4, Chemicon/Millipore Corp (Billerica, MA), followed by a secondary PE-conjugated antibody (1:200; Jackson ImmunoResearch, West Grove, PA). For human cells, we used a PE-conjugated CD133 antibody (1:10 dilution; Clone AC141, Miltenyi Biotec, Auburn, CA). CD15 and A2B5 were detected with conjugated antibodies: CD15-APC (1:5; Clone HI98, Pharmingen/BD Biosciences, San Diego, CA) and A2B5-FITC (1:450; Clone A2B5–105(7), Millipore Corp., Billerica, MA). Marker expression was measured using a Becton Dickinson FACSCalibur (BD Biosciences, Franklin Lakes, NJ). Data was analyzed using FlowJo 7.2.2 software (Tree Star Inc., Ashland, OR).
To analyze cell cycle profiles, cells were fixed with 95% ethanol for at least 24 hrs and washed with apoptosis buffer (PBS, 1% Triton X-100 and 2 mM MgCl2) to permeabilize the cells. The cells were then stained for 30 min prior to analysis with the FACSCalibur with 50 µg/ml propidium iodide (Sigma-Aldrich St. Louis, MO) in PBS, 50 µg/ml RNase and 2 mM MgCl2. Data were analyzed using ModFit LT 3.0 software (Verity Software House, Topsham, ME).
3. Results
3.1 Neurosphere formation is inhibited at lower concentrations of chemotherapy drugs than those required to inhibit bulk cell proliferation
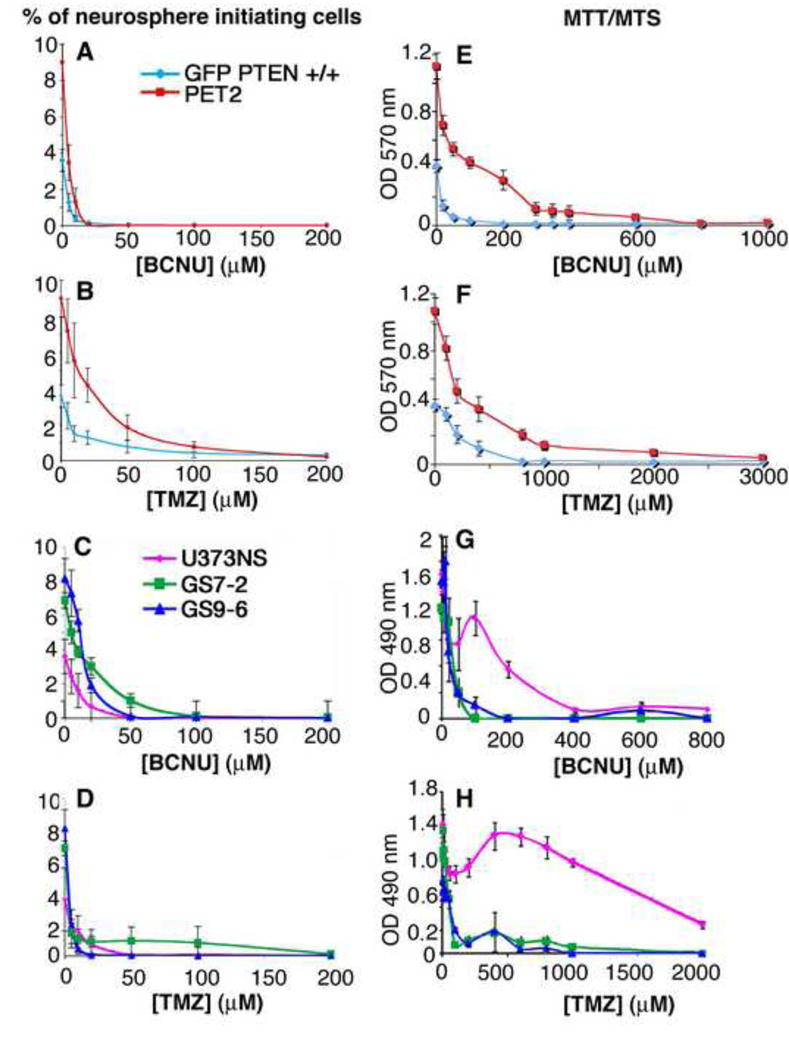
To determine how chemotherapy drugs affect neurosphere formation, we measured neurosphere formation in non-transformed and glioblastoma neurosphere cultures without or with treatment with BCNU or TMZ. Because of their rapid decay in aqueous solution [1; 30], these drugs likely persist only a few hours, but their effects on the tumor cells last longer [31]. After 7 or 10 days, we counted the number of neurospheres that formed without or with drug treatment. For all of these cultures, BCNU and TMZ inhibited neurosphere formation in a dose-dependent manner (Fig. 1A–D).
Fig. 1.
Neurosphere formation is inhibited at a much lower concentration of chemotherapy drug than that required to inhibit bulk cell proliferation. BCNU and TMZ were added to dissociated neurosphere cultures. After 7 or 10 days, we counted the number of neurospheres and calculated the percentage of cells that formed new neurospheres (A–D). We also performed MTT or MTS assays to measure bulk cell proliferation (E–H). Dose-response curves were determined for mouse normal cells (GFP PTEN +/+) and glioblastoma PET2 cells (A–B and E–F). Similar results were obtained with human glioblastoma lines (C–D) and (G–H).
To determine the sensitivity of bulk or total cells to these chemotherapy drugs, we performed MTT or MTS assays to measure the number of viable cells at the time that the neurospheres were counted. Inhibition of bulk cell proliferation, compared with neurosphere formation, required higher doses of chemotherapy drugs for all of these cell cultures (Fig. 1). To facilitate this comparison, we calculated the drug concentrations at which neurosphere formation was inhibited by 50% (IC50), the drug concentrations at which bulk cell numbers were decreased by 50% (MTS/MTT IC50) and the concentration at which bulk cells numbers were decreased by 90% (MTS/MTT IC90)(Fig. 2). GFP PTEN +/+ and PET2 cells had similar sphere IC50 values for treatment with BCNU or TMZ. The MTT IC50 and MTT IC90 values were both 5-fold (BCNU) and 1.4–2.0-fold (TMZ) higher for PET2 than for GFP PTEN +/+, indicating that normal GFP PTEN +/+ bulk cells are more sensitive to BCNU and TMZ than the transformed PET2 cells (Figure 2A–B). The sensitivities of the glioblastoma cultures to BCNU and TMZ varied, but there was one consistent finding. The concentration of chemotherapy drug required to inhibit neurosphere formation is much less than that required to inhibit bulk cell proliferation. The MTS IC50’s for the inhibition of bulk cell proliferation following BCNU treatment were 2.5 to 40 fold higher than those for sphere formation. For TMZ, the MTS IC50’s were 5 to 240 fold higher than sphere IC50’s. Finally, the IC90 values were substantially higher than the sphere IC50’s for both BCNU and TMZ (Fig. 2), indicating that sphere formation is inhibited at much lower doses of drug than that required to induce substantial toxicity.
Fig. 2.
Chemotherapy drugs more efficiently inhibit neurosphere formation than bulk cell proliferation. We calculated the concentrations of chemotherapy drug required to inhibit neurosphere formation by 50% (sphere IC50), the concentrations of drug required to inhibit bulk cell proliferation by 50% (MTS/MTT IC50) and the concentrations required to inhibit bulk cell proliferation by 90% (MTS IC90)(A–D). Also, normal neurosphere bulk cells are more sensitive to chemotherapy drugs than the transformed PET2 cells (A–B).
3.2 Recovery of neurosphere formation after treatment with chemotherapy drugs
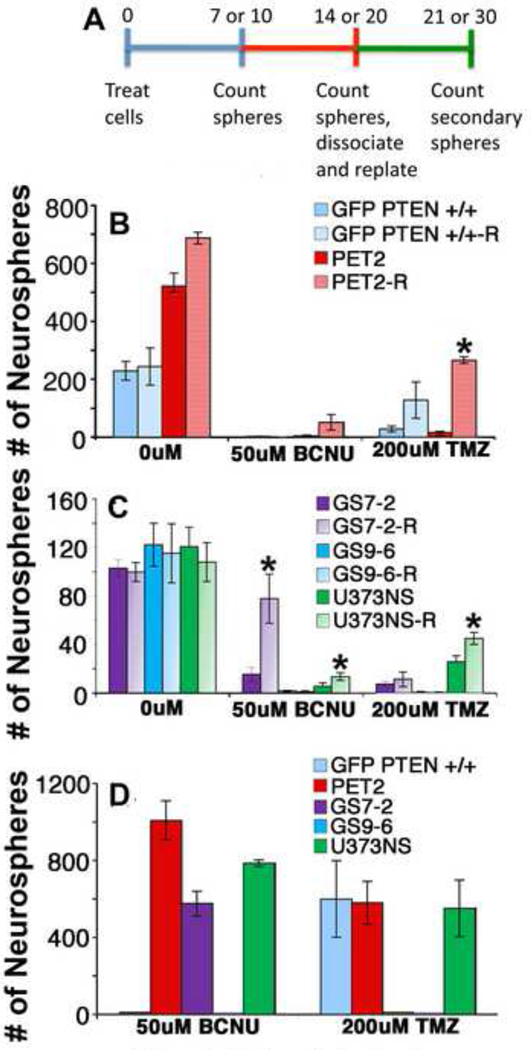
To determine if neurosphere formation resumes after chemotherapy treatment, cultures were counted after a recovery period. Experiments were done with cultures treated with 50 µM BCNU or 200 µM TMZ, which greatly decreased initial neurosphere formation (Fig. 1). Specifically, initial neurospheres were counted 7 or 10 days (based on culture growth rate) after the addition of chemotherapy drugs. Then, fresh medium was added, and the culture was allowed to recover for an additional 7–10 days of in the absence of drugs. Neurospheres counted after the recovery period include both the initial spheres formed by day 7 or 10 and new spheres that formed during the recovery period (Fig. 3A).
Fig. 3.
Inhibition of neurosphere formation by chemotherapy drugs can be reversible. (A) Neurospheres were counted 7 or 10 days after chemotherapy treatment (blue line), and then after an additional 7 or 10 days (recovery period, red line) to allow neurosphere-initiating cells to proliferate in the absence of drug (B–C). The initial measurements from day 7 or 10 are labeled with the cell culture name. Measurements after the recovery period on day 14 or 20 are labeled with the cell culture name and “R”. To analyze self-renewal, these neurospheres were dissociated and replated. Secondary spheres were counted after an additional 7 or 10 days (green line)(D). Although chemotherapy treatment greatly reduced initial sphere formation, some cultures were capable of recovery and secondary sphere formation. *, p<0.05.
The cell cultures varied greatly in their recovery from the two chemotherapy drugs. Normal GFP PTEN +/+ cells did not recover from BCNU treatment, but did recover from TMZ treatment (5-fold increase in sphere formation approaching statistical significance, p<0.13, Fig. 3B), indicating that 200 µM TMZ is less toxic to normal neural precursor cells compared with 50 µM BCNU. The transformed PET2 cells showed dramatic 13- and 17-fold increases in the number of spheres following BCNU and TMZ treatments, respectively (Fig. 3A). For GS7-2, there was a dramatic 5-fold increase in neurospheres during the recovery period after 50 µM BCNU treatment (Fig. 3C). In contrast, the GS7-2 neurospheres did not increase significantly after treatment with 200 µM TMZ. For both chemotherapy drugs, the number of neurospheres for GS9-6 cultures did not increase in the recovery period. For U373NS, the number of neurospheres increased nearly 2-fold during the recovery period for both BCNU and TMZ (Fig. 3C).
As a measure of NIC self-renewal, we also measured secondary sphere formation. The lack of recovery from 50 µM BCNU for GFP PTEN +/+ cells was confirmed by the lack of secondary spheres. GFP PTEN +/+ cells formed secondary spheres following 200uM TMZ treatment, confirming that 200 µM TMZ was less toxic to these normal neurosphere cells compared with 50 µM BCNU (Fig. 3D). PET2 and U373NS cells formed secondary spheres following treatment with both drugs. GS7-2 formed secondary sphere formation following treatment with 50 µM BCNU but not 200 µM TMZ. GS9-6 did not form secondary neurospheres after treatment with 50 µM BCNU or 200 µM TMZ.
In summary, for some cell cultures, inhibition of neurosphere formation by the chemotherapy drugs is reversible. Following a recovery period, there is a robust increase in neurosphere formation, indicating that NICs were viable but did not proliferate immediately after chemotherapy treatment. Secondary sphere formation confirmed that some NICs avoided chemotoxicity and restored the neurosphere cultures.
To further study the effects of chemotherapy drugs on glioblastoma cells, we measured the percentages of cells expressing the stem cell markers CD133, CD15, and A2B5 by flow cytometry (Supplementary Table 1)[5; 32; 33; 34]. The baselines and changes in expression levels of the stem cell markers CD133, A2B5 and CD15 following chemotherapy treatment varied between neurosphere cultures. For untreated cultures, the percentage of CD133+ cells varied between 2 and 91%. Chemotherapy treatment either had no effect or slightly decreased the percentage of CD133+ cells. These results are consistent with other publications demonstrating that the current markers used for labeling glioblastoma stem cells are not sufficient and yield different results [34; 35; 36]. The neurosphere assay, on the other hand, provided a much more consistent finding. Based on decreased neurosphere formation, we conclude that neurosphere formation is more sensitive to chemotherapy drugs than bulk or total cell proliferation.
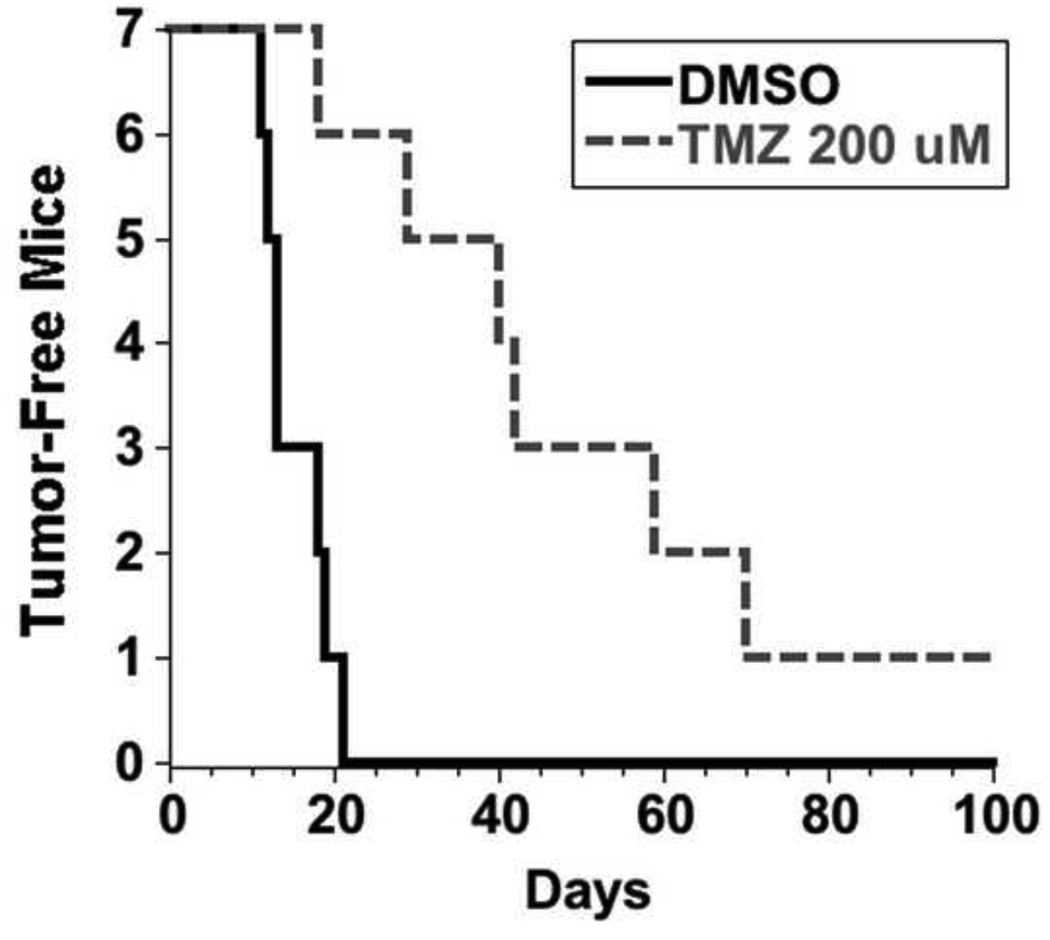
3.3 TMZ-treated cultures maintained their tumorgenicity
After demonstrating that NICs treated with clinically relevant TMZ concentrations are capable of repopulating the culture, we determined whether the cells that persist are still tumorigenic. U373NS cultures were treated with DMSO control or 200 µM TMZ and injected subcutaneously into nude mice. The control DMSO treated cells formed palpable tumors in an average of 15 days for 7/7 xenografts. TMZ treatment increased the latency of tumor formation, however, the tumor incidence was similar to the DMSO control xenografts. Palpable tumors formed for 6/7 TMZ-treated U373NS xenografts in an average of 43 days (Fig. 4).
Fig. 4.
Glioblastoma cells treated in culture with TMZ can form tumors. U373NS cells were treated in culture with a DMSO negative control or 200 µM TMZ. After 7 days, cells (3×106) were injected subcutaneously into immunodeficient mice. The TMZ-treated cells formed tumors in immunodeficient mice with increased latency compared to control-treated cells.
3.4 Normal and transformed neurosphere cultures accumulate DNA damage
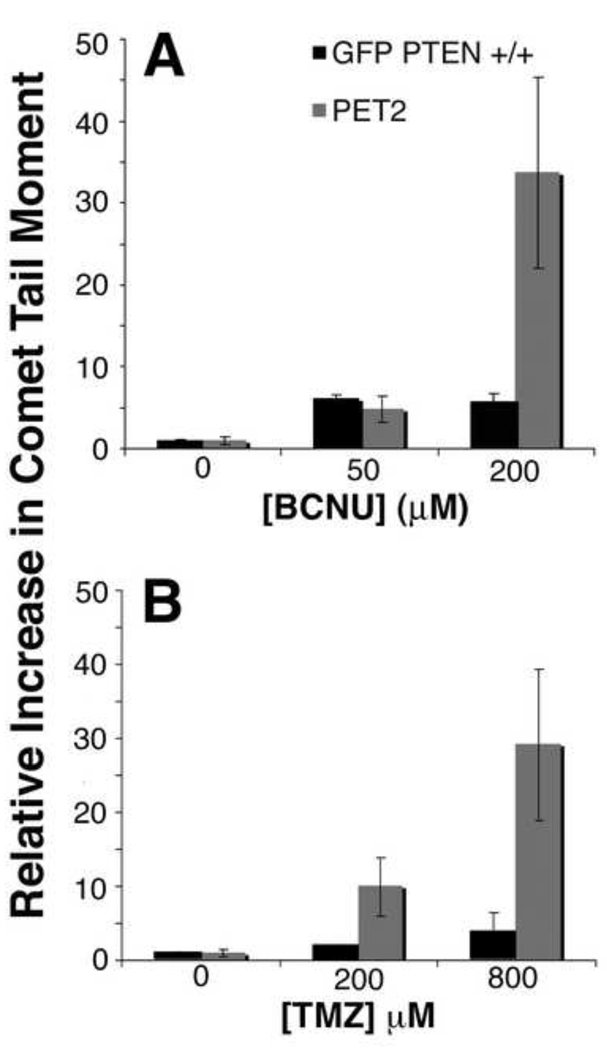
We found that the transformed PET2 neurosphere cultures were more resistant to treatment with chemotherapy drugs than the normal GFP PTEN +/+ neurosphere cells (Fig. 1–3). To determine if this resistance was due to lack of DNA damage, we used the comet assay to measure accumulated DNA damage in untreated GFP PTEN +/+ and PET2 neurosphere cells and cells at 7 days after treatment with BCNU or TMZ. Following treatment with 50 µM BCNU, GFP PTEN +/+ and PET2 cells had 6.1±0.4 and 4.7±1.6 fold increases in comet tail moments, respectively, demonstrating a similar degree of DNA damage (Fig. 5A). Treatment with 200 µM BCNU resulted in even greater increases (34±12 fold) in comet tail moment for PET2 cells. Following treatment with 200 µM TMZ, GFP PTEN +/+ and PET2 cells comet tail moments increased 2.0±0.2 and 10±4 fold, respectively (Fig. 5B). Contrary to the similar extent of DNA damage following 50 µM BCNU treatment, PET2 cells accumulated more DNA damage than GFP PTEN +/+ cells following treatment with 200 µM TMZ. PET2 cells treated with 800 µM TMZ also sustained more damage (29±10 fold) than GFP PTEN +/+ cells (4±2 fold). Therefore, we conclude that the relative resistance of the PET2 cells compared with GFP PTEN +/+ cells is not due to a lack of DNA damage.
Fig. 5.
The comet assay was used to assess DNA breaks in GFP PTEN +/+ and PET2 cells. Data are presented as the ratio of the comet tail moment following treatment to the comet tail moment with no treatment. (A) Following treatment with 50 µM BCNU, GFP PTEN +/+ and PET2 cells accumulated a similar number of DNA breaks. PET2 cells showed more DNA breaks after treatment with 200 µM BCNU. (B) PET2 cells accumulated more DNA breaks compared with GFP PTEN +/+ following treatment with 200 or 800 µM TMZ.
3.5 Glioblastoma neurosphere cells undergo death or cell cycle arrest in response to chemotherapy drugs
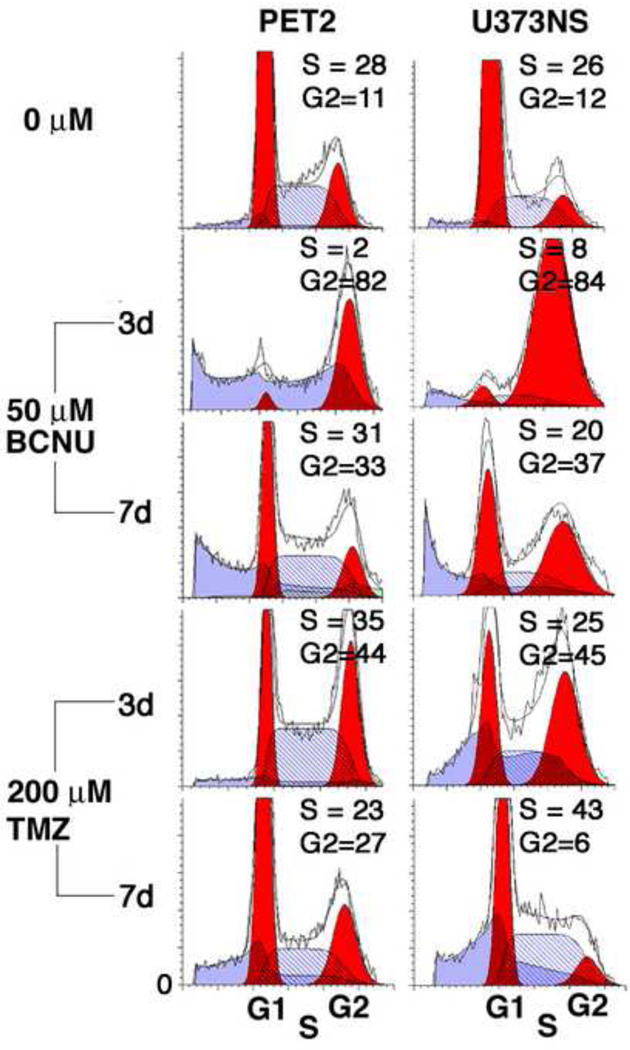
To further characterize the recovery from chemotherapy drugs, we carried out propidium iodide flow cytometry on untreated and treated cultures (Fig. 6). In response to 50 µM BCNU or 200 µM TMZ, PET2 and U373NS cells showed accumulation of cells in S and/or G2/M when analyzed three days post-treatment. We next tested whether cells resumed a normal cell cycle profile. At 7 days post-treatment, the profiles for PET2 cells resembled those for untreated cells except for increased debris. The profile for BCNU-treated U373NS cells 7 days post-treatment showed a smaller fraction of cells accumulated in G2/M as well as increased debris. For TMZ-treated U373NS cells, the G2/M accumulation had dissipated, but there was residual S phase accumulation. Overall, we see a smaller fraction of cells arrested in G2/M at seven days compared with three days for PET2 and U373NS. These results indicate that BCNU and TMZ induce some cells to enter reversible cell cycle arrest. The recovery of neurosphere formation in the recovery week (Fig. 3), suggests that NICs are included in the reversibly arrested fractions.
Fig. 6.
PET2 and U373NS glioblastoma cell cultures show transient cell cycle arrest in response to chemotherapy drugs. Cell cycle profiles were determined 3 and 7 days after addition of drug. PET2 and U373NS cells show S and/or G2/M phase accumulation 3 days post-treatment. This accumulation partially reverses by 7 days post-treatment, and in some cases, debris (sub G1) appears.
4. DISCUSSION
To more effectively treat glioblastomas, we must target the tumor-initiating cells that are resistant to current therapies. To determine the effects of chemotherapy drugs on normal and glioblastoma neurosphere cells, we measured neurosphere formation and bulk cell proliferation after treatment with BCNU or TMZ. The MTT/MTS IC50’s were 2.5–240-fold greater than the neurosphere IC50’s, and the MTT/MTS IC90 values for which there was substantial cell death were 16–400-fold greater than the neurosphere IC50’s. The relative sensitivity of neurosphere formation in response to chemotherapy was consistent for a non-transformed neurosphere culture and four glioblastoma cultures. Hence, we find that chemotherapy drugs efficiently target NICs. For U373NS cells, the MTS dose-response curves were not monotonic. We do not know the origin of this phenomenon, but dose-response curves for chemotherapy drugs are often non-monotonic [37].
To determine if NICs are capable of neurosphere formation after chemotherapy treatment, we devised an assay to measure the recovery of neurosphere formation. We treated neurosphere cultures with doses of chemotherapy drugs that drastically inhibit neurosphere formation, counted the neurospheres after 7 or 10 days, and again counted neurospheres after an additional 7 or 10 days. Surprisingly, although chemotherapy treatment resulted in drastic inhibition of initial sphere formation, some neurosphere cultures substantially increased the number of neurospheres during this recovery period. In addition, neurosphere cultures that recovered after treatment, were dissociated, replated and found to form secondary spheres. Glioblastoma neurosphere cells previously treated with chemotherapy also maintained tumorigenicity. Collectively, these results demonstrate that some NICs treated with clinically relevant doses of chemotherapy avoided chemotoxicity and were capable of restoring the culture.
The expression of stem cell markers used in previous studies to isolate NICs varied greatly among our GBM cultures. In addition, there was no consistent increase or decrease in marker expression following chemotherapy treatment. Finally, even though the percentage of GS9-6 cells expressing CD133 and CD15 is much higher than that for U373NS, the U373NS culture, but not GS9-6, recovers from chemotherapy treatment. The percentage of CD133+ cells for GS7-2 is 18-fold greater than that for U373NS, but does not show enhanced recovery compared to U373NS. In agreement, several recent studies have shown that CD133 is not a reliable marker for cancer stem cells [38; 39; 40; 41]. In summary, stem cell marker expression varies among tumor cultures, does not consistently change following chemotherapy treatment and does not predict which glioblastoma cultures survive treatment with chemotherapy drugs. Neurosphere formation appears to be more accurate than marker expression for evaluation of NIC survival after chemotherapy treatment.
Two GBM neurosphere cultures that resumed neurosphere formation after chemotherapy treatment also underwent reversible cell cycle arrest as assayed by propidium iodide flow cytometry. Flow cytometry indicated that BCNU and TMZ induced a substantial accumulation of cells in G2/M or S phase three days after treatment. By seven days post treatment, some cells died and appeared as debris, but other cells resumed cycling and repopulated the culture. Cell cycle arrest in response to a toxic environment may preserve and maintain viable cell populations that are capable of resuming proliferation [7; 18; 20; 21].
An intriguing question is why in some cases, recovery occurs for one of the drugs and not the other. For example, the GS7-2 neurospheres show a robust recovery from 50 µM BCNU but not from 200 µM TMZ. This may be a result of the substantially different responses to BCNU-induced and TMZ-induced DNA damage [42; 43].
The GFP PTEN +/+ and PET2 cells allow us to make a direct comparison of a normal and transformed pair of cultures. The transformed PET2 cells are more resistant to these chemotherapy drugs. The comet assays showed that following treatment with 200 µM TMZ, the PET2 cells had more DNA breaks than the GFP PTEN +/+ cells. These results suggest that the PET2 cells do sustain DNA damage but are more resistant to the effects of DNA damage than the GFP PTEN +/+ cells. It has previously been noted that expression of the EGFRvIII receptor or loss of PTEN makes cells resistant to apoptosis [44; 45; 46].
A critical question is how these experiments relate to drug treatments used in the clinic. In patients, BCNU is usually administered in a wafer inserted by the surgeon into the tumor bed. The concentrations of BCNU within several centimeters of the wafer are quite high (1–7 mM) and toxic [2; 47]. More distal from the wafers, lower doses of BCNU would be less toxic and may induce reversible cell cycle arrest, enhancing tumor recurrence. In contrast, the in vivo serum concentration of TMZ is about 15 µM, and the concentration in the brain is estimated to be about 5 µM [48; 49; 50], which would inhibit proliferation of tumor-initiating cells but not induce tumor cell death. Indeed, 200 µM TMZ, which is never reached in vivo, was not sufficient to kill all of the NICs. The BCNU levels in the vicinity of the wafer are more than sufficient to kill both NICs and bulk cells. The TMZ levels are only sufficient to inhibit NIC proliferation and, thereby, may slow tumor growth. Hence, BCNU close to the wafer may work by a quite different mechanism compared with BCNU distal from the wafer or TMZ.
The neurosphere recovery observed in this study is clinically relevant. Our finding that the NICs can resume proliferating after treatment with clinically relevant doses of chemotherapy drugs may be similar to the recurrence that occurs in glioblastoma patients. Our experiments provide an in vitro model to analyze the NICs that are capable of recovery after chemotherapy treatment. Because the neurosphere cultures resemble patients’ tumors [17], this model will facilitate experiments testing new drugs to target the chemoresistant population.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Michael Glantz for the gift of the temozolomide and Drs. Stephen Lyle and Lyndon Kim for their comments on this manuscript. The University of Massachusetts Medical School Tumor Bank and Dr. Julian Wu of Tufts University School of Medicine supplied freshly excised glioblastomas. The University of Massachusetts Flow Cytometry Core analyzed marker expression and cell cycle profiles. We thank the National Institutes of Health for support (Grant NS021716).
Abbreviations
- BCNU
1,3-bis(2-chloroethy)-1-nitrosourea
- bFGF
basic fibroblast growth factor
- EGF
epidermal growth factor
- MGMT
O6-methylguanine DNA methyltransferase
- MMT
3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide
- MTS
3-[4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H- tetrazolium
- NIC
neurosphere initiating cells
- PTEN
phosphatase and tensin homologue deleted on chromosome 10
- TMZ
temozolomide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None declared.
REFERENCES
- 1.Friedman HS, Kerby T, Calvert H. Temozolomide and treatment of malignant glioma. Clin Cancer Res. 2000;6:2585–2597. [PubMed] [Google Scholar]
- 2.Grossman SA, Reinhard C, Colvin OM, Chasin M, Brundrett R, Tamargo RJ, Brem H. The intracerebral distribution of BCNU delivered by surgically implanted biodegradable polymers. J Neurosurg. 1992;76:640–647. doi: 10.3171/jns.1992.76.4.0640. [DOI] [PubMed] [Google Scholar]
- 3.Sarkaria JN, Kitange GJ, James CD, Plummer R, Calvert H, Weller M, Wick W. Mechanisms of chemoresistance to alkylating agents in malignant glioma. Clin Cancer Res. 2008;14:2900–2908. doi: 10.1158/1078-0432.CCR-07-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:2960–2965. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 5.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 6.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 7.Hambardzumyan D, Becher OJ, Rosenblum MK, Pandolfi PP, Manova-Todorova K, Holland EC. PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. Genes Dev. 2008;22:436–448. doi: 10.1101/gad.1627008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rietze RL, Valcanis H, Brooker GF, Thomas T, Voss AK, Bartlett PF. Purification of a pluripotent neural stem cell from the adult mouse brain. Nature. 2001;412:736–739. doi: 10.1038/35089085. [DOI] [PubMed] [Google Scholar]
- 9.Reynolds BA, Weiss S. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev. Biol. 1996;175:1–13. doi: 10.1006/dbio.1996.0090. [DOI] [PubMed] [Google Scholar]
- 10.Gritti A, Parati EA, Cova L, Frolichsthal P, Galli R, Wanke E, Faravelli L, Morassutti DJ, Roisen F, Nickel DD, Vescovi AL. Multipotential stem cells from the adult mouse brain proliferate and self-renew in response to basic fibroblast growth factor. J. Neurosci. 1996;16:1091–1100. doi: 10.1523/JNEUROSCI.16-03-01091.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gal H, Makovitzki A, Amariglio N, Rechavi G, Ram Z, Givol D. A rapid assay for drug sensitivity of glioblastoma stem cells. Biochem Biophys Res Commun. 2007;358:908–913. doi: 10.1016/j.bbrc.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 12.Clarke MF. A self-renewal assay for cancer stem cells. Cancer Chemother Pharmacol. 2005;56:64–68. doi: 10.1007/s00280-005-0097-1. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds BA, Rietze RL. Neural stem cells and neurospheres--re-evaluating the relationship. Nat Methods. 2005;2:333–336. doi: 10.1038/nmeth758. [DOI] [PubMed] [Google Scholar]
- 14.Golmohammadi MG, Blackmore DG, Large B, Azari H, Esfandiary E, Paxinos G, Franklin KB, Reynolds BA, Rietze RL. Comparative analysis of the frequency and distribution of stem and progenitor cells in the adult mouse brain. Stem Cells. 2008;26:979–987. doi: 10.1634/stemcells.2007-0919. [DOI] [PubMed] [Google Scholar]
- 15.Louis SA, Rietze RL, Deleyrolle L, Wagey RE, Thomas TE, Eaves AC, Reynolds BA. Enumeration of neural stem and progenitor cells in the neural colony-forming cell assay. Stem Cells. 2008;26:988–996. doi: 10.1634/stemcells.2007-0867. [DOI] [PubMed] [Google Scholar]
- 16.Friedrich J, Seidel C, Ebner R, Kunz-Schughart LA. Spheroid-based drug screen: considerations and practical approach. Nat Protoc. 2009;4:309–324. doi: 10.1038/nprot.2008.226. [DOI] [PubMed] [Google Scholar]
- 17.Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, Park JK, Fine HA. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 18.Mellor HR, Ferguson DJ, Callaghan R. A model of quiescent tumour microregions for evaluating multicellular resistance to chemotherapeutic drugs. Br J Cancer. 2005;93:302–309. doi: 10.1038/sj.bjc.6602710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viale A, De Franco F, Orleth A, Cambiaghi V, Giuliani V, Bossi D, Ronchini C, Ronzoni S, Muradore I, Monestiroli S, Gobbi A, Alcalay M, Minucci S, Pelicci PG. Cell-cycle restriction limits DNA damage and maintains self-renewal of leukaemia stem cells. Nature. 2009;457:51–56. doi: 10.1038/nature07618. [DOI] [PubMed] [Google Scholar]
- 20.Chauffert B, Dimanche-Boitrel MT, Garrido C, Ivarsson M, Martin M, Martin F, Solary E. New insights into the kinetic resistance to anticancer agents. Cytotechnology. 1998;27:225–235. doi: 10.1023/A:1008025124242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishikawa F, Yoshida S, Saito Y, Hijikata A, Kitamura H, Tanaka S, Nakamura R, Tanaka T, Tomiyama H, Saito N, Fukata M, Miyamoto T, Lyons B, Ohshima K, Uchida N, Taniguchi S, Ohara O, Akashi K, Harada M, Shultz LD. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol. 2007;25:1315–1321. doi: 10.1038/nbt1350. [DOI] [PubMed] [Google Scholar]
- 22.Reynolds BA, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J. Neurosci. 1992;12:4565–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li L, Dutra A, Pak E, Labrie JE, 3rd, Gerstein RM, Pandolfi PP, Recht LD, Ross AH. EGFRvIII expression and PTEN loss synergistically induce chromosomal instability and glial tumors. Neuro-oncology. 2009;11:9–21. doi: 10.1215/15228517-2008-081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Ren R. Bcr-Abl efficiently induces a myeloproliferative disease and production of excess interleukin-3 and granulocyte-macrophage colony-stimulating factor in mice: a novel model for chronic myelogenous leukemia. Blood. 1998;92:3829–3840. [PubMed] [Google Scholar]
- 25.Sen A, Kallos MS, Behie LA. New tissue dissociation protocol for scaled-up production of neural stem cells in suspension bioreactors. Tissue Eng. 2004;10:904–913. doi: 10.1089/1076327041348554. [DOI] [PubMed] [Google Scholar]
- 26.Sherry MM, Reeves A, Wu JK, Cochran BH. STAT3 is required for proliferation and maintenance of multipotency in glioblastoma stem cells. Stem Cells. 2009;27:2383–2392. doi: 10.1002/stem.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 28.Ishii N, Maier D, Merlo A, Tada M, Sawamura Y, Diserens AC, Van Meir EG. Frequent co-alterations of TP53, p16/CDKN2A, p14ARF, PTEN tumor suppressor genes in human glioma cell lines. Brain Pathol. 1999;9:469–479. doi: 10.1111/j.1750-3639.1999.tb00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collins AR, Oscoz AA, Brunborg G, Gaivao I, Giovannelli L, Kruszewski M, Smith CC, Stetina R. The comet assay: topical issues. Mutagenesis. 2008;23:143–151. doi: 10.1093/mutage/gem051. [DOI] [PubMed] [Google Scholar]
- 30.Ueda-Kawamitsu H, Lawson TA, Gwilt PR. In vitro pharmacokinetics and pharmacodynamics of 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) Biochem Pharmacol. 2002;63:1209–1218. doi: 10.1016/s0006-2952(02)00878-x. [DOI] [PubMed] [Google Scholar]
- 31.Gunther W, Pawlak E, Damasceno R, Arnold H, Terzis AJ. Temozolomide induces apoptosis and senescence in glioma cells cultured as multicellular spheroids. Br J Cancer. 2003;88:463–469. doi: 10.1038/sj.bjc.6600711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tchoghandjian A, Baeza N, Colin C, Cayre M, Metellus P, Beclin C, Ouafik L, Figarella-Branger D. A2B5 Cells from Human Glioblastoma have Cancer Stem Cell Properties. Brain Pathol. 2009;XXX:XXX. doi: 10.1111/j.1750-3639.2009.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mao XG, Zhang X, Xue XY, Guo G, Wang P, Zhang W, Fei Z, Zhen HN, You SW, Yang H. Brain Tumor Stem-Like Cells Identified by Neural Stem Cell Marker CD15. Transl Oncol. 2009;2:247–257. doi: 10.1593/tlo.09136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogden AT, Waziri AE, Lochhead RA, Fusco D, Lopez K, Ellis JA, Kang J, Assanah M, McKhann GM, Sisti MB, McCormick PC, Canoll P, Bruce JN. Identification of A2B5+CD133- tumor-initiating cells in adult human gliomas. Neurosurgery. 2008;62:505–514. doi: 10.1227/01.neu.0000316019.28421.95. discussion 514–505. [DOI] [PubMed] [Google Scholar]
- 35.Sun Y, Kong W, Falk A, Hu J, Zhou L, Pollard S, Smith A. CD133 (Prominin) negative human neural stem cells are clonogenic and tripotent. PLoS ONE. 2009;4:e5498. doi: 10.1371/journal.pone.0005498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Son MJ, Woolard K, Nam DH, Lee J, Fine HA. SSEA-1 is an enrichment marker for tumor-initiating cells in human glioblastoma. Cell Stem Cell. 2009;4:440–452. doi: 10.1016/j.stem.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calabrese EJ. Cancer biology and hormesis: human tumor cell lines commonly display hormetic (biphasic) dose responses. Crit Rev Toxicol. 2005;35:463–582. doi: 10.1080/10408440591034502. [DOI] [PubMed] [Google Scholar]
- 38.Gilbert CA, Ross AH. Cancer stem cells: cell culture, markers, and targets for new therapies. J Cell Biochem. 2009;108:1031–1038. doi: 10.1002/jcb.22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, Sakariassen PO, Tsinkalovsky O, Immervoll H, Boe SO, Svendsen A, Prestegarden L, Rosland G, Thorsen F, Stuhr L, Molven A, Bjerkvig R, Enger PO. CD133 negative glioma cells form tumors in nude rats and give rise to CD133 positive cells. Int J Cancer. 2008;122:761–768. doi: 10.1002/ijc.23130. [DOI] [PubMed] [Google Scholar]
- 40.Cheng JX, Liu BL, Zhang X. How powerful is CD133 as a cancer stem cell marker in brain tumors? Cancer Treat Rev. 2009;35:403–408. doi: 10.1016/j.ctrv.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Dittfeld C, Dietrich A, Peickert S, Hering S, Baumann M, Grade M, Ried T, Kunz-Schughart LA. CD133 expression is not selective for tumor-initiating or radioresistant cell populations in the CRC cell lines HCT-116. Radiother Oncol. 2009;92:353–361. doi: 10.1016/j.radonc.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 42.Roos WP, Batista LF, Naumann SC, Wick W, Weller M, Menck CF, Kaina B. Apoptosis in malignant glioma cells triggered by the temozolomide-induced DNA lesion O6-methylguanine. Oncogene. 2007;26:186–197. doi: 10.1038/sj.onc.1209785. [DOI] [PubMed] [Google Scholar]
- 43.Batista LF, Roos WP, Christmann M, Menck CF, Kaina B. Differential sensitivity of malignant glioma cells to methylating and chloroethylating anticancer drugs: p53 determines the switch by regulating xpc, ddb2 and DNA double-strand breaks. Cancer Res. 2007;67:11886–11895. doi: 10.1158/0008-5472.CAN-07-2964. [DOI] [PubMed] [Google Scholar]
- 44.Chakravarti A, Dicker A, Mehta M. The contribution of epidermal growth factor receptor (EGFR) signaling pathway to radioresistance in human gliomas: a review of preclinical and correlative clinical data. Int J Radiat Oncol Biol Phys. 2004;58:927–931. doi: 10.1016/j.ijrobp.2003.09.092. [DOI] [PubMed] [Google Scholar]
- 45.Nagane M, Levitzki A, Gazit A, Cavenee WK, Huang HJ. Drug resistance of human glioblastoma cells conferred by a tumor- specific mutant epidermal growth factor receptor through modulation of Bcl-XL and caspase-3-like proteases. Proc Natl Acad Sci U S A. 1998;95:5724–5729. doi: 10.1073/pnas.95.10.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes & Development. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 47.Fung LK, Ewend MG, Sills A, Sipos EP, Thompson R, Watts M, Colvin OM, Brem H, Saltzman WM. Pharmacokinetics of interstitial delivery of carmustine, 4-hydroperoxycyclophosphamide, and paclitaxel from a biodegradable polymer implant in the monkey brain. Cancer Res. 1998;58:672–684. [PubMed] [Google Scholar]
- 48.Baker SD, Wirth M, Statkevich P, Reidenberg P, Alton K, Sartorius SE, Dugan M, Cutler D, Batra V, Grochow LB, Donehower RC, Rowinsky EK. Absorption, metabolism, and excretion of 14C-temozolomide following oral administration to patients with advanced cancer. Clin Cancer Res. 1999;5:309–317. [PubMed] [Google Scholar]
- 49.Spiro TP, Liu L, Majka S, Haaga J, Willson JK, Gerson SL. Temozolomide: the effect of once- and twice-a-day dosing on tumor tissue levels of the DNA repair protein O(6)-alkylguanine-DNA-alkyltransferase. Clin Cancer Res. 2001;7:2309–2317. [PubMed] [Google Scholar]
- 50.Ostermann S, Csajka C, Buclin T, Leyvraz S, Lejeune F, Decosterd LA, Stupp R. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin Cancer Res. 2004;10:3728–3736. doi: 10.1158/1078-0432.CCR-03-0807. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.