Abstract
Taste buds signal the presence of chemical stimuli in the oral cavity to the central nervous system using both early transduction mechanisms, which allow single cells to be depolarized via receptor-mediated signaling pathways, and late transduction mechanisms, which involve extensive cell-to-cell communication among the cells in the bud. The latter mechanisms, which involve a large number of neurotransmitters and neuropeptides, are less well understood. Among neurotransmitters, multiple lines of evidence suggest that norepinephrine plays a yet unknown role in the taste bud. This study investigated the expression pattern of adrenergic receptors in the rat posterior taste bud. Expression of α1A, α1B, α1D, α2A, α2B, α2C, β1, and the β2 adrenoceptor subtypes was observed in taste buds using RT-PCR and immunocytochemical techniques. Taste buds also expressed the biosynthetic enzyme for norepinephrine, dopamine β-hydroxylase (DβH), as well as the norepinephrine transporter. Further, expression of the epinephrine synthetic enzyme, phenylethanolamine N-methyltransferase (PNMT), was observed suggesting a possible role for this transmitter in the bud. Phenotyping adrenoceptor expression patterns with double labeling experiments to gustducin, synaptosomal-associated protein 25 (SNAP-25), and neural cell adhesion molecule (NCAM) suggests they are prominently expressed in subsets of cells known to express taste receptor molecules but segregated from cells known to have synapses with the afferent nerve fiber. Alpha and beta adrenoceptors co-express with one another in unique patterns as observed with immunocytochemistry and single cell RT-PCR. These data suggest that single cells express multiple adrenergic receptors and that adrenergic signaling may be particularly important in bitter, sweet, and umami taste qualities. In summary, adrenergic signaling in the taste bud occurs through complex pathways that include presynaptic and postsynaptic receptors and likely play modulatory roles in processing of gustatory information similar to other peripheral sensory systems such as the retina, cochlea, and olfactory bulb.
Keywords: adrenoceptors, alpha-receptors, beta-receptors, norepinephrine, epinephrine, gustation
Taste buds are collections of 50 to 100 individual cells which act as sensory receptors for chemical stimuli within the oral cavity. These cells have long been understood to be heterogeneous in their anatomical and physiological properties. Anatomically, cells are commonly classified into type I, II, and III based on morphological differences in cytoplasmic translucency, structure, and synaptic connectivity (e.g. Murray, 1973). Physiologically, taste receptor cells (TRCs) respond to unique combinations of chemicals representative of multiple taste qualities (e.g., Herness 2000). These response profiles are at odds with molecular data which demonstrated that tastant receptors for chemicals leading to the generation of sweet, bitter, sour, salty, and umami taste qualities are expressed in restricted non-overlapping cells (e.g., Yarmolinsky et al., 2009; Chandarchekar et al., 2010). Recent advances have elucidated a number of neurotransmitter and neuropeptide signaling agents in the taste bud which may help reconcile the discrepancy of physiological and molecular observations. Current notions suggest that these signaling molecules and their receptors set up hard-wired communication routes across distinct subgroups of cells thus allowing extensive cell-to-cell communication. These changing concepts have lead to the notion of both early and late transduction processes (e.g., Cao et al., 2009). Early events describe interactions with receptors and subsequent downstream transduction events within a single cell. Late events involve processing of information among cells of the taste bud which helps to shape the discharge of afferent nerve fibers. Thus a single cell may be excited not only through apical receptor activation but also through basolateral neurotransmitter release.
Events of late transduction are particularly ill understood and how these hardwired pathways participate in the transduction cascades of taste quality processing is the subject of active investigation. It is now known that multiple neurotransmitters, such as serotonin (Kaya et al., 2004, Huang et al., 2005), norepinephrine (NE, Herness et al., 2002b), acetyl choline (Ogura, 2002), γ-amino butyric acid (GABA, Cao et al., 2009), and adenosine trisphosphate (ATP, Finger et al., 2005), as well as neuropeptides, such as cholecystokinin (CCK, Herness et al., 2002a), neuropeptide Y (NPY, Zhao et al., 2005), and glucagon-like peptide-1 (GLP-1, Shin et al., 2008), participate within the bud. In general, the role of these agents in coding of taste information within the bud isn’t known. Norepinephrine has long been suspected as a possible transmitter in the taste bud though the understanding of the distribution of this catecholamine and its receptors remains rudimentary. The earliest suggestions that subsets of TRCs in rabbits and mice could be adrenergic came from histofluorescence studies (Cano et al., 1982a, Cano et al., 1982b, Gabella 1969, Geerdink and Drukker 1973, Takeda and Kitao 1980, Takeda et al., 1982). Some studies also suggested that there may be adrenergic fibers that surround the taste bud (Paparelli et al., 1986, Paparelli et al., 1988, Sato et al., 2005, Sato et al., 2006).
In the mammalian system, the first physiological studies implicating NE at the level of TRCs demonstrated that chloride currents were enhanced by its exogenous application (Herness and Sun 1999). Subsequently, it was shown that some TRCs are adrenergic and that TRCs express both alpha and beta adrenoceptors (Herness 2002b). A later study (Dvoryanchikov et al., 2007) raised the interesting possibility that TRCs do not synthesize NE, as they lack expression of its synthetic enzyme, dopamine-β-hydroxylase (DβH). These data contrast with those of Ando et al., (2007) who demonstrated DβH-like immunoreactive cells in the frog taste disc. However, TRCs do express molecules involved in the transporting, inactivation, and packaging of NE which include norepinephrine transporter (NET), catechol-O-methyltransferase, monoamine oxidase-A, vesicular monoamine transporter (1 and 2), and chromogranin A.
TRCs have also been documented to release and respond to exogenous NE application. In mouse TRCs, NE is released from type III cells in response to a taste stimuli mixture (Huang et al., 2008). Release was not observed from type II cells. A small subset of these cells co-released both 5HT and NE. Since these cells were isolated using green fluorescent protein expression driven by a glutamate decarboxylase promoter, these data further suggest that this group of cells may actually co-store GABA, serotonin, and NE. In rat TRCs, activation of alpha receptors acts to elevate intracellular calcium levels while activation of beta receptors produces an inhibition of outward potassium currents (Herness et al., 2002b). Both responses would result in overall excitation of the cell.
A role for NE in the peripheral gustatory system has also been suggested in humans. A most intriguing finding is that Heath et al., (2006) demonstrated that human taste thresholds are modulated by NE; enhancing NE levels (using a NE reuptake inhibitor) notably reduced bitter and sour thresholds. Maetsu et al., (2007) found that a human adrenergic α2A receptor polymorphism lead to higher consumption of sweet food. In a similar vein, Young and Mathias (2004) found taste and smell could be disturbed by alpha-adrenoceptor agonist midodrine. These studies share findings of NE as an excitatory modulator in the gustatory system that is involved in more than one taste quality. Further understanding of the role of adrenergic transmission in the taste bud will require examination of the types and pattern of adrenoceptor expression in the bud.
Adrenergic receptors are members of the G protein-coupled receptor (GPCR) superfamily and are classified into three major families: α1-adrenoceptor, α2-adrenoceptor, and β-adrenoceptor (e.g. Bylund 2006; Hein 2006, Philipp and Hein 2004). These families are further subdivided into nine receptor subtypes, α1A, α1B, α1D, α2A, α2B, α2C, β1, β2, and β3. Adrenoceptors are mostly distinct in their G-protein coupled signaling pathways. Alpha1-adrenoceptors are coupled to the Gq signaling pathway and result in activation of phospholipase C. Alpha2-adrenoceptors are coupled with the Gi/Go family of G-proteins and inhibit adenylate cyclase but can also activate the mitogen-activated protein kinase cascade as well as activate K+ channels and stimulate Ca2+ influx. In neurons, alpha-2 ARs serve to suppress neurotransmitter release. Beta receptors mediate their response via the Gs family of G-proteins and act to activate adenylate cyclase. They can also couple to the Gi proteins resulting in the stimulation of mitogen-activated protein kinase pathways. Growing evidence suggests that adrenoceptors are able to form dimers or oliogomers to perform their physiological functions (e.g., Minneman, 2006).
The purpose of this investigation was to examine the expression of adrenergic receptor subtypes in rat TRCs and to elucidate their phenotypic expression pattern across the taste bud.
EXPERIMENTAL PROCEDURES
Subjects
Experiments were performed on adult male Sprague-Dawley rats. All procedures were approved by The Ohio State University’s Laboratory Animal Care and Use Committee and adhered to the NIH “Guide for the Care and Use of Laboratory Animals”. Subjects were brought to a surgical level of anesthesia with a 0.09 ml/100 gm body weight dosage of a 91 mg/ml Ketamine/0.09 mg/ml Acepromazine mixture, sacrificed, and posterior gustatory papillae quickly dissected. For immunocytochemistry, excised papillae were post-fixed by immersion in either 4% paraformaldehyde or Bouin’s fixative for 5 hours at 4°C. Tissue blocks were dehydrated, embedded in paraffin, and sectioned on a rotary microtome at 4 μm thickness. Sections were collected onto Fisher Superfrost Plus slides. For PCR analysis, whole taste buds were mechanically dissociated from lingual epithelium enzymatically isolated using 1 mg/ml collagenase, 1mg/ml trypsin inhibitor and 2.5 mg/ml dispase in mammalian physiological saline (in mM: 120 NaCl, 20 KCl, 10 HEPES, and 2 BAPTA, pH 7.4). Isolated taste buds were collected under microscopic examination.
Immunocytochemistry
Conventional and TSA-amplified immunocytochemistry protocol
Immunocytochemistry was performed on paraffin sections using standard fluorescent protocols as previously described (e.g. Kaya et al., 2004, Zhao et al., 2005, Cao et al., 2009). Sections were deparaffinized, rehydrated, blocked with 10% normal serum diluted in 0.01 M physiologically buffered saline (PBS; pH 7.4) for one hour at room temperature, and incubated in primary antiserum (diluted in PBS containing 2% normal serum) at the appropriate dilutions (Table 1). Slides were housed in a closed moist chamber overnight at 4°C. A citric acid buffer pretreatment was applied for the anti-alpha adrenergic receptor antibodies prior to the blocking step. The following day, sections were rinsed in PBS and then incubated with fluorescein or Cy3-conjugated secondary antibody (1:200 to 1:800 in PBS containing 1.5% normal serum) at room temperature for one hour in the dark. Slides were mounted in Fluoro-Gel. Digital photos of immunofluorescence cells were processed using Metamorph software.
Table I.
List of primary antibodies used in investigation of adrenergic receptors in taste receptor cells.
| Antigen | Immunogen | Manufacturer, species, type, catalog number | Dilution |
|---|---|---|---|
| Gα gust | A peptide mapping within a highly divergent domain of Gα gust of rat origin | Santa Cruz Biotech, rabbit polyclonal, affinity purified IgG, sc-395 | 1:1000 1:5000 |
| SNAP-25 | Human crude synaptic immunoprecipitate that recognizes SNAP-25 protein | Millipore, mouse monoclonal, MAB331 | 1:5000 |
| NCAM | Highly purified chicken NCAM | Chemicon, rabbit polyclonal, affinity purified AB5032 | 1:2000 |
| α1A-AR | Epitope mapping at the C-terminus of α1A-AR of human origin | Santa Cruz Biotech goat polyclonal, affinity purified IgG, sc-1477 | 1:500 1:3000 |
| α1B-AR | Epitope corresponding to a peptide mapping at the C-terminus of α1B adrenergic receptor of human origin, 94% homology with rat | Santa Cruz Biotech, goat polyclonal, affinity purified IgG, sc-1476 | 1:500 |
| α1D-AR | Epitope corresponding to a peptide mapping at the C-terminus of α1D adrenergic receptor of rat origin | Santa Cruz Biotech, goat polyclonal, affinity purified IgG, sc-1475 | 1:200 |
| α2A-AR | Synthetic peptide: C-TERRPNGLGPERS, corresponding to Internal sequence amino acids 246-258 of Human alpha 2a Adrenergic Receptor | Abcam, goat polyclonal, affinity purified IgG, ab45871. | 1:50 |
| α2B-AR | Epitope corresponding to a peptide mapping at the C-terminus of α2B adrenergic receptor of human origin,100% homology with rat | Santa Cruz Biotech, goat polyclonal, affinity purified IgG, sc-1479 | 1:80 |
| α2C-AR | Epitope corresponding to a peptide mapping at the C-terminus of α2C adrenergic receptor of human origin,100% homology with rat | Santa Cruz Biotech, goat polyclonal, affinity purified IgG, sc-30439 | 1:300 |
| β1-AR | Epitope mapping at the C-terminus of β1-AR of mouse origin | Santa Cruz Biotech, rabbit polyclonal, affinity purified IgG, sc-568 | 1:50 1:6000 |
| β1-AR | Synthetic peptide: H(394)GDRPRASGCLARAG(408). Immunizing peptide corresponds to amino acid residues 394-408 of mouse β1-AR. This sequence is completely conserved between mouse and rat β1AR | Abcam, rabbit polyclonal, affinity purified IgG, ab3546 | 1:50 1:750 |
| β2-AR | A synthetic peptide mapping at the C-terminus of β2-AR of mouse origin | Santa Cruz Biotech, rabbit polyclonal, affinity purified IgG, sc-570 | 1:50 1:6000 |
| PNMT | Bovine PNMT | Millipore, rabbit polyclonal, AB110. | 1:600 |
| DβH | Bovine DβH | Milipore, mouse monoclonal, MAB308 | 1:3000 |
For experiments using tyramide signal amplification (TSA; Perkin Elmer), tissue sections were first incubated with a solution of 0.5% hydrogen peroxide in methanol for 30 min to eliminate endogenous peroxidase activity. To reduce nonspecific antibody binding, sections were then incubated for one hour at room temperature in PBS containing 10% normal serum and 0.3% Triton X-100. Primary antiserum (diluted in PBS containing 2% normal serum; Table 1) was subsequently applied to the sections and the slides were housed in a closed moist chamber for 36 hours at 4°C. After PBS washing, sections were incubated with biotin-streptavidin-conjugated IgG Fab fragment (1:800 in PBS containing 1.5% normal serum) for one hour at room temperature and processed according to kit instructions (NEN Life Science Products, Boston, MA). Slides were mounted in Fluoro-Gel and visualized on a fluorescent microscope.
Double label immunocytochemistry
For double labeling experiments using primary antibodies raised in different species, after incubation of the secondary fluorescein antibody following the conventional immunocytochemistry protocol, the sections were rinsed in PBS and then incubated in the second primary antiserum at the appropriate dilutions (diluted in PBS containing 2% normal serum) at 4°C overnight in the dark. After incubation, sections were rinsed in PBS and then incubated for 1 hour at room temperature in a corresponding secondary fluorescein antibody (1:400 or 1:800 in PBS containing 1.5% normal serum) for one hour at room temperature in the dark, rinsed in PBS, and coverslipped with Fluoro-Gel.
An indirect immunofluorescence double-labeling protocol was modified to allow localization of two antigens in the same preparation when both primary antibodies are raised in the same species as previously described (Kaya et al., 2004, Zhao et al., 2005, Cao et al., 2009). This protocol involves using TSA with a Fab fragment secondary antibody for detection of the first primary antibody. With the use of TSA, the first primary antibody can be used at very low concentration so that the antigen can only be detected by TSA but not by a conventional fluorophore-conjugated secondary antibody, which prevents the cross-reaction between the first primary antibody and the second secondary antibody (referred to as interference I), while the use of a Fab fragment instead of the whole IgG molecule or F(ab)2 fragment as the first secondary antibody prevents the capture of the second primary antibody by the first secondary antibody (interference II). Therefore, this modified protocol prevents cross-reactions between a primary antibody and its unintended secondary antibody. Control experiments were performed as previously described (Kaya et al., 2004, Zhao et al., 2005, Cao et al., 2009) to ensure that fluorescent signal did not arise from cross-reactivity.
Cell counting and data analysis
When possible, immunocytochemical data were analyzed quantitatively by cell counting. Sections were chosen from at least three independent experiments repeated identically using different animals. To prevent a TRC from being counted twice, one out of every four consecutive four micron sections (i.e., sections spaced by greater than 8 microns) were selected and adjacent sections were never chosen for analysis. Using either single- or double-labeling protocols, positively stained TRCs were typically observed in a great majority of the taste buds identified on each section. Individual buds were selected for analysis and only those labeled cells that displayed apparent apical processes and/or perinuclear region were counted.
To ensure the consistency between the conventional and the amplification protocols, dilution series of the primary antibodies were performed. The most appropriate dilutions for each antibody were chosen (Table 1) so that when comparing the numbers of labeled cells (per cross section of a taste bud) obtained from either protocol using the same primary antibody, no significant difference was observed between the two. Cell counting results of the experiments using both foliate and circumvallate papillae were pooled. Selected image files were processed in Metamorph software where they were optimized for contrast and brightness and supplemented with scale bars. Image files were then imported into Canvas Illustrator and adjusted for size and cropping to produce illustrative montage figures for publication.
RT-PCR
Standard RT-PCR
RT-PCR experiments were performed on total RNA isolated from individually harvested circumvallate taste buds (20–50) collected into a 1.5 ml microtube containing 100 μl of TRIzol reagent using Totally RNA Isolation Kit (Ambion, Inc., Austin, TX, USA) according to manufacturer’s recommendations. RNA was treated for 30 minutes with amplification grade DNase-I. First strand cDNA was synthesized from total RNA extracted from pure taste buds or control tissue using oligo(dT)12-18 primer. Subsequently, the following components were added to the reaction, with a final total volume of 20 μl: 1x of First Strand Buffer, 10 mM DTT, 500 μM each dNTP, and 200 Units of SuperScript™ II RNase H-Reverse Transcriptase (Invitrogen Life Technologies). The cDNA of pure taste buds was also produced by using Qiagen RNase MiniElute Cleanup Kit (cat No. 74024) and MessageBOOSTER cDNA Synthesis Kit for qPCR (EPICENTRE® Biotechnologies) according to the manufacturer’s instructions.
PCR was performed in a volume of 20 μl using 0.5 μl of cDNAs for each reaction. The standard reaction mixture consisted of 10 μL iQ SYBR Green Supermix (Bio-Rad Laboratories), 0.5 μL each of 100 μM forward and reverse primers, and 8.5 μL nuclease-free water. The PCR profile was 94 °C at 5 min (1 cycle), 94 °C at 30 sec, 55 °C at 30 sec, 72 °C at 45 sec (35 cycles), and 72 °C at 10 min (1 cycle). Primers sequences are described in Table 2. All primers sequences are written 5′ to 3′. PCR products were separated by gel electrophoresis in a 1.5 % agarose gel containing 0.5 μg/ml ethidium bromide, observed under UV light, and photographed. To verify the specificity of the bands, PCR products were either purified by Concert Rapid PCR Purification System (Invitrogen Corp., Carlsbad, CA, USA) and directly sequenced or cloned (Original TA Cloning Kit, Invitrogen Corp., Carlsbad, CA, USA) and sequenced at Plant-Microbe Genomics Facility at The Ohio State University. Identity of the bands was confirmed with BLAST search at the NCBI.
Table II.
Primer Sequences used in RT-PCR reactions for adrenergic receptor subtypes.
| Target | Accession No. | Primers | Product Size | Reference |
|---|---|---|---|---|
| α 1a | NM_017191 | GAATGTCCTGCGAATCCAGT GATTGGTCCTTTGGCACTGT |
237 bp | |
| α1b | NM_016991 | GCTCCTTCTACATCCCGCTCG AGGGGAGCCAACATAAGATGA |
300 bp | (Scofield et al., 1995) |
| α1d | NM_024483 | AGCCTCTGCACCATCTCTGT AAGGAGCACACGGAAGAGAA |
233 bp | (Sun et al., 2007) |
| α2a | NM_012739 | GGTAAGGTGTGGTGCGAGAT CAGCGCCCTTCTTCTCTATG |
229 bp | (Sun et al., 2007) |
| α2b | NM_0138505 | GCACCACAAAACCTGTTCCT TTGTAGATGAGGGGCGGTAG |
320 bp | |
| α2c | NM_0138506 | TACTGTGCTGGTTCCCCTTC CAGAGGCCCAGTTGTCTCTC |
380 bp | (Sun et al., 2007) |
| β1 | NM_012701 | CGCTCACCAAACCTCTTCATCATGTCC CAGCACTTGGGGTCGTTGTAGGAGC |
376 bp | (Troispoux et al., 1998) |
| β2 | NM_012492 | TCTTCGAAAACCTATGGGAACGGC GGATGTGCCCCTTCTGCAAAATCT |
343 bp | (Troispoux et al., 1998) |
| β-arrestin 1 | NM_012910 | GTCAAAGTGAAGCTGGTGGTGTC CCATCATCCTCTTCGTCCTTGTC |
260 bp | (Lymperopoulos et al., 2007) |
| β-arrestin 2 | NM_012911 | TACAGGGTCAAGGTGAAGCTGGT GGTCATCACAGTCGTCATCCTTC |
256 bp | (Lymperopoulos et al., 2007) |
| Gustducin | X65747 | GTTGGCTGAAATAATTAAACG ATCTCTGGCCACCTACATC |
251 bp | (McLaughlin et al., 1992) |
| t1r3 | NM_130818 | CCTCTTCTGCCTCAGTGTCC TAAGCTAGCATGGCGAAGGT |
468bp | |
| t2r9 | NM_023999 | TTTCATGGGCAATCTCCTTC CATGTGGCCCTGAGATCTTT |
514bp | |
| DBH | NM_013158 | GGATCGAGGTGAGATGGAGA CTCCTCCAGGATCCCATACA |
256bp | (Zhu et al., 2005) |
| DBH | NM_013158 | CCTTGAAGGGACTTTAGAGC AGCAGCTGGTAGTCCTGATG |
240 bp | (Giancippoli et al., 2006) |
| PNMT | NM_031526 | TACCTCCGCAACAACTACGC AAGGCTCCTGGTTCCTCTCG |
260bp | (Kubovcakova et al., 2006) |
| NET | NM_031341 | CATCAACTGTGTTACCAGTTTTATT AAACATGGCCAGAAGAAAGGTACC |
334bp | (Bruss et al., 1997, Inazu et al., 2003) |
| β-actin | NM_031144 | GCCAACCGTGAAAAGATGAC GTCTCCGGAGTCCATCACAA |
132bp | |
| CK8 | NM_199370 | ATGCAGAACATGAGCATC ACAGCCACTGAGGCTTTA |
440 bp | (Kishi et al., 2001) |
Single Cell RT-PCR
Phenotyping of expression patterns within single TRCs was accomplished using a strategy that first amplifies RNA in a linear fashion prior to cDNA synthesis (MessageBOOSTER, Epicentre Biotechnologies). RNA was first purified and desalted using RNeasy MinElute Cleanup Kit (Qiagen). Each sample was then concentrated using an Eppendorf Vacufuge to the 2 μl volume required by the MessageBOOSTER cDNA Synthesis Kit for qPCR. RNA was reverse transcribed with MMLV Reverse Transcriptase into 1st-strand cDNA using a synthetic oligo(dT) primer containing a phage T7 RNA polymerase promoter sequence at its 5′ end and producing a cDNA:RNA hybrid. Second-strand cDNA synthesis employed resulted in a double-stranded cDNA containing a T7 transcription promoter in an orientation that generates anti-sense RNA (aRNA) during the subsequent in vitro transcription reaction. The aRNA was purified again by using RNeasy MinElute Cleanup Kit. Purified aRNA was subsequently reverse transcribed into first-strand cDNA using MMLV Reverse Transcriptase with random hexamers primers. PCR was performed on cDNA using standard techniques. For most cells, primers sets for multiple molecules of interest, such as TRPM5, T2R8, T2R9, T1R3, gustducin, as well as the positive controls cytokeratin 8 (CK-8) and β-actin were tested. Each PCR reaction was run independently under its own optimized conditions. Several controls ensured that amplified PCR product arose from cDNA produced from mRNA template and not from either genomic or extraneous sources of DNA. For every reaction, a H2O control lacking template was run to control for carry-over contamination. Another reaction, using extracellular solution collected from close to the cell site, was run to control for extraneous sources of DNA which could arise from the cell lysis during the dissociation procedure.
RESULTS
Multiple adrenoceptors are expressed in the taste bud
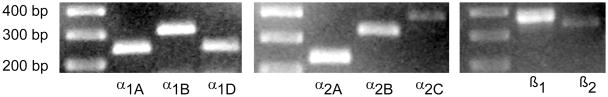
Previous work establishing expression of multiple adrenoceptor subtypes (α1A, α1B, α1D, α2A, α2B, α2C, β1, β2) in the lingual epithelium of rat (Herness et al., 2002) left open the question of which subtypes were specifically expressed in TRCs. To answer this question, RT-PCR was performed using pure taste buds (rather than lingual epithelium) as starting material. Reverse transcribed cDNA was tested with primers to eight subtypes of adrenoceptors: α1A, α1B, α1D, α2A, α2B, α2C, β1, β2. Primers (sequences presented in Table 1) were previously optimized using template cDNA reverse transcribed from positive control tissue (cerebral cortex, heart, liver, and adrenal gland). PCR reactions yielded products of the correct size for all eight tested primers sets (Figure 1). These were α1A (212 base pairs), α1B (300 bp), α1D (304 bp), α2A (312 bp), α2B (456bp), α2C (425bp), β1 (376bp) and β2 (343bp). Amplified products were cloned, sequenced and analyzed by BLAST search at NCBI to verify their identities. Controls for DNA contamination and PCR carryover were performed. These included omission of the reverse transcriptase enzyme (RT-) to control for genomic contamination and omission of template (H2O) control, respectively. PCR was also performed on cDNA reverse transcribed from RNA isolated from positive control tissue that included brain, heart, lung, adipose tissue, liver, adrenal medulla, and kidney. Additionally, reactions using primers for a housekeeping gene (β-actin) and for a robustly expressed taste specific gene (α-gustducin) were performed as positive controls.
Fig. 1.
RT-PCR examination of adrenergic receptor expression in taste buds. RT-PCR was performed on cDNA derived from isolated whole taste buds. PCR reactions to all eight tested adrenoceptor subtypes resulted in product of appropriate size. Markers are in left lane of each gel picture.
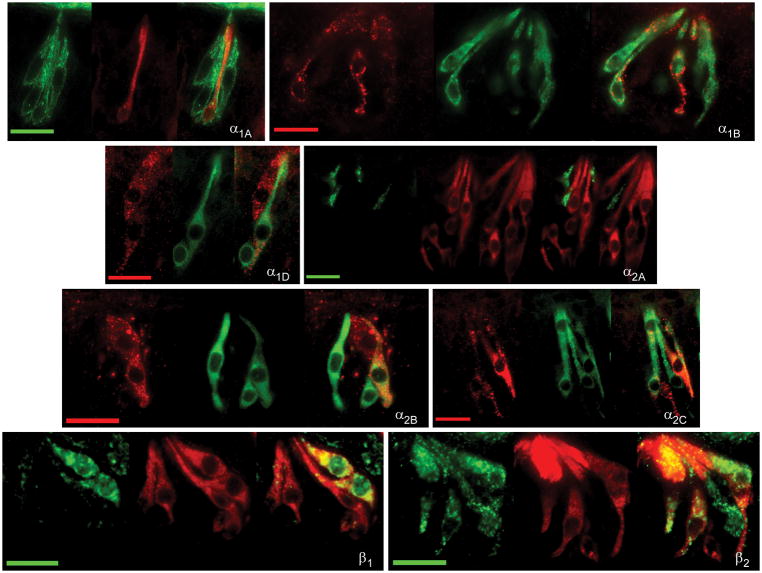
Verification of protein expression for adrenoceptors observed with RT-PCR was performed using immunocytochemistry. Commercial antibodies were obtained for α1A, α1B, α1D, α2A, α2B, α2C, β1, and the β2 adrenergic receptor subtypes (Table 2). Omission of primary antibody was performed as a negative control. No positive staining of the secondary antibody was observed in either conventional or TSA amplified immunocytochemistry experiments. Immunopositive TRCs were observed for each tested antibody (Figure 2) in virtually all observed taste buds. Using an α1A antibody, immunostaining was mainly observed at the TRC membrane with an average of 1.7 ± 0.1 cells per sectioned taste bud (Table 3). With the α1B antibody, a similar membrane bound staining pattern was observed with 2.8 ± 0.2 cells per sectioned taste bud. Alpha 1D immunostaining was observed at a similar frequency to α1B (2.6 ± 0.2 cells per sectioned taste bud).
Fig. 2.
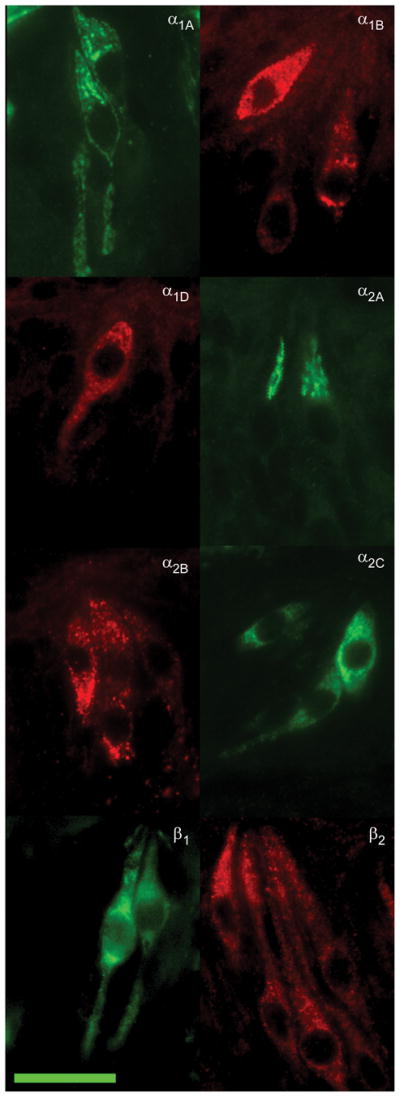
Photomicrographs of immunopositive taste receptor cells to eight expressed adrenoceptor subtypes. Single label fluorescent immunocytochemistry was performed using antibodies specific to varying adrenergic receptor subtypes on tissue samples from foliate or circumvallate papillae. Scale bar in lower right is 20 microns and applies to all examples.
Table III.
Quantitative immunocytochemical single labeling patterns of alpha and beta receptors in taste receptor cells.
| Staining | Total Taste Buds | Stained Taste Buds | % Stained Taste Buds | Stained TRCs | Stained TRCs/Total Taste Buds | Mean (TRCs/Total Taste Buds) ± SE |
|---|---|---|---|---|---|---|
| α1A | 1472 | 1384 | 94.02 | 2411 | 1.64 | 1.71 ± 0.12 |
| α1B | 730 | 663 | 90.82 | 1996 | 2.73 | 2.78 ±0.21 |
| α1D | 726 | 669 | 92.15 | 1904 | 2.62 | 2.64 ± 0.19 |
| α2A | 986 | 933 | 94.62 | 1164 | 1.18 | 1.19 ± 0.10 |
| α2B | 414 | 391 | 94.44 | 1272 | 3.33 | 3.06 ± 0.12 |
| α2C | 663 | 607 | 91.55 | 1567 | 2.36 | 2.37 ± 0.13 |
| β2 | 2184 | 2093 | 95.83 | 5621 | 2.57 | 2.81 ± 0.20 |
Immunocytochemical experiments with an antibody directed against the α2A receptor subtype produced a different staining pattern. Immunopositive α2A TRCs displayed mostly particulate cytoplasmic staining that was mostly confined towards the apical end of the taste cell. An average of 1.2 ± 0.1 immunopositive cells per cross sectioned taste bud was observed. Cells that were immunopositive using an α2B antibody also displayed particulate clustered staining though distributed throughout the entire cytoplasm of the cell. An average of 3.4 ± 0.2 positive cells per sectioned taste bud was observed. With an α2C antibody, immunofluorescence was observed uniformly throughout the cytoplasm in cells with large ovoid nuclei. An average of 2.4 ± 0.1 cells per sectioned taste bud was observed.
Immunostaining for the β1 receptor (using either of two antibodies directed against different antigenic epitopes) was observed in the membrane and cytoplasm of TRCs with large ovoid nuclei and sometimes thin apical processes. These antibodies produced insufficient data for quantification. Immunostaining for the β2 receptor was observed throughout the cytoplasm and the plasma membrane of cells with large ovoid nuclei, similar to those observed in β1-experiments. An average of about three β2-immunopositive TRCs was observed per sectioned taste bud. Thus, the α2B was the most frequently observed receptor subtype in the immunocytochemical experiments followed by (in relative equal observation rates) the α1B, α1D, α2C, and β2, subtypes. The α1A and α2A subtypes appeared to be the least expressed adrenoceptors in the bud.
Expression of adrenergic related molecules
To further explore adrenergic mechanisms in the rat taste bud, expression of key molecules associated with adrenergic signaling was also investigated using RT-PCR. These molecules included the norepinephrine biosynthetic enzyme dopamine β-hydroxylase (DβH), the norepinephrine transporter, NET, beta-arrestin molecules 1 and 2, and the epinephrine biosynthetic enzyme phenylethanolamine N-methyltransferase (PNMT).
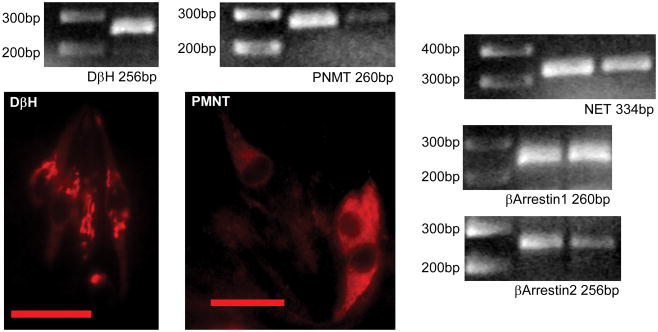
Expression of DβH mRNA was detected in cDNA derived from rat lingual epithelium (Figure 3) using two difference primer pairs directed against different regions of the DβH gene (Table 1). Both primers were tested on adrenal medulla as positive control. To confirm DβH expression, immunocytochemistry was performed. Strong particulate staining in subsets of cells within the taste bud was observed. Cells tended to have large round nuclei and staining mostly confined to the apical cytoplasmic region (Figure 3). Similarly, using RT-PCR on cDNA derived from taste buds, product of appropriate size was observed for NET, β-arrestin 1, and β-arrestin 2. Finally, taste buds were examined for the presence of the epinephrine synthetic enzyme phenylethanolamine N-methyltransferase (PNMT). A band of proper size was produced by RT-PCR using primers specific to this gene reacted with cDNA derived from pure taste buds. To confirm this expression, lingual tissue was also investigated using an antibody specific to PNMT. Immunopositive cells with large round clear nuclei were observed in taste buds which displayed morphology highly reminiscent of the adrenergic immunopositive cells previously observed (Herness et al., 2002).
Fig. 3.
Examination of adrenergic related signaling molecules. Using RT-PCR of cDNA derived from whole taste buds, bands of proper size were observed for reaction with primers for DβH, NET, PNMT, β-arrestin1 and β-arrestin 2. The expected product size for each reaction is listed. Immunopositive taste receptor cells are illustrated using an antibody directed against DβH or PNMT. Scale bar in photomicrographs is 20 microns.
Phenotyping of Adrenergic-receptor expressing cells
Given the well-established heterogeneity of TRCs, experiments were conducted to gain insight into the phenotype of cells expressing adrenoceptors using double-label immunocytochemistry. TRCs expressing specific subtypes of adrenoceptors were either phenotyped against standard gustatory markers that included α-gustducin, SNAP-25 (synaptosome-associated protein of 25,000 daltons), and NCAM (neural cell adhesion molecule) or among themselves, to investigate adrenoceptor co-expression patterns. Due to technical limitations, not all phenotypic combinations were possible.
Gustducin
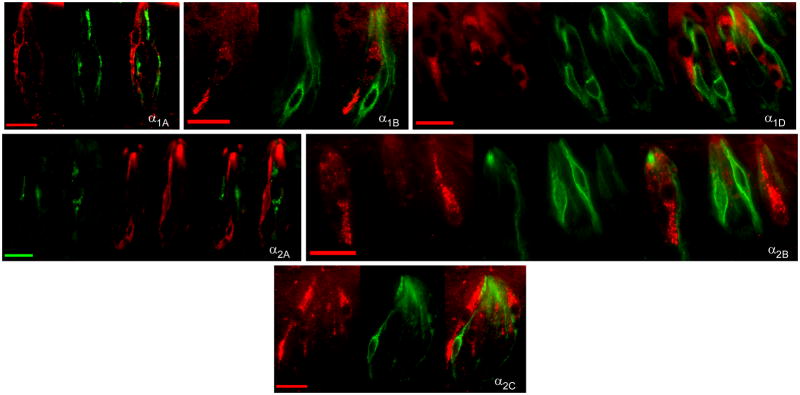
Gustducin, a G-protein first discovered in TRCs, is known to be expressed in a subpopulation of type II cells. In the rat posterior TRCs, it is co-expressed with T2R receptors and mostly excluded from T1R receptors. Double labeling experiments were performed with an antibody directed against the alpha subunit of gustducin with antibodies for α1A, α1B, α1D, α2A, α2B, α2C and β2 subtypes (Figure 4). For each subtype at least 300 to 700 individual taste buds were analyzed (Table 4). Almost no α1A-expressing (6%, n = 629 cells) or α2A-expressing (1%, n = 444) TRCs displayed double-labeling patterns with gustducin. Moderate co-expression was observed for the α1B, α1D and α2B. These cells displayed 40% (n = 815), 35% (n = 953), and 42% (n = 732) co-expression with α-gustducin, respectively, whereas about half of all α2C expressing TRCs co-expressed gustducin (48%; n = 760). The β2-expressing subpopulation of TRCs showed the greatest co-expression pattern with gustducin; about two-thirds of the cells (64%; n = 130). Interestingly, gustducin cells demonstrated almost reciprocal double labeling patterns with receptor subtypes, i.e, about the same percentage of gustducin-positive cells overlapped with an individual adrenoceptor subtype as did that subtype overlap with gustducin (Table 4).
Fig. 4.
Examination of co-expression of adrenergic receptors with gustducin. Representative photomicrographs of double label fluorescent immunocytochemistry with antibodies directed against alpha-gustducin (middle panels) and one of eight tested adrenoceptor subtypes (left panels) are illustrated. Overlaid images are at right of each panel. The scale bar in all photomicrographs represents twenty microns.
Table IV.
Quantitative immunocytochemical double labeling patterns of alpha and beta receptors with phenotypic taste receptor cell markers. Mean 1 represents the mean percentage of all adrenoceptor labeled cells which co-expressed the corresponding phenotypic marker per cross-sectioned taste bud. Mean 2 represents the mean percentage of all phenotypic marker labeled cells which co-expressed the corresponding adrenoceptor per cross-sectioned taste bud. Means are expressed ± SE.
| Staining | Total Taste Buds | Adrenoceptor | Phenotypic Marker | Double Labeled | Mean 1 (Adrenoceptor/Phenotypic Marker) | Mean 2 (Phenotypic Marker/Adrenoceptor) |
|---|---|---|---|---|---|---|
| α1A & Gustducin | 388 buds | 629 cells | 1082 cells | 39 cells | 6.56±1.40 cells | 3.79±0.83 cells |
| α1B & Gustducin | 319 | 815 | 889 | 357 | 40.38±2.06 | 43.97±1.95 |
| α1D & Gustducin | 349 | 953 | 901 | 338 | 35.82±2.04 | 37.82±2.26 |
| α2A & Gustducin | 298 | 444 | 1076 | 4 | 1.01±0.41 | 0.37±0.10 |
| α2B & Gustducin | 414 | 1272 | 1391 | 542 | 42.34±2.20 | 38.52±2.66 |
| α2C & Gustducin | 336 | 760 | 881 | 371 | 48.67±1.00 | 42.02±4.64 |
| β2 & Gustducin | 727 | 1304 | 2109 | 874 | 65.85±7.71 | 43.72±6.99 |
| α1A & SNAP25 | 284 | 613 | 826 | 76 | 12.48±2.97 | 9.52±1.92 |
| α1B & SNAP25 | 289 | 888 | 826 | 423 | 47.60±2.76 | 51.55±3.60 |
| α1D & SNAP25 | 275 | 758 | 822 | 310 | 40.87±2.22 | 37.78±1.88 |
| α2C & SNAP25 | 327 | 807 | 1065 | 424 | 52.35±1.98 | 39.85±3.66 |
| β2 & SNAP25 | 511 | 1315 | 1345 | 784 | 59.49±1.28 | 55.70±5.77 |
| α1A & NCAM | 387 | 608 | 963 | 15 | 2.46±0.23 | 1.99±0.77 |
| α2A & NCAM | 376 | 412 | 872 | 11 | 2.96±1.12 | 1.28±0.34 |
SNAP-25
SNAP-25, a Q-snare protein involved in the exocytotic fusion complex of synaptic vesicles, remains a non-definitive marker in mammalian taste bud. In mouse, SNAP-25 expression is restricted to type III cells (Clapp et al., 06, DeFazio et al., 2006) for which it is concluded to be a specific marker. However, in rat, co-expression of SNAP-25 with transductive elements expressed in type II markers has been reported by several labs including gustducin (Pumplin and Getschman 2000, Ueda et al., 2006), PLCβ2 (Ueda et al., 2006), PLA2-IIA (Oike et al., 2006), and GAD (Cao et al., 2009). Double labeling experiments using an antibody specific to SNAP-25 were performed with antibodies to the α1A, α1D, α2C, and β2 receptor subtypes (Figure 5).
Fig. 5.
Examination of co-expression of adrenergic receptors with SNAP-25. Representative photomicrographs of double label fluorescent immunocytochemistry with antibodies directed against SNAP-25 (middle panel) and one of five tested adrenoceptor subtypes (left panel) are illustrated. Overlaid images are at right of each panel. The scale bar in all photomicrographs represents twenty microns.
All tested adrenoceptor subtypes demonstrated some overlap with SNAP-25 (Table 4). The least overlap, at 10%, was observed for the α1A receptor subtype (n = 613 cells). About half of all α1B receptor expressing TRCs co-expressed SNAP-25 (51%; n = 888). Thirty-eight percent of α1D-expressing cells (n = 758) and 40% of α2C-expressing cells (n = 807) co-expressed SNAP-25. More than half of the β2-expressing cells (56%; n = 1,315) displayed overlapping expression with the SNAP-25 antibody. Additionally, although results could not be quantified, some co-expression of β1-cells was also noted.
NCAM
Co-expression with NCAM, a marker of type III cells (Yee et al., 2001), was tested for both α1A and α2A receptor subtypes. For α1A, in 387 examined taste buds, only 15 cells of either 608 alpha 1A immunopositive or 963 immunopositive NCAM cells appeared as double labeled. Thus 97.6% of alpha 1A cells and 98.5% of NCAM cells did not co-express. For α2A cells, of 376 examined taste buds, only 11 cells of either 412 cells α2A immunopositive cells or 872 immunopositive NCAM cells appeared as double labeled. Thus 98.4% of α2A cells and 98.8% of NCAM cells did not co-express. Hence these alpha receptors are virtually mutually exclusive from NCAM expressing cells.
Alpha-Beta Receptors
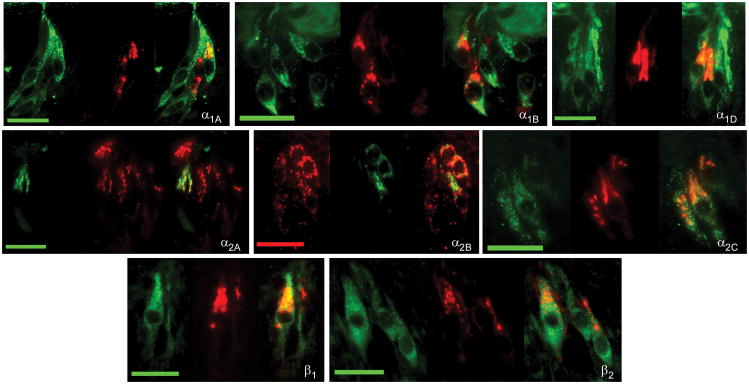
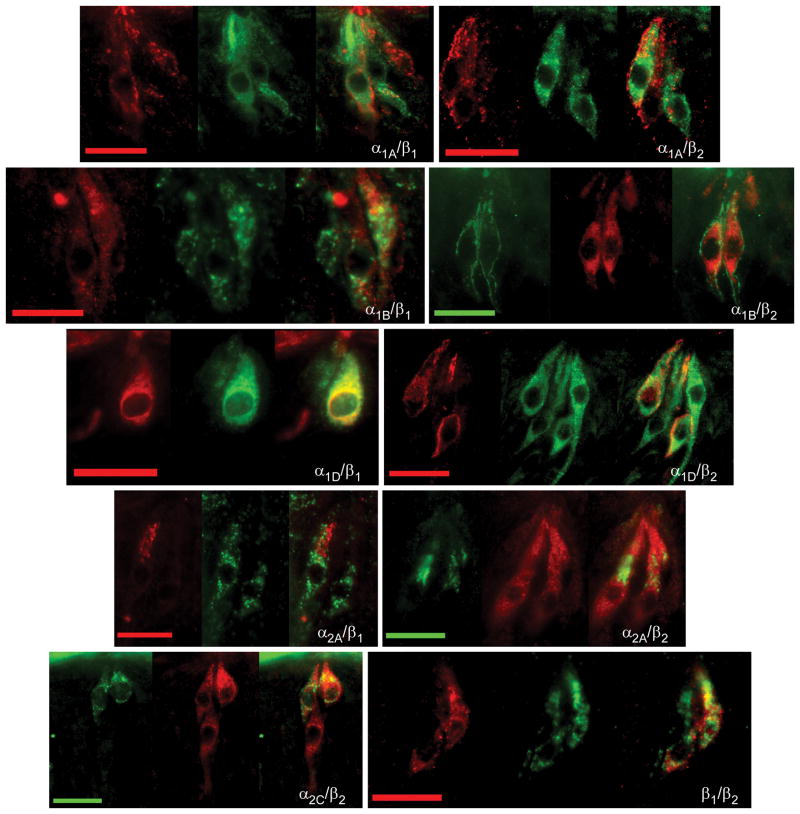
The co-expression patterns of alpha and beta receptors among themselves were also investigated using immunocytochemical double labeling protocols. Since all primary antibodies directed against alpha receptor subtypes were raised in the same animal (goat), double labeling experiments could only be conducted using a TSA amplification protocol. This protocol proved insufficient for quantitative analysis but did provide sufficient qualitative examination to observe the presence or absence of double labeled cells. Double labeling experiments using combinations of α1A/α2C, α1B/α2C, α1D/α2C, and α2A/α2C primary antibodies were conducted. Results of these experiments suggested that both the α1A and α2A were largely segregated from α2C expression as no double labeled cells were observed in these experiments. On the other hand, in experiments using combinations of either the α1B or the α1D antibodies with the α2C antibody, both double labeled and single labeled cells were observed. These data agree with the previous data using phenotypic taste cell markers, since α1B, α1D, and α2C immunopositive cells had similar expression patterns with gustducin, SNAP25 and NCAM. Further the α1A and α2A antibodies segregated from these phenotypic markers and segregated from α2C immunopositive TRCs. These results suggest alpha receptors tended to be expressed in at least two groups: co-expression of α1B, α1D, and α2C or co-expression of α1A and α2A.
Quantitative analysis of co-expression patterns of β2 receptors with α1A, α1B, α1D, and α2A were performed. To varying degrees, β2 receptor subtypes were observed to co-express with these adrenoceptors (Table 5). Of all β2-expressing TRCs, 27% were observed to co-express with α1A whereas 59% of all α1A-positive cells co-expressed β2. In β2 and α1B experiments, 42% of all β2-positive cells overlapped with α1B whereas 59% of all α1B-positive cells overlapped with β2. With α1D, 37% β2-positive cells co-expressed α1D while 57% α1D-positive cells co-expressed β2. Finally, virtually all α2A-positive cells were observed to co-express the β2 subtype. While 25% of β2-cells overlapped with α2A, 94% of α2A-positive cells co-expressed β2. Thus, there was extensive overlap of alpha and beta receptor subtypes. In general, a majority of α1A, α1B, and α1D subtypes co-express the β2 receptor subtype whereas virtually all α2A co-express the β2 subtype. Although quantitative experiments to determine the overlapping expression of beta receptors with one another weren’t possible, preliminary work suggested mostly overlapping expression of β1 and β2 receptors.
Table V.
Quantitative immunocytochemical double labeling patterns of alpha receptors with beta receptors in taste receptor Mean 1 represents the mean percentage of alpha adrenoceptor subtype labeled cells which co-expressed the β-2 receptor per cross-sectioned taste bud. Mean 2 represents the mean percentage of the β-2 receptor subtype labeled cells which co-expressed the corresponding alpha adrenoceptor per cross-sectioned taste bud. Means are expressed ± SE.
| Staining | Total Taste Buds | Alpha Adrenoreceptor | β2 | Double Labeled | Mean 1 (%DL/AR/TBs/sec) ± SE | Mean 2 (%DL/β2/TBs/sec) ± SE |
|---|---|---|---|---|---|---|
| α1A & β2 | 413 buds | 561 cells | 1220 cells | 331 cells | 58.77±4.43 cells | 26.26±3.35 cells |
| α1B & β2 | 210 | 464 | 648 | 288 | 59.25±13.81 | 42.23±7.33 |
| α1D & β2 | 125 | 246 | 360 | 130 | 56.95±7.21 | 37.12±1.96 |
| α2A & β2 | 312 | 308 | 1179 | 290 | 94.29±1.03 | 24.83±2.06 |
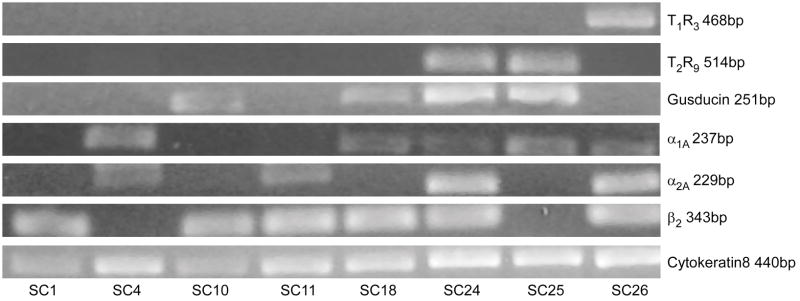
Adrenoceptor mRNA expression in single taste receptor cells
Expression of adrenoceptor mRNA in individual TRCs was investigated using a single cell RT-PCR approach applied to dissociated posterior TRCs. Primer sets for α1A, α2A, α2B, β1, and β2 were investigated in parallel with the signal transduction molecules receptors T1R3, T2R9, and the G-protein gustducin. In addition the positive control cytokeratin 8 (CK8), which is ubiquitously expressed across all taste receptor cells (Knapp et al., 1995), was included in each cell. In some cells a primer set for β-actin, which spanned an intron between exons 3 and 4, was also included as a positive control. This primer set is predicted to amplify a 132bp fragment if reverse-transcribed from mRNA and a 595bp product if genomic DNA serves as its template. It additionally serves, along with the CK8 primer set included in every reaction, as a positive control to ensure that cellular contents were harvested successfully and guard against false negatives with experimental primers. Additionally, an ECF control (no template control) was employed on many cells as a negative control. A small aliquot of ECF, sampled adjacent to the collected cell, was processed through RNA amplification and cDNA production to ensure that PCR products did not result from spurious templates originating from the collected fluid. Results of these controls were as expected. Single cells were only included in the data set if they satisfied criteria for both positive and negative controls.
In initial examination, 73 single TRCs were collected and analyzed. Of these cells, 17 failed to demonstrate a CK8 positive control and were excluded from further analysis. Analysis of the remaining 56 cells for T1R3, T2R9, and gustducin expression demonstrated a faithful representation of known expression pattern of these molecules. A significant population of the tested cells expressed gustducin mRNA (21/51 cells; 41%) comparing favorably with reported gustducin expression in rodent circumvallate from 20% to 40% (Boughter et al., 1997, Wong et al., 1999). One third of the subpopulation expressed T2R9 mRNA (18/55 cells, 33%), of which 83% co-expressed gustducin (15/18). Estimates of T2R expression in rodent posterior taste receptor cells vary from 20 to 30% (Behrens et al., 2006) and others have also observed that not all T2R cells express gustducin (Moon et al., 2009). Fewer cells were positive for T1R3 mRNA (5 of 52 tested cells; 10%) of which 60% were gustducin negative (3/5). T1R3 expression is reported as 15–30% in posterior cells (Montmayeur et al., 2002) of which a majority (about 90%) is thought not to express gustducin (Shigemura et al., 2008). Overall our percentages are close matches to other studies which, it should be emphasized, were not conducted as strictly quantitative.
Expression of α1A, α2A, α2B, β1, and β2 adrenoceptor subtype mRNAs was observed in single TRCs. As observed with immunocytochemistry, expression levels varied across individual cells. Of the alpha receptors, α1D was most represented (5 of 11 tested cells; 45%), followed by α2A (9 of 27 cells; 33%), α1A (9 of 38 cells; 24%) and α2B (1 of 19 cells; 5%). For beta receptors, β2 was well represented (18 of 42 cells; 43%) with fewer β1 subtype positive cells (3 of 30 cells; 10%).
Three of seven tested cells (43%) were positive for expression of PNMT. Of these three cells, two co-expressed gustducin, two co-expressed T2R9, two expressed both α1D and α2A and none expressed β receptors. This limited data set confirms expression of PNMT mRNA at the single cell level and provides a restricted insight into the phenotype of this cell type, suggesting it to be expressed in type II cells.
A few interesting patterns emerged when co-expression patterns among the 56 cells were examined. Similar to immunocytochemical analysis, in single cell RT-PCR experiments α1A showed partial overlapping expression with β2. Of the twenty-five cells that expressed α1A or β2, 36% of α1A cells (4 of 11 cells) expressed β2 while, conversely, 22% of β2 cells (4 of 18) expressed α1A. The α1A expressing cells displayed a partially overlapping expression pattern with gustducin. Of the 22 cells that expressed α1A or gustducin, two-thirds of α1A cells (6 of 9) expressed gustducin whereas one-third of gustducin cells (6 of 18) expressed α1A.
Similarly, β2 and gustducin demonstrated a partially overlapping expression. Of the 32 cells that expressed either gustducin or β2, 14 expressed only gustducin, 14 expressed only β2 and 4 expressed both gustducin and β2. Thus only 22% of each group expressed both receptor subtypes. This co-expression pattern was less than that observed in the immunocytochemical double labeling analysis where about two thirds of all β2 cells co-expressed gustducin.
Finally, α2A and β2 receptors also displayed co-expression. Of 15 cells that were positive for either of the adrenoceptor subtypes, six expressed only α2A, six only β2, and three both species of mRNA. Hence, 33% expressed of either subtype co-expressed both receptors (3 of 9 cells). This is less than observed with immunocytochemistry where almost all α2A co-expressed β2.
Although co-expression patterns differed when immunocytochemical and single cell RT-PCR patterns were compared on a quantitative basis, similarities were apparent when qualitatively compared. For both techniques, the expression pattern of α1D > β2 > α1A ~ α2A was obtained. The most notable exception was the α2B subtype, which was the least expressed subtype observed with immunocytochemistry yet the most expressed when examined at the level of mRNA. Whether this disparity is due to true differences in the expression levels of mRNA and protein or whether technical considerations, such as the efficiency of antibody or primer reactions, contributed to these results isn’t yet known.
DISCUSSION
The present data demonstrate that the taste bud expresses a full array of adrenoceptor subtypes. Expression of all alpha receptor subtypes (α1A, α1B, α1D, α2A, α2B, α2C) as well as both the β1 and β2 subtype was observed. Our previous study documented a lack of expression of the β3 receptor subtype in the taste bud (Kaya et al., 2004). Adrenoceptors are expressed across distinct subpopulations of cells within the taste bud suggesting that these receptors likely have multiple if not complex signaling roles in the processing of peripheral gustatory information. Immunocytochemical analysis revealed a multifaceted, sometimes overlapping, expression pattern across individual cells of the taste bud that integrated with type II cell markers and segregated from a type III marker. Given the known expression of T1R and T2R receptors in type II cells, these data suggest that adrenergic signaling may be important for modulating transduction pathways associated sweet, bitter, and/or umami. As well, these data suggest that norepinephrine may not be the sole adrenergic catecholamine that operates in the taste bud since expression of the synthetic enzyme for epinephrine, PNMT, was observed. When considered with previous observations that a subtype of TRC is immunopositive for NE (Herness et al., 2002b), that TRCs produce physiological responses to adrenergic stimulation mediated by alpha and beta receptors (Herness et al., 2002b), and that NE has been measured to be released from taste buds in response to tastant stimulation (Huang et al., 2008), these data collectively provide strong evidence for adrenergic cell-to-cell communication within the taste bud. This cell-to-cell communication involves adrenergic presynaptic cells, that likely have alpha autoreceptors, and adrenergic postsynaptic cells which are the consequent targets of NE release.
Adrenergic taste receptor cells in the taste bud
There is now substantial evidence to support the notion that subsets of TRCs within vertebrate taste buds are adrenergic. These adrenergic TRCs have been identified by immunocytochemistry, by expression of adrenergic synthetic enzymes and NE transporters, and by physiological studies of NE release. However, since the adrenergic TRC has yet to be phenotyped, only limited information is available regarding its cell type (i.e. type I, II, or III) or co-expression with taste receptors (i.e. T1R, T2R). It is also likely that adrenergic TRCs occur among type II and perhaps type III cells and that they likely express presynaptic adrenoceptors.
The present data corroborate previous immunocytochemical observation of NE in TRCs by demonstrating expression of its synthetic enzyme DβH both at the mRNA and protein level, which has not been previously demonstrated in the rat. In the frog, DβH expression has been observed using immunocytochemistry (Ando et al., 2007). The morphology of frog DβH immunopositive cells suggests they are directly implicated in taste reception. These cells had apical processes reaching the surface of the taste disc and one or more basolateral process, a morphology that is indicative of taste function in the frog. Further, other cells of the frog disc that are presumed to be more glial-like in function (mucous cells, wing cells, and sustentacular cells) were immunonegative for DβH. In rat, DβH immunopositive cells typically appeared with large ovid nuclei, a morphology typical of type II cells, and displayed a strong resemblance to those visualized with a NE-antibody in a prior study (Herness et al., 2002b). As well, NE expression in type III cells cannot be ruled out. In contrast to frog and rat, DβH expression was not observed in mouse (Dvoryanchikov et al., 2007). Mouse TRCs must alternatively uptake NE, presumably released from a nearby source, via transporters. This source of NE remains obscure but nearby sympathetic innervation is a likely and obvious candidate. In the rat, sympathetic fibers, arising from the superior cervical ganglion, are diffusely distributed throughout the tongue, particularly in the submucosal region. These fibers are denser in the posterior tongue and have a regular distribution between muscles and blood vessels as well as submucosal glands (Wang and Chiou, 2004). Presumably, a similar innervation pattern in murine tongue would allow locally released NE to be concentrated in the presynaptic adrenergic TRC via NETs.
TRCs also express other signaling components expected for adrenergic transmission, such as aromatic –amino acid decarboxylase (AADC), NET transporters, and PNMT. AADC, which catalyses the decarboxylation of aromatic L-amino acids in the biosynthetic cascade for both serotonin and norepinephrine, has been detected by RT-PCR and immunocytochemistry in taste buds of mice (Dvoryanchikov et al., 2007, Seta et al., 2007). AADC cells are likely expressed in but not limited to type III cells since they co-localize with serotonin, NCAM, and PGP9.5, which are all expressed in type III cells, but do not co-localize with gustducin (Seta et al., 2007), which is segregated from type III cells. However, expression in type II cells remains equivocal since conflicting information has been reported if AADC and PLCβ2, a marker of type II cells, are co-expressed. The cellular expression of AADC in rat taste buds has not yet been attempted. Because murine adrenergic TRCs may indeed skip this biosynthetic step and 5HT is expressed in type III cells, AADC expression in murine TRCs may be indicative of serotonergic rather than adrenergic cells.
Although NE is presumed to be released from presynaptic adrenergic TRCs in response to tastant stimulation, limited information is available on which qualities might serve as effective stimuli for its release. NE release, using an imaging bioassay, has been measured from presumed type III cells in mouse, (identified using GFP linked to a GAD-promoter as a marker of type III status) in response to direct depolarization with KCl or with acetic acid stimulation. This cell type did not respond to tastant mixture stimulation (Huang et al., 2008). Since GAD expression occurs in both type II and type III cells in the rat taste bud (Cao et al., 2009), the situation may be different across species.
The present study also noted expression of PNMT in TRCs which suggests that epinephrine, in addition to NE, may be operative in adrenergic transmission in the taste bud. In the frog disc, Zancanaro et al. (1995), using HPLC, measured about 20 times more epinephrine than NE in fungiform papillae. However, Dvoryanchikov et al. (2007) failed to note expression of PNMT in murine taste buds. Other components of adrenergic signaling include expression of arrestins (this study) and MAO-b (Xu et al., 2004) in TRCs. The presence of arrestins compliment previous reports of G-protein receptor kinases (GRKs) in TRCs that include GRK 5 and perhaps GRK 2 and GRK6 (Zubare-Samuelov et al., 2005). Both arrestins and GRKs act to control G-protein coupled receptor signaling by mediating desensitization (e.g., Kendall and Luttrell 2009). GRKs have been hypothesized to be important in terminating tastant-mediated signaling at the receptor level. With the further consideration of arrestin participation, it is possible that both early and late transduction pathways are subject to desensitization. TRCs hence express a full repertoire of adrenergic associated molecules within the taste bud.
Additionally, some adrenoceptors are known to have presynaptic functions. In particular, the α2 family of adrenoceptors is often associated with presynaptic feedback inhibition of norepinephrine release (e.g., Hein, 2006). In our analysis, we noted strong segregation of alpha 2A and alpha 2C receptors. If each is serving a presynaptic feedback function, this observation could suggest that adrenergic TRCs may be divided into distinct functional subgroups. Future investigation of α2 receptors with DβH expression may help to elucidate which receptors are serving presynaptic functions in the taste bud. Overall, the rat adrenergic TRC may be similar to neuronal adrenergic cells expressing a full complement of enzymes, and associated molecules for its synthesis, transport, and regulated release.
Postsynaptic adrenergic receptor expression suggests NE may modulate processing of tastant information
The rich expression of adrenergic receptors across subsets of cells within the bud suggests that NE may serve multiple roles in the peripheral processing of taste information. Although these precise functions remain unknown, the receptor expression pattern within the bud combined with the known physiological actions of receptor stimulation provides insight into their function. The present data suggest these receptors are well expressed among type II cells with perhaps little or no expression among type III cells. Two subtypes, tested for co-expression with the type III cells marker NCAM, had almost no overlap, though not all adrenoceptor subtypes were tested. Varying overlapping patterns with SNAP-25, a marker for type II and type III cells in rat TRCs, and gustducin suggest adrenoceptors are well expressed in type II cells. These cell types predict that adrenergic signaling patterns would likely be involved in processing of sweet, bitter, and umami qualities. However, a lack of expression on type III cells, thought to be sour detectors in the taste bud, does not preclude adrenergic transmission from modulating sour taste as modulation of other non-type III cells could potentially influence the excitability of the type III cells itself. Future studies that more directly examine expression of taste receptors (e.g. T1R and T2R) and adrenoceptors will be useful.
At present, only limited information on the physiological consequences of alpha or beta receptor stimulation in TRCs is available. In rat posterior cells, stimulation of alpha receptors both inhibited outward potassium current and effectively increased intracellular calcium using the alpha 2 agonist clonidine. Beta receptor stimulation, using isoproterenol, both increased a calcium-dependent chloride current and reduced an outward potassium current (Herness and Sun, 1999; Herness et al., 2002b). In general, the actions of either alpha or beta stimulation would act to place the cell in a state of greater excitability. Additionally, in the same study, expression of alpha and beta receptors was examined using pharmacology. In recording of isolated cells, seven of fourteen cells responded to an alpha agonist (clonidine) and a beta agonist (ISP), five responded only to the alpha agonist and two responded only to the beta agonist. Hence these previous physiological observations are in agreement with the heterogeneous expression pattern of alpha and beta receptors observed with immunocytochemistry and RT-PCR.
A qualitative Venn diagram summarizing the expression patterns of adrenoceptors in TRCs is presented in Figure 9. This diagram consolidates double labeling data obtained with phenotypic markers or adrenoceptors subtypes using either immunocytochemistry or single cell RT-PCR. For the most part, alpha receptors tended to fall into two segregated groups. In one, co-expression of α1B, α1D, α2B, α2C adrenoceptors was noted that partially overlapped with gustducin (about 40%) and β2 (about 60%). Segregated from this group were the α2A cells, which partially overlapped with the α1A group. These cells typically did not express gustducin but did co-express β2 receptors (with α2Aa cells doing so exclusively). In total, the α1A/α2A group of cells does not co-express gustducin and therefore are likely to be expressed in Type I or non-gustducin Type II cells. Consequently, these cells would be segregated from T2R receptors. The possibility that the α2A/β2 expressing TRC co-express T1R receptors seems likely since these cells did not co-express gustducin. Other alpha receptors do co-express gustducin suggesting some combination of α1B, α1D, α2B, α2C subtypes may co-express T2R receptors. How activation of these receptors may modulate taste qualities mediated by T1R or T2R receptors remains an open question. The issue of co-localization patterns is further complicated by adrenergic receptor dimerization (e.g. Hein, 2006). For example, there is evidence that the heterodimerization of β1 and β2 subtypes or α1D and α1B subtypes may be essential for their function. Our observations that there may be extensive overlap of β1 and β2 receptors or α1B and α1D receptors are aligned with the notion of heterodimerization. These observations would suggest that fewer functional subtypes of adrenergic receptors may exist within the taste bud than might, in a rote manner, be predicted based on immunocytochemical co-localization patterns.
Fig. 9.
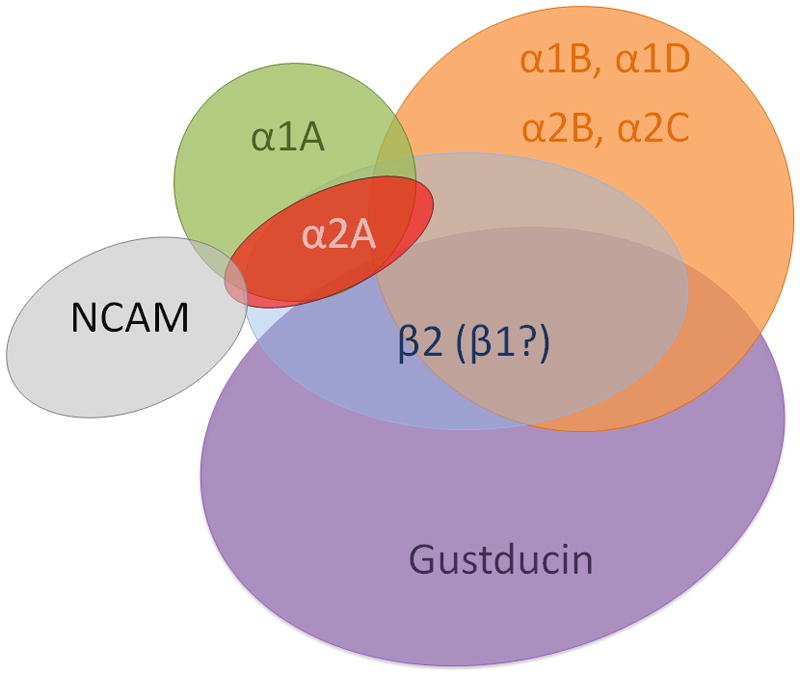
Qualitative Venn diagram summarizing the co-expression patterns of adrenergic receptor subtypes observed in rat posterior taste receptor cells with two additional gustatory phenotypic markers.
The adrenergic system may modulate peripheral sensory information as occurs in other sensory systems
The expression of multiple adrenergic receptor subtypes in taste buds parallels findings in other sensory systems such as the retina (Dong et al., 2007, Kalapesi et al., 2005, Zarbin et al., 1986), the organ of Corti (Fauser et al., 2004, Khan et al., 2007), and the olfactory bulb (Doucette et al., 2007, Ennis et al., 2007, Nai et al., 2009). In each, the expression of both alpha and beta receptors across peripheral receptor cells or output neurons appear to share a common general conclusion: adrenergic receptors play a modulatory rather than primary functional role in sensory stimulation. In the main olfactory bulb, norepinephrine is thought to play a strong neuromodulatory role via projections from the locus coeruleus. Olfactory stimuli can increase the output of locus coeruleus and increase NE levels in the main olfactory bulb. Adrenoceptors are expressed in multiple layers and on multiple cell types throughout the bulb. For example, the mitral cell may express multiple NE receptor subtypes that include α1A, α1B, α1D, α2A and α2C. However, although NE inputs to olfactory bulb are discussed as critical to olfactory function, effects at cellular and network levels are somewhat discrepant. There are reports (e.g., Ennis et al., 2007) that NE may enhance responses of mitral cells to weak but not strong stimuli, perhaps improving detection of weak odorants. Other suggestions include the formation and/or recall of olfactory memories, pheromonal regulation of pregnancy, maternal behavior, and olfactory learning in young animals.
Similarly, the role of the adrenergic stimulation in the gustatory system remains obscure. There are limited available data on the role of NE in gustatory processing of taste stimuli. All suggest a modulatory role that acts to enhance the gustatory signal within the taste bud. An early physiological study, measuring frog neural responses with perfusion of the lingual artery, observed that spontaneous activity was enhanced by NE and suppressed by agents that depleted monoamines (Morimoto 1982). Similar results were observed in a subsequent independent study (Nagahama 1985) where injection of the catecholamine depleting agents resperpine or guanetidine reduced the taste nerve response to CaCl2. This inhibition was almost completely reversed with injection of NE. A role for NE in the peripheral gustatory system has also been suggested in human where taste thresholds were documented to change with treatments that alter system adrenergic levels. Heath and colleagues (2006) demonstrated that the human taste threshold to bitter and sour threshold were reduced by 39% and 22% respectively with treatments that enhanced NE levels. Taken together these studies suggest NE acts, across a variety of species, to enhance taste function in a quality specific manner.
Fig. 6.
Examination of co-expression of adrenergic receptors with NCAM. Representative photomicrographs of double label fluorescent immunocytochemistry with antibodies directed against NCAM (middle panel) and one of five tested adrenoceptor subtypes (left panel) are illustrated. Overlaid images are at right of each panel. The scale bar in all photomicrographs represents twenty microns.
Fig. 7.
Immunocytochemical double labeling combinations of alpha and beta receptors. Each triplet of photomicrographs is arranged as the alpha receptor image, beta receptor image, and overlay (left to right). All scale bars represent 20 microns.
Fig. 8.
Gel electrophoresis illustrating single cell RT-PCR products from eight taste receptor cells tested with a variety of primers sets to adrenoceptors or taste-related genes. Each row represents PCR results from a single primer set; each column represents results from a single cell.
Acknowledgments
Supported by NIH NIDCD R0100401 awarded to S.H. Y.Z. was supported by an Exchanging Scholarship from China Scholarship Council of the Ministry of Education of China. This work is in partial fulfillment of the PhD degree awarded to Y.Z. from Xi’an Jiantong University.
List of Abbreviations
- AADC
Aromatic –amino acid decarboxylase
- ATP
Adenosine triphosphate
- CCK
Cholecystokinin
- DβH
Dopamine-β-hydroxylase
- E
Epinephrine
- GABA
Gamma-amino butyric acid
- GLP-1
Glucagon-like peptide-1
- GRK
G-protein coupled receptor kinase
- NCAM
Neural cell adhesion molecule
- NE
Norepinephrine
- NET
Norepinephrine transporter
- NPY
Neuropeptide Y
- PBS
Physiologically buffered saline
- PNMT
Phenylethanolamine N-methyltransferase
- RT-PCR
Reverse transcription polymerase chain reaction
- SNAP-25
Synaptosomal-associated protein 25
- TSA
Tyramide signal amplification
- TRCs
Taste receptor cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ando H, Tomida M, Inoue K, Asanuma N. Dopamine β-hydrolyase-like immunoreactive cells in the frog taste disc. Chemical Senses. 2007;32:825–832. doi: 10.1093/chemse/bjm051. [DOI] [PubMed] [Google Scholar]
- Behrens M, Meyerhof W. Bitter taste receptors and human bitter taste perception. Cell Mol Life Sci. 2006;63:1501–1509. doi: 10.1007/s00018-006-6113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughter JD, Pumplin DW, Yu C, Christy RC, Smith DV. Differential expression of α-gustducin in taste bud populations of the rat and hamster. J Neurosci. 1997;17:2852–2858. doi: 10.1523/JNEUROSCI.17-08-02852.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruss M, Porzgen P, Bryan-Lluka LJ, Bonisch H. The rat norepinephrine transporter: molecular cloning from PC12 cells and functional expression. Brain Res Mol Brain Res. 1997;52:257–262. doi: 10.1016/s0169-328x(97)00267-2. [DOI] [PubMed] [Google Scholar]
- Bylund DB. Adrenergic receptors: Historical perspectives from the 20th century. In: Perez D, editor. The adrenergic receptors: in the 21st century. Ch1. Humana Press Inc; Totowa NJ: 2006. [Google Scholar]
- Cano J, Lobera B, Rodriguez-Echandia EL, Machado A. Influence of innervation on the levels of noradrenaline and serotonin in the circumvallate papilla of the rat. Journal of Neurobiology. 1982a;13:1–7. doi: 10.1002/neu.480130102. [DOI] [PubMed] [Google Scholar]
- Cano J, Machado A, Roza C, Rodriguez-Echandia E. Effect of testosterone on serotonin and noradrenaline concentrations and taste bud cell number of rat circumvallate papilla. Chemical Senses. 1982b;7:109–116. [Google Scholar]
- Cao Y, Zhao FL, Kolli T, Hivley R, Herness S. GABA expression in the mammalian taste bud functions as a route of inhibitory cell-to-cell communication. PNAS. 2009;106:4006–4011. doi: 10.1073/pnas.0808672106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Rybe NJP, Zuker CS. The cells and peripheral representation of sodium taste in mice. Nature. 2010;464:297–301. doi: 10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp T, Medler K, Damak S, Margolskee R, Kinnamon S. Mouse taste cells with G protein-coupled taste receptors lack voltage-gated calcium channels and SNAP-25. BMC Biology. 2006;4:7. doi: 10.1186/1741-7007-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFazio RA, Dvoryanchikov G, Maruyama Y, Kim JW, Pereira E, Roper SD, Chaudhari N. Separate populations of receptor cells and presynaptic cells in mouse taste buds. Journal of Neuroscience. 2006;26:3971–3980. doi: 10.1523/JNEUROSCI.0515-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C-J, Guo Y, Wheeler L, Hare WA. α2 adrenergic receptor-mediate modulation of cystolic Ca++ signals at the inner plexiform layer of the rat retina. Investigative Ophthalmology & Visual Science. 2007;48:1410–1415. doi: 10.1167/iovs.06-0890. [DOI] [PubMed] [Google Scholar]
- Doucette W, Milder J, Restrepo D. Adrenergic modulation of olfactory bulb circuitry affects odor discrimination. Learning & Memory. 2007;14:539–547. doi: 10.1101/lm.606407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoryanchikov G, Tomchik SM, Chaudhari N. Biogenic amine synthesis and uptake in rodent taste buds. J Comp Neurol. 2007;505:302–313. doi: 10.1002/cne.21494. [DOI] [PubMed] [Google Scholar]
- Ennis M, Hamilton KA, Hayar A. Neurochemistry of the main olfactory system. In: Lajtha A, editor. Handbook of Neurochemistry and Molecular Neurobiology. 3. Vol. 20 2007. [Google Scholar]; Johnson DA, editor. Sensory Neurochemistry. pp. 137–204. [Google Scholar]
- Springer Fauser C, Schimanski S, Wangemann P. Localization of β1-adrenergic receptors in the cochlea and the vestibular labyrinth. Journal of Membrane Biology. 2004;201:25–32. doi: 10.1007/s00232-004-0703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- Gabella G. Taste buds and adrenergic fibers. Journal of the Neurological Sciences. 1969;9:237–242. doi: 10.1016/0022-510x(69)90073-2. [DOI] [PubMed] [Google Scholar]
- Geerdink HG, Drukker J. Uptake of l-dopa by cells in the taste buds of the vallate papilla of the mouse. Histochemie. 1973;36:219–223. doi: 10.1007/BF00306311. [DOI] [PubMed] [Google Scholar]
- Giancippoli A, Novara M, de Luca A, Baldelli P, Marcantoni A, Carbone E, Carabelli V. Low-threshold exocytosis induced by cAMP-recruited CaV3.2 (alpha1H) channels in rat chromaffin cells. Biophys J. 2006;90:1830–1841. doi: 10.1529/biophysj.105.071647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath TP, Melichar JK, Nutt DJ, Donaldson LF. Human taste thresholds are modulated by serotonin and noradrenaline. J Neurosci. 2006;26:12664–12671. doi: 10.1523/JNEUROSCI.3459-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein L. Adrenoceptors and signal transduction in neurons. Cell Tissue Res. 2006;326:541–551. doi: 10.1007/s00441-006-0285-2. [DOI] [PubMed] [Google Scholar]
- Herness S. Coding in Taste Receptor Cells: The Early Years of Intracellular Recordings. Physiology & Behavior. 2000;69:17–27. doi: 10.1016/s0031-9384(00)00186-4. [DOI] [PubMed] [Google Scholar]
- Herness MS, Sun XD. Characterization of chloride currents and their noradrenergic modulation in rat taste receptor cells. J Neurophysiol. 1999;82:260–271. doi: 10.1152/jn.1999.82.1.260. [DOI] [PubMed] [Google Scholar]
- Herness S, Zhao FL, Lu SG, Kaya N, Shen T. Expression and physiological responses of cholecystokinin in taste receptor cells. Journal of Neuroscience. 2002a;22:10018–10029. doi: 10.1523/JNEUROSCI.22-22-10018.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herness S, Zhao FL, Lu SG, Kaya N, Shen T, Sun XD. Adrenergic signaling between rat taste receptor cells. Journal of Physiology (London) 2002b;543:601–614. doi: 10.1113/jphysiol.2002.020438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y-J, Maruyama Y, Lu KS, Pereira E, Plonsky I, Baur JE, Wu D, Roper SD. Mouse taste buds use serotonin as a neurotransmitter. Journal of Neuroscience. 2005;25:843–847. doi: 10.1523/JNEUROSCI.4446-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y-J, Maruyama Y, Roper SD. Norepinephrine is coreleased with serotonin in mouse taste buds. J Neurosci. 2008;28:13088–13093. doi: 10.1523/JNEUROSCI.4187-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Devilbiss DM, Waterhouse BD. A matter of focus: monoaminergic modulation of stimulus coding in mammalian sensory networks. Curr Opin Neurobiol. 2004;14:488–495. doi: 10.1016/j.conb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Inazu M, Takeda H, Matsumiya T. Functional expression of the norepinephrine transporter in cultured rat astrocytes. J Neurochem. 2003;84:136–144. doi: 10.1046/j.1471-4159.2003.01514.x. [DOI] [PubMed] [Google Scholar]
- Kalapesi FB, Coroneo MT, Hill MA. Human ganglion cells express the alpha-2 adrenergic receptor: relevance to neuroprotection. Br J Ophthalmol. 2005;89:758–763. doi: 10.1136/bjo.2004.053025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya N, Shen T, Lu SG, Zhao FL, Herness S. Expression and localization of serotonin receptor subtypes in rat taste buds. American Journal of Physiology: Regulatory, Integrative, and Comparative Physiology. 2004;286:R649–R658. doi: 10.1152/ajpregu.00572.2003. [DOI] [PubMed] [Google Scholar]
- Kendall RT, Luttrell LM. Diversity in arrestin function. Cell Mol Life Sci. 2009;66:2953–2973. doi: 10.1007/s00018-009-0088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan KM, Drescher MJ, Hatfield JS, Ramakrishnan NA, Drescher DG. Immunohistochemical localization of adrenergic receptors in the rat organ of Corti and spiral ganglion. Journal of Neuroscience Research. 2007;85:3000–3012. doi: 10.1002/jnr.21404. [DOI] [PubMed] [Google Scholar]
- Kishi M, Emori Y, Tsukamoto Y, Abe K. Primary culture of rat taste bud cells that retain molecular markers for taste buds and permit functional expression of foreign genes. Neuroscience. 2001;106:217–225. doi: 10.1016/s0306-4522(01)00184-1. [DOI] [PubMed] [Google Scholar]
- Knapp L, Lawton A, Oakley B, Wong L, Zhang C. Keratins as markers of differentiated taste cells of the rat. Differentiation. 1995;58:341–349. doi: 10.1046/j.1432-0436.1995.5850341.x. [DOI] [PubMed] [Google Scholar]
- Kubovcakova L, Micutkova L, Bartosova Z, Sabban EL, Krizanova O, Kvetnansky R. Identification of phenylethanolamine N-methyltransferase gene expression in stellate ganglia and its modulation by stress. J Neurochem. 2006;97:1419–1430. doi: 10.1111/j.1471-4159.2006.03832.x. [DOI] [PubMed] [Google Scholar]
- Lu B, Yan J, Yang X. Effects of sodium depletion on detection thresholds for salty taste in rats. Physiology & Behavior. 2009;97:463–469. doi: 10.1016/j.physbeh.2009.03.022. [DOI] [PubMed] [Google Scholar]
- Lymperopoulos A, Rengo G, Funakoshi H, Eckhart AD, Koch WJ. Adrenal GRK2 upregulation mediates sympathetic overdrive in heart failure. Nature medicine. 2007;13:315–323. doi: 10.1038/nm1553. [DOI] [PubMed] [Google Scholar]
- Maestu J, Villa I, Parik J, Paaver M, Merenäkk L, Eensoo D, Harro M, Harro J. Human adrenergic alpha 2A receptor C-1291G polymorphism leads to higher consumption of sweet food products. Mol Psychiatry. 2007;12(6):520–1. doi: 10.1038/sj.mp.4001976. [DOI] [PubMed] [Google Scholar]
- McLaughlin SK, McKinnon PJ, Margolskee RF. Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature. 1992;357:563–569. doi: 10.1038/357563a0. [DOI] [PubMed] [Google Scholar]
- Minneman KP. New signal transduction paradigms. In: Perez D, editor. The adrenergic receptors: in the 21st century. Ch 3. Humana Press Inc; Totowa NJ: 2006. [Google Scholar]
- Montmayeur J-P, Matsunami H. Receptors for bitter and sweet taste. Curr Opin Neurobiol. 2002;12:366–371. doi: 10.1016/s0959-4388(02)00345-8. [DOI] [PubMed] [Google Scholar]
- Morimoto K, Sato Z. Role of monoamines in afferent synaptic transmission in frog taste organ. Japanese Journal of Physiology. 1982;32:855–871. doi: 10.2170/jjphysiol.32.855. [DOI] [PubMed] [Google Scholar]
- Moon YW, Lee J, Yoo SB, Jahng JW. Capsaicin receptors are colocalized with sweet/bitter receptors in the taste sensing cells of circumvallate papillae. Genes Nutr. 2009 doi: 10.1007/s12263-009-0164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RG. The ultrastructure of taste buds. In: Friedmann I, editor. The ultrastructure of sensory organs. Ch 1. Elsevier; New York: 1973. pp. 1–82. [Google Scholar]
- Nagahama S, Kurihara K. Norepinephrine as a possible transmitter involved in synaptic transmission in frog taste organs and Ca dependence of its release. J Gen Phyisol. 1985;85:431–442. doi: 10.1085/jgp.85.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nai Q, Dong H-W, Hayar A, Linster C, Ennis M. Noradrenergic regulation of GABAergic inhibition of main olfactory bulb mitral cell varies as a function of concentration and receptor subtype. J Neurophysiol. 2009;101:2472–2484. doi: 10.1152/jn.91187.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura T. Acetylcholine increases intracellular Ca2+ in taste cells via activation of muscarinic receptors. J Neurophysiol. 2002;87:2643–2649. doi: 10.1152/jn.2002.87.6.2643. [DOI] [PubMed] [Google Scholar]
- Oike H, Matsumoto I, Abe K. Group IIA phospholipase A2 is co-expressed with SNAP-25 in mature taste receptor cells of rat circumvallate papillae. Journal of Comparative Neurology. 2006;494:876–886. doi: 10.1002/cne.20848. [DOI] [PubMed] [Google Scholar]
- Paparelli A, Soldani P, Pellegrini A. Noradrenergic innervation of the lingual papillae in certain rodents pre-treated with adriblastin. I. Comparative study on the filiform and fungiform papillae. Int J Tissue Reac. 1986;8:527–531. [PubMed] [Google Scholar]
- Paparelli A, Pellegrini A, Soldani P. Noradrenergic innervation of the lingual papillae in certain rodents pre-treated with adriblastin. II. A comparative study on circumvallate and foliate papillae. Bollettino della Società italiana di biologia sperimentale. 1988;64:907–913. [PubMed] [Google Scholar]
- Philipp M, Hein L. Adrenergic receptor knockout mice: distinct functions of 9 receptor subtypes. Pharmacology & Therapeutics. 2004;101:65–74. doi: 10.1016/j.pharmthera.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Pumplin DW, Getschman E. Synaptic proteins in rat taste bud cells: Appearance in the golgi apparatus and relationship to α-gustducin and the Lewisb and A antigens. J Comp Neurol. 2000;427:171–184. doi: 10.1002/1096-9861(20001113)427:2<171::aid-cne1>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Sato T, Nishishita K, Kato Y, Okada Y, Toda K. Tonic activity of parasympathetic efferent nerve fibers hyperpolarizes the resting membrane potential of frog taste cells. Chem Sens. 2006;31:307–313. doi: 10.1093/chemse/bjj034. [DOI] [PubMed] [Google Scholar]
- Sato T, Okada Y, Miyazaki T, Kato Y, Toda K. Taste cell responses in the frog are modulated by parasympathetic efferent nerve fibers. Chem Sens. 2005;30:761–769. doi: 10.1093/chemse/bji068. [DOI] [PubMed] [Google Scholar]
- Scofield MA, Liu F, Abel PW, Jeffries WB. Quantification of steady state expression of mRNA for alpha-1 adrenergic receptor subtypes using reverse transcription and a competitive polymerase chain reaction. J Pharmacol Exp Ther. 1995;275:1035–1042. [PubMed] [Google Scholar]
- Seta Y, Kataoka S, Toyono T, Toyoshima K. Immunohistochemical localization of aromatic l-amino acid decarboxylase in mouse taste buds and developing taste papillae. Histochem Cell Biol. 2007;127:415–422. doi: 10.1007/s00418-006-0257-3. [DOI] [PubMed] [Google Scholar]
- Shigemura N, Nakao K, Yasuo T, Murata Y, Yasumatsu K, Nakashima A, Katsukawa H, Sako N, Ninomiya Y. Gurmarin sensitivity of sweet taste responses is associated with co-expression patterns of T1R2, T1R3, and gustducin. Biochem Biophys Res Comm. 2008;367:356–363. doi: 10.1016/j.bbrc.2007.12.146. [DOI] [PubMed] [Google Scholar]
- Shin Y-K, Martin B, Golden E, Dotson CD, Maudsley S, Kim W, Jang H-J, Mattson MP, Drucker DJ, Egan JM, Munger SD. Modulation of taste sensitivity by GLP-1 signaling. Journal of Neurochemistry. 2008;106:455–463. doi: 10.1111/j.1471-4159.2008.05397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Kintsurashvili E, Ona D, Ignjacev-Lazich I, Gavras I, Gavras H. Inhibition of the alpha(1D)-adrenergic receptor gene by RNA interference (RNAi) in rat vascular smooth muscle cells and its effects on other adrenergic receptors. Vascul Pharmacol. 2007;46:367–372. doi: 10.1016/j.vph.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda M, Kitao K. Effect of monoamines on the taste buds in the mouse. Cell Tiss Res. 1980;210:71–78. doi: 10.1007/BF00232142. [DOI] [PubMed] [Google Scholar]
- Takeda M, Shishido Y, Kitao K, Suzuk Y. Monoamines of taste buds in the fungiform and foliate papillae of the mouse. Arch histol japon. 1982;45:239–246. doi: 10.1679/aohc.45.239. [DOI] [PubMed] [Google Scholar]
- Troispoux C, Reiter E, Combarnous Y, Guillou F. Beta2 adrenergic receptors mediate cAMP, tissue-type plasminogen activator and transferrin production in rat Sertoli cells. Mol Cell Endocrinol. 1998;142:75–86. doi: 10.1016/s0303-7207(98)00115-4. [DOI] [PubMed] [Google Scholar]
- Ueda K, Ichimori Y, Okada H, Honma S, Wakisaka Immunolocalization of SNARE proteins in both type II and type III cells of rat taste buds. Archives of Histology and Cytology. 2006;69:289–296. doi: 10.1679/aohc.69.289. [DOI] [PubMed] [Google Scholar]
- Wang H-W, Chiou W-Y. Sympathetic innervation of the tongue in rats. ORL Journal for Oto-Rhino-Laryngology, Head and Neck Surgery. 2004;66:16–20. doi: 10.1159/000077228. [DOI] [PubMed] [Google Scholar]
- Wong GT, Ruiz-Avilla L, Margolskee RF. Directing gene expression to gustducin-positive taste receptor cells. Journal of Neuroscience. 1999;19:5802–5809. doi: 10.1523/JNEUROSCI.19-14-05802.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Ito A, Arai R. Immunohistochemical localization of monoamine oxidase type B in the taste bud of the rat. NeuroToxicology. 2004;25:149–154. doi: 10.1016/S0161-813X(03)00116-5. [DOI] [PubMed] [Google Scholar]
- Yarmolinsky DA, Zuker CS, Ryba NJP. Common sense about taste: from mammals to insects. Cell. 2009;139:234–244. doi: 10.1016/j.cell.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee CL, Yand R, Bottger B, Finger TE, Kinnamon JC. “Type III” cells of rat taste buds: immunohistochemical and ultrastructural studies of neuron-specific enolase, protein gene product 9.5, and serotonin. J Comp Neurol. 2001;440:97–108. doi: 10.1002/cne.1372. [DOI] [PubMed] [Google Scholar]
- Young TM, Mathias CJ. Taste and smell disturbance with the α-adrenoceptor agonist midodrine. The Annals of Pharmacotherapy. 2004;38:1868–1870. doi: 10.1345/aph.1E171. [DOI] [PubMed] [Google Scholar]
- Zarbin MA, Wamsley JK, Palacious JM, Kuhar MJ. Autoradiographic localization of high affinity GABA, benzodiazepine, dopaminergic, adrenergic, and muscarinic cholinergic receptors in rat, monkey, and human retina. Brain Res. 1986;374:75–92. doi: 10.1016/0006-8993(86)90396-3. [DOI] [PubMed] [Google Scholar]
- Zancanaro C, Sbarbati A, Bolner A, Accordini C, Piedmonte G, Osculati F. Biogenic amines in the taste organ. Chem Senses. 1995;20:329–335. doi: 10.1093/chemse/20.3.329. [DOI] [PubMed] [Google Scholar]
- Zhao FL, Shen T, Kaya N, Lu SG, Cao Y, Herness S. Expression, physiological action, and co-expression patterns of NPY in rat taste bud cells. PNAS. 2005;102:11100–11105. doi: 10.1073/pnas.0501988102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MY, Wang WP, Iyo AH, Ordway GA, Kim KS. Age-associated changes in mRNA levels of Phox2, norepinephrine transporter and dopamine beta-hydroxylase in the locus coeruleus and adrenal glands of rats. J Neurochem. 2005;94:828–838. doi: 10.1111/j.1471-4159.2005.03245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubare-Samuelov M, Shaul ME, Peri I, Aliluiko A, Tirosh O, Naim M. Inhibition of signal termination-related kinases by membrane-permeant bitter and sweet tastants: potential role in taste signal termination. Am J Physiol Cell Physiol. 2005;289:483–492. doi: 10.1152/ajpcell.00547.2004. [DOI] [PubMed] [Google Scholar]