Abstract
The tumor suppressor protein p53 plays a key role in regulation of negative cellular growth in response to EGCG. To further explore the role of p53 signaling and elucidate the molecular mechanism, we employed colon cancer HCT116 cell line and its derivatives in which a specific transcriptional target of p53 is knocked down by homologous recombination. Cells expressing p53 and p21 accumulate in G1 upon treatment with EGCG. In contrast, same cells lacking p21 traverse through the cell cycle and eventually undergo apoptosis as revealed by TUNEL staining. Treatment with EGCG leads to induction of p53, p21 and PUMA in p21 wild-type, and p53 and PUMA in p21−/− cells. Ablation of p53 by RNAi protects p21−/− cells, thus indicating a p53-dependent apoptosis by EGCG. Furthermore, analysis of cells lacking PUMA or Bax with or without p21 but with p53 reveals that all the cells expressing p53 and p21 survived after EGCG treatment. More interestingly, cells lacking both PUMA and p21 survived ECGC treatment whereas those lacking p21 and Bax did not. Taken together, our results present a novel concept wherein p21-dependent growth arrest pre-empts and protects cells from otherwise, in its absence, apoptosis which is mediated by activation of pro-apoptotic protein PUMA. Furthermore, we find that p53-dependent activation of PUMA in response to EGCG directly leads to apoptosis with out requiring Bax as is the case in response to agents that induce DNA damage. p21, thus can be used as a molecular switch for therapeutic intervention of colon cancer.
Keywords: Cell cycle, Bax, HCT116 cells, colon cancer, isogenic cells
1. Introduction
The epidemeological and animal model studies show that epigallocatechin-3-gallate (EGCG), a major ingredient of tea exhibits remarkable chemopreventive properties [1–3]. Work from several laboratories including ours has shown that treatment of mammalian cells with EGCG modulates and activates the expression of several proteins involved in cell signaling, invasion, tumor angiogenesis and vascular tumor growth [3–8]. Among others, the modulation of two major proteins, p53 and NF-κB, results in negative cellular growth regulation by cell cycle arrest and/or apoptosis [9, 10]. Disruption of either p53 or any of the components that form the so called, “p53-network” is a key event in the etiology and development of virtually all human cancers [11, 12]. It is, therefore, important to devise strategies whereby cancer cells (defective in functional p53) are eliminated and simultaneously, normal cells (containing functional p53) are protected [13]. p53 functions as a double-edged sword, inducing cell death by initiating apoptosis in response to some stimuli or when overexpressed and protects cells by inducing transient, reversible growth arrest in response to other stimuli, such as starvation of DNA precursors [14–17].
In an effort to identify agents that exhibit differential growth regulation based on presence or absence of p53 and furthermore investigate the role of this key protein we have made use of a number of isogenic cell lines. While testing many compounds we found that cells expressing wild-type p53 were much more sensitive to EGCG-induced growth inhibition compared to p53-null cells. However, cells lacking p53 also exhibit a certain degree of growth inhibition at a concentration of EGCG that was physiologically relevant [9, 10]. Based on our published and the work presented here we infer that the effects of EGCG in negative regulation of cellular growth are manifested primarily via two pathways, one involving p53 (p53-dependent) and the other that operates in the absence of functional p53 (mediated primarily via p73) where a tyrosine phosphatase, SHP-2 plays a critical role leading to differential apoptosis [8].
Despite a wealth of information on the efficacy of green tea in prevention of cancer and characterization of biochemical pathways, genetic evidence is lacking that establish a role of a specific protein. We have begun to utilize genetic approaches such as the use of isogenic cell lines and shRNA technology to investigate and define the role of p53 and the members of its so called “p53-network” in differential growth regulation by EGCG. Previous studies suggest that although most cell lines were sensitive to EGCG induced apoptosis at concentrations ranging 30–100 µM, HCT116 colon cancer cell line showed relatively resistance to EGCG and even low apoptosis was observed at 200 µM of EGCG (18). In the present study, we investigated the mechanism of such resistance of HCT116 cells to EGCG and found a critical role of p53 and two of its target proteins, p21 and PUMA in the anti-proliferative and apoptotic effects of EGCG in HCT116 colon cancer cells. Our results indicate that colon cancer cells are resistant to EGCG due to p53-dependent induction of p21 which mediates cell cycle arrest. However, in the absence of p21 these cells switch to apoptosis. Interestingly, we find that the apoptosis in response to EGCG is directly mediated by PUMA and does not require Bax as reported for agents that cause DNA damage [19].
2. Materials and Methods
2.1. Materials
Colon cancer HCT116 cells and its derivatives lacking p21, Bax, PUMA and p21/PUMA or p21/Bax double knockout cells were a kind gift from Dr. Bert Vogelstein, The Johns Hopkins Kimmel Cancer Center. EGCG was purchased from Sigma-Aldrich.
2.2. Cell culture and treatment
Monolayer cultures of colon cancer cell line HCT116 or its derivatives were grown in DMEM growth medium containing 10% heat inactivated fetal bovine serum (FBS), 50 U/ml penicillin and 50 µg/ml streptomycin. 5×105 cells were incubated in 10-cm tissue culture plates at 37°C in a humidified atmosphere (5% CO2). Cells were plated at required seeding density allowed to settle for 24 h. Media was replaced and cells were then treated with 100 µM EGCG for indicated times. At the end of the incubation, cells were used for various assays.
2.3. Methylene blue staining and quantification
Cells were plated at a density of 5×105 cells per 10-cm plate and treated with 100 µM EGCG. 96 h after treatment, media was replaced with methylene blue solution and kept on shaker for 4–6 hr. Dye was removed and plates were gently washed to remove excess dye. Plates were dried and scanned. After scanning, the dye taken up by the live cells is eluted using 0.1N HCl and its optical density was measured. Considering the optical density of the dye taken up by untreated cells as 100%, the optical density of the dye taken by treated cells was calculated. As only live cells take up the dye, the ratio of the dye between untreated vs treated plates gives the ratio of live cells present in the treated vs untreated plates and is represented as % of live cells at the end of 96 h after treatment in untreated plates.
2.4. Transfection of packaging cells for viral production and infection of cells with virus
293T packaging cells were plated in 10-cm plates with cell density of 5×106 the day prior to transfection in DMEM containing 10% heat inactivated FBS without penicillin-streptomycin. 6 µg of shp53 or shGFP (control) RNA along with second generation packaging constructs (pCMV-dR8.74 and pMD2G) was transfected using lipofectamin Plus reagent (Invitrogen Corp) as per the protocol supplied by the manufacturer. Media was collected for two subsequent days and layered onto the cells to be infected with virus after adding 10 µl of 4 mg/ml polybrene per 10 ml and sterilize through filtering.
2.5. Western blotting
Cells were lysed in the lysis buffer {Tris-HCl-50mM, NaCl-150mM, Triton X-100-1%, EGTA-1mM, Sodium pyrophosphate-20mM, pH-7.4 containing protease inhibitors cocktail (10ul/ml), NaF (10 mM), DTT(1 mM), PMSF (0.1 mM) and sodium vanadate (1 mM)} on ice. To confirm equal loadings total protein concentration was determined using Bradford method (Biorad). Proteins were resolved using SDS-PAGE and then transferred to polyvinylidene diflouride (PVDF) membrane. Non specific binding sites on membrane were blocked using 5% non-fat skimmed milk and incubated with the primary antibody followed by the incubation with a secondary antibody. Proteins were detected using ECL Plus kit (Perkin Elmer).
2.6. TUNEL assay
The cells (both attached and floaters) were harvested, washed with PBS then incubated in 1% paraformaldehyde for 30–60 minutes and fixed in 70% ethanol. DNA breaks were labeled using TdT enzyme, bromodeoxyuridine triphosphate (BrdUTP) and fluorescein labeled anti BrdU antibody and total DNA was counter stained using propidium iodide/RNase A solution as per manufacturer’s protocol using APO-BRDU apoptosis kit (Phoenix Flow Systems, Inc, San Diego, CA.). For each determination, a minimum of 10,000 cells were analyzed.
2.7. Cell cycle analysis
After EGCG treatment, cells were harvested at indicated time intervals, washed with PBS, fixed in ice-cold 90% methanol, again washed with PBS and DNA was stained using propidium iodide/RNase A solution at 37°C for 60 min. Flow cytometry analyses were performed on Coulter EPICS-XL MCL flow cytometer and analyzed using Cell Quest analysis software modfit. For each determination, a minimum of 20,000 cells was analyzed.
2.8. Detection of oxidative damage to DNA
For the detection of oxidative damage to DNA in vitro, we used the Oxy DNA assay kit (Calbiochem, San Diego, CA), which is based on the direct binding of a fluorescent probe to 8-oxoguanine moieties in the DNA of fixed cells. Cells were grown at a density of 5 × 105 cells in 100-mm culture dishes for overnight, fresh media were added followed by the addition of EGCG, camptothecin and nutlin-3 at the concentrations of 100 µM, 100 ng/ml and 10 µM respectively for 24 h. After exposure to test agents, cells were washed with ice-cold PBS and then fixed with 2% paraformaldehyde and 70% ethanol. Untreated cells were taken as controls through all the experiments. Cells were blocked and stained with FITC conjugate. Fluorescence was analyzed using the Coulter Epics XL Flow Cytometer (Beckman Coulter, Inc. Fullerton, CA).
2.9. Measurement of ROS
Intracellular ROS generation was measured by flow cytometry following staining with hydroethidium (HE), which is oxidized to ethidium bromide. Briefly, after treatment with the indicated agents for 24 h, cells were stained with HE for 30 minutes at 37°C in an incubator and analyzed using a Coulter Epics XL Flow Cytometer (Beckman Coulter, Inc. Fullerton, CA).
3. Results
3.1. EGCG treatment selectively induces apoptosis of HCT116 colon cancer cells lacking p21
Previous studies from our laboratory established a role of p53 in EGCG mediated growth arrest and apoptosis in prostate cancer cells. To further elucidate the role of p53 and its downstream signaling, we made use of the colon cancer HCT116 cells expressing wild-type p53 and its targets p21, Bax and PUMA (herein referred as WT) and its derivatives where expression of a specific known target of p53 such as p21, Bax or PUMA were knocked down by homologous recombination [19]. While comparing the response of WT cells with those lacking p21, we observed a remarkable difference in the sensitivity of these two cell types towards EGCG. WT and p21−/− cells were treated with a wide range of concentrations of EGCG for different times and plates were stained with methylene blue. Although EGCG strongly inhibited the growth of both WT and p21−/− cells, p21−/− cells were much more sensitive to EGCG and 100 µM of EGCG clearly induced differential growth inhibition depending on the p21 status of the cells. Therefore, we used 100 µM of EGCG throughout the study. A representative data for 96 h of 100 µM EGCG treatment was shown in Fig. 1A. In order to further investigate the mode of cell growth inhibition, WT and p21−/− cells were treated with EGCG and apoptosis and cell cycle distribution were measured as described in the materials and methods. As shown in Fig. 1B, a great majority of cells lacking p21 stained positive in the TUNEL assay, whereas WT cells had much reduced TUNEL staining. In contrast, cell cycle analysis suggest that WT cells expressing p21 were arrested in G1 whereas cells lacking p21 continued to progress through cell cycle and eventually underwent apoptosis as reflected by subG1 amount of DNA contents (Fig. 1C). All the data together thus indicate that p21 plays a key role in conferring protection of colon cancer cells to EGCG-induced apoptosis.
Figure 1. EGCG selectively induces apoptosis of HCT116 cells lacking p21.
(A) WT and p21−/− cells were treated with 100 µM EGCG for 96 h and stained with methylene blue. The intensity of methylene blue take-up by live cells was measured calorimetrically after eluting the dye in 0.1N HCl and compared with untreated cells. (B) Cells were treated with 100 µM EGCG for 96 h, harvested, treated with 1% para-formaldehyde for 1h, fixed in 70% ethanol and stained for TUNEL positive cells. (C) Cell treated with 100 µM EGCG for 24, 48 and 72 h were harvested, stained with PI and analyzed by flow cytometry to measure Sub-G1 and G1 population. (D) Cells were treated with 100 µM EGCG for 48 and 96 h. Total cell lysates were subjected to Western blotting for p53, p21, and PUMA. All the data are representative of three independent experiments with highly reproducible results.
In order to elucidate the mechanism associated with cell cycle arrest and apoptosis, cells were treated with 100 µM EGCG for 48 and 96 h and cell lysates were analyzed by Western blot using antibodies that recognize p53 and its downstream effectors p21, or PUMA, (Fig. 1D). As expected, EGCG treatment resulted in an appreciable increase in the levels of p53 and its downstream targets p21, and PUMA in WT cells, whereas EGCG-induced the expression of p53 and PUMA in cells lacking p21.
3.2. p53-dependent expression of p21 or PUMA determine the biological response of cells to EGCG
In order to unequivocally implicate the roles of p53 and its two targets, p21 and PUMA in the biology associated with the effects of EGCG, expression of p53 was ablated in both WT and p21−/− cells using shRNA specific for p53 [20]. Cells transduced with vector containing shGFP were used as control. These cells were treated with EGCG and subjected to Western analysis. As shown in Fig. 2A, 2B & 2C, the ablation of p53 led to substantial inhibition of both basal and EGCG-induced p21 and PUMA levels. These results suggest that p53 is important for the expression of p21 and PUMA by EGCG. The residual expression of p21 and PUMA after p53 ablation might be from incomplete p53 ablation or from p53-independent expression. Particularly, we have previously reported that EGCG could also induced p21 in a p73-dependent manner [8]. Next, the cells after treatment with EGCG were subjected to cell cycle analysis to measure the sub G1 population as apoptotic cells. As shown in Fig. 2D and 2E respectively, WT cells transduced with shGFP showed minimal (7.3%) sub G1 DNA content, whereas p21−/− cells following EGCG treatment were readily killed (55.9% sub G1). WT cells transduced with shp53, which inhibited the expression of p53, p21 and PUMA was also resistant to apoptosis (4.8% sub G1), primarily due to absence of p53 and PUMA. However, cells lacking p21 transduced with shp53 (i.e., silenced expression of p53 and PUMA) again became resistant (7.9% sub G1) to EGCG likely due to absence of p53-dependent expression of PUMA and suggesting p53-dependent apoptosis in the absence of p21. Moreover, all these observations together suggest that expression of p21 and PUMA and their respective roles in cell cycle arrest and apoptosis in response to EGCG are strictly p53-dependent. Furthermore, p21-dependent cell cycle arrest confers a protective mechanism from otherwise p53-mediated apoptosis.
Figure 2. p53 dependent expression of p21 or PUMA determine the cellular response to EGCG.
(A) Total cell lysate from Wild type and p21−/− cells transduced with shGFP or shp53 RNA vector were used for Western blot analysis for p53. (B and C) Total cell lysates from WT or p21−/− cells transduced with shp53 or GFP and treated with 100 µM EGCG for 48 and 96 h were subjected to Western blotting for p21 or PUMA respectively. (D and E) WT or p21−/− HCT116 cells with shp53 or shGFP were harvested after 96 h of 100 µM EGCG treatment and subjected to cell cycle analysis by flow cytometry, respectively. Reproducibility of the data were confirmed by at least three independent experiments.
3.3. p21-mediated cell cycle arrest predominates and protects cells from PUMA-mediated apoptosis
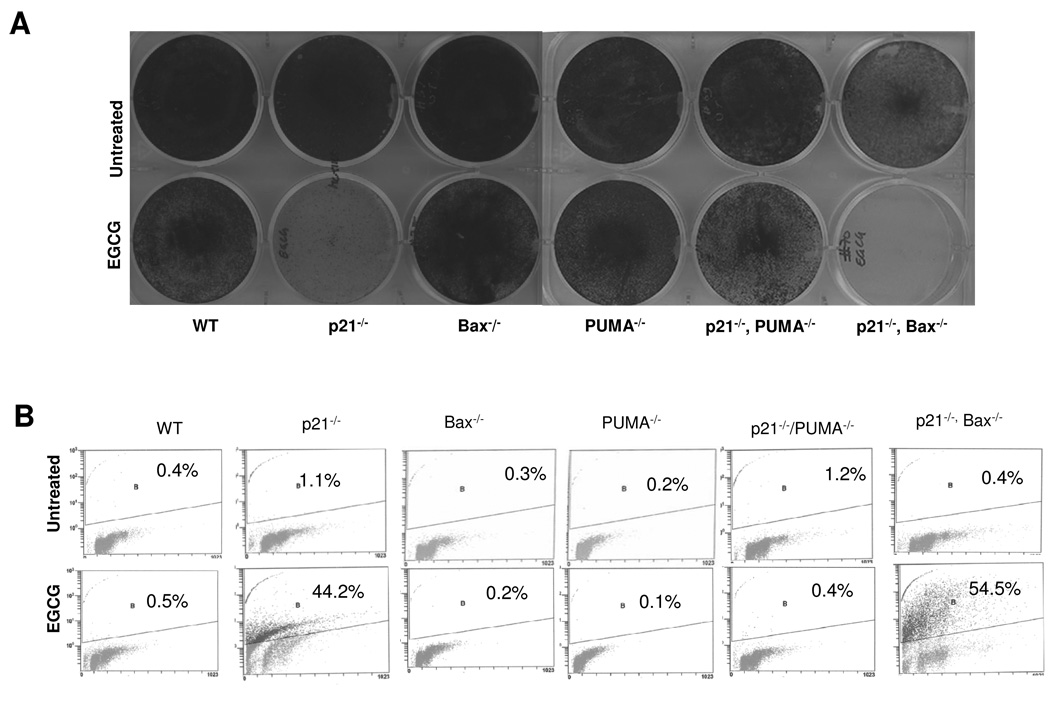
p53-dependent expression of p21 and its role in cell cycle arrest in G1 in response to a wide array of stimuli is well established. However, cells eventually undergo apoptosis likely via induction of proapoptotic BH3 only Bcl2 family members such as PUMA and Bax. To further dissect the intricate signaling mechanism of growth inhibition by EGCG we made use of different variants of HCT116 cells whereby p21, Bax or PUMA were genetically deleted by homologous recombination [19]. To verify the expression of respective proteins cells were treated with 100 µM EGCG and analyzed by Western blotting. The expression of p53, p21, PUMA or Bax was evident in all cell types except in their respective knock-out counterparts (data not shown). To further implicate the role of p21, PUMA and Bax in the cellular response to EGCG cells were treated with 100 µM EGCG, visualized under phase contrast microscope and stained with methylene blue. As shown in Fig. 3A, EGCG treatment greatly inhibited the growth of cells lacking p21, but having PUMA. Similar observation was made in case of cells lacking both p21 and Bax, but having PUMA. In contrast, no remarkable growth inhibition was observed in cells lacking either PUMA or Bax. Interestingly, cells lacking both p21 and PUMA exhibited a remarkable protection. These observations were also confirmed by TUNEL staining of the EGCG-treated cells (Fig. 3B). Cells lacking p21 but expressing PUMA underwent apoptosis, whereas cells lacking both p21 and PUMA were apoptosis resistant. This result suggests that PUMA is required for EGCG-induced apoptosis of cells lacking p21. In contrast, cells expressing p21 but lacking either PUMA or Bax were apoptosis resistant, suggesting that p21-mediated cell cycle arrest predominates over PUMA-mediated apoptosis. Again, cells lacking both p21 and Bax underwent apoptosis, suggesting that PUMA alone is sufficient to induce apoptosis without co-operation from Bax.
Figure 3. p21-mediated cell cycle arrest predominates and prevent cells from PUMA-dependent, Bax-independent apoptosis.
(A) Cells were treated with 100 µM EGCG for 96 h and stained with methylene blue. (B) Cells were treated with 100 µM EGCG for 96 h, harvested, treated with 1% paraformaldehyde for 1 h, fixed in 70% ethanol and stained for TUNEL positive cells.
3.4. No oxidative damage or DNA damage induced by EGCG
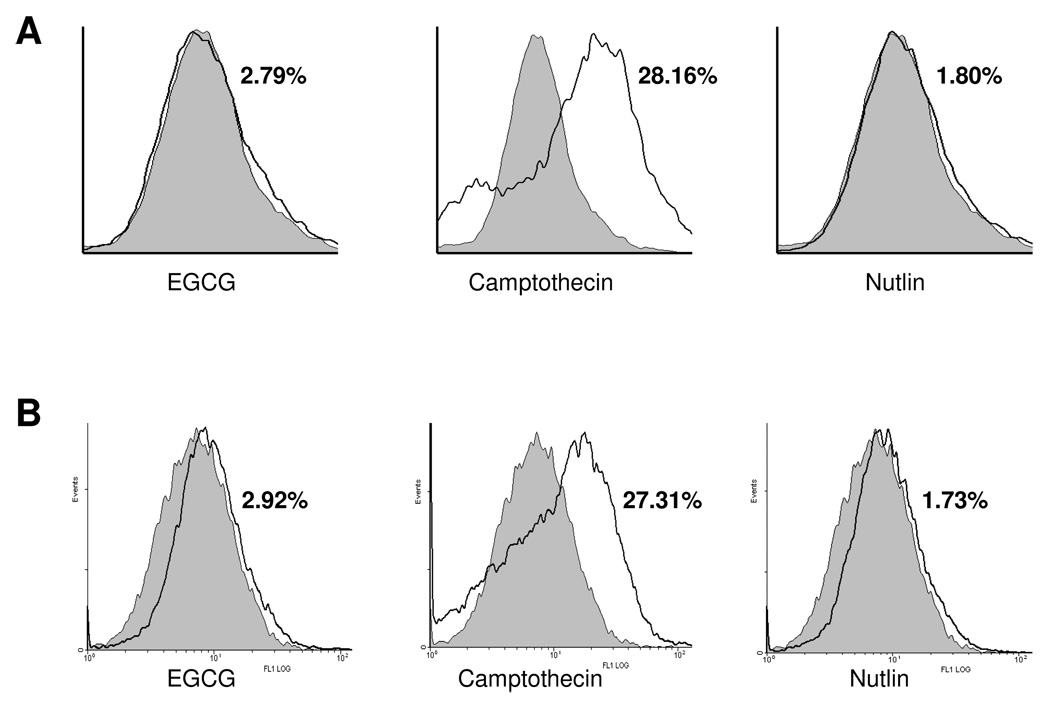
The two important modes of p53 activation are generation of reactive oxygen species (ROS) or induction of DNA damage. Since EGCG is well known for its anti-oxidant property, we next examined the generation of ROS after EGCG treatment. The DNA-damaging agent camptothecin is used as a positive control, whereas nutlin-3 is used as negative control. As shown in Fig. 4A, the DNA damaging agent Camptothecin efficiently induced ROS generation, whereas both EGCG and nutlin-3 did not generate ROS. We also measured DNA damage after EGCG, camptothecin and nutlin-3 treatments and found that although camptothecin efficiently induced DNA damage but neither EGCG nor nutlin-3 had any effect on DNA damage (Fig. 4B). These results suggest that EGCG-induced p53 activation is independent of DNA damage or ROS generation.
Figure 4. EGCG does not cause DNA damage or ROS generation.
(A) ROS generation in cells treated with 100 µM EGCG after 24 h. Camptothecin and nutlin were used as positive and negative controls, respectively. Treated (unshaded) are superimposed on untreated (shaded) samples. (B) DNA damage was measured after 24 h of 100 µM of EGCG treatment, using Oxy DNA assay kit (Calbiochem, San Diego, CA) as described in the methods. Camptothecin and nutlin were used as positive and negative controls, respectively, for DNA damage. Reproducibility of the data were confirmed by multiple independent experiments.
Taken together, all these observations thus suggest that colon cancer HCT116 cells expressing p53 and p21 are growth arrested and the ones lacking p21 escape cell cycle arrest and continue progress through the cell cycle. Furthermore, in absence of p21, p53 activates PUMA-mediated apoptosis which is independent of Bax, in sharp contrast to DNA damaging agents where Bax is required for p53-dependent PUMA-mediated apoptosis [19]. Simplified models for the mechanism of action of EGCG and that of DNA damaging agent are shown in Fig. 5.
Figure 5. Proposed working model.
(A) EGCG treatment leads to induction of p53 followed by induction of p21 and PUMA. Induction of p21 causes cell cycle arrest thereby protecting cells from EGCG induced apoptosis, however in the absence of p21 cells continue in cell cycle and undergo PUMA mediated apoptosis. (B) DNA damaging agents require both PUMA and Bax for apoptosis. Unlike apoptosis caused by DNA damaging agents, apoptosis caused by EGCG does not require Bax, and PUMA is sufficient to cause apoptosis.
4. Discussion
Colorectal cancers are more resistant to chemotherapy as compared to other cancer types [21]. It is, therefore, important to devise strategies making the chemo-resistant tumor cells more sensitive to drugs. Understanding the underlying mechanisms of drug resistance is thus critical for developing new agents effective against colon cancers. Cancer cells may escape drug-induced apoptosis either by arresting in a particular phase of cell cycle or by disabling the apoptotic signaling [22, 23]. In the present report, we show that p53-dependent induction of p21 plays a predominant role and protects the HCT116 colon cancer cells from EGCG-induced apoptosis. Our results suggest that in cells containing wild-type p53, EGCG stabilizes and activates p53, thus induces both the pro-apoptotic protein PUMA and check-point protein p21. However, rather than undergoing apoptosis, cells are arrested at G1. In contrast, genetic ablation of p21 by homologous recombination makes these cells sensitive to EGCG-induced apoptosis. Moreover, ablation of p53 by shRNA or knockout of PUMA by homologous recombination in cells lacking p21 renders them resistant to EGCG. All these results suggest that EGCG induces either p53-dependent cell cycle arrest by inducing p21 or p53-dependent apoptosis by inducing PUMA. However, p21-dependent cell cycle arrest is the predominant signal. These findings are consistent with those previously reported that p53-dependent induction of p21 protects HCT116 cells from apoptosis in response to DNA damaging agents and that ablation of p21 induces Bax/PUMA-dependent apoptosis utilizing p53 [19, 24]. In contrast, using the same very HCT116 colon cancer cells and its derivatives, a recent study reported that p53-dependent expression of p21 confers a pro-apoptotic role instead of its well characterized growth arrest effect in response to the natural alkaloid, noscapine [25].
p53 can be activated and stabilized in response to different stimuli, including DNA damage, oncogenic stimulation, generation of reactive oxygen species and negative regulators such as Mdm2 and ARF [26, 27]. An increasing body of evidence suggests that EGCG can act either as an anti-oxidant or induces oxidative stress [28, 29]. Moreover, some reports suggest that EGCG causes DNA damage [29]. In order to study the mode of p53 activation by EGCG in colon cancer cells, we examined both DNA damage and ROS generation and find no evidence of DNA damage or ROS (Fig.4). Thus, our results suggest a different mechanism of p53 activation by EGCG that does not involve DNA damage or ROS generation. The pathways involved in activation of p53 by EGCG and other natural chemopreventive agents remain to be deciphered.
The downstream targets of p53 to execute apoptosis are wide and vary with the cell type and stimuli. The Bcl2 family proapoptotic proteins, Bax and BH3 only Bcl2 family proteins, PUMA and NOXA play important role in mitochondrial apoptotic pathways induced in a p53-dependent and p53-independent manner. Earlier work from our laboratory reported that two p53 targets, p21 and Bax cooperate in facilitating EGCG-induced apoptosis of prostate cancer cells [10]. In addition, we found that treatment of MEFs with EGCG induced a number of these genes, including Perp, Wig1 and Pig11 in a p53-independent manner in cells undergoing apoptosis [8]. In the current study, we find that EGCG-treatment induces PUMA which plays a critical role in p53-dependent apoptosis of colon cancer cells. Both genetic and biochemical evidence implicate an important role of PUMA in p53-dependent apoptosis where another target protein of p53, Bax cooperates and is essential [19, 30]. Moreover, PUMA-dependent apoptosis also requires generation of ROS [31]. In contrast, a recent study reports that p53-dependent apoptosis by noscapine requires p21 but not Bax [25]. The observations in the present study strongly support the notion that PUMA alone is sufficient for inducing apoptosis without the cooperation of Bax or p21 and does not depend on ROS generation. In conclusion, our results identify p21 as a protective factor for colon cancer cells from EGCG-induced apoptosis and a novel role for PUMA in this process in the absence of p21. Modulation of p21, therefore, could be exploited as an effective strategy for colon cancer therapeutics by EGCG.
Acknowledgement
We are grateful to Dr. Bert Vogelstein for providing HCT116 and its knock-out derivative cell lines. This work was supported by NIH grant R01 CA98916 to MLA. ARA is a recipient of SPORE Career Development Award (P50 CA128613).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
References
- 1.Mukhtar H, Ahmad N. Tea polyphenols: prevention of cancer and optimizing health. Am J Clin Nutr. 2000;71:1698S–1702S. doi: 10.1093/ajcn/71.6.1698S. discussion 703S-4S. [DOI] [PubMed] [Google Scholar]
- 2.Chung FL, Schwartz J, Herzog CR, Yang YM. Tea and cancer prevention: studies in animals and humans. J Nutr. 2003;133:3268S–3274S. doi: 10.1093/jn/133.10.3268S. [DOI] [PubMed] [Google Scholar]
- 3.Amin AR, Kucuk O, Khuri FR, Shin DM. Perspectives for cancer prevention with natural compounds. J Clin Oncol. 2009;27:2712–2725. doi: 10.1200/JCO.2008.20.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garbisa S, Sartor L, Biggin S, Salvato B, Benelli R, Albini A. Tumor gelatinases and invasion inhibited by the green tea flavanol epigallocatechin-3-gallate. Cancer. 2001;91:822–832. doi: 10.1002/1097-0142(20010215)91:4<822::aid-cncr1070>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 5.Jung YD, Kim MS, Shin BA, Chay KO, Ahn BW, Liu W, et al. EGCG, a major component of green tea, inhibits tumour growth by inhibiting VEGF induction in human colon carcinoma cells. Br J Cancer. 2001;84:844–850. doi: 10.1054/bjoc.2000.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fassina G, Vene R, Morini M, Minghelli S, Benelli R, Noonan DM, et al. Mechanisms of inhibition of tumor angiogenesis and vascular tumor growth by epigallocatechin-3-gallate. Clin Cancer Res. 2004;10:4865–4873. doi: 10.1158/1078-0432.CCR-03-0672. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu M, Deguchi A, Lim JT, Moriwaki H, Kopelovich L, Weinstein' IB. (-)-Epigallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clin Cancer Res. 2005;11:2735–2746. doi: 10.1158/1078-0432.CCR-04-2014. [DOI] [PubMed] [Google Scholar]
- 8.Amin AR, Thakur VS, Paul RK, Feng GS, Qu CK, Mukhtar H, et al. SHP-2 tyrosine phosphatase inhibits p73-dependent apoptosis and expression of a subset of p53 target genes induced by EGCG. Proc Natl Acad Sci U S A. 2007;104:5419–5424. doi: 10.1073/pnas.0700642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hastak K, Gupta S, Ahmad N, Agarwal MK, Agarwal ML, Mukhtar H. Role of p53 and NF-kappaB in epigallocatechin-3-gallate-induced apoptosis of LNCaP cells. Oncogene. 2003;22:4851–4859. doi: 10.1038/sj.onc.1206708. [DOI] [PubMed] [Google Scholar]
- 10.Hastak K, Agarwal MK, Mukhtar H, Agarwal ML. Ablation of either p21 or Bax prevents p53-dependent apoptosis induced by green tea polyphenol epigallocatechin-3-gallate. FASEB J. 2005;19:789–791. doi: 10.1096/fj.04-2226fje. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal ML, Taylor WR, Chernov MV, Chernova OB, Stark GR. The p53 network. J Biol Chem. 1998;273:1–4. doi: 10.1074/jbc.273.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 13.Amin AR, Paul RK, Thakur VS, Agarwal ML. A novel role for p73 in the regulation of Akt-Foxo1a-Bim signaling and apoptosis induced by the plant lectin, Concanavalin A. Cancer Res. 2007;67:5617–5621. doi: 10.1158/0008-5472.CAN-07-0655. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal MK, Hastak K, Jackson MW, Breit SN, Stark GR, Agarwal ML. Macrophage inhibitory cytokine 1 mediates a p53-dependent protective arrest in S phase in response to starvation for DNA precursors. Proc Natl Acad Sci U S A. 2006;103:16278–16283. doi: 10.1073/pnas.0607210103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Efeyan A, Serrano M. p53: guardian of the genome and policeman of the oncogenes. Cell Cycle. 2007;6:1006–1010. doi: 10.4161/cc.6.9.4211. [DOI] [PubMed] [Google Scholar]
- 16.Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–283. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- 17.Hastak K, Paul RK, Agarwal MK, Thakur VS, Amin AR, Agarwal S, et al. DNA synthesis from unbalanced nucleotide pools causes limited DNA damage that triggers ATR-CHK1-dependent p53 activation. Proc Natl Acad Sci U S A. 2008;105:6314–6319. doi: 10.1073/pnas.0802080105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inaba H, Nagaoka Y, Kushima Y, Kumagai A, Matsumoto Y, Sakaguchi M, et al. Comparative examination of anti-proliferative activities of (-)-epigallocatechin gallate and (--)-epigallocatechin against HCT116 colorectal carcinoma cells. Biol Pharm Bull. 2008;31:79–84. doi: 10.1248/bpb.31.79. [DOI] [PubMed] [Google Scholar]
- 19.Yu J, Wang Z, Kinzler KW, Vogelstein B, Zhang L. PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc Natl Acad Sci U S A. 2003;100:1931–1936. doi: 10.1073/pnas.2627984100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson MW, Agarwal MK, Yang J, Bruss P, Uchiumi T, Agarwal ML ML, et al. p130/p107/p105Rb-dependent transcriptional repression during DNA-damage-induced cell-cycle exit at G2. J Cell Sci. 2005;118:1821–1832. doi: 10.1242/jcs.02307. [DOI] [PubMed] [Google Scholar]
- 21.Blijham GH. Chemotherapy of colorectal cancer. Anticancer Drugs. 1991;2:233–245. doi: 10.1097/00001813-199106000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Shah MA, Schwartz GK. Cell cycle-mediated drug resistance: an emerging concept in cancer therapy. Clin Cancer Res. 2001;7:2168–2181. [PubMed] [Google Scholar]
- 23.Brown JM, Wilson G. Apoptosis genes and resistance to cancer therapy: what does the experimental and clinical data tell us? Cancer Biol Ther. 2003;2:477–490. doi: 10.4161/cbt.2.5.450. [DOI] [PubMed] [Google Scholar]
- 24.Javelaud D, Besancon F. Inactivation of p21WAF1 sensitizes cells to apoptosis via an increase of both p14ARF and p53 levels and an alteration of the Bax/Bcl-2 ratio. J Biol Chem. 2002;277:37949–37954. doi: 10.1074/jbc.M204497200. [DOI] [PubMed] [Google Scholar]
- 25.Aneja R, Ghaleb AM, Zhou J, Yang VW, Joshi HC. p53 and p21 determine the sensitivity of noscapine-induced apoptosis in colon cancer cells. Cancer Res. 2007;67:3862–3870. doi: 10.1158/0008-5472.CAN-06-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brooks CL, Gu W. p53 ubiquitination: Mdm2 and beyond. Mol Cell. 2006;21:307–315. doi: 10.1016/j.molcel.2006.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallagher SJ, Kefford RF, Rizos H. The ARF tumour suppressor. Int J Biochem Cell Biol. 2006;38:1637–1641. doi: 10.1016/j.biocel.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Hu C, Kitts DD. Evaluation of antioxidant activity of epigallocatechin gallate in biphasic model systems in vitro. Mol Cell Biochem. 2001;218:147–155. doi: 10.1023/a:1007220928446. [DOI] [PubMed] [Google Scholar]
- 29.Furukawa A, Oikawa S, Murata M, Hiraku Y, Kawanishi S. (-)-Epigallocatechin gallate causes oxidative damage to isolated and cellular DNA. Biochem Pharmacol. 2003;66:1769–1778. doi: 10.1016/s0006-2952(03)00541-0. [DOI] [PubMed] [Google Scholar]
- 30.Chen L, Jiang J, Cheng C, et al. p53 dependent and independent apoptosis induced by lidamycin in human colorectal cancer cells. Cancer Biol Ther. 2007;6:965–973. doi: 10.4161/cbt.6.6.4193. [DOI] [PubMed] [Google Scholar]
- 31.Liu Z, Lu H, Shi H, Du Y, Yu J, Gu S, et al. PUMA overexpression induces reactive oxygen species generation and proteasome-mediated stathmin degradation in colorectal cancer cells. Cancer Res. 2005;65:1647–1654. doi: 10.1158/0008-5472.CAN-04-1754. [DOI] [PubMed] [Google Scholar]