Abstract
Recent evidence suggests that a genetic polymorphism in the promoter region (5-HTTLPR) of the serotonin transporter gene (SLC6A4) mediates stress reactivity in adults. Little is known, however, about this gene-brain association in childhood and adolescence, generally conceptualized as a time of heightened stress reactivity. The present study examines the association between 5-HTTLPR allelic variation and responses to fearful and angry faces presented both sub- and supraliminally in participants, ages 9–17. Behaviorally, carriers of the 5-HTTLPR short (s) allele exhibited significantly greater attentional bias to subliminally presented fear faces than did their long (l)-allele homozygous counterparts. Moreover, s-allele carriers showed greater neural activations to fearful and angry faces than did l-allele homozygotes in various regions of association cortex previously linked to attention control in adults. These results indicate that in children and adolescents, s-allele carriers can be distinguished from l-allele homozygotes on the basis of hypervigilant behavioral and neural processing of negative material.
Introduction
Serotonin plays a critical role in the modulation of emotion1. A common functional variant in the human serotonin transporter gene (5-HTTLPR; SLC6A4) produces a short or long nucleotide repeat chain that alters serotonin availability. Compared to the long (l) allele, the short (s)-allele gene variant is associated with reduced serotonin reuptake in vitro2, 3, which appears to have adverse behavioral consequences3, 4 5–9. Indeed, although results have been equivocal 10, 11, 5-HTTLPR has been found to interact with stress to predict the onset of depression 9.
In an effort to elucidate the mechanisms that may underlie the association between the 5-HTTLPR polymorphism and depression, researchers have examined associations between 5-HTTLPR and markers of stress reactivity. For example, Gotlib et al. (2008) found that adolescent girls who were homozygous for the s allele secreted more cortisol over a longer period of time in response to an acute stressor than did l-allele carriers 12. Other investigators have examined the relation between 5-HTTLPR and brain function and structure. Compared with l-allele homozygotes, s-allele carriers have been found to exhibit reduced grey matter volume 13, 14, increased amygdala activity to threatening faces (reviewed in 15), and altered functional coupling of emotion-regulation brain circuits encompassing the amygdala and associated prefrontal cortical (PFC) projection zones14, 16, 17. These findings of increased cortisol and increased neural responsivity to threat suggest that exacerbated stress or arousal responses to environmental threat underlie the association between the s allele and increased trait negative affect 18–20.
Cognitive attention theories may be relevant to our understanding of how the 5-HTTLPR gene may exert its effects, particularly implicating modulation of attention. In the clinical domain, cognitive theories linking enhanced detection of potentially threatening cues in the environment to anxiety have been influential. Thus, individuals who prioritize attention allocation 21 towards negative or threatening material are more susceptible to mood and anxiety disorders 22, 23. Indeed, heightened attention to negative stimuli has been observed across emotional disorders: in children and adolescents who are diagnosed with anxiety disorders 24–26, who are at risk for depression 27, 28, and/or who have high levels of trait anxiety 29. By altering serotonin levels, the 5-HTTLPR gene may change the sensitivity to processing negative information on the environment. This formulation is supported by recent research indicating that children with the s allele of the 5-HTTLPR gene have faster responses to angry faces 30. It is possible, therefore, that individuals with the 5-HTTLPR s allele have a greater tendency to direct processing resources toward danger-relevant stimuli. If persistent hypervigilant orienting toward negative material in the environment does characterize s-allele carriers, then it is important to elucidate patterns of neural processing that are associated with this allocation difference, and to learn whether these behaviors are observable from a young age, before significant or chronic life stressors have been likely to exert their effects.
The present functional magnetic resonance imaging (fMRI) study was designed to examine whether 5-HTTLPR genotype affects the neural substrates of spatial orienting of attention in a sample of unselected, healthy children and adolescents. We focused on this age range because childhood and early adolescence are times when individuals may experience their first onset of depression and other disorders, as well as a time of heightened stress reactivity 31. Consequently, we were interested in whether at a young age, this gene would affect cognitive biases, presumably training the brain for environmental processing that will shape later life experiences. In particular, we expected differences in regions of the parietal cortex and lateral frontal cortex that interact with sensory brain structures to control human visual attention 32, 33, and that are postulated to control enhanced processing of emotionally salient material (reviewed in 34). During fMRI image acquisition, healthy children and adolescents completed the dot-probe task, one of the most widely used tasks in the assessment of behavioral and neural aspects of attentional biases 24–26, 29 35. We assess responses to both subliminal and supraliminal stimulus presentation durations because whereas briefly presented stimuli have been found to be associated with biases in anxiety, depression has been found to be characterized by biases for stimuli that are presented for longer durations 27. Moreover, neural responses have been shown to differ with presentation rate 24, 25.
We hypothesized that carriers of the s allele would exhibit behavioral and neural responses reflecting a cognitive system that prioritizes processing of negative emotional material. Specifically, we predicted that s-allele carriers would demonstrate (1) enhanced neural processing (i.e., greater BOLD signal response) during the presentation of fear and angry (compared with neutral) faces than would l-allele homozygotes; and (2) faster behavioral responses to dot probes when they replace fear and angry (compared with neutral) faces.
Materials and methods
Participants
Participants were 51 adolescents (24 females) between the ages of 9 and 17 years (M=13.12, SD= 2.75). Participants were recruited through local advertisements and parent networks and scanned either at the National Institute of Mental Health (NIMH) functional MRI Facility (fMRIF) (n = 17) or at Stanford University's Richard M. Lucas Center for Imaging (n = 34). All participants had no reported history of brain injury, no behavioral indications of possible mental impairment, no past or present Axis I disorder, and were fluent in English. Three participants were left-handed, and one participant had questionable lifetime diagnosis of Attention/Deficit Hyperactivity Disorder. At both sites, the participants were compensated for their time. Parents and adolescents gave informed consent and assent, respectively, as approved by the NIMH and Stanford Institutional Review Boards.
Measures
Trained interviewers assessed the diagnostic status of the adolescents by administering the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL), which has been shown to generate reliable and valid psychiatric diagnoses 36. Any adolescent who received a current or past Axis-I diagnosis was eliminated from the study. To assess inter-rater reliability, for Stanford participants an independent trained rater evaluated 30% of all K-SAD-PL interviews by randomly selecting audiotapes. In all cases, these diagnoses matched the diagnoses made by the original interviewer, κ=1.00, indicating excellent inter-rater reliability. Similarly, at the NIMH site, all raters had to complete standardized training and then demonstrate acceptable reliability in interviews conducted with 10 individuals.
To ensure that participants did not differ in current levels of depressive symptomatology, all participants completed the short form (10-item) of the Children’s Depression Inventory (CDI-S), a self-report measure of depressive symptoms developed for children and adolescents between the ages of 8 and 17 37. The CDI-S has been demonstrated to have acceptable internal consistency (α = .80) and to correlate highly with the full CDI (r = .89) 38. In addition, to assess levels of anxiety, participants also completed the 41-item version of the Screen for Child Anxiety Related Emotional Disorders (SCARED). The SCARED, too, has been demonstrated to have good internal consistency (α = .74 to .93) and test-retest reliability (correlation coefficients = .7 to .9) 39.
Procedure
The study consisted of two separate sessions. In the first session, all parent-child dyads participated in diagnostic interviews to assess DSM-IV current and lifetime diagnostic status using the K-SADS-PL 40, 41 . During this session, adolescents also provided saliva samples for genetic testing and viewed a video or visited a mock scanner to prepare them for the MRI scan session. In the second session, brain-imaging data were acquired from the adolescents using a whole-brain MRI scanner.
Stimuli
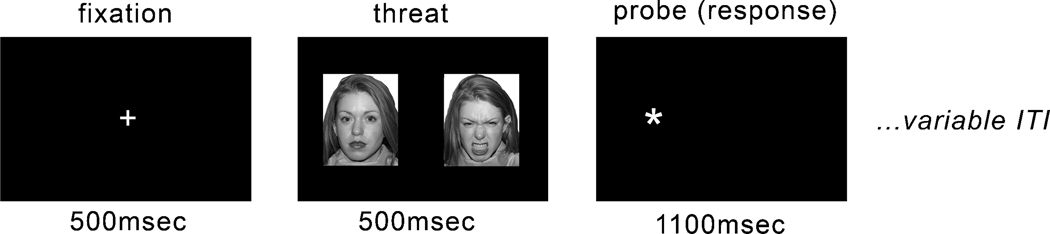
The dot-probe task has been previously used in adolescents with generalized anxiety disorder 24. The task involved viewing a central fixation point, then viewing two faces presented side-by-side, and finally indicating the location of an asterisk (probe) via a left or right button press (Fig. 1). In this way, the participant was not instructed to do any deliberate processing of the face stimuli, but instructed simply to press on the side that the asterisk appeared following the pair of faces, or following faces + scrambled images (subliminal trials). Faces from the NIMSTIM set 42 were presented in pairs of neutral/neutral, neutral/angry (as seen in Figure 1), and neutral/fearful expressions; each pair contained a single actor with two different expressions, except neutral trials, in which the same picture appeared on the right and left. Trials were balanced to have the emotion and target equally presented on the left and right. In addition, an equal number of congruent (asterisk appears on the same side as the expressive emotion face) and incongruent (asterisk appears on the same side as the neutral face) trials were presented.
Figure 1.
Example of a supraliminal fear-neutral trial in the dot-probe task.
The task included an additional manipulation of face-stimuli presentation duration, supraliminal and subliminal, in order to assess automatic processing of emotional material. Participants were not told that the faces were being presented subliminally. Instead, they were simply taught the task required pressing when a single probe asterisk appeared regardless of which preceded it: a pair of faces or scrambled picture pairs. For subliminal trials, the timing of events was: fixation: 500 msec; face-pair: 17 msec; mask (scrambled image): 68 msec; probe: 1100 msec; blank screen: 415 msec (with timing informed by previous designs 43). For supraliminal trials, the timing of events was: fixation: 500 msec; face-pair: 500 msec; probe: 1100 msec. Although for both subliminal and supraliminal trials the total trial length was held constant (2100 msec), the structure of the two trial types were different due to the two face exposure durations; consequently, supra- and subliminal trials were analyzed independently. In this rapid-event-related design, trials were separated by a jittered (variable length) ITI that was between 750–1250 msec (average ITI = 1000 msec). The ITI was jittered to vary the timing of presentations for psychological purposes, so that subjects could not completely anticipate the timing of stimulus presentations, rather than for purely hemodynamic purposes. To improve hemodynamic response estimation, blank or “null” trials were included to help deconvolve the BOLD signal. Each of four functional runs consisted of 96 trials (16 of which were blank/null trials) in random order. Runs lasted 5 minutes and 18 seconds, for a total functional scan duration of approximately 21 minutes.
Participants were given a dot-probe practice task outside of the scanner until they understood how to perform the task, and performed 12 practice trials in the scanner immediately preceding the first run of the task. During scanning, visual stimuli were generated using Eprime (http://www.pstnet.com) on a PC computer, and were presented onto a screen viewed by the participant inside the fMRI machine. Participants used a button box interfaced to the Eprime computer to make their responses.
Genetic analysis
DNA through saliva samples were analyzed using the Oragene Kit (DNA Genotek, Inc. Ottawa, Ontario, Canada), an all-in-one system for the collection, preservation, transportation and purification of DNA from saliva. This procedure is minimally invasive. DNA extracted by this method is of high quality and allows for genotyping with a high success rate 44. To examine the 5-HTTLPR polymorphism, oligonucleotide primers flanking the 5-HTT-linked polymorphic region 2 and corresponding to the nucleotide positions -1416 to -1397 (stpr5, 5'-GGC GTT GCC GCT CTG AAT GC) and -910 to -888 (stpr3, 5'-GAG GGA CTG AGC TGG ACA ACC AC) of the 5-HTT gene 5'-flanking regulatory region were used to generate 484bp or 528bp fragments. The PCR products were electrophoresed through 5% Polyacrylamide gel (Acrylamide/bis-Acrylamide ratio 19:1) at 60 V for 60 min.
Following this genotyping procedure, two groups of children were identified: those possessing at least one copy of the 5-HTTLPR s allele (n = 31) and those carrying two l alleles (n = 20). Classifiying participants based on the triallelic classification of 5- HTTLPR (i.e., considering the A–G single nucleotide substitution in the l allele 45, 46) resulted in 10 participants with two lA alleles and 41 participants with at least one lG or s-allele. Because of both the small number of lA participants in the sample and the considerably greater body of research the bi-allelic classification, we conducted analyses using the biallelic (ss/sl vs ll) classification that yielded more balanced numbers in each gene group.
Behavioral analysis
Attentional bias scores were calculated from the latency data for each type of emotional face (fear and angry) and for exposure duration (subliminal [17 msec] and supraliminal – [500 msec]) as described by Joormann and colleagues 27. Briefly, the bias score was calculated by subtracting the mean reaction time for identifying probes appearing on the same side as the emotion face from the mean reaction time for identifying probes appearing on the opposite side as the emotion face, after excluding error trials. Thus, positive scores indicate greater attentional capture by the emotional face, and negative scores reflect the tendency to avoid the emotional face. Attentional bias scores were analyzed using a three-way (genotype group [l-allele homozygotes, s-allele carriers], emotion [fear, angry], presentation duration [subliminal, supraliminal]) analysis of variance (ANOVA). Alpha=.05 was used to test significant main effects and interactions. Bias scores were also submitted to one sample t-tests in order to determine if bias scores were significantly different than zero.
fMRI data acquisition
Magnetic resonance imaging was performed on a 3.0 T GE whole-body scanner at both sites. A purpose-built single channel T/R head was used at Stanford and an 8-channel head coil was used at NIH. To reduce motion-related artifacts during scanning, participants were stabilized by clamps and a bite bar formed with dental impression wax at Stanford (made of Impression Compound Type I, Kerr Corporation, Romulus, MI) and with expandable cushions surrounding the head at NIH. Senior physicists at each of the two sites optimized institution-specific scanning parameters; therefore, the scan parameters were consistent at both sites unless described otherwise.
High-resolution T2-weighted fast spin-echo structural images (TR = 3000ms; TE = 68ms) were acquired for anatomical reference. A T2*-sensitive gradient echo spiral in/out pulse sequence 47 was used for all functional imaging at Stanford and NIH used a gradient echo single-shot bottom-up interleaved sequence (TR = 2100 ms; TE = 30 ms; flip angle = 77° at Stanford, 78° at NIH; FOV = 22 cm; 64 × 64; 29 axial slices with 4mm slice thickness and no skip). An automated high-order shimming procedure, based on spiral acquisitions, was used to reduce B0 heterogeneity 48. High-resolution volume scans (140 slices at Stanford, 144 slices at NIH; 1mm slice thickness) were collected for every participant using a spoiled grass gradient recalled (SPGR) sequence for T1 contrast (TR = 3000 ms at Stanford, 700 ms at NIH; TE = 68 ms at Stanford, minimum at NIH; TI = 500 ms; flip angle = 11°; FOV = 25 cm at Stanford, 22 cm at NIH; 256 × 256). During the functional scans, heart-rate and respiration waveforms were recorded.
fMRI analysis
fMRI data were preprocessed using Analysis for Functional Neuroimages (AFNI, http://afni.nimh.nih.gov/afni) 49 and custom MATLAB routines. Preprocessing included slice-timing correction, realignment, smoothing (4mm), and bandpass filtering (.011 < f < .15). Four runs of the experiment were concatenated into one long run, for which task vectors specific to each randomized run were generated from participants’ behavioral files. Once convolved with a canonical hemodynamic response function (HRF) the extracted task vectors for each emotion condition (fear, angry, neutral) within each of supra- and subliminal, were then used to model BOLD response to each condition of interest. We created contrasts for fear > neutral and angry > neutral and submitted these to between-group (l-allele homozygotes vs. s-allele carriers) t-tests to assess neural response to emotion-face vs neutral-face baseline for each of four conditions: 1) fear > neutral subliminal; 2) angry > neutral subliminal; 3) fear > neutral supraliminal; and 4) angry > neutral supraliminal. Results were spatially constrained to a grey matter mask image and transformed to Talairach space for reporting. Results are reported for p < .01, corrected. Multiple testing correction was performed using the AFNI subroutine, AlphaSim, which uses Monte Carlo simulation to estimate the number of contiguous voxels one would expect to observe in a significant cluster given the p threshold used and number of comparisons made.
Results
Group characteristics
The two genotype groups (l-allele homozygotes, s-allele carriers) did not differ significantly with respect to age, t(49) = 1.18, p = .25, CDI-S scores, t(47) = .27, p = .79, or SCARED scores, t(46) = .78, p = .43. In addition, the observed frequency of the short and long alleles were in Hardy-Weinberg equilibrium, χ2 = 4.62, p = .1.
Dot-probe behavioral data
Four participants were excluded from analysis because of errors in data collection. For all participants, trials with errors were discarded. The mean percentage of data loss to participant errors was low (less than 5%) and did not differ between gene groups, t(45) = 1.16, p = .25
The ANOVA conducted on the behavioral data did not yield significant main effects for gene group, F(1,49) = .19, p = .67, for face emotion, F(1,49) = .90, p = .35, or for the duration of presentation, F(1,49) = 3.21, p = .08. Moreover, none of the two-way interactions or the three-way interaction of gene group, face emotion, or presentation duration were significant, all ps>.05. Because we hypothesized that s-allele carriers would demonstrate greater attentional bias to fear and angry emotional faces than would l-allele homozygotes, we conducted separate t-tests on participants’ bias scores for each of the four conditions: subliminal fear, subliminal angry, supraliminal fear, supraliminal angry to examine whether the gene groups differed significantly in any of these four conditions. These analyses indicated that the gene groups differed significantly in their attentional bias only in the subliminal fear condition, t(49) = 2.28, p = .03. The group difference in response to subliminal fear resulted from a significant positive mean attentional bias score t(30) = 2.97, p < .01 within s-allele carriers and a non-significant negative attentional bias score in l-allele homozygotes, reflecting greater attentional capture by subliminally presented fear faces in s-allele carriers; see Table 1.
Table 1.
Means and attentional bias scores for each gene group.
| ll carriers (N= 20) | s-allele carriers (N=31) | Between groups statistic | ||
|---|---|---|---|---|
| Age | 12.6 (2.7) | 13.5 (2.8) | t(49) = 1.18, p = .25 | |
| Average reaction times across trials | Subliminal fear | 554 (127) | 557 (120) | t(49) = .11, p = .92 |
| Supraliminal fear | 532 (116) | 537 (123) | t(49) = .15, p = .88 | |
| Subliminal angry | 546 (122) | 557 (126) | t(49) = .31, p = .76 | |
| Supraliminal angry | 527 (120) | 537 (117) | t(49) = .29, p = .77 | |
| Subliminal neutral | 551 (126) | 555 (118) | t(49) = .12, p = .91 | |
| Supraliminal neutral | 532 (116) | 541 (131) | t(49) = .24, p = .81 | |
| Attentional bias scores | Subliminal fear | −4.05 (28) | 12.14 (22)** | t(49) = 2.28, p = .027 |
| Supraliminal fear | 5.89 (45) | 4.95 (37) | t(49) = .08, p = .94 | |
| Subliminal angry | 2.12 (38) | −0.47 (23) | t(49) = .30, p = .76 | |
| Supraliminal angry | 18.56 (32)* | 15.35 (45) | t(49) = .28, p = .78 |
Means and standard deviations are provided for each comparison. Significant between group differences in attentional bias were observed for the subliminal fear condition (p value shown in bold text).
Asterisks are used to denote the significance of one-sample t-tests (**at the .01 level or *at the .05 level) aimed at answering whether within-group means are significantly different than zero.
fMRI results
Fear > neutral subliminal
Between-groups comparisons for fear > neutral subliminal revealed that s-allele carriers had significantly (p < .01, corrected) greater BOLD activation than did l-allele-homozygotes in regions of the parietal and occipital cortices, in particular, medial as well as lateral aspects of the precuneus, and the posterior cingulate. The reverse contrast at the same threshold (l-allele homozygotes > s-allele carriers) did not yield any significant clusters; see Table 2.
Table 2.
Regions of significant differences between 5-HTTLPR polymorphism groups for both subliminal and supraliminal contracts.
| BA | x | y | z | Volume (voxels) | T score | Fig. 2 label | |
|---|---|---|---|---|---|---|---|
| Subliminal fear > neutral | |||||||
| S-carriers > LL | |||||||
| Parietal | |||||||
| Precuneus | R7 | 23 | −70 | 36 | 18 | 3.97 | C |
| Precuneus | R3I | 16 | −50 | 35 | 10 | 3.89 | D |
| Precuneus | L7 | −24 | −61 | 38 | 24 | 2.56 | E |
| Posterior cingulated | L23 | −6 | −31 | 26 | 13 | 3.48 | B |
| Occipital Middle |
L18 | −13 | −88 | 17 | 40 | 3.27 | A |
| LL > S-carriers | |||||||
| For this contrast, no clusters survived threshold | |||||||
| Suhliminal angry > neutral | |||||||
| S-carriers > LL | |||||||
| Limbic | |||||||
| Cingulate | R24 | 12 | 1 | 32 | 27 | 3.69 | G |
| Cingulate | R23 | 7 | −24 | 28 | 11 | 2.24 | F |
| LL > S-carriers | |||||||
| For this contrast, no chusters survived threshold | |||||||
| Supraliminal fear > neutral | |||||||
| S-carriers > LL | |||||||
| Parietal | |||||||
| Inferior | R40 | 56 | −37 | 42 | 16 | 3.26 | 1 |
| LL > S-carriers | |||||||
| Temporal | |||||||
| Superior | R21 | 55 | −24 | −1 | 12 | 3.14 | H |
| Supraliminal angry > neutral | |||||||
| S-carriers > LL | |||||||
| Frontal | |||||||
| Inferior | R44/45 | 57 | 10 | 22 | 13 | 3.10 | K |
| Superior | R1O | 25 | 49 | 19 | 11 | 3.91 | L |
| Inferior | L44 | −48 | 12 | 11 | 10 | 4.20 | |
| Parietal | |||||||
| Inferior | L40 | −40 | −32 | 41 | 120 | 2.78 | O |
| Inferior | R7 | 32 | −53 | 45 | 47 | 3.42 | N |
| Interior | R40 | 51 | −36 | 40 | 37 | 2.82 | M |
| Limbic | |||||||
| Insula | L13 | −35 | −1 | 5 | 9 | 2.79 | J |
| LL > S-carriers | |||||||
| For this contrast, no clusters survived threshold |
Coordinates are given in Talairach and Tournoux convention.
BA = Brodmann’s area. Results provided for p < .01, corrected. LL = ll allele homozygotes; S-carriers = carriers of at least one s-allele; R = right; L = left.
Angry > neutral subliminal
The between-groups comparison for angry > neutral subliminal contrast images resulted in greater activation in the s-allele carriers than in the l-allele homozygotes participants in the cingulate gyrus, compared to no significant clusters of greater response in l-allele homozygotes.
Fear > neutral supraliminal
Between-groups analysis of fear > neutral supraliminal trials showed that s-allele carriers exhibited significantly greater (p < .01, corrected) activation in an inferior parietal region encompassed by Brodmann’s area 40. For the reverse contrast, there was a cluster in the superior temporal gyrus that showed greater BOLD response to fear > neutral supraliminal in l-allele homozygotes than in s-allele carriers; see Table 2.
Angry > neutral supraliminal
The between-groups comparison of angry > neutral supraliminal statistical maps revealed numerous regions, including frontal, parietal, and paralimbic (insula cortex) regions, in which response was significantly greater (p < .01, corrected) in s-allele carriers than in l-allele homozygotes. In contrast, for the same statistical threshold, there were no brain regions in which l-allele homozygotes exhibited greater response in this contrast than did the s-allele carriers; see Table 2.
Consistencies across contrasts
In sum, there was consistency in the whole-brain between-groups comparisons; s-allele carriers exhibited greater activation than did l-allele homozygotes in numerous parietal and paralimbic regions; see Figure 2 and Table 2. There was only one region (superior temporal cortex) in which l-allele homozygotes exhibited significantly (p < .01; cluster min, or k ≥ 9) greater BOLD response than did s-allele carriers.
Figure 2.
Between-group whole brain t-tests (p < .01 corrected) show that, overall, carriers of the 5-HTTLPR s allele demonstrate greater neural response to emotion faces across conditions than l-allele homozygotes. Regions where neural response was greater in s-allele carriers are shown in blue; regions where neural response was greater in l-allele homozygotes are shown in orange. Letters correspond to clusters provided in Table 2. sc = s-allele carrier; ll = l-allele homozygotes.
Discussion
Recent studies suggest that attentional biases to threat play a causal role in the development of anxiety in both children 50–52 and adults 53. There is also mounting evidence in children and adolescents that common variations in the serotonin transporter gene are associated with biases to threat 30, 54. Recent work has added to this formulation, demonstrating that cortisol responses (indicative of biological stress reactivity) are greater in children who are homozygous for 5-HTTLPR s-allele than for l-allele carriers 12. The neural mechanisms underlying these relations, however, are not yet well understood. We examined whether 5-HTTLPR polymorphism is associated with behavioral biases in processing of emotional material and investigated the neural bases of these responses. Importantly, we recruited a sample of healthy adolescents with no current psychopathology or history of any disorder.
We found that the s allele was associated with significantly greater attentional bias to subliminally presented fear faces. This behavioral observation supports the formulation that attentional mechanisms are altered in s-allele carriers. In the present study, we show for the first time that even when emotional information is presented too briefly to reach awareness, biased information processing is associated with variation in 5-HTTLPR. Therefore, individual differences in the processing of rapidly presented emotional stimuli that do not reach awareness may underlie the association between the s-allele and the emergence of difficulties in emotion regulation. Our observation that differences in early attentional biases are related to the serotonin transporter gene, as well as recent work by other groups also examining behavioral consequences of this gene in children 30, 54, suggest that early automatic orienting toward or away from threatening or appetitive stimuli is affected by the 5-HTTLPR gene. This might be compared, for example, to the observation that aggressive individuals show misattribution bias, such as teenagers with conduct disorder.
We found that healthy children and adolescents who carry the 5-HTTLPR s allele showed greater neural responses in parietal, frontal and limbic regions than did youth who were homozygous for the l allele. There was only one region (superior temporal cortex) in which l-allele homozygotes exhibited greater BOLD response to the emotion face > neutral face contrast than did s-allele carriers.
In the present study, we describe genotype-associated differences in neural processing that reflect differences in perceptual processing that have previously been reported 30. In a review of dot-probe neuroimaging studies, Pourtois and Vuilleumier (2006) reviewed dot-probe studies and showed that the attentional bias towards fearful faces was modulated by posterior parietal and intraparietal control over extrastriate regions. Dot-probe studies that used either event-related potentials (ERPs) and/or fMRI methods and found that the temporal dynamics of ERP responses indicated a positive relation between parietal control regions and sensory cortices 34. The differences in behavior observed between the 5-HTTLPR groups in our study and in past investigations may result from upregulation of perceptual responses to these emotional faces by regions that control attentional allotment to afferent sensory pathways. It is as if a gain mechanism in the processing of negative emotion faces is ‘boosted’ in carriers of the s allele, a neurobiological finding supported by the behavior observed in the subliminal neutral condition: that s-allele carriers were more likely to respond faster when targets and negative-emotion faces appeared in the same visual field.
It is important to consider the findings in light of several limitations of this study. First, because of the small number of lA participants, we conducted analyses using a biallelic (ss/sl vs ll) classification that yielded more balanced numbers in each gene group. Because the lG allele shows transporter expression closer to the s-allele, the effect of classifying lG along with lA may have reduced the ability to detect differences between gene groups. On the other hand, the study enrolled a wide range of ages (9–17) and as a result the sample possessed additional variability due to staging in neural development. Second, the combination of a small sample size and multiple testing may have caused some of the findings purely by change and the independent replication is needed and desired. Third, we conducted our scans at two centers. We did examine the effect of site on the neural results and found that scan site did not moderate the obtained gene group differences. Finally, we conducted this study with diagnostically healthy individuals who volunteered to participate in this research. It is possible that this “self-selecting” class of participants represents a relatively narrow range of individuals by virtue of volunteering to participate in neuroimaging research studies. Also, by having studied healthy individuals there was little variability in anxiety propensity; consequently, the findings cannot be related to behavior. Thus, implications of these findings for the development of affective clinical symptoms is not yet clear.
Studying a sample of children and adolescents, we have shown that neural networks that support visual selective attention operate distinctly in different 5-HTTLPR gene variants, even in the early years of life, before the experience of major chronic stress and in the absence of a history of psychopathology. We posit that the s-allele carrier gain in perceptual response to threatening stimuli in childhood and adolescence is part of a tendency to exhibit maladaptive behaviors in response to threatening stimuli, and to experience anxiety, depression, and other mood disorders. We expect that this early life feature will persist into adulthood and be formative in development. Our work fits with a growing body of literature that suggests that these genotype-associated differences in neural function and structure mediate individuals’ capacity to deal with stress 55. Future studies might examine how the general attentional system responsible for allocating resources is altered under stress conditions, and investigate whether and how this attentional style interacts with 5-HTTLPR genotype. Future studies should also examine how this cognitive system may be similarly altered in individuals who carry other risk factors for the development of mood and anxiety disorders.
Acknowledgements
This project was supported by awards from the National Institute of Mental Health [MH081583 to MET, and MH074849 to IHG], and by a NARSAD Young Investigator Award to MET, and by funding from the NIMH-Intramural Research Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank Melissa Henry, Sarah Victor, Emily Dennis, Rebecca Johnson, Meggy Wang, and Hannah Kang for their assistance in acquiring the scan data, and Yamanda Wright, Kirsten Gilbert, and Lindsey Sherdell for their assistance in participant recruitment, screening, and conducting structured behavioral interviews.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998;44:151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- 2.Heils A, et al. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- 3.Lesch KP, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 4.Mazzanti CM, et al. Role of the serotonin transporter promoter polymorphism in anxiety-related traits. Arch Gen Psychiatry. 1998;55:936–940. doi: 10.1001/archpsyc.55.10.936. [DOI] [PubMed] [Google Scholar]
- 5.Zalsman G, et al. Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. Am J Psychiatry. 2006;163:1588–1593. doi: 10.1176/ajp.2006.163.9.1588. [DOI] [PubMed] [Google Scholar]
- 6.Taylor SE, et al. Early family environment, current adversity, the serotonin transporter promoter polymorphism, and depressive symptomatology. Biol Psychiatry. 2006;60:671–676. doi: 10.1016/j.biopsych.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 7.Kendler KS, et al. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. Arch Gen Psychiatry. 2005;62:529–535. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- 8.Lohmueller KE, et al. Meta-analysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nat Genet. 2003;33:177–182. doi: 10.1038/ng1071. [DOI] [PubMed] [Google Scholar]
- 9.Caspi A, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 10.Risch N, et al. Interaction Between the Serotonin Transporter Gene (5-HTTLPR), Stressful Life Events, and Risk of Depression A Meta-analysis. Jama-Journal of the American Medical Association. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belsky J, et al. Vulnerability genes or plasticity genes? Mol Psychiatry. 2009;14:746–754. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gotlib IH, et al. HPA axis reactivity: A mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biological Psychiatry. 2008;63:847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canli T, et al. Beyond affect: a role for genetic variation of the serotonin transporter in neural activation during a cognitive attention task. Proc Natl Acad Sci U S A. 2005;102:12224–12229. doi: 10.1073/pnas.0503880102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pezawas L, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 15.Munafo MR, et al. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biol Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heinz A, et al. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci. 2005;8:20–21. doi: 10.1038/nn1366. [DOI] [PubMed] [Google Scholar]
- 17.Canli T, et al. Neural correlates of epigenesis. Proc Natl Acad Sci U S A. 2006;103:16033–16038. doi: 10.1073/pnas.0601674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munafo MR, et al. Genetic polymorphisms and personality in healthy adults: a systematic review and meta-analysis. Mol Psychiatry. 2003;8:471–484. doi: 10.1038/sj.mp.4001326. [DOI] [PubMed] [Google Scholar]
- 19.Schinka JA, et al. A meta-analysis of the association between the serotonin transporter gene polymorphism (5-HTTLPR) and trait anxiety. Mol Psychiatry. 2004;9:197–202. doi: 10.1038/sj.mp.4001405. [DOI] [PubMed] [Google Scholar]
- 20.Sen S, et al. Meta-analysis of the association between a serotonin transporter promoter polymorphism (5-HTTLPR) and anxiety-related personality traits. Am J Med Genet B Neuropsychiatr Genet. 2004;127B:85–89. doi: 10.1002/ajmg.b.20158. [DOI] [PubMed] [Google Scholar]
- 21.Mathews A. Why worry? The cognitive function of anxiety. Behav. Res. Ther. 1990;28:455–468. doi: 10.1016/0005-7967(90)90132-3. [DOI] [PubMed] [Google Scholar]
- 22.Williams JMG, et al. Wiley Series in Clinical Psychology: Cognitive Psychology and Emotional Disorders. John Wiley and Sons; 1988. [Google Scholar]
- 23.Eysenck MW. Anxiety: The cognitive perspective. Erlbaum Ltd; 1992. [Google Scholar]
- 24.Monk CS, et al. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am J Psychiatry. 2006;163:1091–1097. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- 25.Monk CS, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65:568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waters AM, et al. Attentional bias toward fear-related stimuli: an investigation with nonselected children and adults and children with anxiety disorders. J Exp Child Psychol. 2004;89:320–337. doi: 10.1016/j.jecp.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Joormann J, et al. Biased processing of emotional information in girls at risk for depression. J Abnorm Psychol. 2007;116:135–143. doi: 10.1037/0021-843X.116.1.135. [DOI] [PubMed] [Google Scholar]
- 28.Monk CS, et al. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am J Psychiatry. 2008;165:90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- 29.Telzer EH, et al. Relationship between trait anxiety, prefrontal cortex, and attention bias to angry faces in children and adolescents. Biol Psychol. 2008;79:216–222. doi: 10.1016/j.biopsycho.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez-Edgar K, et al. Variations in the serotonin-transporter gene are associated with attention bias patterns to positive and negative emotion faces. Biol Psychol. 2009 doi: 10.1016/j.biopsycho.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dahl RE, Gunnar MR. Heightened stress responsiveness and emotional reactivity during pubertal maturation: implications for psychopathology. Dev Psychopathol. 2009;21:1–6. doi: 10.1017/S0954579409000017. [DOI] [PubMed] [Google Scholar]
- 32.Corbetta M, et al. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nature Neuroscience. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- 33.Hopfinger JB, et al. The neural mechanisms of top-down attentional control. Nature Neuroscience. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- 34.Pourtois G, Vuilleumier P. Dynamics of emotional effects on spatial attention in the human visual cortex. Understanding Emotions. 2006;156:67–91. doi: 10.1016/S0079-6123(06)56004-2. [DOI] [PubMed] [Google Scholar]
- 35.Vasey MW, MacLeod C. Information-processing factors in childhood anxiety: a review and developmental perspective. In: Vasey MW, Dadds MR, editors. The developmental psychopathology of anxiety. Oxford University Press; 2001. pp. 27–42. [Google Scholar]
- 36.Kaufman J, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 37.Kovacs M. The Childrens Depression, Inventory (CDI) Psychopharmacology Bulletin. 1985;21:995–998. [PubMed] [Google Scholar]
- 38.Kovacs M. Children’s depression inventory. Multi-Health Systems; 1992. [Google Scholar]
- 39.Birmaher B, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry. 1997;36:545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- 40.Geller B, et al. WASH-U-KSADS (Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia) St. Louis (Missouri: Washington University; 1996. [DOI] [PubMed] [Google Scholar]
- 41.Geller B, et al. Reliability of the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) mania and rapid cycling sections. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:450–455. doi: 10.1097/00004583-200104000-00014. [DOI] [PubMed] [Google Scholar]
- 42.Tottenham N, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mogg K, Bradley B. Selective orienting of attention to masked threat faces in social anxiety. Behavioral Research Therapy. 2002;40:1403–1414. doi: 10.1016/s0005-7967(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 44.Rylander-Rudqvist T, et al. Quality and quantity of saliva DNA obtained from the self-administrated oragene method--a pilot study on the cohort of Swedish men. Cancer Epidemiol Biomarkers Prev. 2006;15:1742–1745. doi: 10.1158/1055-9965.EPI-05-0706. [DOI] [PubMed] [Google Scholar]
- 45.Neumeister A, et al. Differential effects of 5-HTTLPR genotypes on the behavioral and neural responses to tryptophan depletion in patients with major depression and controls. Arch Gen Psychiatry. 2006;63:978–986. doi: 10.1001/archpsyc.63.9.978. [DOI] [PubMed] [Google Scholar]
- 46.Wendland JR, et al. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Mol Psychiatry. 2006;11:224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- 47.Glover G, Law C. Spiral-in/out BOLD fMRI for increased SNR and reduced suceptibility artifacts. Magn Reson Med. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- 48.Kim D, et al. Regularized higher-order in vivo shimming. Man Reson Med. 2002;48:715–722. doi: 10.1002/mrm.10267. [DOI] [PubMed] [Google Scholar]
- 49.Cox R. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 50.Brotman MA, et al. Attention bias to threat faces in children with bipolar disorder and comorbid lifetime anxiety disorders. Biological Psychiatry. 2007;61:819–821. doi: 10.1016/j.biopsych.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 51.Eldar S, et al. Plasticity in attention: Implications for stress response in children. Behav. Res. Ther. 2008;46:450–461. doi: 10.1016/j.brat.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 52.Roy AK, et al. Attention bias toward threat in pediatric anxiety disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:1189–1196. doi: 10.1097/CHI.0b013e3181825ace. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Macleod C, et al. Attentional bias in emotional disorders. Journal of Abnormal Psychology. 1986;95:15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- 54.Gibb BE, et al. Children’s attentional biases and 5-HTTLPR genotype: Potential mechanisms linking mother and child depression. Journal of Clinical Child and Adolescent Psychology. 2009;38:415–426. doi: 10.1080/15374410902851705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn Sci. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]