Abstract
Background & Aims
The influence of age on the presentation, clinical course, and therapeutic response of patients with adult-onset ulcerative colitis (UC) is understudied. Given potential age-related differences in risk factors and immune function, we sought to determine if disease behavior or clinical outcomes differed between patients diagnosed with UC in later vs. earlier stages of adulthood.
Methods
We performed a retrospective cohort study of 295 patients with UC seen at a tertiary care center from 2001 to 2008. Adult subjects newly diagnosed with UC between the ages of 18 and 30 years were defined as early-onset; those newly diagnosed at age 50 or older were defined as late onset. The 2 groups were analyzed for differences in medication use and clinical endpoints, including disease extent, severity at the time of diagnosis, and steroid-free clinical remission at 1 year after disease onset.
Results
Disease extent and symptom severity were similar between groups at the time of diagnosis. One year after diagnosis, more patients in the late-onset group achieved steroid-free clinical remission (64% vs. 49%, p=0.01). Among those that required systemic steroid therapy, more late-onset patients achieved steroid-free remission by 1 year (50% vs. 32%, p=0.01). Former smoking status was a more common risk factor in the late-onset cohort (p<0.001), whereas more early-onset patients had a positive family history (p=0.008).
Conclusions
Patients with early- and late-adult-onset UC have similar initial clinical presentations, but differ in disease risk factors. Late-onset patients have better responses to therapy 1 year after diagnosis.
Keywords: Ulcerative Colitis, Late-onset, age, Therapy and disease outcomes
Introduction
Epidemiologic studies of ulcerative colitis (UC) reveal a bimodal distribution of disease onset with an initial peak in the third decade and a smaller second peak between the ages of 50 and 80. An estimated 12% of all patients diagnosed with UC present during the latter period,1 though late-onset UC has been inconsistently defined as onset of disease anytime between the ages of 40 and 70.2-5 With the aging of the population, the incidence of late-onset UC is expected to increase; therefore, an appreciation of the disease features unique to this cohort is essential for optimizing medical management.
The clinical course and therapeutic response in late-onset UC are not well defined as most studies addressing this population predate contemporary treatment paradigms and rarely provide comprehensive details of medication use and outcomes. Reports from the 1970's and 1980's suggested that the older individuals tend to have UC limited to the rectum or left colon yet with a more aggressive clinical course, more frequent hospitalizations, and earlier need for steroids compared to younger patients.2, 3, 5-8 Furthermore, mortality was reported to be higher within the first year of diagnosis in these older patients, often attributed to higher rates of fulminant colitis, toxic megacolon, emergency surgery and post-surgical complications.3-5, 9-13 In contrast, more recent studies have found no differences in clinical behavior and medical responsiveness between older and younger patients presenting with an initial UC flare.14-17 Some have even suggested that older age colitis may have a more benign clinical course18-20 and that long-term prognosis is similar to the general UC population.11, 21 These contradictory results may relate to advances in management of UC in recent years, particularly with the increased use of immunomodulators and availability of biologics.
Other age-related factors also may differentially influence the presentation and course of disease in late-onset UC. Other diseases more prevalent in an older population such as diverticulitis, infections, microscopic colitis, ischemia or neoplasia may present with similar symptoms as colitis making the initial differential diagnosis more broad.21 As co-morbidities, some of these diseases may also impact subsequent clinical UC management. The behavior of UC may also be affected by the relative immune senescence of aging, an age-related decline and dysfunction in immunity, which is characterized by a less robust peripheral immune system response and alterations in mucosal barrier function.22, 23 With increasing age, infections of mucosal surfaces become more common because of an age-related decline in immunity. Generation of cell-mediated immune responses to new antigens is also impaired due to a less robust peripheral immune system.24, 25 The immune dysregulation seen with aging may also lead to differential response to therapy or contribute to the development of autoimmunity and malignancies.26, 27
In light of these senescence-associated changes in the immune system and potential age related differences in disease risk factors and/or response to current medical therapeutics, we sought to determine if disease behavior or clinical outcomes differed between patients diagnosed with UC in later versus earlier adulthood. For this comparison we used a large contemporary cohort of UC patients and examined disease characteristics at the time of diagnosis, medical therapeutic interventions, and one-year clinical outcomes.
Methods
Patients with an established diagnosis of UC were identified using a clinical database maintained in the Inflammatory Bowel Disease clinics at Washington University in St. Louis School of Medicine (WUSM) over a 7-year period from 2001-2008. The date of initial UC diagnosis was confirmed by review of initial diagnostic endoscopic data, pathology reports, and radiographic studies. If the diagnosis was not made initially at WUSM, outside hospital records were accessed for confirmation. Additional data sources reviewed included outpatient clinical records, inpatient history and physical exam, hospital discharge summaries, and operative reports when applicable. Complete medical records for at least 1-year after initial diagnosis was required for inclusion into the study. Patients with indeterminate colitis, Crohn's disease, segmental colitis associated with diverticulosis, ischemic colitis, and primary neoplasia or lost to follow-up were excluded from the study. Demographic information included: gender; smoking history, categorized as current, former, or non-smoker; family history of IBD, defined as having a first or second degree relative with a history of IBD; and age of UC diagnosis. All IBD-related medication usage, hospitalizations for symptomatic flares of colitis, emergent and elective colectomy and mortality during the first year of disease were documented.
Disease extent and symptom severity at the time of diagnosis were categorized using the Montreal classification with symptom severity based on the modified Truelove and Witts Severity Index (MTWSI).28-30 Clinical remission was defined as absence of corticosteroids and complete relief of colitis symptoms based on physician's global assessment and patient report. Age at date of initial diagnosis was used to categorize patients into early-onset and late-onset UC. Patients diagnosed between the ages of 18 and 30 were defined as “early-onset,” while those diagnosed at or after age 50 were designated “late-onset.” The primary clinical endpoint for comparison between these cohorts was steroid-free clinical remission one year after disease onset. Secondary clinical endpoints included symptom severity at one year of disease and medication usage during the first year of disease. Medication use was included if prescribed at least once during the first year. Immunomodulator (IMM) and infliximab therapy were included only when use was consistently documented including blood counts monitoring (IMM) or induction doses were given (IFX). Additional clinical comparisons included disease extent and severity at time of diagnosis, hospitalizations, rates of surgery, and one-year mortality. Unless otherwise specified, we included all UC patients captured during the study periods and excluded from analysis those with incomplete data.
Descriptive statistics are reported as percentages, means and standard errors of the mean. Categorical data were compared between cohorts using two-sided Fisher's exact test or Pearson's chi-square test as appropriate. P-values of ≤ 0.05 were considered to be significant. The Human Research Protection Office (Institutional Review Board) at the Washington University in St. Louis School of Medicine approved this study.
Results
Patient demographics
A total of 467 UC patients were identified during the 7-year study period. The age distribution at diagnosis showed a bimodal distribution with the two peaks corresponding to the study definitions of early- and late-onset UC (Figure 1). Of all patients, 155 (33.2%) fulfilled study criteria for inclusion in the early-onset UC cohort and 140 (30.0%) for the late-onset UC. No statistically significant differences in gender or race were present between the early-onset and late-onset groups (Table 1). Patients with early-onset UC were more likely to be non-smokers and have a family history of IBD compared to late-onset patients, while more patients with late-onset UC were former smokers. No statistically significant differences were identified in disease extent, symptom severity at diagnosis, remission rates or medication usage between the different smoking categories (former, current or non-smokers).
Figure 1.
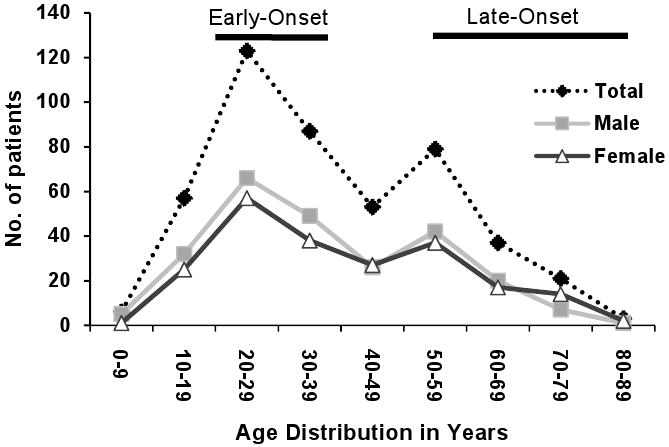
Age at diagnosis of UC study population
Table 1.
Demographics and patient characteristics.
| Early-onset | Late-onset | P-value | |
|---|---|---|---|
| Number of patients | 155 | 140 | |
| Gender (M:F) | 85:70 | 70:70 | |
| Mean age (yrs) | 23.8±0.2 | 60.2±0.7 | |
| Range (yrs) | 18-30 | 50-87 | |
| Ethnicity | |||
| Caucasian | 133 (85.8%) | 126 (90.0%) | 0.27 |
| African-American | 18 (11.6%) | 12 (8.6%) | 0.39 |
| Other | 4 (2.6%) | 2 (1.4%) | 0.48 |
| Smoking history | |||
| Current | 12 (7.7%) | 4 (2.9%) | 0.06 |
| Never | 122 (78.7%) | 63 (45.3%) | <0.001* |
| Former | 21 (13.5%) | 72 (51.8%) | <0.001* |
| Family history of IBD | 33 (21.3%) | 14 (10.7%) | 0.008* |
Clinical comparisons and primary outcome
Disease presentation and symptom severity at the time of diagnosis were similar between the early-onset and late-onset UC (Table 2). There were no gender differences in either cohort. Almost two thirds of patients in the late-onset group achieved steroid-free clinical remission at 1-year compared to half of early-onset patients (p=0.0096, Table 3). When stratified by gender, late-onset females were more likely than early-onset females to be in clinical-remission at one year (74.3% vs. 55.7% respectively, p=0.03). Remission rates for males trended similarly (57.1% vs. 43.5% respectively, p=0.10). At one-year after diagnosis, the trend of more early-onset UC patients either having had a colectomy or persistence of severe symptoms (7.8% early vs 3.5% late-onset) was non-significant (p=0.14). There were a similar number of UC-related hospitalizations among early-onset (n=57) and late-onset patients (n=66) during the first year of disease (p=0.09); this difference became significant only when a later-onset cohort (those diagnosed at age ≥60) was considered (p=0.03). No cases of toxic megacolon or fatalities occurred in either group.
Table 2.
Disease behavior at time of diagnosis
| Early-onset N (%) | Late-onset N (%) | P-value | |
|---|---|---|---|
| Disease extent | |||
| Proctitis | 21 (13.6) | 11 (8.0) | 0.12 |
| Left-sided | 68 (44.2) | 69 (50.3) | 0.35 |
| Extensive | 65 (42.2) | 57 (41.6) | 0.83 |
| Symptom severity | |||
| Mild | 34 (22.5) | 25 (18.0) | 0.38 |
| Moderate | 98 (64.9) | 99 (71.2) | 0.17 |
| Severe | 19 (12.6) | 15 (10.5) | 0.68 |
Extent and severity based on Montreal Classification: Silverberg et al. Can J Gastro 2005;19 (Suppl A):9A-13A.
Table 3.
Symptom severity at 1 year of disease
| Early-onset N (%) of 153 | Late-onset N (%) of 140 | P-value | |
|---|---|---|---|
| Clinical Remission | 75 (49.0) | 90 (64.3) | <0.01* |
| Mild | 18 (11.8) | 8 (5.7) | 0.10 |
| Moderate | 48 (31.3) | 37 (26.4) | 0.37 |
| Severe | 8 (5.2) | 3 (2.1) | 0.22 |
| Colectomy | 4 (2.6) | 2 (1.4) | 0.69 |
Medication use during 1st year of disease
Overall the types of medications used during the first year of disease were similar between the two groups (Table 4). However, while common in both groups, more late-onset patients received oral 5-aminosalicylate (5-ASA) therapy. 5-ASAs as mono-therapy were used to achieve and maintain remission in a similar number of early (42/153) and late-onset (41/140) patients. An additional 31 early-onset and 29 late-onset patients maintained remission at 1-year on 5-ASAs after a single course of systemic or rectal steroids. These rates were statistically similar.
Table 4.
Medication usage during 1st year of disease for all patients.
| Early-onset N (%) | Late-onset N (%) | P value | |
|---|---|---|---|
| 5-ASA compounds | |||
| Oral | 137 (88.4) | 135 (96.4) | 0.01* |
| Rectal | 53 (34.2) | 56 (40.0) | 0.30 |
| Steroids | |||
| Oral | 98 (63.2) | 94 (67.8) | 0.48 |
| Rectal | 12 (7.7) | 19 (13.6) | 0.10 |
| IV | 21 (13.5) | 21 (15.0) | 0.72 |
| Immunomodulators | 40 (25.8) | 41 (29.3) | 0.50 |
| Infliximab | 7 (4.5) | 8 (5.7) | 0.64 |
The majority of early-onset (97/153, 63.4%) and late-onset (95/140, 67.9%) patients required an initial course of oral or intravenous steroid therapy during their first year of disease. However, late-onset patients were more likely to be successfully tapered off steroids and maintain steroid-free clinical remission (p=0.02) than the early-onset patients at one year after diagnosis (Table 5).
Table 5.
Symptom severity 1 year after diagnosis in patients requiring systemic steroids
| Early-onset N (%) of 97 | Late-onset N (%) of 95 | P-value | |
|---|---|---|---|
| Clinical Remission | 31 (32.0) | 47 (49.5) | 0.02* |
| Mild | 12 (12.4) | 6 (6.3) | 0.22 |
| Moderate | 42 (43.3) | 37 (38.9) | 1.00 |
| Severe | 8 (8.2) | 3 (3.2) | 0.21 |
| Colectomy | 4 (4.1) | 2 (2.1) | 0.68 |
Immunomodulator (azathioprine and 6-mercaptopurine) and infliximab (IFX) therapy was used in a similar number of patients in each group. However, of those who used IMMs, more late-onset (18/41, 43.9%) than early-onset patients (7/40, 17.5%) were in maintained clinical remission at 1-year (p=0.016). Clinical remission rates of those treated with IFX during the first year was not statistically different between late (3/8, 37.5%) and early-onset (1/7, 14.3%) UC patients.
Discussion
In this study, a retrospective cohort analysis was used to compare early verses late-adult onset ulcerative colitis patients including assessment of disease risk factors, extent, severity, treatment and outcomes at 1-year. To our knowledge, this is the largest, US based study to provide this comprehensive comparison using modern patient cohorts inclusive of current treatment paradigms. No significant differences were found between cohorts in terms of disease extent or severity at time of initial diagnosis. However, one year after diagnosis, the late-onset cohort exhibited a better disease profile, with more patients achieving and maintaining steroid-free clinical remission. Even among those requiring systemic steroid therapy, more late-onset patients were in remission at one-year. Prominent risk factors for UC also differed between age groups. Whereas family history of IBD was more prevalent in the early onset cohort, former smoking status was significantly more common in the late onset cohort.
Our study differs from previously published studies in several ways. A validated UC classification system (Montreal) was used for disease extent and severity at diagnosis and disease severity at 1-year. 28 The population was US based and the cohort size was larger 14, 31 and more current 18 than published studies providing similar components of comparison. Earlier studies had reported older patients more likely to present with limited disease extent, but more severe symptoms at the time of diagnosis.2, 3 Additionally, some of their results also suggested that late-onset UC was associated with earlier surgical intervention and higher associated mortality.3, 4, 8, 9, 12 In contrast, our study found >90% of late-onset patients presented with left-sided or extensive disease and only 10.5% had symptoms categorized as severe. Furthermore, the overall 1-year outcome was no worse in the late-onset patients, with no deaths or emergent surgeries for fulminant colitis in either group, and no differences in the overall colectomy rate during the first year. The presence of severe symptoms or colectomy was more common numerically, though not significantly, at one-year in the early-onset cohort. While colitis-related hospitalizations were more frequent in the late-onset group, the difference compared to the early-onset group was significant only in the cohort diagnosed over the age of 60 years, a finding reflective of a recent report examining patients >65 years-old.32 Our findings are more consistent with studies published in the last decade that have included data for late-onset UC patients.14, 15, 17, 19, 20 Contemporary data thus indicate that disease severity, extent and clinical course over the first year are at least no worse in patients newly diagnosed with UC at an age >50 years-old.
There has been an expansion of therapies available for UC in the decades since the first publications describing late-onset UC.33 In the United States, newer 5-ASA compounds have largely supplanted sulfasalazine as first-line treatment in mild to moderate UC. Moreover, the availability of infliximab and the increased utilization of weight-based immunomodulator therapy have enhanced the ability to induce and maintain long-term response in UC. 34, 35 However, there has been a paucity of literature investigating clinical response and remission when used in an older patient population. Earlier studies reporting medication use in late-onset UC patients reported only on steroids +/- sulfasalazine/5-ASA.18, 20 In this study, medication use during the first year was similar between early- and late onset UC cohorts with the exception of higher 5-ASA use among the late-onset cohort. However, similar numbers of patients in both groups were maintained in clinical remission with 5-ASA monotherapy at one year. The increased prescribing of 5-ASAs for the older patient population may be due to the favorable adverse effect profile especially when considering a group of patients who are more likely to have comorbid conditions and polypharmacy.
Published studies the reporting on the efficacy and safety of immunomodulators in IBD have included older patients 36, 37, though a study specifically examining clinical outcomes with immunomodulators or biologics for this group does not exist. In our study, nearly 30% of each cohort had received infliximab or immunomodulator therapy. While the number of patients who received immunomodulators was similar in both groups (n=41 late vs n=40 early), a greater percentage of late-onset patients achieved steroid-free clinical-remission after one year with immunomodulators (43.9% late vs. 17.5% early p=0.016). A study designed to address this difference as a primary endpoint would be required to confirm this intriguing finding and authenticate the safety of immunomodulators use in this older group. However, we believe the findings from this study provide some insight into overall use and therapeutic response rates of late-onset UC patients following current treatment paradigms.
A population-based study of the corticosteroid response in UC patients found that approximately one-third of UC patients required steroids during their first year of disease with 49% achieving complete or partial remission at one year.38 Notable characteristics of this study compared with our study were a smaller number of patients but in a population based study, one-year outcomes that included both complete and partial remission, and fewer available effective maintenance medications during their study period (1970-1993). In the current study, more patients in both groups received steroids during their first year of disease. However, more than half of the late-onset group were in complete steroid-free remission compared to only one third of the younger cohort (P=0.019), with a trend toward more early-onset patients experiencing clinically severe disease activity or colectomy at one-year. Possible explanations for the superior outcome in late-onset patients include earlier diagnosis, better adherence to therapy, and perhaps a more effective response to medications afforded by age related differences in the immune system.39, 40
Some authors have suggested that late-onset UC may represent a different disease process than early-onset UC.21 In support of this notion, we found that a family history of inflammatory bowel disease was more common in the early versus late-onset cohort (p=0.008), suggesting that genetic factors may predominate with regard to development of early-onset UC. As additional genetic markers are identified 41, examination of polymorphism presence by age at diagnosis may confirm this finding providing novel insight into disease pathogenesis. In contrast, late-onset UC may manifest as a result of an accumulation of environmental exposures over time, in addition to the effects of age-related changes in the immune system and intestinal barrier function. In this study, we found tobacco exposure to be an important risk factor for the development UC in the late- but not early-adult-onset UC (p<0.001) confirming findings reported in earlier studies.42, 43
Aging is associated with relative systemic immunodeficiency; however, little research has focused on immune system changes that accompany aging.16 As we age, the production of new lymphocytes decreases due to thymic atrophy. Additionally, there are decreased CD4 and CD8 mediated responses, incomplete T-cell differentiation, and overall decreased cell-mediated immunity in the aging immune system.44-46 The combination of age-related immune compromise and a higher susceptibility to infections may predispose older patients to dysregulation of the T-cell response leading to ulcerative colitis. However, this relative state of immunodeficiency in the older population may attenuate the immune response that would typify unremitting disease flares, and perhaps facilitate improved response to medical therapy resulting in an improved disease course.
There are certain limitations to this study. This was a retrospective cohort study analyzing patients who were seen at a large tertiary-care center. While referral centers tend to see sicker patients, our group of gastrointestinal specialist serves as primary referral for many area primary care physicians and both of the study cohorts compared here were drawn from the same institutional database. Variability among the treating physicians at the study site may also have affected patient outcomes at one year; however, the majority of the patients in the study were under the care of only two inflammatory bowel disease specialists. The criteria to determine disease severity and extent rely heavily on the accuracy of the medical record and the description of the colonoscopic findings at the time of diagnosis. However, the classification systems utilized in this study have been validated in published clinical trials, retrospective cohort, and population-based studies. To maximize the accuracy of our data, patient demographics, endoscopic findings and pathology results were confirmed across multiple clinical data systems and by direct data review whenever possible. Lastly, the study is limited by the lack of endoscopic criteria to corroborate clinical remission at one year. Strengths of our study, however, include the use of a large, well-characterized UC population, specifically examining the clinical outcomes of late-onset UC and directly comparing these patients to a defined early-onset population.
The number of patients diagnosed with late-onset UC will likely continue to increase with the aging of the population. In our study, 30% of adult onset UC patients seen in a 7-year period were diagnosed at age 50 or older. Despite comparable disease characteristics at initial diagnosis and similar medical therapy, patients diagnosed with UC after age 50 demonstrated better outcomes and improved steroid- free remission rates at one year after diagnosis. Treatment of the older UC patient is often challenged by concomitant medication use and comorbid conditions that may affect gastroenterologists' choice of initial induction and maintenance therapies. Given that older patients are at increased risk for steroid related complications, this work underscores the importance of early initiation of maintenance therapy with 5-aminosalicylates and consideration to immunomodulator therapy. Additional research focusing on the therapeutic response to these medications in the late-onset UC population is needed to improve our understanding of management of these complex patients. Consideration to the age of disease onset may also be of value in the design and/or analysis of future clinical trials. Finally, further investigation into the environmental and genetic factors that differentiate late- versus early-onset UC may explain the underlying causes for the observed differences between these two populations.
Supplementary Material
Acknowledgments
Grant support: Matthew Ciorba is the recipient of a career development award from the Crohn's and Colitis Foundation of America. Rodney Newberry is supported by the National Institutes of Health (5R21AG028309-02). The Washington University School of Medicine Digestive Diseases Research Core Center (DDRCC) provided clinical database management and is supported by grant (P30-DK52574).
Abbreviations
- 5-ASA
5-aminosalicylates
- IBD
inflammatory bowel disease
- IMM
Immunomodulators
- IFX
infliximab
- MTWSI
modified Truelove and Witts Severity Index
- SEM
standard error of the mean
- UC
ulcerative colitis
Footnotes
Author Contributions: Christina Ha: data collection and interpretation, manuscript preparation.
Rodney Newberry: study conception and critical revision of the manuscript for important intellectual content.
Christian Stone: critical revision of the manuscript for important intellectual content and review of statistical analysis.
Matthew Ciorba: study conception and design, data interpretation and manuscript preparation. All authors approved the final draft for submission.
Disclosures: Christina Ha, Christian Stone, Rodney Newberry and Matthew Ciorba have no specific conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Loftus CG, Loftus EV, Harmsen WS, et al. Update on the incidence and prevalence of Crohn's Disease and Ulcerative Colitis in Olmsted County, Minnesota, 1940-2000. Inflamm Bowel Dis. 2007;13:254–261. doi: 10.1002/ibd.20029. [DOI] [PubMed] [Google Scholar]
- 2.Zimmerman J, Gavish D, Rachmilewitz D. Early and late onset ulcerative colitis: distinct clinical features. J Clin Gastroenterol. 1985;7:492–498. doi: 10.1097/00004836-198512000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Carr N, Schofield PF. Inflammatory bowel disease in the older patient. Br J Surg. 1982;69:223–225. doi: 10.1002/bjs.1800690418. [DOI] [PubMed] [Google Scholar]
- 4.Brandt LJ, Boley SJ, Mitsudo S. Clinical characteristics and natural history of colitis in the elderly. Am J Gastroenterol. 1982;77:382–386. [PubMed] [Google Scholar]
- 5.Jones HW, Hoare AM. Does ulcerative colitis behave differently in the elderly? Age Ageing. 1988;17:410–414. doi: 10.1093/ageing/17.6.410. [DOI] [PubMed] [Google Scholar]
- 6.Gupta S, Saverymuttu SH, Hodgson HJF. Is the pattern of inflammatory bowel disease different in the elderly? Age Ageing. 1985;14:366–370. doi: 10.1093/ageing/14.6.366. [DOI] [PubMed] [Google Scholar]
- 7.Myren J, Bouchier IA, Watkinson G, et al. The OMGE multinational inflammatory bowel disease survey 1976-1986. A further report on 3175 cases. Scand J Gastroenterol. 1988;144:11–19. [PubMed] [Google Scholar]
- 8.Stonnington CM, Phillips SF, Melton LJ, Zinsmeister AR. Chronic ulcerative colitis: inicidence and prevalence in a community. Gut. 1987;1987:4. doi: 10.1136/gut.28.4.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watts JM, De Dombal FT, Watkinson G, Goligher JC. Early course of ulcerative colitis. Gut. 1966;7:16–31. doi: 10.1136/gut.7.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinclair TS, Brunt PW, Mowat NA. Nonspecific proctocolitis in northeastern Scotland: a community study. Gastroenterology. 1983;85:1–11. [PubMed] [Google Scholar]
- 11.Winther KV, Jess T, Langholz E, et al. Survival and cause-specific mortality in Ulcerative Colitis: follow-up of a population-based cohort in Copenhagen County. Gastroenterology. 2003;125 doi: 10.1053/j.gastro.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 12.Toghill PJ, Benton P. Ulcerative colitis in elderly patients. Gerontol Clin. 1973;15:65–73. doi: 10.1159/000245435. [DOI] [PubMed] [Google Scholar]
- 13.Edwards FC, Truelove SC. The course and prognosis of ulcerative colitis. Gut. 1963;4:299–315. doi: 10.1136/gut.4.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Triantafillidis JK, Emmanouilidis A, Pomonis E, et al. Ulcerative colitis in the elderly: clinical patterns and outcome in 51 Greek patients. J Gastroenterol. 2001;36:312–316. doi: 10.1007/s005350170096. [DOI] [PubMed] [Google Scholar]
- 15.Piront P, Louis E, Latour P, et al. Epidemiology of inflammatory bowel diseases in the elderly in the province of Liege. Gastroenterol Clin Biol. 2001;25:157–161. [PubMed] [Google Scholar]
- 16.Robertson DJ, Grimm IS. Inflammatory bowel disease in the elderly. Gastrointest Clin North Am. 2001;30:409–426. doi: 10.1016/s0889-8553(05)70188-6. [DOI] [PubMed] [Google Scholar]
- 17.Tremaine WJ, Timmons LJ, Loftus EV, et al. Age at onset of inflammatory bowel disease and the risk of surgery for non-neoplastic bowel disease. Aliment Pharmacol Ther. 2007;25 doi: 10.1111/j.1365-2036.2007.03341.x. [DOI] [PubMed] [Google Scholar]
- 18.Moum B, Ekbom A, Vatn MH, et al. Clinical course during the 1st year after diagnosis in ulcerative colitis and Crohn's disease. Scand J Gastroenterol. 1997;32:1005–1012. doi: 10.3109/00365529709011217. [DOI] [PubMed] [Google Scholar]
- 19.Hadithi M, Cazemier M, Gerrit AM, et al. Retrospective analysis of old-age colitis in the Dutch inflammatory bowel disease population. World J Gastroenterol. 2008;14:3183–3187. doi: 10.3748/wjg.14.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riegler G, Tartaglione MT, Carratu R, et al. Gruppo Italiano Studio Colon-Retto (GISC). Age-related clinical severity at diagnosis in 1705 patients with Ulcerative Colitis. Dig Dis Sci. 2000;45:462–465. doi: 10.1023/a:1005424603085. [DOI] [PubMed] [Google Scholar]
- 21.Grimm IS, Friedman LS. Inflammatory bowel disease in the elderly. Gastroenterol Clin North Am. 1990;19:361–389. [PubMed] [Google Scholar]
- 22.Castle SC. Clinical relevance of age-related immune dysfunction. Clin Infect Dis. 2000;31:578–585. doi: 10.1086/313947. [DOI] [PubMed] [Google Scholar]
- 23.Pawelec G. Immunosenescence: impact in the young as well as the old? Mech Ageing Dev. 1999;108:1–7. doi: 10.1016/s0047-6374(99)00010-x. [DOI] [PubMed] [Google Scholar]
- 24.Castle SC. Clinical relevance of age-related immune dysfunction. Clin Infect Dis. 2000;31:578–85. doi: 10.1086/313947. [DOI] [PubMed] [Google Scholar]
- 25.Hakim FT, Gress RE. Immunosenescence: deficits in adaptive immunity in the elderly. Tissue Antigens. 2007;70:179–89. doi: 10.1111/j.1399-0039.2007.00891.x. [DOI] [PubMed] [Google Scholar]
- 26.Johnson SA, Cambier JC. Ageing, autoimmunity and arthritis: senescence of the B cell compartment - implications for humoral immunity. Arthritis Res Ther. 2004;6:131–9. doi: 10.1186/ar1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang HG, Grizzle WE. Aging, immunity, and tumor susceptibility. Immunol Allergy Clin North Am. 2003;23:83–102. vi. doi: 10.1016/s0889-8561(02)00085-1. [DOI] [PubMed] [Google Scholar]
- 28.Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19:9A–13A. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- 29.Lichtiger S, Present DH. Preliminary report: cyclosporin in treatment of severe active ulcerative colitis. Lancet. 1990;336:16–19. doi: 10.1016/0140-6736(90)91521-b. [DOI] [PubMed] [Google Scholar]
- 30.Truelove SC, Witts LT. Cortisone in ulcerative colitis: final report on a therapeutic trial. Br Med J. 1955;2:1041–1048. doi: 10.1136/bmj.2.4947.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jang ES, Lee DH, Kim J, et al. Age as a clinical predictor of relapse after induction therapy in ulcerative colitis. Hepatogastroenterology. 2009;56:1304–9. [PubMed] [Google Scholar]
- 32.Ananthakrishnan AN, McGinley EL, Binion DG. Inflammatory bowel disease in the elderly is associated with worse outcomes: a national study of hospitalizations. Inflamm Bowel Dis. 2009;15:182–9. doi: 10.1002/ibd.20628. [DOI] [PubMed] [Google Scholar]
- 33.Ng SC, JKamm MA. Therapeutic strategies for the management of ulcerative colitis. Inflamm Bowel Dis. 2009;15:935–950. doi: 10.1002/ibd.20797. [DOI] [PubMed] [Google Scholar]
- 34.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 35.Gisbert JP, Nino P, Cara C, Rodrigo L. Comparative effectiveness of azathioprine in Crohn's disease and ulcerative colitis: prospective, long-term, follow-up study of 394 patients. Aliment Pharmacol Ther. 2008;28:228–38. doi: 10.1111/j.1365-2036.2008.03732.x. [DOI] [PubMed] [Google Scholar]
- 36.Present DH, Meltzer SJ, Krumholz MP, et al. 6-Mercaptopurine in the management of inflammatory bowel disease: short- and long-term toxicity. Ann Intern Med. 1989;111:641–9. doi: 10.7326/0003-4819-111-8-641. [DOI] [PubMed] [Google Scholar]
- 37.Bouhnik Y, Lemann M, Mary JY, et al. Long-term follow-up of patients with Crohn's disease treated with azathioprine or 6-mercaptopurine. Lancet. 1996;347:215–9. doi: 10.1016/s0140-6736(96)90402-x. [DOI] [PubMed] [Google Scholar]
- 38.Faubion WA, Loftus EV, Harmsen WS, et al. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255–260. doi: 10.1053/gast.2001.26279. [DOI] [PubMed] [Google Scholar]
- 39.Cerveny P, Bortlik M, Kubena A, et al. Nonadherence in inflammatory bowel disease: results of factor analysis. Inflamm Bowel Dis. 2007;13:1244–1249. doi: 10.1002/ibd.20189. [DOI] [PubMed] [Google Scholar]
- 40.Higgins PD, Rubin DT, Kaulback K, et al. Systematic review: impact of nonadherence to 5-aminosalicylic acid products on the frequency and cost of ulcerative colitis flares. Aliment Pharmacol Ther. 2009;29:247–257. doi: 10.1111/j.1365-2036.2008.03865.x. [DOI] [PubMed] [Google Scholar]
- 41.Barrett JC, Lee JC, Lees CW, et al. Genome-wide association study of ulcerative colitis identifies three new susceptibility loci, including the HNF4A region. Nat Genet. 2009;41:1330–4. doi: 10.1038/ng.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Regueiro M, Kip KE, Cheung O, Hegazi RA, Plevy S. Cigarette smoking and age at diagnosis of inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:42–47. doi: 10.1097/00054725-200501000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Aldhous MC, Drummond HE, Anderson N, et al. Smoking habit and load influence age at diagnosis and disease extent in Ulcerative Colitis. Am J Gastroenterol. 2007;102:589–597. doi: 10.1111/j.1572-0241.2007.01065.x. [DOI] [PubMed] [Google Scholar]
- 44.Dorshkind K, Montecino-Rodriguez E, Signer RAJ. The ageing immune system: is it ever too old to become young again? Nat Rev Immunol. 2009;9:57–62. doi: 10.1038/nri2471. [DOI] [PubMed] [Google Scholar]
- 45.Gruver AL, Hudson LL, Sempowski GD. Immunosenescence of aging. J Pathol. 2007;211:144–156. doi: 10.1002/path.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


