Abstract
Chronic exposure to cocaine increases the activity of extracellular signal-regulated kinase (ERK1/2) in the ventral tegmental area (VTA), a neural substrate for drugs of abuse. However, the functional significance of changes in ERK1/2 activity in this brain region is unknown. Using herpes simplex virus-mediated gene transfer to regulate ERK2 activity within the VTA in male rats, we show that overexpressing ERK2 increases preference for environments previously paired with low doses of cocaine and enhances cocaine-induced locomotion, whereas blocking ERK2 activity blocks cocaine-induced place conditioning and locomotor activity. These results demonstrate that ERK2-signaling within the VTA is a key modulator of functional responses to cocaine.
Keywords: Extracellular signal-regulated kinase, cocaine, ventral tegmental area, place preference conditioning, behavioral sensitization
Repeated exposure to drugs of abuse results in long lasting cellular, molecular and behavioral adaptations in mesolimbic dopamine neurons–originating from the ventral tegmental area (VTA) and projecting to target regions such as the nucleus accumbens (NAc)–that have been implicated in the transition from drug use to abuse [24, 38]. Within this VTA-NAc neural pathway, it has been demonstrated that neurotrophins and their signaling cascades play an important role in mediating cellular and behavioral responses to drugs of abuse [7, 19, 33]. Among other adaptations, chronic exposure to cocaine or morphine causes changes in VTA neurotrophin signaling proteins, including the upregulation of extracellular signal-regulated kinase (ERK) signaling [6, 40]. ERK, a member of the mitogen-activated protein kinase (MAPK) family, consists of two isoforms (ERK 1 and 2) and has been implicated in responses to drugs of abuse [19, 29]. Specifically, global ERK1/2 signaling has been implicated in the development of preference for environments previously paired with cocaine [30, 40], the sensitized locomotor responses to the stimulant [35, 41], and the self-administration and seeking of the drug [15, 28]. However, it is not known how discrete modulation of ERK activity within specific brain regions, such as the VTA per se, prior to drug exposure may influence behavioral responsivity to drugs of abuse. Determining the specific role this protein kinase plays in response to drugs of abuse has proven difficult given that the pharmacological tools available to inhibit ERK lack specificity [13, 32], and the use of knockout models presents additional limitations as well, since genetic inactivation of ERK2 is lethal [1], while ERK1 knockouts display altered basal locomotor activity [37] accompanied by compensations of enhanced ERK2 signaling in the brain [18], thus making behavioral interpretation difficult. Consequently, the present study was designed to directly assess the functional consequences of ERK2 modulation on behavioral responses to cocaine using locomotor activity and conditioned place preference (CPP) paradigms, after selectively increasing or blocking ERK2 activity in the VTA by microinjecting a herpes simplex virus (HSV) vector encoding a wild type (HSV-wtERK2) or a dominant negative mutant form (HSV-dnERK2) of this protein.
Adult male Sprague-Dawley rats were anesthetized with an intramuscular injection of a ketamine/xylazyne cocktail (80/10 mg/kg), and given atropine (0.25 mg/kg) subcutaneously to minimize bronchial secretions and given bilateral microinjections (1.0 μl per side over 10 min) of and HSV vector encoding green fluorescent protein (HSV-GFP), HSV-wtERK2, or HSV-dnERK2, in established VTA coordinates, AP: −4.9, Lat: +2.2, DV: −7.6 mm below dura, angled at 10° from the midline [23]. Only needle placements ranging from −4.9 and −5.5 mm from Bregma were included in this study. The construction of the HSV vectors has been described [31], and the ERK2 virus have been validated in vivo and in vitro [22, 26]. Behavioral testing commenced 3 days after viral surgery, a time at which maximal transgene expression is observed [10]. No detectable HSV expression was seen in either efferent (e.g., NAc) or afferent (e.g., dorsal raphé) regions of the injected area. Histological assessment of microinjections and transgene detection was performed as described [22].
Place preference conditioning was carried out in a three-chamber apparatus as described [9, 23]. On the preconditioning day (day 0), rats were allowed to freely explore the entire testing apparatus for 30 min to obtain baseline preference to any of the three compartments (side compartments: 35 × 27 × 25 cm; middle compartment: 10 × 27 × 25 cm). Only rats showing no spontaneous preference to either compartment were used (unbiased procedure); this accounted for more than 90% of all of the rats tested. Rats then received bilateral microinjections of HSV vectors into the VTA, and were allowed to recover for two days. After recovery, conditioning trials (two per day) were given on two consecutive days (day 3 and 4). During the conditioning trials, rats received an intraperitoneal (IP) saline injection (1 ml/kg) and were confined to one of the compartments of the apparatus for 1 hr. After 3 hr, rats received cocaine (0, 2.5, 5, or 10 mg/kg, IP; Sigma-Aldrich, St. Louis, MO) and were confined to the opposite side compartment for 1 hr. Conditioning trials were counterbalanced such that half the rats received drug in one compartment and the other half received the drug in the opposite compartment. On the final day (day 5), rats were again allowed to freely explore the entire apparatus for 30 min.
Two experiments were conducted to assess how ERK activity within the VTA influences cocaine-induced locomotor activity. In experiment one, locomotor activity was measured each day for 2 hr in automated (75 cm diameter × 15 cm wide, 4 photocell beams) circular activity chambers (Med Associates, St. Albans, VT). Rats were exposed to the chambers after one saline injection each day for 3 consecutive days and underwent HSV surgery at the end of day 3. On day 5, rats received an acute saline injection saline to assess whether surgery itself changed baseline locomotion. Rats were then randomly assigned to receive cocaine (2.5, 5.0 or 10 mg/kg) once daily for 5 consecutive days (starting on day 6). One day after the last cocaine injection (day 11), rats were given a saline injection to assess whether they were responding to the injection itself or to cocaine, and were rested for 1 week. At the end of the rest period (day 18), rats were challenged with 2.5, 5.0 or 10 mg/kg cocaine (according to original group assignment). The second experiment was conducted in a similar fashion, with the exception that on day 15 (i.e., 3 days before challenge day) rats received viral infusions (HSV-GFP, -wtERK2, or -dnERK2). Only rats showing enhanced behavioral responding to cocaine on the last day of cocaine treatment (day 10) were assigned to various viral conditions. Three days after viral infusion (day 18) all rats were challenged with 10 mg/kg cocaine.
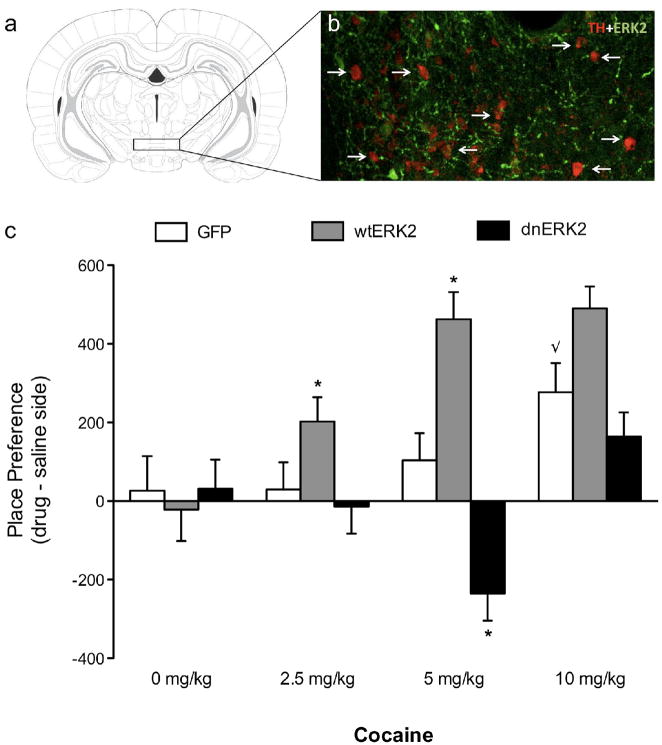
Figure 1a shows the region of the VTA to which microinjections of HSV vectors (HSV-GFP, -wtERK2, or -dnERK2) were targeted. Confocal microscopy (Fig. 1b) revealed that the percentage of tyrosine hydroxylase (TH)-positive neurons overexpressing GFP-wtERK2 in the VTA was similar (~53%) to previous findings [26]. The effects of HSV treatments on cocaine (0, 2.5, 5, and 10 mg/kg) CPP are shown in Figure 1c (N=97). Time spent in the cocaine-paired compartment varied as a function of virus- (F(2,85)=19.08, p<0.0001) and by drug-treatment (F(3,85)=10.61, p<0.0001), as well as their interaction (virus × drug interaction: F(6,85)=4.69, p<0.0001). Rats receiving viral-vector treatments [HSV-GFP (n=7/group), HSV-wtERK2 (n=9/group), dnERK2 (n=8–9/group)] and conditioned to saline did not show preferences for either side of the compartments. Conversely, rats receiving HSV-wtERK2 microinjections into the VTA spent significantly more time in environments paired with moderate doses (2.5 and 5 mg/kg) of cocaine (p<0.05), whereas rats receiving HSV-dnERK2 microinjections did not consistently approach the cocaine-paired environments. In fact, microinjecting HSV-dnERK2 into the VTA resulted in avoidance of the cocaine-paired compartment (5 mg/kg; p<0.05).
Figure 1.
ERK2 activity in the VTA regulates cocaine-mediated place conditioning. (a) Rostral region of the VTA to which microinjections of HSV vectors were targeted. Adapted from The Rat Brain in Stereotaxic Coordinates (3rd ed), Paxinos and Watson, 1997. (b) Merged confocal photomicrograph (magnification, 400×) of a representative brain slice from the rostral VTA (~5 mm caudal to Bregma) double-labeled for TH (red: Cy3) and GFP-wtERK2 (green: Cy2) fluorescence. Arrows indicate double-labeled cells. (c) HSV-wtERK2 overexpression enhanced sensitivity to 2.5 and 5 mg/kg of cocaine, whereas expression of HSV-dnERK2 resulted in place aversion at the 5 mg/kg dose (*p<0.05). HSV-GFP, and HSV-wtERK2 rats treated with 10 mg/kg cocaine showed reliable place conditioning (√p<0.05: different from HSV-GFP group exposed to 0, 2.5, and 5 mg/kg cocaine). HSV-dnERK2-treated rats did not show place conditioning or aversion to 10 mg/kg cocaine. Error bars indicate mean ± SEM.
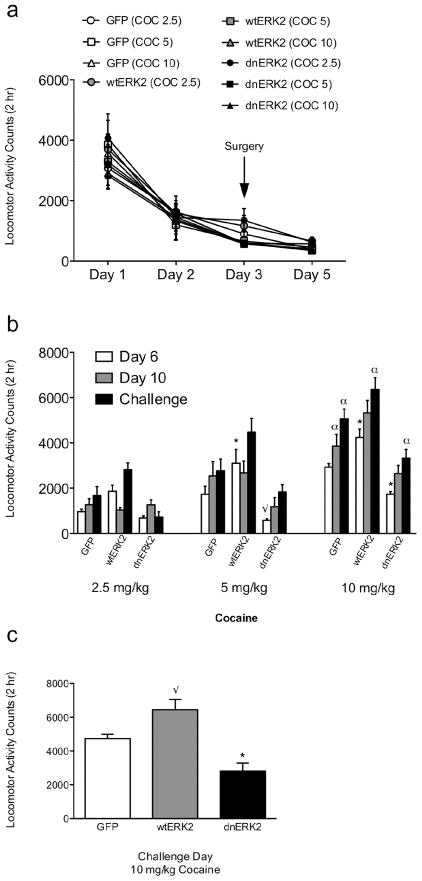
Figure 2a–c shows the effects of virus treatment [-GFP (n=9–10/group), -wtERK2 (n=8–9group), -dnERK2 (n=9/group)] on cocaine-induced (2.5, 5, or 10 mg/kg) locomotor activity (N=81). Figure 2a shows that baseline locomotion was similar across the groups before and 2 days after surgery, thus indicating that surgery alone, or the virus treatments, did not affect spontaneous locomotor activity. Figure 2b compares behavioral responding to cocaine (2.5, 5, and 10 mg/kg) on day 6 (first cocaine exposure), day 10 (last day of cocaine before rest period), and day 18 (challenge day) in all groups. These rats also received a saline injection on day 11 to assess for potential conditioned locomotor activity effects. No differences in behavioral responding to saline in any of the groups were revealed (data not shown). A three way ANOVA yielded significant main effects of virus (F(2,216)=44.38, p<0.0001), drug (F(2,216)=83.28, p<0.0001), and day (F(2,216)=18.52, p<.0001). Behavioral responses to cocaine also varied as a function of virus × drug interaction (F(4,216)=2.87, p<0.02). No differences were observed between the groups treated with 2.5 mg/kg cocaine. Conversely, post hoc analysis revealed significant differences between the groups treated with 5 mg/kg cocaine on day 6, indicating that behavioral responses were significantly higher for the HSV-wtERK2-treated rats (p<0.05), and trended to be lower for the HSV-dnERK2-treated rats (p=0.056), when compared to controls (HSV-GFP). As expected for the higher dose of cocaine (10 mg/kg), the HSV-GFP group had enhanced locomotor responding on days 10 and 18 when compared to day 6 (p<0.05), indicating the expression of locomotor sensitization. Also, rats microinjected with HSV-wtERK2 had a greater overall behavioral responding to cocaine (10 mg/kg) than controls on day 6, which suggest that overexpression of ERK2 increases behavioral sensitivity to acute cocaine. In addition, these rats showed enhanced sensitivity to the stimulant on day 18, as compared with their behavioral responding on day 6 (p<0.05), but not when compared to day 10. Interestingly, the magnitude of their behavioral responding (wtERK2) did not significantly differ from that in the HSV-GFP group on day 18 (challenge day), suggesting that wtERK2 did not enhance the degree of locomotor sensitization compared to control animals. Conversely, no differences were apparent between day 6 and day 10 in the dnERK2-treated rats receiving 10 mg/kg cocaine. Furthermore, these rats showed significantly blunted responses to cocaine as compared with the other groups on day 6 (p<0.05) and day 10 (p<0.05; as compared to wtERK2). On the other hand, there was a significant increase in locomotor activity in these animals on day 18, although their behavioral responding to the cocaine challenge was still significantly lower (p<0.05) than that observed in the other treatment groups. In the second locomotor sensitization experiment (n=8/group), which was designed to assess how ERK2 activity after cocaine exposure influenced the expression of behavioral sensitization, a one-way ANOVA revealed a significant virus main effect (F(2,21)=14.89, p<0.0001), indicating that HSV-dnERK2-treated rats had a diminished response to cocaine (p<0.05), whereas the GFP- and wtERK2-treated groups had the expected behavioral responding to cocaine (Fig. 2c). Interestingly, the HSV-wtERK2-treated group trended towards a higher response than the GFP-treated rats (p=0.054).
Figure 2.
ERK2 activity in the VTA regulates cocaine-induced locomotion. (a) Baseline locomotor activity for 3 consecutive days prior to and following 2 days after surgery (day 5). (b) Data representing all groups on Day 6, 10, and 18. Administration of cocaine (5 mg/kg) enhanced behavioral responding acutely (day 6) in the wtERK2-treated rats (*p<0.05: compared to controls), while treatment with HSV-dnERK2 blunted behavioral responses (√p=0.05). Repeated cocaine (10 mg/kg) treatment resulted in behavioral sensitization in all groups (αp<0.05), though the magnitude displayed by the HSV-dnERK2-treated group was significantly reduced. (c) HSV-dnERK2 significantly (p<0.05) blunted behavioral responses to the cocaine challenge on day 18. *Significantly different from HSV-GFP; √p=0.054 from HSV-GFP. Error bars indicate mean ± SEM.
This study was designed to assess behavioral responses to cocaine in two behavioral tasks designed to examine the rewarding and locomotor activating properties of cocaine [2, 36] after discrete manipulation of ERK2 within the VTA using viral-mediated gene transfer to locally increase or decrease ERK2 activity in this brain region. Rats treated with HSV-wtERK2 reliably approached environments previously paired with doses of cocaine (2.5 mg/kg) that did not induce place conditioning in control rats, thus directly demonstrating that elevated levels of ERK2 activity prior to cocaine administration facilitated place conditioning. In contrast, HSV-dnERK2-treated rats avoided environments paired with a threshold dose of cocaine (5 mg/kg), while showing no reliable preference, or avoidance, to environments paired with the higher (10 mg/kg) dose of cocaine, findings that parallel those showing that ERK1/2 inhibition within the mesolimbic VTA-NAc circuit prevents the establishment of drug-induced CPP [27, 30, 40]. These findings are in agreement with demonstrations that molecular manipulations that reduce the rewarding effects of cocaine often lead to cocaine-induced place aversions [3, 10, 23]. The biphasic properties of cocaine are well documented, and it is possible that the aversion is caused by a decrement in cocaine’s rewarding effects (i.e., tolerance to the rewarding effects of the drug), which, in turn, unmasks other aversive effects of the drug [17, 25, 39].
A parallel behavioral phenotype was observed when increased ERK2 activity in the VTA lead to an enhancement in the locomotor-activating effects induced by acute and repeated cocaine exposure, while blocking ERK2 activity blunted cocaine-induced locomotion. However, HSV-dnERK2-treated rats still showed a modest, though significant, increase in locomotor activity to repeated cocaine exposure (at the 10 mg/kg dose) on the challenge day. It is conceivable that this modest increase in locomotor activity may be mediated via the NAc due to the systemic administration of cocaine [34, 42]. These results are consistent with those showing that repeated exposure to cocaine enhances ERK activity within the VTA [5] and that pharmacological inhibition of ERK1/2 prior to cocaine administration decreases the development of psychomotor sensitization to cocaine [35, 41]. Given that no viral expression can be detected a week after virus infusion, these data suggest that ERK2 activity in the VTA may also be important for the expression of drug-induced behavioral sensitization [29], because rats previously sensitized to cocaine and then treated with HSV-dnERK2 prior to drug challenge showed a significant decrease in response to cocaine, although others report opposite results using pharmacological ERK inhibitors [41]. Alternatively, it is conceivable that ERK2 activity levels simply enhance or block cocaine-induced behaviors, as seen with the CPP procedure, without regulating the intricate aspects of ‘induction’ and ‘expression’ of behavioral sensitization, a hypothesis that now needs further assessment in future studies.
The mechanism(s) underlying these results are not fully known. It has been previously shown that topographical differences within the VTA can mediate differential responding to the rewarding and locomotor-activating properties of drugs [8, 11, 21], thus it is conceivable that distinct populations of dopamine neurons within the VTA might mediate the effects observed in this study [12, 14, 20]. Though anatomical characterization of the VTA is not yet complete, this assumption is consistent with findings suggesting that dopamine neurons from rostral portions of the VTA innervate primarily, but not exclusively, the NAc shell (i.e., mesolimbic), while dopamine neurons from more caudal VTA regions project predominantly, but not exclusively, to cortical areas [i.e., mesocortical regions] [16]. These neural projections also show differential responding to drugs of abuse by increasing extracellular dopamine levels in the NAc shell as compared to prefrontal cortical areas [4]. In this study we specifically targeted rostral portions of the VTA to regulate ERK2 activity. Thus it is conceivable, within this framework, that increasing ERK2 activity within dopamine neurons in rostral VTA increases sensitivity to cocaine, resulting in increased reward and locomotor responding, while resulting in opposite effects when ERK2 activity is blocked in this brain region.
Our findings are in agreement with evidence implicating the ERK pathway as facilitator for development of cocaine-induced plasticity that may contribute to addiction [19, 29]. Our data further establish the functional importance of ERK2 expression in the VTA as mediating behavioral responses to cocaine. Further assessment of the mechanisms underlying the downstream targets of VTA ERK2 will lead to better understanding of the neural and molecular basis of drug addiction.
Acknowledgments
This work was supported by grants from the National Institute on Drug Abuse (CABG and EJN) and the National Institute of Mental Health (EJN). SDI was supported by a McKnight Fellowship from the Florida Education Fund and a NRSA (F31DA027300) from NIDA. BLW was supported by a Neuroscience Fellowship from the Florida State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aouadi M, Binetruy B, Caron L, Le Marchand-Brustel Y, Bost F. Role of MAPKs in development and differentiation: lessons from knockout mice. Biochimie. 2006;88:1091–8. doi: 10.1016/j.biochi.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- 3.Barot SK, Ferguson SM, Neumaier JF. 5-HT(1B) receptors in nucleus accumbens efferents enhance both rewarding and aversive effects of cocaine. Eur J Neurosci. 2007;25:3125–31. doi: 10.1111/j.1460-9568.2007.05568.x. [DOI] [PubMed] [Google Scholar]
- 4.Bassareo V, Tanda G, Petromilli P, Giua C, Di Chiara G. Non-psychostimulant drugs of abuse and anxiogenic drugs activate with differential selectivity dopamine transmission in the nucleus accumbens and in the medial prefrontal cortex of the rat. Psychopharmacology (Berl) 1996;124:293–9. doi: 10.1007/BF02247433. [DOI] [PubMed] [Google Scholar]
- 5.Berhow MT, Hiroi N, Nestler EJ. Regulation of ERK (extracellular signal regulated kinase), part of the neurotrophin signal transduction cascade, in the rat mesolimbic dopamine system by chronic exposure to morphine or cocaine. J Neurosci. 1996;16:4707–15. doi: 10.1523/JNEUROSCI.16-15-04707.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berhow MT, Russell DS, Terwilliger RZ, Beitner-Johnson D, Self DW, Lindsay RM, et al. Influence of neurotrophic factors on morphine- and cocaine-induced biochemical changes in the mesolimbic dopamine system. Neuroscience. 1995;68:969–79. doi: 10.1016/0306-4522(95)00207-y. [DOI] [PubMed] [Google Scholar]
- 7.Bolaños CA, Nestler EJ. Neurotrophic mechanisms in drug addiction. Neuromolecular Med. 2004;5:69–83. doi: 10.1385/NMM:5:1:069. [DOI] [PubMed] [Google Scholar]
- 8.Bolaños CA, Neve RL, Nestler EJ. Phospholipase C gamma in distinct regions of the ventral tegmental area differentially regulates morphine-induced locomotor activity. Synapse. 2005;56:166–9. doi: 10.1002/syn.20136. [DOI] [PubMed] [Google Scholar]
- 9.Bolaños CA, Perrotti LI, Edwards S, Eisch AJ, Barrot M, Olson VG, et al. Phospholipase Cgamma in distinct regions of the ventral tegmental area differentially modulates mood-related behaviors. J Neurosci. 2003;23:7569–76. doi: 10.1523/JNEUROSCI.23-20-07569.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlezon WA, Jr, Boundy VA, Haile CN, Lane SB, Kalb RG, Neve RL, et al. Sensitization to morphine induced by viral-mediated gene transfer. Science. 1997;277:812–4. doi: 10.1126/science.277.5327.812. [DOI] [PubMed] [Google Scholar]
- 11.Carlezon WA, Jr, Haile CN, Coppersmith R, Hayashi Y, Malinow R, Neve RL, et al. Distinct sites of opiate reward and aversion within the midbrain identified using a herpes simplex virus vector expressing GluR1. J Neurosci. 2000;20:RC62. doi: 10.1523/JNEUROSCI.20-05-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000;20:3864–73. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Chiara G. Cortical and limbic dopamine (on opiate addiction): do not mix before use! Trends Pharmacol Sci. 1997;18:77–8. [PubMed] [Google Scholar]
- 15.Edwards S, Graham DL, Bachtell RK, Self DW. Region-specific tolerance to cocaine-regulated cAMP-dependent protein phosphorylation following chronic self-administration. Eur J Neurosci. 2007;25:2201–13. doi: 10.1111/j.1460-9568.2007.05473.x. [DOI] [PubMed] [Google Scholar]
- 16.Emson PC, Koob GF. The origin and distribution of dopamine-containing afferents to the rat frontal cortex. Brain Res. 1978;142:249–67. doi: 10.1016/0006-8993(78)90634-0. [DOI] [PubMed] [Google Scholar]
- 17.Ettenberg A. Opponent process properties of self-administered cocaine. Neurosci Biobehav Rev. 2004;27:721–8. doi: 10.1016/j.neubiorev.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson SM, Fasano S, Yang P, Brambilla R, Robinson TE. Knockout of ERK1 enhances cocaine-evoked immediate early gene expression and behavioral plasticity. Neuropsychopharmacology. 2006;31:2660–8. doi: 10.1038/sj.npp.1301014. [DOI] [PubMed] [Google Scholar]
- 19.Girault JA, Valjent E, Caboche J, Herve D. ERK2: a logical AND gate critical for drug-induced plasticity? Curr Opin Pharmacol. 2007;7:77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikemoto S, Kohl RR, McBride WJ. GABA(A) receptor blockade in the anterior ventral tegmental area increases extracellular levels of dopamine in the nucleus accumbens of rats. J Neurochem. 1997;69:137–43. doi: 10.1046/j.1471-4159.1997.69010137.x. [DOI] [PubMed] [Google Scholar]
- 22.Iñiguez SD, Vialou V, Warren BL, Cao J-L, Alcantara LF, Davis LC, et al. Extracellular Signal-Regulated Kinase-2 Within the Ventral Tegmental Area Regulates Responses to Stress. J Neurosci. 2010 doi: 10.1523/JNEUROSCI.0951-10.2010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iñiguez SD, Warren BL, Neve RL, Nestler EJ, Russo SJ, Bolanos-Guzman CA. Insulin receptor substrate-2 in the ventral tegmental area regulates behavioral responses to cocaine. Behav Neurosci. 2008;122:1172–7. doi: 10.1037/a0012893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–80. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- 25.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 26.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Lin X, Wang Q, Ji J, Yu LC. Role of MEK-ERK pathway in morphine-induced conditioned place preference in ventral tegmental area of rats. J Neurosci Res. 2010;88:1595–604. doi: 10.1002/jnr.22326. [DOI] [PubMed] [Google Scholar]
- 28.Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005;8:212–9. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- 29.Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of ERK in cocaine addiction. Trends in neurosciences. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–84. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Neve RL, Howe JR, Hong S, Kalb RG. Introduction of the glutamate receptor subunit 1 into motor neurons in vitro and in vivo using a recombinant herpes simplex virus. Neuroscience. 1997;79:435–47. doi: 10.1016/s0306-4522(96)00645-8. [DOI] [PubMed] [Google Scholar]
- 32.Nishimoto S, Nishida E. MAPK signalling: ERK5 versus ERK1/2. EMBO Rep. 2006;7:782–6. doi: 10.1038/sj.embor.7400755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pierce RC, Bari AA. The role of neurotrophic factors in psychostimulant-induced behavioral and neuronal plasticity. Rev Neurosci. 2001;12:95–110. doi: 10.1515/revneuro.2001.12.2.95. [DOI] [PubMed] [Google Scholar]
- 34.Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- 35.Pierce RC, Pierce-Bancroft AF, Prasad BM. Neurotrophin-3 contributes to the initiation of behavioral sensitization to cocaine by activating the Ras/Mitogen-activated protein kinase signal transduction cascade. J Neurosci. 1999;19:8685–95. doi: 10.1523/JNEUROSCI.19-19-08685.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 37.Selcher JC, Nekrasova T, Paylor R, Landreth GE, Sweatt JD. Mice lacking the ERK1 isoform of MAP kinase are unimpaired in emotional learning. Learn Mem. 2001;8:11–9. doi: 10.1101/lm.37001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Self DW, Nestler EJ. Molecular mechanisms of drug reinforcement and addiction. Annu Rev Neurosci. 1995;18:463–95. doi: 10.1146/annurev.ne.18.030195.002335. [DOI] [PubMed] [Google Scholar]
- 39.Solomon RL, Corbit JD. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychol Rev. 1974;81:119–45. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- 40.Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci. 2000;20:8701–9. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valjent E, Corvol JC, Trzaskos JM, Girault JA, Herve D. Role of the ERK pathway in psychostimulant-induced locomotor sensitization. BMC neuroscience. 2006;7:20. doi: 10.1186/1471-2202-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]