Abstract
Cardiac dysfunction and hypothalamic activation during a social crowding stressor in prairie voles. – Negative social interactions produce several detrimental consequences in humans and non-human animals; and conversely, positive social interactions may have stress-buffering effects on both behavior and physiology. However, the mechanisms underlying specific stressor-responsiveness in the context of the social environment are not well understood. The present study investigated the integration of behavior, cardiac function, and Fos-immunoreactivity in the hypothalamic paraventricular nucleus during an acute social stressor in female, socially monogamous prairie voles exposed to previous long-term pairing (control conditions) or isolation. Animals previously exposed to social isolation displayed increased heart rate, attenuated heart rate variability, and increased incidence of cardiac arrhythmias during an acute crowding stressor versus animals previously exposed to social pairing; these cardiac alterations were not secondary to behavioral changes during the crowding stressor. Furthermore, social isolation was associated with increased c-Fos-immunoreactivity in the hypothalamic paraventricular nucleus following the crowding stressor, versus social pairing. The prairie vole provides a useful model for understanding how the social environment contributes to changes in behavior, cardiac function, and central stress-regulatory processes in humans.
Keywords: Arvicolinae, Autonomic nervous system, Brain, Fos, Heart rate variability, Hypothalamus, Immunohistochemistry, Paraventricular nucleus, Prairie vole, Respiratory sinus arrhythmia, Social behavior, Social isolation, Social stress
Introduction
Negative social interactions and social stressors are posited to have several detrimental consequences in humans and non-human animals, including (but not limited to) effects on behavior, emotion, endocrine and autonomic functions, and central nervous system processes (see for instance Anisman & Zacharko, 1982; Kim & Kirkpatrick, 1996; Blanchard et al., 2001; McCabe et al., 2002; Weihs et al., 2005; Grant et al., 2009). Conversely, the presence of (or perception of) social bonds positively affects general health and buffers against environmental stressors (House et al., 1988; Kiecolt-Glaser & Newton, 2001; MacMahon & Lip, 2002; Adams et al., 2004; Aron et al., 2005; Lim & Young, 2006). For example, in humans feelings of loneliness were associated with detrimental behavioral and cardiovascular effects including increased ratings of hopelessness, reduced self-esteem, and increased diastolic blood pressure (in women) (Steptoe et al., 2004). Also, a combination of oxytocin treatment and social support reduced cortisol responses and subjective anxiety reactions following a social stressor in men (Heinrichs et al., 2003). Similarly, in male rats maternal separation (as pups) was associated with a greater stress-induced corticosterone response in adulthood, versus a handled control group (Kalinichev et al., 2002). In studies with socially monogamous rodents, social pairing with a family member or an opposite-sex partner (versus social isolation) was protective against several negative behavioral and physiological alterations, including depression- and anxiety-relevant behaviors, autonomic and cardiac dysfunction, and increased stressor reactivity (Grippo et al., 2007d; Bosch et al., 2009).
These previous findings suggest that behavioral and neurobiological responses to negative social interactions may be associated with the established link between psychological disorders and cardiovascular diseases (see also Grippo et al., 2007d; Grippo, 2009). Although the processes that mediate reactions to the social context are receiving increased attention among researchers (Sgoifo et al., 2001; Cacioppo, 2002; Carter et al., 2008), the precise neurobiological mechanisms underlying responses to negative social experiences are not well understood. Therefore, studies that focus on the consequences of social stressors using valid and relevant animal model systems will promote a greater understanding of the social factors that contribute to behavioral and neurobiological dysregulation. The prairie vole (Microtus ochrogaster) is a highly social rodent species that shares several behavioral and physiological features with humans and other primates. For instance, prairie voles exhibit social behaviors paralleling those of primates, including an active engagement in and reliance on the social environment, living in pairs or family groups, and forming enduring social bonds (Carter et al., 1995; Getz & Carter, 1996; Carter & Keverne, 2002). Prairie voles, like other socially monogamous mammals such as humans, are extremely sensitive to disruptions of the social environment – and in particular, social isolation (Ruscio et al., 2007; Grippo et al., 2008; Bosch et al., 2009). In addition to the behavioral similarities, this species also exhibits resting autonomic characteristics similar to humans (but unlike rats and mice), including a large contribution from the parasympathetic nervous system to regulate resting cardiac function (Grippo et al., 2007c). Given these unique behavioral and autonomic characteristics, the prairie vole provides a useful translational model for investigating mechanisms through which social factors influence both cardiovascular function and behavior.
Recent studies with the prairie vole model have indicated that this species is sensitive to negative social environmental changes (Ruscio et al., 2007; Grippo et al., 2008; Bosch et al., 2009). Following short- or long-term social isolation, prairie voles showed several behavioral alterations including immobility in a forced swim test, reduced responsiveness to a rewarding stimulus, reduced time spent in the open arms of the elevated plus maze, and increased aggressive behavior toward an unrelated and unfamiliar prairie vole pup (Grippo et al., 2007b; Grippo et al., 2008; Bosch et al., 2009). Long-term social isolation in prairie voles (versus pairing with a sibling of the same sex) also produced several autonomic and cardiac disruptions indicative of potential cardiovascular pathophysiology, including increased resting heart rate (HR), reduced HR variability, increased cardiac responsiveness to acute stressors, and sympathovagal imbalance due to withdrawal of vagal tone and increased sympathetic drive to the heart (Grippo et al., 2007d; Grippo et al., 2009). Finally, varying periods of social isolation (including short- and long-term isolation in juvenile or adult prairie voles) were associated with dysregulation of stressor-responsive neurons in the brain, altered circulating stress hormones, and exaggerated behavioral and neuroendocrine reactivity to novel environmental stressors (Ruscio et al., 2007; Grippo et al., 2007a; Grippo et al., 2007b; Bosch et al., 2009).
While some recent studies (Ruscio et al., 2007; Grippo et al., 2007d; Bosch et al., 2009) have focused on short- and long-term behavioral and physiological consequences of social environmental disruptions in prairie voles, the integration of behavioral, cardiac, and central nervous system responses to stressors has received little attention in this animal model. Given the utility of this species for the investigation of behavior and autonomic function, the purpose of the present study was to examine acute stressor reactivity in prairie voles exposed to different long-term social experiences. Specifically, we investigated the hypothesis that chronic social isolation (4 weeks) in adult, female prairie voles would predispose animals to altered behavioral, cardiac, and hypothalamic responses during an acute social crowding stressor.
Materials and Methods
Animals
Thirteen adult (60–90 days), female prairie voles (35–55 grams), descendants of a wild stock caught near Champaign, Illinois, were maintained on a 14/10 h light/dark cycle (lights on at 0630 h), with a temperature of 25 ± 2° C and relative humidity of 21 ± 4 g/m3. Animals were allowed food (Purina rabbit chow) and water ad libitum. Offspring were removed from breeding pairs at 21 days of age and housed in same-sex sibling pairs. All procedures were conducted in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and were approved by the local university Institutional Animal Care and Use Committees.
Females were used in these experiments because they may be especially sensitive to the effects of negative social interactions, and they have been investigated extensively in studies of short- and long-term social stressors (Cushing & Carter, 2000; Grippo et al., 2007a; Grippo et al., 2007b; Grippo et al., 2008). Additionally, female prairie voles do not show a spontaneous puberty or estrous cycle; the ovaries remain inactive until the female has physical contact with a male, allowing for the use of reproductively intact animals without the need for controlling the estrous cycle (Carter et al., 1987).
Telemetric Transmitter Implantation
Wireless radiofrequency transmitters [Data Sciences International (DSI), St. Paul, MN; model TA10ETA-F20] were implanted for continuous electrocardiographic (ECG) and activity recordings, under aseptic conditions, during the light period, using procedures described previously (Grippo et al., 2009). Care was taken to ensure that animals did not experience unnecessary pain and distress during the procedures. Briefly, transmitters were implanted intraperitoneally into anesthetized animals (ketamine 67mg/kg, sc; xylazine 13.33mg/kg, sc; NLS Animal Health, Owings Mills, MD), and the leads were directed rostrally (subcutaneously) and anchored in place with permanent sutures (DII positioning). Subcutaneous sterile saline was administered as necessary. Following the surgical procedures, all animals were housed for 5 days in custom-designed divided cages (Scientific Instrument Shop, University of Illinois at Chicago, Chicago, IL) to permit adequate healing of suture wounds (see Grippo et al., 2007c), and then were returned to the home cages to recover for an additional 5–7 days.
Radiotelemetric Recordings
ECG signals were recorded with a radiotelemetry receiver (DSI, St. Paul, MN; sampling rate 5 kHz, 12-bit precision digitizing). Activity level was monitored via the receiver (sampling rate 256 Hz). The radiotelemetry receiver was controlled by the vendor software (Dataquest ART, Version 4.1 Acquisition software; DSI, St. Paul, MN).
Social Isolation
Following recovery from surgery, animals were randomly divided into paired (control; n=6) or isolated (n=7) conditions; only one animal from each sibling pair was studied. Isolated animals were separated from the sibling and housed individually (in a separate room, without visual or olfactory cues) for 4 weeks; paired animals were continually housed with the siblings during this period. Handling and cage changing were matched between the two groups.
Acute Social Crowding
Following 4 weeks of social isolation or pairing, all animals were exposed to a period of social crowding, using a variation of procedures described previously (see Djordjevic et al., 2005; Herzog et al., 2009). The paired or isolated animal was placed into a cage of 19 × 28 × 13 cm (equivalent to the size of the home cage), containing 5 unrelated and unfamiliar female prairie voles of approximately the same age and size as the experimental animal, for a total of 10 minutes. Behaviors, ECG, and activity variables were recorded continuously during the 10-minute stressor, and brain tissue was removed 2 hours following the end of the stressor (methods described below).
Quantification of Behaviors
Behaviors were video-recorded continuously during the 10-minute crowding stressor using a JVC Everio digital camcorder, and were then scored by two trained, experimentally-blind observers for aggressive and social behaviors. Aggressive behaviors were defined as aggressive grooming or posture, swatting, biting, thrusting, pulling, and/or attack behavior directed toward the other animals (Mitchell et al., 2003). General social behavior was defined as sniffing other animals, and positive social behavior was defined as sitting in physical (side-by-side) contact with other animals (Williams et al., 1992). General locomotor activity was recorded via the radiotelemetry receiver and vendor software (DSI, St. Paul, MN), and was measured in counts/minute.
Quantification of Radiotelemetric Recordings
Quantification of Telemetric Variables
Telemetric variables were recorded continuously during the crowding stressor, and quantification was conducted according to procedures described elsewhere (Grippo et al., 2007c; Grippo et al., 2007d). Briefly, the data were evaluated for HR, HR variability, ventricular and supraventricular arrhythmias, and activity. All ECG and activity parameters were evaluated using continuous data that were not confounded by movement artifact.
Quantification of Cardiac Variables
HR was evaluated using Dataquest ART, Version 4.1 Analysis software (DSI, St. Paul, MN), and R-wave detections were verified with custom-designed software (Department of Psychiatry, University of Illinois at Chicago, Chicago, IL).
The R-R intervals were analyzed for variations (i.e., HR variability) using custom-designed software (Department of Psychiatry, University of Illinois at Chicago), and included the standard deviation of all R-R (normal-to-normal; N-N) intervals (SDNN index) and amplitude of respiratory sinus arrhythmia (RSA). The SDNN index was evaluated according to procedures described previously (Task Force of the European Society of Cardiology & North American Society of Pacing and Electrophysiology, 1996), and represents the convergence of both sympathetic and parasympathetic innervation to the cardiac pacemaker. RSA was assessed with a modification of procedures described elsewhere (Yongue et al., 1982), which has been validated in prairie voles and other species (Porges et al., 1982; Grippo et al., 2007c). The amplitude of RSA represents the influence of myelinated vagal efferent pathways originating in the nucleus ambiguus (see Porges, 2007). Briefly, preliminary spectral analyses (Grippo et al., 2007c) identified spectral peaks associated with breathing of 1.0–4.0 Hz (Ishii et al., 1996; Gehrmann et al., 2000). The R-R intervals were resampled at 20 Hz and, to comply with the assumption of stationarity, detrended with a 21-point cubic moving polynomial to remove low frequency (trend) components below 0.5 Hz. The residuals of this procedure were free of aperiodic and slow periodic processes that may have violated the assumption of stationarity. A bandpass filter was applied to define RSA by extracting only the variance in the HR spectrum between the frequencies of 1.0–4.0 Hz.
Quantification of Arrhythmias
The incidence of the most representative types of rhythm disturbance in this species, including ventricular and supraventricular premature beats, was quantified according to procedures described previously (Sgoifo et al., 1997). The occurrence of cardiac arrhythmias was determined off-line, based on the classical definition of arrhythmias in man (Catalano, 1993) and on the Lambeth Conventions for the study of experimental arrhythmias (Walker et al., 1988).
Collection of Tissue
Two hours following the end of the crowding stressor, all animals were sacrificed under anesthesia via cervical dislocation for the quantification of neural activation in the hypothalamic paraventricular nucleus (PVN). This time course of stressor-induced PVN activation has been described previously in prairie voles (Grippo et al., 2007b). Brains were removed carefully from the skulls and processed with a passive perfusion (i.e., spin immersion) technique described previously (Cushing et al., 2001). Brains were immersed in a fixative solution consisting of 4% paraformaldehyde containing 5% acrolein for a total of 4 hours. Brains were postfixed for 24 hours in 4% paraformaldehyde, and sunk in 25% sucrose. Tissue was stored in 25% sucrose at 4° C until it was sectioned at 40 μm on a freezing sliding microtome. Sliced serial brain sections were stored at −20° C, in cryoprotectant antifreeze solution, until assayed for c-Fos-immunoreactivity in the PVN.
Stressor-Induced Immunohistochemistry and Image Analysis
Serial brain slices (40 μm) from the PVN were assayed for the c-Fos protein using standard avidin:biotinylated enzyme complex (ABC) immunohistochemistry, using commercially-available materials, with procedures that have been employed previously in prairie voles (Cushing et al., 2003; Grippo et al., 2007b). Anti-c-Fos (Oncogene Science, Cambridge, MA; generated in rabbit) was used at a concentration of 1:100,000. c-Fos is a non-specific marker of cellular activity, and is a useful tool for identifying neural responses to environmental and social stimuli, including short-term stressors. Single-label immunohistochemical staining procedures were identical to those described in Grippo et al. (2007b).
Stained sections were mounted on gelatin coated slides, air-dried, dehydrated in a series of ethanol dilutions, cleared with Histoclear (National Diagnostics, Atlanta, GA), and protected with coverslips using Histomount mounting medium (National Diagnostics, Atlanta, GA).
Images were captured using an Olympus BH-2 microscope (Lombard, IL) equipped with a Pixera Penguin 600CL camera, and Pixera Studio 3.0 imaging software (San Jose, CA). The density of c-Fos-positive nuclei was determined manually in the PVN using a 20x objective, within a standardized sampling area, according to procedures described previously (Ruscio et al., 2007; Grippo et al., 2007b). Measurements within the PVN were taken in a section of the nucleus approximate to Fig. 49 in Paxinos and Watson (2005), similar to previous studies (Wang et al., 1996; Ruscio et al., 2007), and included sections matched in rostral-caudal and medial-lateral orientation to minimize variability. Density measures were averaged across multiple hemispheres, brain slices, and two trained, experimentally blind raters to provide an accurate estimation of cell density in the PVN. Damaged sections (from one subject in the isolated group) were excluded from analysis.
Data Analyses
Data are presented as means ± (or +) standard error of the mean (SEM) for all analyses, tables and figures, and a probability value of P < 0.05 was considered to be statistically significant. Behavioral, cardiac, and tissue data were analyzed with single-factor analyses of variance (ANOVA), mixed-design ANOVAs (for repeated measures variables), a priori Student’s t-tests, and/or z-tests for a difference between two proportions where relevant. A Bonferroni correction was used for all multiple comparisons involving Student’s t-tests. Arrhythmia analyses were conducted using Student’s t-tests assuming unequal variances, due to the lack of arrhythmia occurrence in the paired group. For all cardiac quantifications, care was taken not to include periods of ECG involving animal movement artifact. Relevant correlations between behaviors and cardiac function were computed using Pearson’s r. One brain from the isolated group was excluded from the tissue analyses, due to damage to the PVN tissue during the immunohistochemical staining procedures.
Results
Behavioral Analyses
Social isolation (versus pairing) was not associated with a difference in behaviors during the crowding stressor (Table 1; p > 0.05 for all comparisons).
Table 1.
Mean (± SEM) behavioral responses exhibited by paired and isolated prairie voles during a 10-minute social crowding stressor.
| Aggressive Behaviors (# of Episodes) | General Social Behaviors (# of Episodes) | Positive Social Behaviors (# of Episodes) | General Physical Activity (counts/minute) | |
|---|---|---|---|---|
| Paired | 31.7 ± 7.7 | 38.0 ± 6.3 | 8.8 ± 3.1 | 9.4 ± 2.2 |
| Isolated | 33.5 ± 7.6 | 36.5 ± 3.0 | 5.3 ± 1.5 | 7.0 ± 1.5 |
Note: Behavioral responses are shown for the entire 10-minute stressor.
Cardiac Parameter Analyses
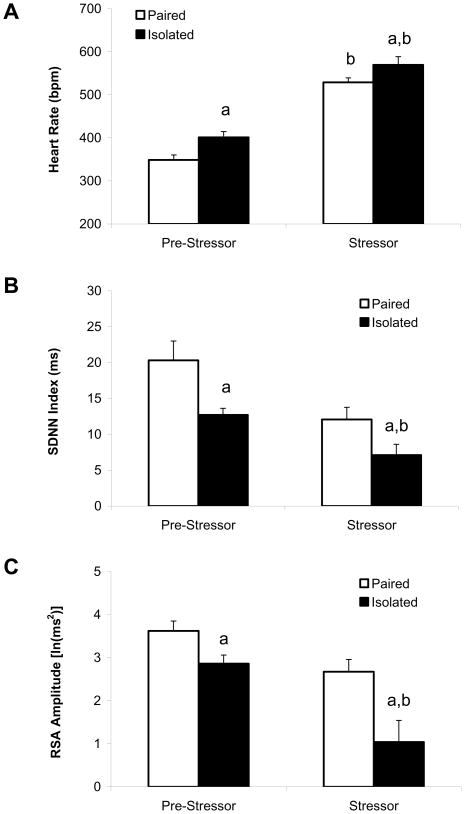
Cardiac parameters (HR, SDNN index, and RSA amplitude) recorded during the stressor were compared to pre-stressor values recorded 24 hours prior to the stressor presentation. Compared to control conditions (social pairing), social isolation was associated with higher pre-stressor HR, lower pre-stressor HR variability, and altered cardiac responses during the crowding stressor (Fig. 1). The ANOVA for HR yielded main effects of group [F(1,11) = 10.45, p < 0.05] and condition [F(1,11) = 143.20, p < 0.05]. Both groups displayed a higher stressor-induced HR vs. their respective pre-stressor HR values [t(5) = 14.73, p < 0.05; and t(6) = 6.81, p < 0.05 for paired and isolated groups, respectively]. Versus the paired group, the isolated group displayed a significantly higher pre-stressor HR [t(11) = 2.92, p < 0.05] and a higher stressor-induced HR [t(11) = 1.78, p = 0.05].
Fig. 1.
Mean (+ SEM) heart rate (Panel A), SDNN index (Panel B), and amplitude of respiratory sinus arrhythmia (Panel C) in paired and isolated prairie voles prior to and during a 10-minute social crowding stressor. Note the scale differences among the three panels. aP < 0.05 vs. respective paired value; bP < 0.05 vs. respective pre-stressor value. SDNN, standard deviation of normal-to-normal intervals; RSA, respiratory sinus arrhythmia.
The ANOVA for SDNN index yielded main effects of group [F(1,11) = 21.74, p < 0.05] and condition [F(1,11) = 10.93, p < 0.05]. The stressor-induced SDNN index in the paired group did not differ from this group’s respective pre-stressor SDNN index (p > 0.05); however the stressor-induced SDNN index in the isolated group was significantly lower than this group’s respective pre-stressor SDNN index [t(6) = 2.85, p < 0.05]. Versus the paired group, the isolated group displayed a significantly lower pre-stressor SDNN index [t(11) = 2.81, p < 0.05] and a lower stressor-induced SDNN index [t(11) = 2.19, p = 0.05].
The ANOVA for RSA amplitude yielded main effects of group [F(1,11) = 10.60, p < 0.05] and condition [F(1,11) = 20.68, p < 0.05]. The stressor-induced RSA amplitude in the paired group did not differ from this group’s respective pre-stressor RSA amplitude (p > 0.05); however the stressor-induced RSA amplitude in the isolated group was significantly lower than this group’s respective pre-stressor RSA amplitude [t(6) = 4.03, p < 0.05]. Versus the paired group, the isolated group displayed a significantly lower pre-stressor RSA amplitude [t(11) = 2.55, p < 0.05] and a lower stressor-induced RSA amplitude [t(11) = 2.70, p = 0.05].
Arrhythmia Analyses
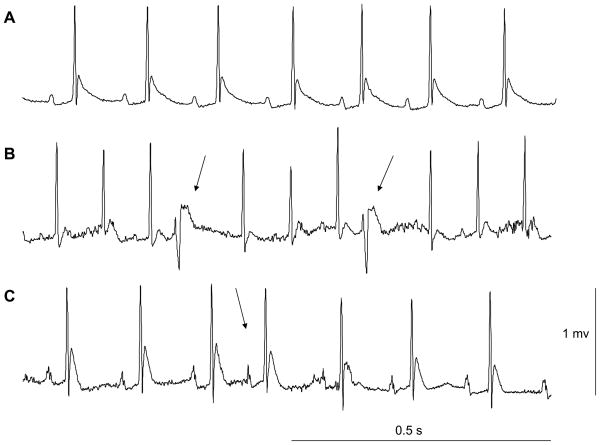
Social isolation was associated with an increased vulnerability toward arrhythmic events during the crowding stressor, versus social pairing (Table 2). Fig. 2 shows an example of the arrhythmic events exhibited by the isolated group during the crowding stressor. Isolated animals were significantly more likely to display both ventricular (z = 2.22, p < 0.05) and supraventricular arrhythmias (z = 3.61, p < 0.05) during the crowding stressor. Given the significant difference in the proportion of animals that displayed stressor-induced arrhythmic events, the total number of arrhythmic events was analyzed with a paired t-test assuming unequal variances. The isolated group displayed a significantly greater number of stressor-induced supraventricular arrhythmias [t(6) = 3.63, p < 0.05] and a tendency toward a greater number of stressor-induced ventricular arrhythmias [t(6) = 1.70, p < 0.07].
Table 2.
Mean (± SEM) ventricular and supraventricular arrhythmias exhibited by paired and isolated prairie voles during a 10-minute social crowding stressor.
| Ventricular Premature Beats (% of each group) | Supraventricular Premature Beats (% of each group) | Incidence of Ventricular Premature Beats | Incidence of Supraventricular Premature Beats | |
|---|---|---|---|---|
| Paired | 0 | 0 | 0 ± 0 | 0 ± 0 |
| Isolated | 57a | 100a | 2.7 ± 0.9b | 2.8 ± 0.9a |
Note: Arrhythmic responses are shown for the entire 10-minute stressor.
P < 0.05 vs. paired group in the same column;
P < 0.07 vs. paired group in the same column.
Fig. 2.
Electrocardiographic tracings showing sinus rhythm (Panel A), ventricular premature beats (Panel B), and a supraventricular premature beat (Panel C) in an isolated prairie vole during a 10-minute social crowding stressor. Arrows in Panels B and C are denoting the arrhythmic events.
Tissue Analyses
Social isolation (versus pairing) led to increased neural activation in the PVN following the crowding stressor. Fig. 3 depicts sample brain sections showing c-Fos-immunoreactivity in a paired and isolated prairie vole. Isolated animals displayed increased c-Fos-positive neuronal density in the PVN (271 ± 19 immunoreactive nuclei/sampling area) compared with paired animals (152 ± 17 immunoreactive nuclei/sampling area) at 2 hours following the crowding stressor [t(10) = 2.25, p < 0.05].
Fig. 3.
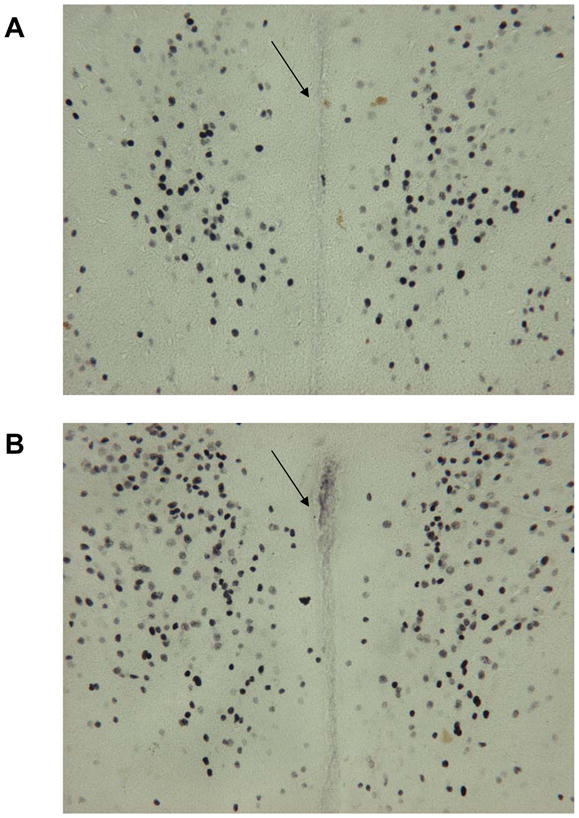
Brain sections (40 μm thickness; 20x magnification) showing c-Fos-immunoreactive cell density in the hypothalamic paraventricular nucleus in a paired (Panel A) and isolated (Panel B) prairie vole 2 hours after a 10-minute social crowding stressor. Arrows are denoting the 3rd ventricle.
Correlational Analyses
Correlational analyses indicated that social bonding was negatively associated with HR in both the paired (r = −0.85, significantly different from 0 at p < 0.05) and isolated groups (r = −0.80, significantly different from 0 at p < 0.05), but was not associated with the number of arrhythmic events in either group (p > 0.05, data not shown).
Discussion and Conclusions
The purpose of the present study was to investigate the integration of behavioral, cardiac, and neural consequences of chronic and acute social stressors. The prairie vole was chosen as a model for this investigation due to the unique social behaviors, reliance on the social context, and resting sympathovagal balance exhibited by this species (Carter et al., 1995; Getz & Carter, 1996; Carter & Keverne, 2002; Grippo et al., 2007c). The integration of acute stressor-induced behavioral, cardiac, and neural parameters in prairie voles exposed to different social experiences has not been investigated previously. The findings from the current study indicate that social isolation in female prairie voles sensitizes animals to acute stressor-induced cardiac dysfunction, including both rate and rhythm disturbances during a social crowding stressor. Additionally, social isolation is associated with increased PVN activation during the same behavioral stressor, which may represent a mechanism by which the brain interacts with the autonomic nervous system and the heart during exposure to acute social stressors.
While some studies have investigated the effects of social crowding in other rodents such as rats and mice (Bugajski et al., 1995; Bugajski et al., 2003; Dronjak et al., 2004; Reber et al., 2006), this study is the first to describe specifically behavioral, cardiac, and neural responses to acute social crowding in prairie voles. The present findings indicate that social isolation (versus social pairing) was associated with an increased tachycardic response, and also with reduced HR variability (both SDNN index and RSA amplitude), during the crowding stressor. These cardiac rate and rhythm disturbances were not secondary to behavioral changes, as the paired and isolated groups did not differ in the amount of physical activity, aggressive behaviors, or social behaviors exhibited during the crowding stressor. These data are consistent with previous findings showing that social isolation does not produce increased aggressive behaviors during a 5-minute resident-intruder stressor in prairie voles; yet isolation is associated with altered neuroendocrine, autonomic, and cardiac responses to this social stressor (Grippo et al., 2007b; Grippo et al., 2007d). Because long-term isolation has been associated with increased resting HR and decreased resting HR variability in prairie voles (Grippo et al., 2007d), a finding which was confirmed in the present study (see Fig. 1), it is possible that the increased HR and reduced SDNN index and RSA amplitude observed during the crowding stressor were the result of altered basal autonomic tone in the isolated group.
Social isolation (versus pairing) also was associated with an increased vulnerability to ventricular and supraventricular arrhythmias during the crowding stressor. Isolated prairie voles were significantly more likely than paired prairie voles to experience an arrhythmic event during the crowding stressor, and exhibited a greater number of arrhythmic events versus paired animals (as shown in Table 2, the paired group did not show any occurrence of arrhythmic events during the stressor). To our knowledge, the present study is the first to demonstrate cardiac arrhythmias in prairie voles exposed to a behavioral stressor. Disruptions in autonomic function – which may manifest as increases in HR, reductions in HR variability, or increases in the incidence of cardiac arrhythmias – are common in cardiovascular disease, predicting mortality in myocardial infarction and heart failure (Frasure-Smith et al., 1995; Ferrari et al., 2003; Guzzetti et al., 2005). The prairie vole may therefore be a useful model for investigating specifically social mechanisms underlying autonomic and cardiac pathophysiology.
Although social isolation did not predispose animals to changes in behavior during the crowding stressor (see Table 1), correlational analyses suggested that social bonding behaviors during the crowding stressor were negatively associated with HR in both the paired and isolated groups. These data indicate that increased positive social behaviors were related to attenuated HR responses to the stressor, and highlight the need for investigating individual differences in behavioral strategies and cardiac responses to stressors using rodent models.
The mechanisms by which social isolation leads to altered cardiac responsiveness to acute social stressors are not well understood. The cardiac changes observed in isolated prairie voles may be the result of altered neural control of cardiac function (e.g., mediating HR and HR variability) or the result of structural or functional changes at the level of the myocardium (e.g., mediating vulnerability to arrhythmic events). To investigate a potential neural mechanism, the present study attempted to elucidate whether altered activity of stressor-responsive neurons in the hypothalamic PVN was associated with dysfunctional cardiac responses to stress. The findings indicate that social isolation, versus social pairing, is associated with greater activation of PVN neurons following the crowding stressor. The PVN projects to the intermediolateral cell column of the spinal cord, rostral ventrolateral medulla, and dorsal vagal complex to influence both sympathetic and parasympathetic outflow (Swanson & Sawchenko, 1980; Badoer, 2001). Activation of this nucleus may alter the autonomic and cardiac responsiveness in animals that have been exposed to social isolation, thereby producing increased HR, reduced HR variability, and increased cardiac arrhythmias during acute environmental stressors. This hypothesis is consistent with previous findings showing sympathovagal imbalance (including a significant withdrawal of vagal tone and an increase in sympathetic tone to the heart) following 4 weeks of social isolation in adult prairie voles (Grippo et al., 2007d).
The precise signaling pathways between the PVN and specific hindbrain or brainstem structures in isolated prairie voles (and humans) remain to be elucidated. A limitation of the present study is that it was not possible to investigate the activation of specific populations of PVN-containing neurons following the social crowding stressor. However, previous evidence suggests that both oxytocin and corticotropin-releasing hormone (CRH) neurons, as well as CRH receptors, are altered in the PVN following social isolation in this species (Grippo et al., 2007b; Pournajafi-Nazarloo et al., 2009). Additionally, exposure of prairie voles to an acute swim stressor altered both oxytocin mRNA (decreased) and CRH mRNA labeling (increased) in the PVN (Liu et al., 2001). Therefore, dysfunction of PVN oxytocin and/or CRH as a result of social isolation is a candidate mechanism for altered communication with hindbrain autonomic nuclei and the disruption of downstream autonomic and cardiovascular processes. Further investigation of behavioral, physiological, and central nervous system responses to the social context using relevant animal model systems will improve our understanding of interactions among the social environment, behavior, and neurobiological processes in humans.
Acknowledgments
The investigators would like to thank Ms. Iman Hassan for assistance. This research was funded by the National Institutes of Health, MH-077581 (Grippo); and by the Italian Ministry for Education, University and Research, Project 2007WB35CW_004 (Sgoifo).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams KB, Sanders S, Auth EA. Loneliness and depression in independent living retirement communities: risk and resilience factors. Aging Ment Health. 2004;8:475–485. doi: 10.1080/13607860410001725054. [DOI] [PubMed] [Google Scholar]
- Anisman H, Zacharko RM. Depression: the predisposing influence of stress. Behav Brain Sci. 1982;5:89–137. [Google Scholar]
- Aron A, Fisher H, Mashek DJ, Strong G, Li H, Brown LL. Reward, motivation, and emotion systems associated with early-stage intense romantic love. J Neurophysiol. 2005;94:327–337. doi: 10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- Badoer E. Hypothalamic paraventricular nucleus and cardiovascular regulation. Clin Exp Pharmacol Physiol. 2001;28:95–99. doi: 10.1046/j.1440-1681.2001.03413.x. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, McKittrick CR, Blanchard DC. Animal models of social stress: effects on behavior and brain neurochemical systems. Physiol Behav. 2001;73:261–271. doi: 10.1016/s0031-9384(01)00449-8. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Nair HP, Ahern TH, Neumann ID, Young LJ. The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacology. 2009;34:1406–1415. doi: 10.1038/npp.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugajski J, Borycz J, Glod R, Bugajski AJ. Crowding stress impairs the pituitary-adrenocortical responsiveness to the vasopressin but not corticotropin-releasing hormone stimulation. Brain Res. 1995;681:223–228. doi: 10.1016/0006-8993(95)00297-4. [DOI] [PubMed] [Google Scholar]
- Bugajski J, Gadek-Michalska A, Bugajski AJ. Effect of the cyclooxygenase inhibitors on the CRH-induced pituitary-adrenocortical activity during crowding stress. J Physiol Pharmacol. 2003;54:99–108. [PubMed] [Google Scholar]
- Cacioppo JT. Social neuroscience: understanding the pieces fosters understanding the whole and vice versa. Am Psychologist. 2002;57:819–831. doi: 10.1037/0003-066x.57.11.819. [DOI] [PubMed] [Google Scholar]
- Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci Biobehav Rev. 1995;19:303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- Carter CS, Grippo AJ, Pournajafi-Nazarloo H, Ruscio MG, Porges Oxytocin, vasopressin and sociality. Prog Brain Res. 2008;170:331–336. doi: 10.1016/S0079-6123(08)00427-5. [DOI] [PubMed] [Google Scholar]
- Carter CS, Keverne EB. The neurobiology of social affiliation and pair bonding. Horm Brain Behav. 2002;1:299–337. [Google Scholar]
- Carter CS, Witt DM, Schneider J, Harris ZL, Volkening D. Male stimuli are necessary for female sexual behavior and uterine growth in prairie voles (Microtus ochrogaster) Horm Behav. 1987;21:74–82. doi: 10.1016/0018-506x(87)90032-8. [DOI] [PubMed] [Google Scholar]
- Catalano JT. Guide to ECG Analysis. Philadelphia, PA: Lippincott; 1993. [Google Scholar]
- Cushing BS, Carter CS. Peripheral pulses of oxytocin increase partner preferences in female, but not male, prairie voles. Horm Behav. 2000;37:49–56. doi: 10.1006/hbeh.1999.1558. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Klein D, Hoffman GE, Carter CS, Le WW, De Vries GJ. Comparison of fixation techniques: immersion versus perfusion. Horm Behav. 2001;39:329. [Google Scholar]
- Cushing BS, Mogekwu N, Le WW, Hoffman GE, Carter CS. Cohabitation induced Fos immunoreactivity in the monogamous prairie vole. Brain Res. 2003;965:203–211. doi: 10.1016/s0006-8993(02)04199-9. [DOI] [PubMed] [Google Scholar]
- Djordjevic J, Cvijic G, Petrovic N, Davidovic V. Effect of the acute crowding stress on the rat brown adipose metabolic function. Comp Biochem Physiol. 2005;142:433–438. doi: 10.1016/j.cbpa.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Dronjak S, Gavrilovic L, Filipovic D, Radojcic MB. Immobilization and cold stress affect sympatho-adrenomedullary system and pituitary-adrenocortical axis of rats exposed to long-term isolation and crowding. Physiol Behav. 2004;81:409–415. doi: 10.1016/j.physbeh.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Ferrari R, Censi S, Mastrorilli F, Boraso A. Prognostic benefits of heart rate reduction in cardiovascular disease. Eur Heart J Suppl. 2003;5 (Suppl G):G10–G14. [Google Scholar]
- Frasure-Smith N, Lespérance F, Talajic M. Depression and 18-month prognosis after myocardial infarction. Circulation. 1995;91:999–1005. doi: 10.1161/01.cir.91.4.999. [DOI] [PubMed] [Google Scholar]
- Gehrmann J, Hammer PE, Maguire CT, Wakimoto H, Triedman JK, Berul CI. Phenotypic screening for heart rate variability in the mouse. Am J Physiol Heart Circ Physiol. 2000;279:H733–H740. doi: 10.1152/ajpheart.2000.279.2.H733. [DOI] [PubMed] [Google Scholar]
- Getz LL, Carter CS. Prairie-vole partnerships. Am Scientist. 1996;84:56–62. [Google Scholar]
- Grant N, Hamer M, Steptoe A. Social isolation and stress-related cardiovascular, lipid, and cortisol responses. Ann Behav Med. 2009;37:29–37. doi: 10.1007/s12160-009-9081-z. [DOI] [PubMed] [Google Scholar]
- Grippo AJ. Mechanisms underlying altered mood and cardiovascular dysfunction: the value of neurobiological and behavioral research with animal models. Neurosci Biobehav Rev. 2009;33:171–180. doi: 10.1016/j.neubiorev.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Cushing BS, Carter CS. Depression-like behavior and stressor-induced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosom Med. 2007a;69:149–157. doi: 10.1097/PSY.0b013e31802f054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, Carter CS. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 2007b;32:966–980. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Lamb DG, Carter CS, Porges SW. Cardiac regulation in the socially monogamous prairie vole. Physiol Behav. 2007c;90:386–393. doi: 10.1016/j.physbeh.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Lamb DG, Carter CS, Porges SW. Social isolation disrupts autonomic regulation of the heart and influences negative affective behaviors. Biol Psychiatry. 2007d;62:1162–1170. doi: 10.1016/j.biopsych.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Trahanas DM, Zimmerman RR, II, Porges SW, Carter CS. Oxytocin protects against negative behavioral and autonomic consequences of long-term social isolation. Psychoneuroendocrinology. 2009;34:1542–1553. doi: 10.1016/j.psyneuen.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Wu KD, Hassan I, Carter CS. Social isolation in prairie voles induces behaviors relevant to negative affect: toward the development of a rodent model focused on co-occurring depression and anxiety. Depress Anxiety. 2008;25:E17–E26. doi: 10.1002/da.20375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzzetti S, La Rovere MT, Pinna GD, Maestri R, Borroni E, Porta A, Mortara A, Malliani A. Different spectral components of 24 h heart rate variability are related to different modes of death in chronic heart failure. Eur Heart J. 2005;26:357–362. doi: 10.1093/eurheartj/ehi067. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Herzog CJ, Czéh B, Corbach S, Wuttke W, Schulte-Herbrüggen O, Hellweg R, Flügge G, Fuchs E. Chronic social instability stress in female rats: a potential animal model for female depression. Neuroscience. 2009;159:982–992. doi: 10.1016/j.neuroscience.2009.01.059. [DOI] [PubMed] [Google Scholar]
- House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- Ishii K, Kuwahara M, Tsubone H, Sugano S. Autonomic nervous function in mice and voles (Microtus arvalis): investigation by power spectral analysis of heart rate variability. Lab Animals. 1996;30:359–364. doi: 10.1258/002367796780739880. [DOI] [PubMed] [Google Scholar]
- Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG. Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long-Evans rats. Pharmacol Biochem Behav. 2002;73:131–140. doi: 10.1016/s0091-3057(02)00781-5. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Newton TL. Marriage and health: his and hers. Psychol Bull. 2001;127:472–503. doi: 10.1037/0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- Kim JW, Kirkpatrick B. Social isolation in animal models of relevance to neuropsychiatric disorders. Biol Psychiatry. 1996;40:918–922. doi: 10.1016/0006-3223(95)00546-3. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm Behav. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Liu Y, Curtis JT, Fowler CD, Spencer C, Houpt T, Wang ZX. Differential expression of vasopressin, oxytocin and corticotrophin-releasing hormone messenger RNA in the paraventricular nucleus of the prairie vole brain following stress. J Neuroendocrinol. 2001;13:1059–1065. doi: 10.1046/j.1365-2826.2001.00729.x. [DOI] [PubMed] [Google Scholar]
- MacMahon KMA, Lip GYH. Psychological factors in heart failure: a review of the literature. Arch Int Med. 2002;162:509–516. doi: 10.1001/archinte.162.5.509. [DOI] [PubMed] [Google Scholar]
- McCabe PM, Gonzales JA, Zaias J, Szeto A, Kumar M, Herron AJ, Schneiderman N. Social environment influences the progression of atherosclerosis in the Watanabe heritable hyperlipidemic rabbit. Circulation. 2002;105:354–359. doi: 10.1161/hc0302.102144. [DOI] [PubMed] [Google Scholar]
- Mitchell PJ, Fairhall SJ, Fletcher A, Redfern PH. Effects of single and repeated electroconvulsive shock on the social and agonistic behaviour of resident rats. Neuropharmacology. 2003;44:911–925. doi: 10.1016/s0028-3908(03)00075-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5. New York: Elsevier; 2005. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biol Psychol. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, McCabe PM, Yongue BG. Respiratory-heart rate interactions: psychophysiological implications for pathophysiology and behavior. In: Cacioppo J, Petty R, editors. Perspectives in cardiovascular psychophysiology. New York: Guilford Publications, Inc; 1982. pp. 223–264. [Google Scholar]
- Pournajafi-Nazarloo H, Partoo L, Sanzenbacher L, Paredes J, Hashimoto K, Azizi F, Carter CS. Stress differentially modulates mRNA expression for corticotrophin-releasing hormone receptors in hypothalamus, hippocampus and pituitary of prairie voles. Neuropeptides. 2009;43:113–123. doi: 10.1016/j.npep.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Reber SO, Obermeier F, Straub HR, Falk W, Neumann ID. Chronic intermittent psychosocial stress (social defeat/overcrowding) in mice increases the severity of an acute DSS-induced colitis and impairs regeneration. Endocrinology. 2006;147:4968–4976. doi: 10.1210/en.2006-0347. [DOI] [PubMed] [Google Scholar]
- Ruscio MG, Sweeny T, Hazelton J, Suppatkul P, Carter CS. Social environment regulates corticotropin releasing factor, corticosterone and vasopressin in juvenile prairie voles. Horm Behav. 2007;51:54–61. doi: 10.1016/j.yhbeh.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Sgoifo A, De Boer SF, Westenbroek C, Maes FW, Beldhuis H, Suzuki T, Koolhaas JM. Incidence of arrhythmias and heart rate variability in wild-type rats exposed to social stress. Am J Physiol Heart Circ Physiol. 1997;273:H1754–H1760. doi: 10.1152/ajpheart.1997.273.4.H1754. [DOI] [PubMed] [Google Scholar]
- Sgoifo A, Koolhaas J, Alleva E, Musso E, Parmigiani S. Social stress: acute and long-term effects on physiology and behavior. Physiol Behav. 2001;73:253–254. doi: 10.1016/s0031-9384(01)00544-3. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Owen N, Kunz-Ebrecht SR, Brydon L. Loneliness and neuroendocrine, cardiovascular, and inflammatory stress responses in middle-aged men and women. Psychoneuroendocrinology. 2004;29:593–611. doi: 10.1016/S0306-4530(03)00086-6. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology. 1980;31:410–417. doi: 10.1159/000123111. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology, North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Walker MJA, Curtis MJ, Hearse DJ, Campbell RFW, Janse MJ, Yellon DM, Cobbe SM, Coker SJ, Harness JB, Harron DWG, Higgins AJ, Julian DG, Lab MJ, Manning AS, Northover BJ, Parratt JR, Riemersma RA, Riva E, Russell DC, Sheridan DJ, Winslow E, Woodward B. The Lambeth Conventions: guidelines for the study of arrhythmias in ischaemia, infarction, and reperfusion. Cardiovasc Res. 1988;22:447–455. doi: 10.1093/cvr/22.7.447. [DOI] [PubMed] [Google Scholar]
- Wang ZX, Zhuo L, Hulihan TJ, Insel TR. Immunoreactivity of central vasopressin and oxytocin pathways in microtine rodents: a quantitative comparative study. J Comp Neurol. 1996;366:726–737. doi: 10.1002/(SICI)1096-9861(19960318)366:4<726::AID-CNE11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Weihs KL, Simmens SJ, Mizrahi J, Enright TM, Hunt ME, Seigel RS. Dependable social relationships predict overall survival in stages II and III breast carcinoma patients. J Psychosom Res. 2005;59:299–306. doi: 10.1016/j.jpsychores.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Williams JR, Catania KC, Carter CS. Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Horm Behav. 1992;26:339–349. doi: 10.1016/0018-506x(92)90004-f. [DOI] [PubMed] [Google Scholar]
- Yongue BG, McCabe PM, Porges SW, Rivera M, Kelley SL, Ackles PK. The effects of pharmacological manipulations that influence vagal control of the heart on heart period, heart-period variability and respiration in rats. Psychophysiology. 1982;19:426–432. doi: 10.1111/j.1469-8986.1982.tb02499.x. [DOI] [PubMed] [Google Scholar]