Abstract
Idiopathic Parkinson’s disease (PD) is a neurodegenerative disorder of mature and older individuals. Since all aged individuals do not develop PD, predisposing conditions may exist that pair with the stress placed on the basal ganglia during aging to produce the symptoms of PD. In this project we used 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) to test the hypothesis that a sensitization stage and a precipitating stage underlie idiopathic PD. To induce the sensitization stage, pregnant C57BL/6J mice were treated with MPTP (10mg/kg/dy) during gestation days 8–12 to target the emerging fetal nigrostriatal dopamine neurons. For the precipitating stage, the 3-months old offspring were administered MPTP for 7 days, to simulate the changes that occur during aging. The weights and motor activity of the offspring, HPLC striatal dopamine and its metabolites and Western blot for tyrosine hydroxylase (TH) were determined. Offspring exposed to prenatal MPTP showed lower birth weights that eventually recovered. Prenatal MPTP also reduced motor activity by 10–30%, striatal TH by 38%, dopamine by 14%, homovanillic acid by 16.5% and 3-methoxytyramine by 66%. The postnatal MPTP was more potent in the prenatal MPTP-exposed offspring. MPTP at 10, 20 and 30mg/kg, dose-relatedly, reduced striatal TH by 9.4%, 48.6% and 82.4% in the prenatal-PBS mice and by 48%, 78.7% and 92.7% in the prenatal-MPTP groups. More importantly, postnatal MPTP at 10mg/kg that showed slight effects on DA, DOPAC, HVA and 3-MT in the prenatal-PBS offspring, showed 69.9%, 80.0%, 48.4% and 65.4% reductions in the prenatal-MPTP mice. The study may identify a new model for PD, and the outcome suggests that some cases of idiopathic PD may have a fetal basis in which early subtle nigrostriatal impairments occurred and PD symptoms are precipitated later by deteriorating changes in the nigrostriatum, that would not caused symptoms in individuals with normal nigrostriatal system.
Keywords: Parkinson’s disease; 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP); striatum; dopamine; tyrosine hydroxylase
INTRODUCTION
Parkinson’s disease (PD) is the second most prevalent age-associated neurodegenerative disorder, characterized by the degeneration of dopaminergic neurons in the substantia nigra, leading to decreased dopamine and tyrosine hydroxylase (Nagatsu et al., 1979, Heikkila and Sonsalla, 1992) in the neostriatum. PD may be initiated and/or precipitated by environmental or endogenous toxins by a mechanism similar to that of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in genetically predisposed individuals (Jenner et al., 1992 and Aoyama et al., 2000). Several studies show pesticide exposure as a risk factor for PD (Kanthasamy et al. 2005) and fetal cocaine exposure in an animal model produces long-term alterations in the morphology, structure and organization of the CNS, including the dopaminergic system (Lidow 2003, Lloyd et al. 2006, Landrigan et al. 2005), which generates the hypothesis that exposure of the developing brain to toxic environmental agents during a window of vulnerability in utero and in early postnatal life may be important causative factor for PD. It was also proposed that the vast majority of the PD cases are caused by the interaction of genetic susceptibility with exposure to environmental agents (Di Monte 2003, Kennedy et al., 2003), and it has been shown that the exposure of pregnant rats to the bacteriotoxin lipopolysaccharide (LPS) resulted in a significant reduction of dopamine neurons in the offspring (Ling et al., 2002), showing that the fetal dopaminergic system is vulnerable. Among the neurotoxins, 1-methyl-4-phenyl pyridnium (MPP+), the active component of the pro-toxin MPTP, stands out, because this agent has been shown to cause symptoms that mimic PD. MPP+ is proposed to covalently react with DNA (Chung et al., 2004), to reduce total DNA and to cause oxidative stress. MPP+ freely crosses tissue barriers (Markey et al. 1986, Perry et al. 1986, Bagetta et al. 1992). Whatever is its mechanism of action, MPTP via MPP+, kills DA neurons and depletes striatal dopamine (Davis et al. 1979, Langston et al. 1983, Jarvis and Wagner 1985, Jackson–Lewis et al. 1995). Other toxins that target the NS DA neurons and cause basal ganglia toxicity (Aziz et al., 2002) may accumulate as ubiquitous contaminants in the environment (Tipton and Singer; 1993). The cause of Parkinson’s disease is currently unknown. Familial PD is caused by genetic changes, but it represents only about 5–10% of all PD cases. Therefore, as suggested by Calne and Langston (1983), the great proportion of PD is likely due to the exposure to environmental toxins, and MPTP serves as the best example of agents that cause PD.
This study is based on the hypothesis that toxic substances are involved in the cause of idiopathic Parkinson’s disease (PD) by inducing early in life, a less resilient but functional set of nigrostriatal dopamine neurons that cannot withstand the stress placed on them latter in life. Two stages of afflictions are therefore involve, the predisposing, susceptible, vulnerable or sensitizing stage that occurs early in life and causes changes that impair the phenotypic expression or reduce the number of nigrostriatal dopamine neurons. The second stage is the precipitating or the inducing stage that occurs when age-related “wear-and-tear” or other interventions damage the already susceptible nigrostriatal dopamine neurons and precipitate the symptoms of PD.
In this project we administered MPTP to pregnant mice during the vulnerable period of the differentiation or neurogenesis of the nigrostriatal dopamine neurons of the fetuses. The intent is to modify the nigrostriatal dopamine system but not significantly impairing motor functions. This is regarded as the first stage of affliction in the PD model. To induce the second or precipitating stage the young adults at three months of age were challenged with MPTP to cause further and above threshold-level harm to the nigrostriatal dopamine neurons. The 2nd stage represents or mimics the changes that occur during the aging process that make PD an age-related disorder. So the second administration of MPTP is a short-term substitution for the age-related changes.
MATERIALS AND METHODS
Animals
C57BL/6J timed pregnant mice weighing around 20g were purchased from Jackson Laboratory, Bar Harbor, Maine, USA. The animal use was approved by the Institutional Animal Care and Use Committee (IACUC) of Meharry Medical College. They were housed 3 per cage under a 12 -12 h light-dark cycle from 6 AM to 6 PM in a temperature-controlled room with standard food and water available ad libitum. The mice were allowed to acclimatize for about six days before use. The dams were divided into two groups, phosphate buffered saline (PBS) group and MPTP group. MPTP was purchased form Sigma-Aldrich, St Louis, MO. MPTP was dissolved in PBS and was administered intraperitoneally (ip). PBS was given at 0.1 ml/100g body weight and MPTP at 10 mg/kg at gestation day 8–12 (G8–G12). After delivery the pups were cross-nurtured, and their weights determined, as a way of accessing the global toxic effects of MPTP. At 28 days the pups were weaned and separated according to sex and their weights recorded until 12th weeks. At 12 weeks the young adult pups from both the prenatal PBS and MPTP groups were randomly divided into four groups each and were treated with either PBS, or MPTP, 10, 20 or 30 mg/kg body weight, for 7 days (i.p). MPTP was used to simulate a short-term manipulation that can lead to PD-type changes, in order to understand if the prenatal exposure to MPTP makes the mice more vulnerable to PD-type changes. At 7 days after the last MPTP injection the motor activity of the mice was determined and then a sub-group was sacrificed by decapitation, their brains dissected and the striatum used for Western blot analysis for TH or for the HPLC determination of dopamine and its metabolites, 3,4-dihydroxyphenylacetic acid (DOPAC), 3-methoxytyramine (3-MT) and homovanillic acid (HVA).
Measurement of Locomotor Activities
Seven days after the last post-natal injection of PBS or MPTP the mice were placed in the locomotor activity monitor and the measurement was started 3 min later and continued for 30 minutes. The changes in motor activity of the animals were measured in an activity monitor (Versamax analyzer, Accusccan Instruments, Inc, Columbus OH. The measurements were performed in a quiet isolated room with dim light. Movement time (MT), total distance (TD) and the number of movements (NM) were measured.
Western blot Analysis of TH protein expression
Immunoblotting was used to quantify the amount of TH protein in the striatum. After decapitation, the brains were dissected and the sections were homogenized in lysis buffer (Ambion, Austin, TX, USA). Protein concentration was determined using Bio-Rad protein reagent (Bio-Rad, USA). The proteins were precipitated by adding 100% methanol and centrifuged at 10,000 rpm for 10 minutes in a Sorvall refrigerated centrifuge. Then the supernatants were decanted and the precipitates washed with 90% methanol for 10 minutes. Proteins were dissolved in Lamelli sample buffer and transferred to nitrocellulose membrane. The membranes were blocked in 5% BSA for one hour. Membranes were incubated with primary rabbit TH antibody (1: 2,000, Chemicon International, CA, USA) and then exposed to the secondary antibody 1: 10,000 (HRP conjugated anti-rabbit IgG, Sigma Chemical Co, Saint Louis MO) and visualized by chemiluminescence (Muthian et al., 2006). All the experiments were repeated three times to confirm the results.
HPLC–electrochemical (EC) determination of catecholamines
The dissected brain tissues were homogenized in 750 μl of 0.1M trichloro-acetic acid (TCA), which contains 10−2 M sodium acetate, 10−4 M EDTA and 10.5% methanol (pH 3.8) using a tissue ‘dismembrator’ (Fisher Scientific). Samples were spun in a microcentrifuge at 10,000 x g for 20 minutes. Samples of the supernatant were then analyzed for biogenic monoamines in the core analytical facility at Vanderbilt University.
The biogenic amines were determined by specific HPLC assay utilizing an Antec Decade 11 (oxidation: 0.5) electrochemical detector operated at 33°C. Supernatant samples of 20 μl were injected, using a Water’s 717+ autosampler, onto a Phenomenex Nucleosol (5U, 100 A) C18 HPLC column (150×4.60 mm). Biogenic amines were eluted with a mobile phase consisting of 89.5% 0.1M TCA, 10−2 M sodium acetate, 10−4 M EDTA and 10.5% methanol (PH 3.8). Solvent is delivered at 0.8 ml/min using Water’s 515 HPLC pump. Using this HPLC solvent the following biogenic amines elute in the following order: noradrenaline, adrenaline, di-hydroxyphenylacetic acid (DOPAC), dopamine (DA), 5-hydroxy indole acetic acid (5-HIAA), 5-hydroxytryptamine (5-HT), and 3-methoxy tyramine (3-MT). HPLC control and data acquisition were managed by Water’s Empower software.
Statistical Analysis
The data were analysed by using the student t test. All values represent mean ± SEM. P values less than 0.05 were considered significant.
RESULTS
A. Effects of Prenatal Exposure to MPTP: Producing the Sensitization/Vulnerable Stage
1. Prenatal exposure to MPTP reduced birth weights of the mice pups
The body weights of the pups of C57BL/6J mice were measured and recorded once weekly before weaning and for every two weeks after weaning up to 12 weeks of age. This was done to determine if in utero exposure to 10 mg/kg of MPTP, as compared to in utero PBS, administered by the intraperitoneal (i.p) route to pregnant dams, caused effects that are expressed as changes in body weights and growth. As compared to the group exposed to prenatal PBS, the body weights of the offspring that were exposed to prenatal MPTP were significantly lower during the first 3 wks of life (figure 1A). The 1–4 week average body weights for the prenatal PBS-exposed pups were 2.2gm, 4.4gm, 7.4gm and 8.7gm and for the prenatal MPTP-exposed pups 1.8gm, 3.7gm, 6.3gm and 8.3gm. Thus, the weights of the prenatal MPTP-exposed offspring were 85.1, 82.9, 85.7 and 95.0% of the weights for the prenatal PBS-exposed pups. The data suggest that at birth the prenatal MPTP-exposed pups were smaller than the PBS control offspring, but the MPTP-exposed offspring gained weights at a slightly higher rate, because the deficit of 14.9% during the 1st week was reduced to 5.0% by the 4th week of age. The body weights were not significantly different during the post-weaning period and were closely alike by the 11–12 week period (figure 1B). In summary, the results show that the exposure of the fetus to MPTP (10 mg/kg), via its injection into the dams during G8–G12 resulted in pups with reduced birth weights. The MPTP exposure, however, may not affect the nurturing and development process, because the body weights for both groups were almost identical at weaning and became statistically the same by the 11–12 week period.
Figure 1.
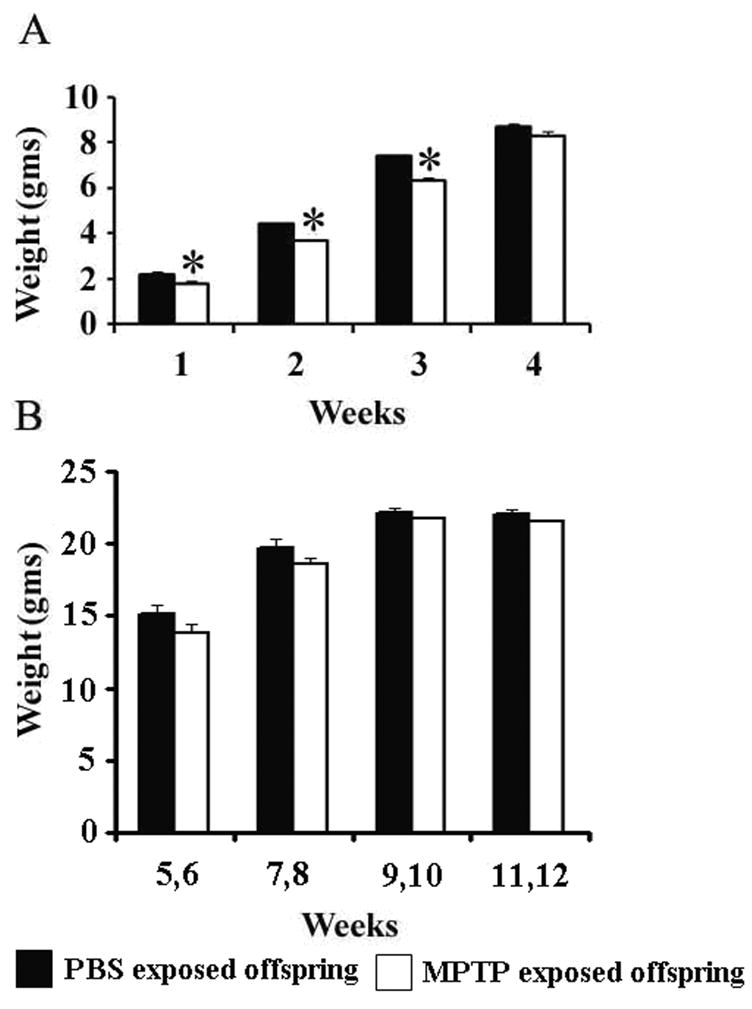
The body weights of the prenatal PBS-exposed and the prenatal MPTP- exposed C57BL/6J mouse offspring. ‘A’ shows the weekly pre-weaning weights and ‘B’ shows the two-weekly post-weaning weights. The lower birth weights of the MPTP exposed offspring seen during the first 3 weeks of the life (A) recovered in the post weaning stage (B), which suggests a normal biological state for the MPTP exposed offspring. Values are mean ± SEM of 15 pups each. P≤0.05.
2. Prenatal exposure to MPTP reduced motor activities in the adult offspring
The spontaneous motor activities of the mice offspring exposed to PBS or to 10 mg/kg of MPTP during G8-12 prenatal period were determined at 12 wks of age. The parameters measured were the total distance travel (TD), the number of movements (NM) and the movement time (MT). As shown in figure 2, the spontaneous activities of the mice were affected by the prenatal MPTP exposure, as compared to prenatal PBS exposure (figure 2, 1st pair of columns). For the PBS versus the MPTP groups, the TD were 1850 ± 62.4 cm vs 1322 ± 84.3 cm; NM: 150.9 ± 5.0 vs 123.2 ± 3.5 and MT: 182.2 ± 7.1 vs 130.8 ± 19.4, so the data show that the exposure to MPTP during the prenatal development period caused the reduction of spontaneous motor activities of the 12 weeks old adult offspring.
Figure 2.
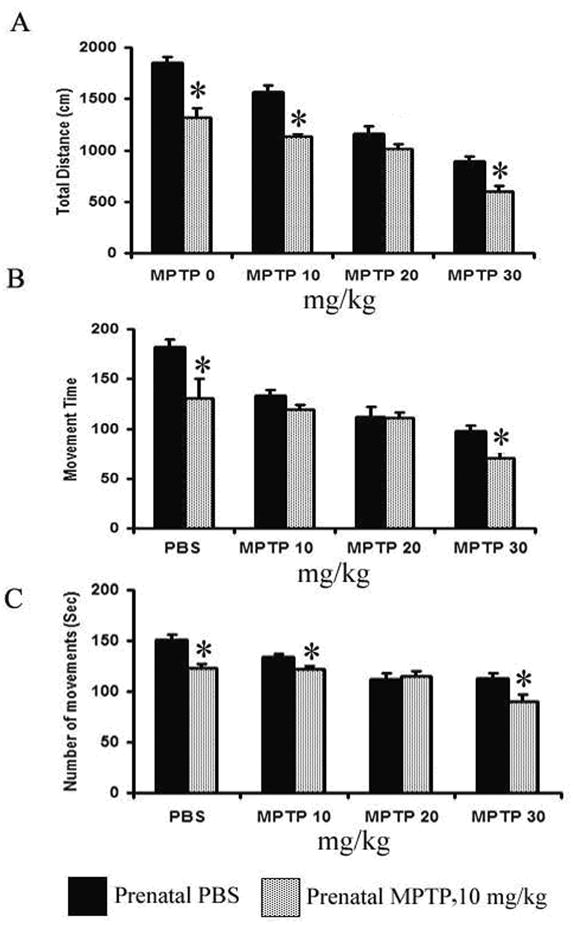
The effects of prenatal and postnatal MPTP on motor activities in C57BL/6J mice. The measures were made 24 hours after the last postnatal injection of MPTP (10, 20 or 30 mg/kg) or PBS in the 12 weeks prenatal PBS-exposed and prenatal MPTP-exposed offspring. The total distance traveled (TD, A), the number of movements (NM, B) and the movement time (MT, C) are shown and were determined using an activity monitor (Degiscan Instruments Inc., Columbus, OH, USA). Values are expressed as means ± SEM of 15 animals. Data were analyzed by using student-t test with P≤ 0.05. Dose-response effects are seen for both prenatal PBS and MPTP mice. Asterisks (*) indicate significant differences for the MPTP group from the respective PBS controls.
3. The effects of prenatal MPTP-exposure on the levels of striatal DA, DOPAC, HVA and 3-MT in the adult mouse offspring
The concentrations of striatal dopamine (DA), dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA) and 3-methoxytyramine (3-MT) were measured in the striatum of 12 week old C57BL/6J offspring that were exposed in utero to 10 mg/kg MPTP or to PBS, as the control (table 1, 3rd column). DA, HVA and 3-MT were reduced by 13.80%, 16.48% and 66.25%, respectively, in the striatum of the prenatal MPTP-exposed offspring as compared to the prenatal PBS-exposed offspring (table 1, column 3; 2nd, 4th and 5th rows). DOPAC level was statistically unchanged (table 1, column 3; 3rd row).
Table 1.
Effects of postnatal MPTP (10, 20 and 30 mg/kg) on striatial DA, DOPAC, HVA and 3-MT in offspring exposed to prenatal MPTP or prenatal PBS. The percent changes based on the population (PBS mice) levels are enclosed by brackets below the respective concentrations. The results show that postnatal MPTP was more effective in reducing DA and its metabolites in the prenatal MPTP mice. However, for the 20 and 30 mg/kg doses of MPTP the significance was masked because those doses of MPTP also reduced DA and its metabolites in the prenatal PBS offspring.
| DA and Metabolites (ng/mg protein) | Prenatal Exposure. | Postnatal MPTP Challenges (mg/kg) |
|||
|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 mg/kg | ||
| DA | PBS | 157.3 ± 17.3 [0.0] | 141.0 ± 5.50 [10.35] | 34.5 ± 1.7 [78.06] | 16.40 ± 2.0 [89.57] |
| MPTP 10mg/kg | 135.6 ± 4.80 [13.80] | 48.0 ± 7.10 [69.96] | 28.0 ± 2.0 [82.20] | 3.95 ± 1.0 [97.49] | |
| DOPAC | PBS | 5.2 ± 0.76 [0.0] | 6.00 ± 1.00 [15.38] | 3.3 ± 0.4 [36.53] | 1.95 ± 0.41 [62.5] |
| MPTP 10mg/kg | 5.9 ± 0.88 [+13.46] | 1.04 ± 0.96 [80.0] | 0.46 ± 0.58 [91.15] | 0.41 ± 0.33 [92.11] | |
| HVA | PBS | 18.2 ± 0.80 [0.0] | 17.5 ± 1.00 [3.85] | 9.84 ± 0.6 [45.93] | 6.0 ± 0.47 [67.03] |
| MPTP 10mg/kg | 15.2 ± 0.80 [16.48] | 9.4 ± 0.66 [48.35] | 8.3 ± 2.1 [54.39] | 4.7 ± 0.70 [74.17] | |
| 3-MT | PBS | 1.6 ± 0.20 [0.0] | 1.2 ± 0.15 [25.0] | 0.75 ± 12 [53.22] | 0.54 ± 0.11 [66.25] |
| MPTP 10mg/kg | 0.54 ± 0.12 [66.25] | 0.45 ± 0.11 [65.38) | 0.32 ± 0.05 [80.0] | 0.32 ± 0.06 [80.0] | |
4. Prenatal MPTP reduced Striatal Tyrosine Hydroxylase (TH) in the adult offspring
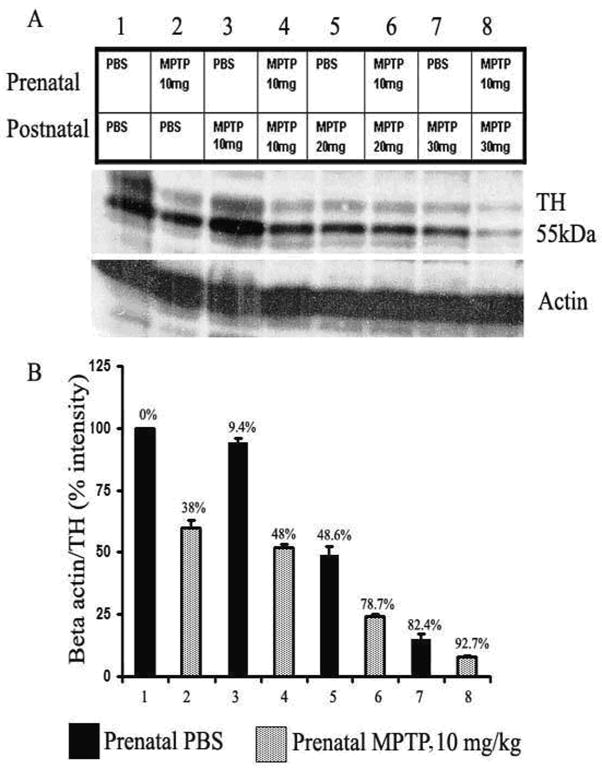
Western blot analysis of TH shows that the concentration of 10 mg/kg MPTP administered to the pregnant mice during G8-12 reduced the protein level for striatal TH by 38% in the 12 wks old offspring, when compared with the animals exposed to PBS (figure 6, lane 2 as compared to lane). The quantitative values are shown in figure 6B (column 2 versus column 1).
Figure 6.
Immunoblot and densitometric analysis of striatal TH for mice exposed in utero to PBS (blocks 1, 3, 5 and 7) or 10 mg/kg MPTP (blocks 2, 4, 6, and 8). The mice were treated postnatal at 12 weeks with PBS or 10, 20 or 30 mg/kg of MPTP. The bands indicate the quantities of TH proteins for the 4 treatment pairs. Postnatal MPTP caused dose-related reduction of TH that was more severe in the offspring that were exposed prenatally to 10 mg/kg of MPTP (columns 2, 4, 6 and 8, as compared to the matching prenatal PBS-exposed group (columns 1, 3, 5 and 7). Whereas, the 10 mg/kg of prenatal MPTP exposure reduced TH by 38 % (block 2 vs 1), the postnatal 10 mg/kg of MPTP reduced TH by only 9.4% (block 3 vs 1), which shows that the fetus is vulnerable to the MPTP toxin as compared to the 12 weeks offspring. TH density was expressed as TH/β-actin ratio with reference to total protein.
B. Postnatal MPTP challenges in offspring exposed to prenatal PBS or MPTP: Simulating the Precipitating/Inducing Stage
1. Effects of postnatal MPTP challenges on spontaneous motor activities in the prenatal MPTP-exposed and PBS-exposed offspring
The behavioral effects of 10, 20 and 30 mg/kg of postnatal MPTP or postnatal PBS were determined in mice offspring exposed in utero to PBS or 10 mg/kg of MPTP. First, note that the prenatal MPTP-exposed offspring showed a reduction in total distance (TD), movement time (MT) and number of movements (NM) as viewed in the first pair of columns in figure 2. The postnatal challenges with 3 different doses of MPTP decreased motor activities for both groups in dose-dependent manner for all parameters; the TD, MT and NM (figure 2). It should be noted further that for the TD the reductions were more severe for the offspring that were exposed to prenatal MPTP, as compared to the prenatal PBS (Figure 2, total distance), especially for the 10 and 30 mg/kg groups. Moreover, the reduction of MT and MN also were more for the prenatal MPTP-exposed offspring at the 30 mg/kg dose level of postnatal MPTP (figure 2). When the TD/NM (the average distance traveled for each movement made) was calculated it shows that for the prenatal PBS-exposed offspring, the postnatal administration of 0, 10, 20 and 30 mg/kg MPTP produced TD/NM values of 12.3, 10.0, 10.0, and 7.5 cm, as compared to the values for the prenatal MPTP-exposed offspring of 10.7, 8.3, 7.8 and 6.9 cm. The overall outcome shows, therefore, that spontaneous motor activities of the prenatal MPTP-exposed offspring, as compared to the prenatal PBS-exposed group, overall, were more affected by the postnatal MPTP challenges. The reduction in TD/NM may be relevant to the reduction in the ability to initiate movements in PD. It is of interest, also, that MPTP dose-dependently reduced motor activity in both groups, but the rate of change caused by the 10, 20 and 30 mg/kg of MPTP is steeper for the prenatal PBS group. The determination of the slopes for the lines that depict the changes produces values of 0.480, 0.325 and 0.238 for TD, MT and NM for the prenatal PBS animals as compared to 0.288, 0.127 and 0.086 for the prenatal MPTP offspring. These indicate that the mice pre-exposed to MPTP were somewhat resistant to the reduction in motor activity caused by the postnatal MPTP challenges, which suggests that a form of behavioral tolerance occurred for the mice that were exposed prenatal to MPTP. This phenomenon may be related to compensatory processes (Zigmond et al 1984; Zigmond, 1997) and may be relevant to the conservation of motor function in humans that results in a reduction of about 80% of dopamine and dopamine neurons before PD motor symptoms are seen.
2. Postnatal MPTP depletes striatal DA, DOPAC, HVA and 3-MT in offspring exposed to prenatal MPTP as compared to prenatal PBS
The study shows that the prenatal exposure to MPTP (10 mg/kg), as compare to the prenatal exposure to PBS, renders the nigrostriatal dopaminergic system in the 12 weeks old offspring more susceptible to the effects of postnatal MPTP. The postnatal administration of MPTP was used to test the susceptibility level of the basal ganglia of the offspring exposed to prenatal MPTP or prenatal PBS. The postnatal MPTP challenges also serve as the short-term way of simulating the age-related changes that precipitate PD in human.
First, the outcome shows that the postnatal MPTP administration causes dose-related changes in DA, DOPAC, HVA and 3-MT in both the prenatal PBS-exposed offspring and the prenatal MPTP-exposed group (table 1). The 10, 20 and 30 mg/kg of postnatal MPTP reduced striatal DA in the prenatal PBS offspring by 10.35, 78.06 and 89.57% of the normal control (table 1, 2nd row, upper line). The same doses of postnatal MPTP reduced DA by 69.96, 82.20 and 97.49% in the offspring exposed to prenatal MPTP (table 1, 2nd row, lower line). So, when the prenatal PBS-exposed and prenatal MPTP-exposed offspring were compared the data show that the 10 mg/kg of postnatal MPTP significantly reduced DA, DOPAC, HVA and 3-MT in the prenatal MPTP-exposed offspring (figure 3; 2nd column compared with the 4th column). Lines drawn to bridge the values for the prenatal PBS and prenatal MPTP show meaning differences for all parameters, DA, DOPAC, HVA and 3-MT (figure 3).
Figure 3.
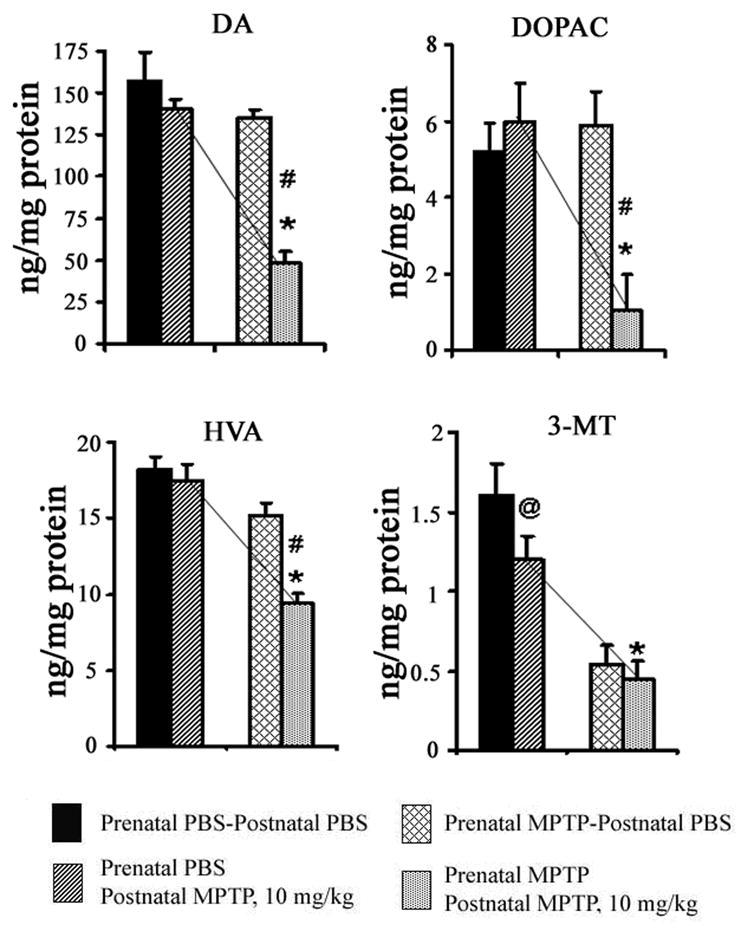
The effects of postnatal 10 mg/kg of MPTP on striatal DA, DOPAC, HVA and 3-MT in C57Bl/6J mice offspring that were exposed in utero to PBS or MPTP. The 1st pair of columns for each represents offspring exposed to prenatal PBS and received postnatal PBS (blocked columns) or 10 mg/kg MPTP (diagonally striped columns). The 2nd pair of columns represents offspring exposed to prenatal MPTP and received postnatal PBS (diagonally hatched columns) or 10 mg/kg MPTP (dotted columns). Note that the postnatal MPTP showed no significant effect in the prenatal PBS offspring (the 2nd vs the 1st columns), but the same dose of MPTP significantly reduced DA, DOPAC, HVA and 3-MT in the offspring that were exposed to prenatal MPTP (the 4th vs the 2nd columns). This highlights the susceptibility caused by the prenatal MPTP exposure. The slopes of the lines that connect column 2 and 4 dramatize the differences. Data were analyzed by using student-t test with P≤ 0.05. Asterisks (*) indicate significant differences between the prenatal PBS-postnatal MPTP mice (2nd column) vs prenatal MPTP-postnatal MPTP mice (4th column). The pound (#) sign shows that significant changes for DA, DOPAC and HVA occur when prenatal MPTP offspring were challenged with postnatal MPTP (4th column), as compared to prenatal MPTP that were exposed to postnatal PBS (3rd column). Only 3-MT was significantly reduced (@) by treating the prenatal PBS offspring with the 10 mg/kg of postnatal MPTP. The values are expressed in ng/mg protein and as mean ± SEM for 6 animals. Data were analyzed by using Student-t test.
The 20 mg/kg of postnatal MPTP reduced DA, DOPAC, HVA and 3-MT in the prenatal PBS-exposed offspring as well as the prenatal MPTP-exposed offspring (table 1 and figure 4). For example, using the population mean as the basis, DA was reduced by 78.06% in the prenatal PBS offspring and by 82.20% in the prenatal MPTP-exposed offspring (table 1). So, due to the toxicity of the 20 mg/kg dose of MPTP in the prenatal PBS-exposed offspring the comparative significance of the effects of this dose level in the prenatal PBS and prenatal MPTP mice is diminished; showing a mere 4.14% higher level of effect in the prenatal MPTP-exposed offspring (table 1). Figure 4, highlights the relative differences in the effects of the 20 mg/kg of postnatal MPTP in depleting DA and its metabolites in the prenatal PBS-exposed offspring versus the prenatal MPTP-exposed offspring. Lines drawn to bridge the key values show that meaning differences occur for only DOPAC and 3-MT (figure 4).
Figure 4.
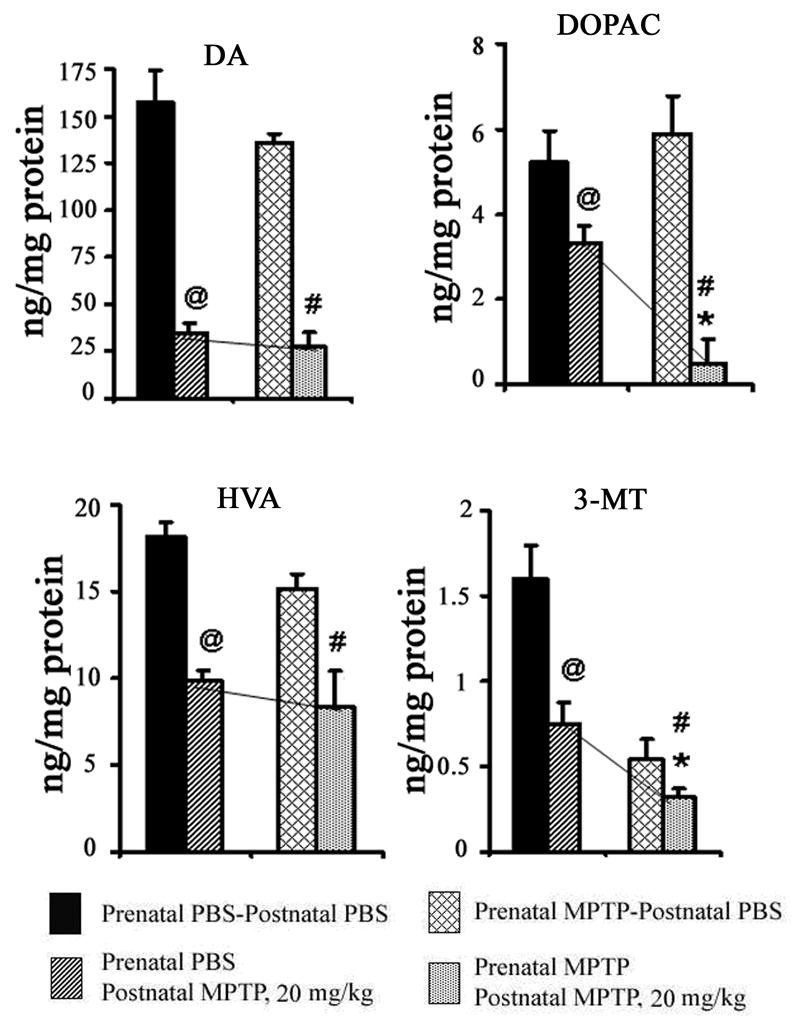
The effects of postnatal 20 mg/kg of MPTP on striatal DA, DOPAC, HVA and 3-MT in C57Bl/6J mice offspring that were exposed in utero to PBS or MPTP. The 1st pair of columns for DA, DOPAC, HVA and 3-MT represents offspring exposed to prenatal PBS and received postnatal PBS (blocked columns) or 20 mg/kg MPTP (diagonally striped columns). The 2nd pair of columns represents offspring exposed to prenatal MPTP and received postnatal PBS (diagonally hatched columns) or 20 mg/kg MPTP (dotted columns). The 20 mg/kg of postnatal MPTP significantly (@) decreased DA, DOPAC, HVA and 3-MT in the PBS-exposed offspring as shown in the 1st vs the 2nd columns. It also reduced DA, DOPAC, 3-MT and HVA in the prenatal MPTP-exposed offspring, as shown in the 3rd vs the 4th columns (#). The toxicity of the postnatal 20 mg/kg MPTP in the prenatal PBS-exposed offspring masked the relative effects of postnatal MPTP in the prenatal MPTP-exposed offspring. However, using the normal levels as the basis, DA, DOPAC, HVA and 3-MT were comparatively lower in the prenatal MPTP–exposed offspring by 4.14, 54.62, 8.46 and 26.78%, respectively, showing significance (*) for only DOPAC and 3-MT, also as illustrated by the slope of the bridging lines. P≤ 0.05. The values are expressed in ng/mg protein and as mean ± SEM for 6 animals. Data were analyzed by using Student-t test.
The 30 mg/kg of postnatal MPTP was also toxic to both prenatal PBS-exposed and prenatal MPTP-exposed groups (table 1), causing, respectively, DA reduction of 89.57% and 97.49%. The comparative outcome of the 30 mg/kg postnatal MPTP in the prenatal PBS-exposed offspring and the MPTP-exposed offspring is shown in figure 5, and draws attention to the fact that postnatal MPTP depletes DA and its metabolites to a greater extent in the prenatal MPTP-exposed offspring, however the toxicity of the 30 mg/kg in the prenatal PBS mice masked any sensitivities that may occur. Only the effects on DA and DOPAC were markedly more severe in the prenatal MPTP mice (figure 5).
Figure 5.
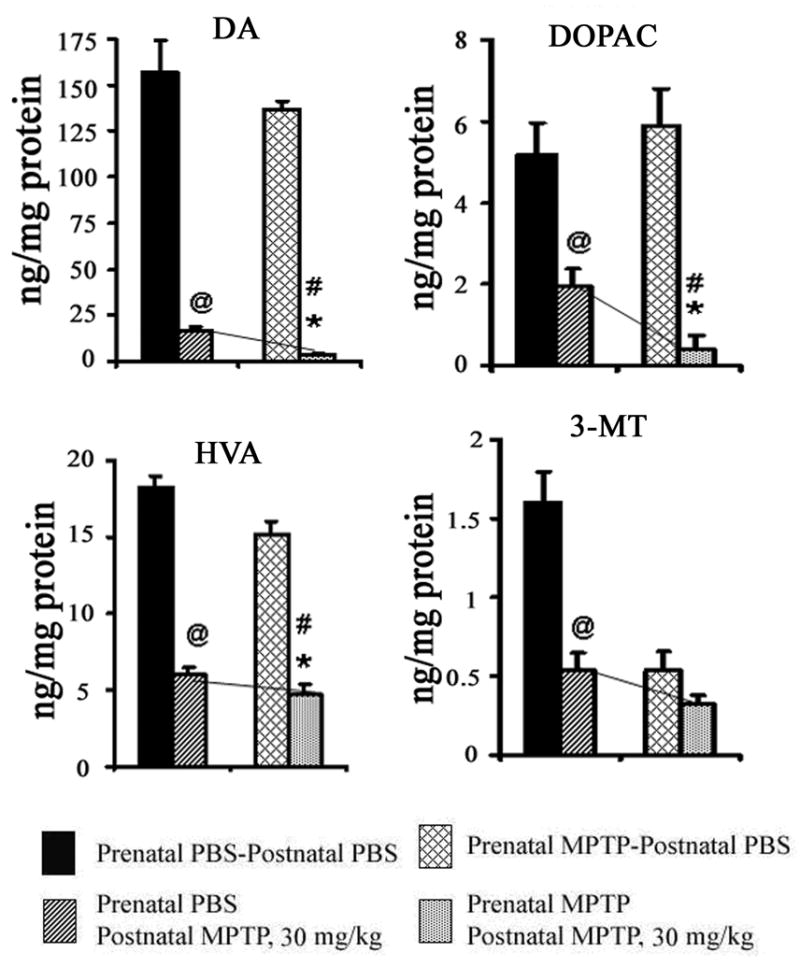
The effects of postnatal 30 mg/kg of MPTP on striatal DA, DOPAC, HVA and 3-MT in C57Bl/6J mice offspring that were exposed in utero to PBS or MPTP. The 1st pair of columns for DA, DOPAC, 3-MT and HVA represents offspring exposed to prenatal PBS and received postnatal PBS (blocked columns) or 30 mg/kg MPTP (diagonally striped columns). The 2nd pair of columns represents offspring exposed to prenatal MPTP and received postnatal PBS (diagonally hatched columns) or 30 mg/kg MPTP (dotted columns). The 30 mg/kg of postnatal MPTP significantly decreased DA, DOPAC, HVA and 3-MT in the PBS-exposed offspring as indicated by the ‘at’ sign (@) and as shown in the 1st vs the 2nd columns. It also reduced DA, DOPAC, 3-MT and HVA in the prenatal MPTP-exposed offspring, as shown in the 3rd vs the 4th columns (#). The toxicity of the postnatal 30 mg/kg MPTP in the prenatal PBS-exposed offspring masked the relative effects of postnatal MPTP in the prenatal MPTP-exposed offspring. However, using the normal levels as the basis, DA, DOPAC, HVA and 3-MT, the concentrations were comparatively lower in the prenatal MPTP–exposed offspring by 7.92, 29.61, 7.14 and 13.75%, respectively, showing significance (*) for only DOPAC, as illustrated, also, by the slope of the bridging lines. P≤ 0.05. The values are expressed in ng/mg protein and as mean ± SEM for 6 animals. Data were analyzed by using Student-t test.
3. Effects of MPTP challenges on striatal tyrosine hydroxylase expression in offspring exposed to prenatal MPTP and prenatal PBS
Figure 6 shows the effect of MPTP administration on the expression of tyrosine hydroxylase (TH) in the offspring of C57BL/6J mice. The pregnant dams were treated with PBS or 10 mg/kg of MPTP during gestation day 8–12 (G8-G12) to target the nigrostriatal TH containing dopaminergic system. The prenatal MPTP reduced striatal TH by 38% (figure 6, column 2 versus 1). Shown also in figure 6 are the effects of 0, 10, 20 and 30 mg/kg of MPTP administered in the 12 weeks old offspring that were exposed to prenatal PBS (filled columns 1, 3, 5 and 7) or to prenatal MPTP (striped columns 2, 4, 6 and 8). The postnatal MPTP caused dose-dependent reduction of TH in both the prenatal PBS-exposed and prenatal MPTP-exposed offspring. In addition, the challenges with 10, 20 and 30 mg/kg of MPTP, administered to the prenatal PBS-exposed 12 weeks old offspring reduced striatal TH by 9.4%, 48.6% and 82.4%, respectively (figure 6, lanes 3, 5, 7). In the offspring that were exposed to prenatal MPTP the same MPTP doses reduced striatal TH by 48.%, 78.7% and 92.7%, respectively, (figure 6, lanes 4, 6, 8). The outcomes show that the administration of the 10, 20 and 30 mg/kg of postnatal MPTP caused 38.6, 30.1 and 10.3% higher percentage of toxicity to TH in the offspring that were exposed to prenatal MPTP as compared to the offspring exposed to prenatal PBS. So, as reported earlier for the changes in DA and its metabolites (table 1), the lower postnatal dose of 10 mg/kg of MPTP was comparatively more discriminatory (independently reducing TH by 38.6%), because that dose level caused only slight reduction of striatal TH in the control offspring exposed to prenatal PBS. On the other hand the 20 and 30 mg/kg of MPTP appreciable reduced TH in both the prenatal PBS-exposed and the prenatal MPTP-exposed offspring, which diminishes the effectiveness of the doses to 30.1 and 10.3% reduction of TH in the offspring exposed to prenatal MPTP. It is of interest also that the 10 mg/kg dose of MPTP showed greater effect in reducing TH by 38% in the offspring (figure 6, #2) when it was administered prenatally to the pregnant dams, whereas, the administration of the same dose to the 12 weeks old prenatal PBS offspring caused only 9.4% reduction in TH (figure 6, #3). These outcomes show that the fetus is sensitive to the toxic effects of MPTP and that the concentration of chemical agents, like MPTP, that are apparently safe in the postnatal normal population could be very harmful during the fetal stage.
DISCUSSION
MPTP causes parkinsonism (Davis et al., 1979; Langston et al., 1983; Burns et al., 1983; Schneider et al., 1983; and Hallman, et al., 1984), by impairing and killing dopaminergic cells (Wesemann et al., 1993; Kowall et al., 2000; Chassain et al., 2001; Przedborski et al., 2001; Tillerson et al., 2002). So, MPTP was used in this study to target the nigrostriatal (NS) dopamine (DA) neurons during their development in the mouse fetus. The goal was to cause subtle changes to the neuronal system that will make the dopaminergic neurons susceptible to toxic changes that occur later in life. We also used MPTP, administered to the adult offspring at 12 weeks of age, as the postnatal toxin. In that way MPTP also serves as the short-term changes that mimic the phenomenon in aging that precipitates PD. So, in essence the study tests the hypothesis that two stages are involved in PD; (1) a sensitization stage that occurs early in life, causing subtle harm to the nigrostriatal system so that they become susceptible or vulnerable and (2) the second stage, which is the precipitating or inducing stage that occurs later in life when additional interventions cause further or additional harm to the NS dopaminergic system equivalent to the level of changes that cause PD in human.
The results obtained are indicative of a positive outcome for the studies. First, the toxicological outcome shows that postnatal MPTP caused a dose-related reduction of tyrosine hydroxylase (figure 6) as well as DA, DOPAC, HVA and 3-MT in the striatum (table 1). More importantly, the studies support the hypothesis. The results showed that the prenatal exposure to MPTP caused no major visible impairments or abnormalities to the offspring, but there were appreciable reduction in TH expression and in DA and its metabolites in the striatum, the site that is also impaired in PD. Furthermore, when the adult mice offspring were challenged with MPTP to mimic the precipitating stage further impairment in TH, DA and its metabolites occurred; that is, the offspring that were exposed to prenatal MPTP, as compared to prenatal PBS, were more seriously affected. These sets of results related to DA, DOPAC, HVA and 3-MT are summarized in table 1, and show that the exposure to prenatal MPTP makes the NS susceptible to the postnatal administration of MPTP in the adult offspring.
It was of interest that the 10 mg/kg dose of MPTP resulted in the more significant differences between the prenatal MPTP-exposed and the prenatal PBS-exposed offspring, because that dose level of 10 mg/kg of MPTP showed no significant effect in the prenatal PBS-exposed offspring, but in the MPTP-exposed offspring the same dose of 10 mg/kg of MPTP caused the reduction of DA, DOPAC, HVA and 3-MT by 69.96, 80.0, 48.35 and 65.38% of the population value (table 1). The table also shows that the dose levels of 20 and 30 mg/kg of MPTP were more toxic in the prenatal MPTP-exposed offspring, but appreciable reduction of DA, DOPAC, HVA and 3-MT also occurred in the prenatal PBS-exposed offspring (table 1). A comparatively more severe effect was also shown for TH (figure 6). These observation are indeed important, because the data show that the low concentration of a toxin, in this case the 10 mg/kg of MPTP, that does not harm the nigrostriatal neurons in normal individuals will harm the same set of cells in individuals in which the nigrostriatal dopaminergic neurons were made sensitive. So, prenatal sensitization of neurons, as a general rule, may be a major underpinning that endows the severity of response to a neurotoxin. When the concentrations are calculated as a percentage of the value for the normal population (the PBS mice) the dose response effects of MPTP are clearly seen for all parameters. For example, the 10, 20 and 30 mg/kg dose levels of postnatal MPTP reduced DA by 69.49, 82.20 and 97.49%, but the 20 and 30 mg/kg dose levels of MPTP also reduced DA in both the PBS and the MPTP groups, Using the high doses, therefore, masked the sensitization that were developed in the prenatal-MPTP exposed offspring. So, the 10 mg/kg dose level of MPTP and lower are the preferred concentrations for testing vulnerability. So, overall, the studies support the hypothesis that a fetally occurred predisposing/sensitization condition may exist for PD and that postnatal stress imposed on the basal ganglia later in life can cause additive deleterious effects that precipitates the symptoms of PD.
The recovery of body weights in the MPTP-exposed offspring indicates that these animals were not adversely harmed. In fact, empirical observation shows that the MPTP-exposed offspring were apparently physically normal. The lower birth weight for the MPTP exposed pups and the slight reduction in spontaneous motor activity, as compared to the PBS control, though, may be subtle external markers of early impairment of the NS system, that may be also true for human, especially since the 10 mg/kg of MPTP also caused a reduction of TH, DA and its metabolites in the striatum. The results also show that in utero exposure to MPTP, at a concentration that has no visual toxic effect on the dams, has long-lasting effect in the offspring.
Conclusion
The results are apparently a positive outcome for the hypothesis that was tested in these experiments. The objective of the studies was to test the hypothesis that two sets or two stages of impairments are involved in neurodegenerative disorder, such as Parkinson’s disease (PD). To produce the first stage, MPTP was administered to pregnant mice during the period of the differentiation of the basal ganglia dopamine neurons. The intent was to allow MPTP to cause sub-threshold impairments to the basal ganglia dopaminergic system. For the second stage, the prenatal MPTP exposed offspring were challenged with MPTP, to determine whether MPTP would be more toxic in the prenatal MPTP-exposed offspring to the point of causing changes equivalent to those that occur in parkinsonism. Thus, the MPTP challenge would mimic, in a short period of time, the age-related and protracted changes that cause PD in the adult human. The results show that prenatal MPTP exposure caused sub-threshold deficiencies to the nigrostriatal dopamine neuronal system. This is an apparently positive test for the hypothesis and representing the first stage of affliction, the susceptible, sensitizing or vulnerable stage. The severe toxicity caused by the postnatal MPTP challenge in the prenatal MPTP-exposed offspring, furthermore, seems to represent the second stage of affliction, the precipitating or inducing stage. Together, both stages may serve as a model for the cause of PD and other neurodegenerative disorders. Even in PD disorder with a genetic basis, such as ‘Juvenile PD’ that has its onset at about 40 years, the sensitization-precipitation hypothesis will stand, because at about 25 years of age the patient that developed Juvenile PD was free from the symptoms of the disorder, and was merely predisposed.
So, if it turns out that these two stages are involved in the cause of PD, it means that protective measures for the basal ganglia will be beneficial in utero and that postnatal measures such as antioxidants to protect the vulnerable neurons may be useful during postnatal development and aging. The postnatal neuroprotective measures may be useful also in Juvenile PD, since Juvenile PD is also an age-related disorder that manifests its symptoms at about 40 years of age in a patient that was apparent healthy at 25 years.
The study shows that TH was reduced by 38% by the prenatal MPTP exposure, but the same dose of MPTP, when given postnatally, reduced TH by only 9.4% in the offspring. This outcome points to the sensitivity of the fetus to toxin, as compared to the adults. The 38% reduction in striatal TH was much greater than the 13.80% reduction in striatal DA, which suggests that homeostasis and compensatory adjustment in the production and storage of striatal DA may have occurred. Our preliminary histological studies indicate that the prenatal MPTP caused the failure of some larger nigrostriatal DA neurons to assemble into the compacta zone of the nigrostriatum (Charlton, unpublished results), which may have resulted in the failure of some DA neuronal axons to reach the striatum. These could occur as a result of the reduction in cytoskeleton proteins by prenatal MPTP that was observed (Muthian et al., SfN Abstract, 2009). Since in the normal adult mice the low dose of 10 mg/kg of MPTP did not cause serious toxicity, a major lesson that may be learned from this study, also, is that dose levels of an agent thought to be too low to cause toxic effects in the adult may cause serious effects if exposure occurred prenatally, rendering vulnerable neurons sensitive to exposure to the same low levels that may be encountered later in life.
Acknowledgments
This work was supported by NIH grant # RO1NS041674 and NIH R21NS049623.
List of abbreviations Used
- CNS
Central Nervous System
- DA
Dopamine
- DOPAC
3,4-Dihydroxyphenylacetic acid
- EC
Electro Detection
- 5-HIAA
5-Hydroxy indole acetic acid
- 5-HT
-5-Hydroxytryptamine
- HVA
Homovanillic acid
- HPLC
High Performance Liquid Chromatography
- LPS
Lipopolysaccharide
- MPP+
-1-Methyl-4-phenyl pyridnium
- MPTP-
1- Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- 3-MT
3-Methoxytyramine
- MT
Movement time
- NM
Number of Movements
- NS DA
Nigrostriatal dopamine
- PBS
Phosphate Buffered Saline
- PD
Parkinson’s disease
- TCA
Trichloro acetic acid
- TD
Total distance
- TH
Tyrosine hydroxylase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aoyama K, Matsubara M, Kondo Y, Murakawa M, Suno K, Yamaguchi S. N- Methylation ability for azaheterocyclic amines in higher in Parkinson’s disease: Nicotinamide loading test. J Neural Transm. 2000;107:985–995. doi: 10.1007/s007020070047. [DOI] [PubMed] [Google Scholar]
- Aziz MH, Agarwal AK, Adhami VM, Ali MM, Baig MA, Seth PK. Methanol induced neurotoxicity in pups exposed during lactation through mother: role of folic acid. Neurotoxicol Teratol. 2002;24:519–27. doi: 10.1016/s0892-0362(02)00231-3. [DOI] [PubMed] [Google Scholar]
- Bagetta G, Corasaniti MI, Iannone M, Nistico G, Stephenson JD. Production of limbic motor seizures and brain damage by systemic and intracerebral injections of paraquat in rats. Pharmacol Toxicol. 1992;71:443–48. doi: 10.1111/j.1600-0773.1992.tb00575.x. [DOI] [PubMed] [Google Scholar]
- Burns RS, Chiueh CC, Marley SP, Elbert MH, Jacobowitz DM, Kopin IJ. A primate model of Parkinsonism: Selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N – methyl-4- phenyl- 1, 2, 3, 6- tetrahydropyridine. Proc Natl Acad Sci USA. 1983;80:4546–4550. doi: 10.1073/pnas.80.14.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calne DB, Langston JW. A etiology of Parkinson’s disease. Lancet. 1983;2:1457–1459. doi: 10.1016/s0140-6736(83)90802-4. [DOI] [PubMed] [Google Scholar]
- Chassain C, Eschalier A, Durif F. Assessment of motor behavior using a video system and a clinical rating scale in parkinsonian monkeys lesioned by MPTP. J Neurosci Methods. 2001;111:9–16. doi: 10.1016/s0165-0270(01)00425-3. [DOI] [PubMed] [Google Scholar]
- Chung KKK, Thomas B, Li X, Pletnikova O, Troncoso JC, Marsh L, Dawson VL, Dawson TM. S-Nitrosylation of parkin reugulates ubiquitination and compromises parkin’s protective function. Science. 2004;28(304):1328–1331. doi: 10.1126/science.1093891. [DOI] [PubMed] [Google Scholar]
- Davis GC, Williams AC, Markey SP, Elbert MH, Caine ED, Reichert CM, Kopin IJ. Chronic Parkinsonism secondary to intravenous injection of meperidine analogues. Psychiatry Res. 1979;1:249–254. doi: 10.1016/0165-1781(79)90006-4. [DOI] [PubMed] [Google Scholar]
- Di Monte DA. The environment and Parkinson’s disease is the nigrostriatal system preferentially targeted by neurotoxins? Lancet Neurol. 2003;2:531–538. doi: 10.1016/s1474-4422(03)00501-5. [DOI] [PubMed] [Google Scholar]
- Hallman H, Olson L, Jonsson G. Neurotoxicity of the meperidine analog N- methyl -4- phenyl – 1,2,3, 6 – tetrahydropyridine on brain catecholamine neurons in mouse. Eur J Pharmacol. 1984;97:133–136. doi: 10.1016/0014-2999(84)90521-1. [DOI] [PubMed] [Google Scholar]
- Heikkila RE, Sonsalla PK. The MPTP treated mouse as a model of Parkinsonism: How good is it? Neurochem Int. 1992;20:299s–303s. doi: 10.1016/0197-0186(92)90256-q. [DOI] [PubMed] [Google Scholar]
- Jackson- Lewis V, Jakowec M, Burke RE, Przedborski S. Time course and morphology of dopaminergic neuronal death caused by the neuro toxin 1- methyl- 4- phenyl- 1.2.3, 6 – tetrahydropyridine. Neurodegeneration. 1995;4:257–269. doi: 10.1016/1055-8330(95)90015-2. [DOI] [PubMed] [Google Scholar]
- Jarvis MF, Wagner GC. Age-dependent effects of 1-methyl-4-phenyl-1,2,5,6- tetrahydropyridine (MPTP) Neuropharmacology. 1985;24(6):581–3. doi: 10.1016/0028-3908(85)90068-1. [DOI] [PubMed] [Google Scholar]
- Jenner PHV, Shapira A, Marsden CD. New insight in to the cause of Parkinson’s disease. Neurol. 1992;42:2241–2250. doi: 10.1212/wnl.42.12.2241. [DOI] [PubMed] [Google Scholar]
- Kanthasamy AG, Kitazawa M, Kanthasamy A, Anantharaman V. Dieldrin- induced neuro toxicity: relevance to Parkinson’s disease pathogenesis. Neurotoxicol. 2005;26:701–19. doi: 10.1016/j.neuro.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Kennedy JL, Farrer LA, Andreason NC, Mayeux R, St George Hyslop P. The genetics of adult- onset neuropsychiatric disease: complexities and conundra? Science. 2003;302:822–826. doi: 10.1126/science.1092132. [DOI] [PubMed] [Google Scholar]
- Kowall NW, Hantraye P, Brouillet E, Beal MF, McKee AC, Ferrante RJ. MPTP induces alpha– synuclein aggregation in the substantia nigra of baboons. NeuroReport. 2000;11:211–213. doi: 10.1097/00001756-200001170-00041. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Sonawane B, Butler R, Leonardo T Rasande, Callan R, Droller D. Early environmental origins of neurodegenerative disease in latter life. Environ Health Perspectives. 2005;113:1230–1233. doi: 10.1289/ehp.7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Tetrub JW, Irvin I. Chronic Parkinsonism in humans due to a product of meperidine- analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Lidow MS. Consequences of prenatal cocaine exposure in nonhuman primates. Dev Brain Res. 2003;147:23–36. doi: 10.1016/j.devbrainres.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Ling Z, Gayle DA, Ma SY, Lipton JW, Tong CW, Hong JS, Carvey PM. In utero bacterial endotoxin exposure causes loss of tyrosine hydroxylase neurons in the postnatal rat midbrain. Mov Disord. 2002;17:116–124. doi: 10.1002/mds.10078. [DOI] [PubMed] [Google Scholar]
- Lloyd SA, Faherty C, Smeyne RJ. Adult and in utero exposure to cocaine alters sensitivity to the parkinsonian toxin 1- methyl-4 – phenyl-1, 2, 3, 6-tetrahydropyridine. Neurosci. 2006;137:905–913. doi: 10.1016/j.neuroscience.2005.09.035. [DOI] [PubMed] [Google Scholar]
- Markey SP, Weisz A, Bacon JP. Reduced paraquat does not exhibit MPTP- like neurotoxicity. J Anal Toxicol. 1986;10:257–61. doi: 10.1093/jat/10.6.257. [DOI] [PubMed] [Google Scholar]
- Muthian G, Raikwar HP, Rajasingh J, Bright JJ. 1, 25 Dihydroxyvitamin D3 modulates JAK-STAT pathway in IL-12/IFN gamma axis leading to TH1 response in experimental allergic encephalomyelitis. J N eurosci Res. 2006;15:1299–309. doi: 10.1002/jnr.20826. [DOI] [PubMed] [Google Scholar]
- Muthian G, King J, Mackey C, Charlton CG. The reduction in cytoskeleton proteins may underlie the sensitization of basal ganglia neurons in Parkinson’s disease Program # 731.22. Society for Neuroscience. 2009 (Abstract) [Google Scholar]
- Nagatsu I, Karasawa N, Kondo Y, Inagaki S. Immunohistochemical localization of tyrosine hydroxylase, dopamine –beta hydroxylase and phenylethanolamine- N-methyltransferase in the adrenal glands of the frog and rat by a peroxidase – antiperoxidase method. Histochemistry. 1979;4:131–144. doi: 10.1007/BF00490094. [DOI] [PubMed] [Google Scholar]
- Perry TL, Young VW, Wall RA, Jones K. Paraquat and two endogenous analogues of the neurotoxic substance N- methyl-4-phenyl-1,2,3,6- tetrahydropyridine do not damage dopaminergic nigrostriatal neurons in the mouse. Neurosci Lett. 1986;69:285–89. doi: 10.1016/0304-3940(86)90495-7. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Jackson-Lewis V, Naini AB, Jakowec M, Petzinger G, Miller R, Akram M. The parkinsonian toxin 1 methyl – 4 phenyl- 1,2,3,6 tetrahydropyridine MPTP: a technical review of its utility and safety. J Neurochem. 2001;76:1265–1274. doi: 10.1046/j.1471-4159.2001.00183.x. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Yuwiler A, Markham CH. Production of Parkinson-like syndrome in the cat with N- methyl-4- phenyl-1,2,3,6- trtrahydropyridine. Proc Natl Acad Sci USA. 1983;80:293–307. doi: 10.1016/0014-4886(86)90070-1. [DOI] [PubMed] [Google Scholar]
- Tillerson JL, Caudle WM, Reveron ME, Miller GW. Detection of behavioral impairments correlated to neuro chemical deficits in mice treated with moderate doses of 1- methyl-4 phenyl-1, 2, 3, 6 tetrahydropyridine. Exp Neurol. 2002;178:80–90. doi: 10.1006/exnr.2002.8021. [DOI] [PubMed] [Google Scholar]
- Tipton KF, Singer TP. Advances in our understanding of the mechanism of the neurotoxicity of MPTP and related compounds. J Neurochem. 1993;61:1191–206. doi: 10.1111/j.1471-4159.1993.tb13610.x. [DOI] [PubMed] [Google Scholar]
- Wesemann W, Grote C, Clement HW, Block F, Sontag KH. Functional studies on monoaminergic transmitter release in Parkinsonism. Prog Neuropsychopharmacol Biol Psychiatry. 1993;17:487–499. doi: 10.1016/0278-5846(93)90081-3. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, Acehson AL, Stachowiak MK, Striker EM. Neurochemical compensation after nigrostriatal bundle injury in an animal model of preclinical Parkinsonism. Arch Neurol. 1984;41:856–861. doi: 10.1001/archneur.1984.04050190062015. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ. Do compensatory processes underlie the preclinical phase of neurodegenerative disease? Insights from an animal model of parkinsonism. Neurobiol Dis. 1997;4:247–253. doi: 10.1006/nbdi.1997.0157. [DOI] [PubMed] [Google Scholar]