Abstract
mTOR, the mammalian target of rapamycin, is a serine-threonine kinase known to regulate cell proliferation and growth. mTOR has also been implicated in neuronal synaptic plasticity as well as in pain transmission in models of chemically induced and neuropathic pain. To date, the role of mTOR as a modulator of inflammatory pain has not been examined. In this study, we investigated the role of mTOR in Sprague Dawley rats using the carrageenan model of inflammatory pain. mRNA of Ras homolog enriched in brain (Rheb), a GTPase that positively regulates mTOR activation, was significantly increased 2 hours following carrageenan injection. Four hours after induction of inflammation phosphorylation (p) of p70S6 kinase (S6K), ribosomal protein S6 (S6) and eukaryotic initiation factor 4E-binding protein 1 (4E-BP1) was increased, indicating mTOR activation. Inhibition of spinal mTOR with intrathecal (i.t.) injection of rapamycin (0.1–3 μg) led to a dose-dependent decrease in carrageenan-induced thermal hyperalgesia and a reduction of mechanical allodynia. In vitro studies confirmed rapamycin inhibition of the mTOR pathway. Carrageenan-induced activation of the mTOR pathway in rats was localized predominantly to dorsal horn neurons in the superficial lamina. Taken together, these data show that the mTOR pathway is activated in dorsal horn neurons during inflammatory pain, and that inhibition of spinal mTOR attenuates inflammation-induced thermal and tactile hypersensitivity. Hence, our study indicates that spinal mTOR is an important regulator of spinal sensitization and suggests that targeting mTOR may provide a new avenue for pain therapy.
Increased sensitivity to both noxious and non-noxious stimuli is a hallmark of persistent pain states following tissue injury and inflammation. This hypersensitivity is associated with both peripheral and spinal neuronal plasticity (Hunt and Mantyh, 2001; Woolf, 2007), leading to a reduction of activation threshold in peripheral nociceptive sensory neurons, as well as an increase in the synaptic activity between sensory nerve endings and second-order neurons in the dorsal spinal cord. It is also clear that spinal glia such as microglia and astrocytes can modulate pain transmission (McMahon et al., 2005; Milligan and Watkins, 2009).
The serine-threonine kinase mammalian target of rapamycin (mTOR) is the core of an important intracellular signaling hub that regulates cell growth, cell proliferation and synaptic plasticity downstream of multiple stimuli such as glutamate, growth factors and cytokines (Gingras et al., 1999; Takei et al., 2001; Hay and Sonenberg, 2004; Lenz and Avruch, 2005). mTOR is comprised by two distinct protein complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2) (Laplante and Sabatini, 2009). mTORC1 and mTORC2 are characterized by different protein components and cell functions. mTORC1 activity, which is the focus of the current study, is modulated by the small GTPase Rheb. Once activated, Rheb binds and sequesters the endogenous inhibitor of mTOR, FKBP-38, resulting in mTORC1 activation (Bai et al., 2007). mTORC1 has several downstream targets and phosphorylation of S6K and 4E-PB1, as well as S6, the substrate of S6K, are commonly used for assessment of mTOR activation. 4E-BP1 and S6K are involved in the regulation of cell physiology through modulation of protein synthesis (Jaworski and Sheng, 2006). 4E-BP1 inhibits the cap-binding translation initiation factor eIF4E from interacting with other elongation factors, which is a key regulatory process in translation. mTOR-mediated phosphorylation of 4E-BP1 releases this inhibition, allowing translation initiation to proceed. S6K-mediated phosphorylation of S6 promotes unwinding and initiation of translation of a subgroup of messenger RNAs (mRNAs) called 5′ terminal oligopyrimidine tract (TOP) mRNAs. TOP mRNAs encode for ribosomal proteins and elongation factors 1a and 2, which are important in translational control (Ma and Blenis, 2009). Thus, mTOR regulates protein translation through multiple factors. Of importance, the mTOR-regulated translation machinery is ubiquitously expressed in the central nervous system and is present both in neurons and glia (Tang et al., 2002; Uhlmann et al., 2004; Codeluppi et al., 2009).
mTOR has recently been linked to pain processing. mTOR, S6 and 4E-BP1 are mainly expressed and constitutively phosphorylated in myelinated A-fibers in the peripheral nerve and dorsal roots, though a small number of C-fibers also express mTOR (Jimenéz-Diaz et al., 2008; Géranton et al., 2009). In addition, inhibition of mTOR activity through intraplantar injection of rapamycin, an mTOR-specific inhibitor, blocks hypersensitivity evoked by local injection of capsaicin or formalin, as well as neuropathic pain induced by spinal nerve ligation (Jimenéz-Diaz et al., 2008; Price et al., 2007). These findings suggest that mTOR-mediated local protein translation is a key component in peripheral sensitization. Shortly after current work was submitted for publication, Géranton et al. (2009) reported that mTOR, S6 and 4E-BP1 are constitutively phosphorylated in dorsal horn projection neurons and glial cells and that injection of capsaicin to the paw evokes an increase in the number of p-S6 immunoreactive neurons. Blocking mTOR activity in the spinal cord through i.t. injection of rapamycin attenuates formalin, capsaicin and nerve ligation-induced nociceptive behavior (Price et al., 2007; Asante et al, 2009; Géranton et al., 2009) and direct application of rapamycin on the spinal cord reduces formalin-induced neuronal Aδ and C-fiber activity during electrophysiological recordings from lamina V wide dynamic dorsal horn neurons (Asante et al. 2009). Thus, mTOR also appears to play a critical role in spinal sensitization via the regulation of neuronal protein synthesis. While no direct link has been made between pain processing and mTOR activation in spinal glia, it has been shown that astrocyte hypertrophy and proliferation in response to spinal cord injury is attenuated by rapamycin treatment, suggesting mTOR activity may also regulate glial function (Codeluppi et al, 2009).
To date, the role of spinal mTOR has not been examined in pain models driven by pronounced inflammation. Therefore, the aims of the current work were to i) assess if the mTOR signaling pathway is activated in the spinal cord in the carrageenan model of inflammation-induced pain, ii) evaluate the effects of mTOR inhibition on inflammation-induced hypersensitivity and iii) determine the cellular location of mTOR activity in the spinal cord. We found that peripheral inflammation drives activation of the mTOR pathway in spinal neurons and that inhibition of spinal mTOR attenuates inflammatory pain.
EXPERIMENTAL PROCEDURES
Animals
All experiments were carried out according to protocols approved by the Institutional Animal Care Committee of the University of California, San Diego and the local Ethical Committee for animal experiments (Stockholms Norra Djurförsöksetiska Nämnd).
Male Holtzman and Scanbur Sprague-Dawley rats (250–350g) were housed in standard cages (4–5 rats/cage) and maintained on a 12-h light/dark cycle with free access to food and water. To permit bolus i.t. drug delivery, chronic lumbar i.t. injection catheters (single lumen PE-5, 8.5 cm in length) were implanted through a cisternal exposure under isoflurane anesthesia (2–4%) and externalized as previously described (Yaksh and Rudy, 1976). The rostral end of each cannula was heat fixed to PE-10 tubing that was externalized on the head for bolus injection. Studies involving rats with chronic single lumen injection catheter were undertaken 4–5 days after surgery. Rats were monitored daily and removed from the study if any neurological dysfunction was noted, if there was greater than 10% weight loss over 5 days or if the catheter became occluded. Fewer than 5% of the animals prepared were so excluded.
Drug administration
Rapamycin was delivered via i.t. catheter in 10 μl vehicle followed by 10 μl saline flush. Rapamycin was dissolved in 0.5% ethanol in saline and sonicated prior to i.t. injection.
Carrageenan-induced inflammation and behavioral testing
To induce a state of local inflammation, carrageenan (lambda, Sigma, St. Louis, MO, USA; 100 μl of 2% solution (w/v) in physiological saline) was injected subcutaneously into the plantar surface of the left hind paw of a rat under brief isoflurane anesthesia. Heat-evoked paw withdrawal response, was assessed using a device similar to that described by Hargreaves et al., (1988). The apparatus consists of a glass surface (maintained at 25 °C) on which the rats are placed individually in Plexiglas cubicles. The thermal nociceptive stimulus originates from a focused projection bulb positioned below the glass surface. A timer is actuated by the light source, and latency was defined as the time required for the paw to show a brisk withdrawal as detected by phosphodiode motion sensors that stopped the timer and terminated the stimulus. Basal paw withdrawal latencies were assessed at time = −60 min. At time = −15 min the animals received i.t. vehicle or rapamycin and the carrageenan was injected in the hind paw at time = 0 min. Withdrawal latencies were then assessed at time = 90, 120, 150, 180, 240 and 360 min. The data were also presented as area under the curve (AUC), a calculation that defines the magnitude of carrageenan-induced sensitization. AUC represents the area under the time-effect curve after stimulation, in which the percent reduction from baseline (e.g., pre-carrageenan) response latency is plotted against time. The resulting metric is % change × min. The formula for calculating the percent change is (baseline latency – post drug latency) × 100 (baseline latency)−1, where latency is expressed in seconds. Increasing values indicate increasing hyperalgesia.
For assessment of tactile allodynia (mechanical probability withdrawal threshold), rats were placed in individual Plexiglas compartments with wire mesh bottoms. Following a 30 min acclimation period, mechanical allodynia was assessed using von Frey filaments and the Dixon up-down method as previously described (Chaplan et al., 1994). Briefly, calibrated filaments (Stoelting, Wood Dale, IL, USA) with buckling forces between 0.41 and 15.2 g were applied perpendicular to the mid-paw plantar surface until the filament was slightly bent and held there for 4–6 s. Stimuli were separated by several seconds or until the animal was calm with both hind paws placed on the grid. A positive response was noted if the paw was sharply withdrawn. Testing always began with the 2.0-g filament. The 50% probability withdrawal threshold was determined and data are reported as such. Tests were performed on animals prior to and 60, 120, 150, 180, 240 and 360 min after injection of carrageenan. These data are also expressed as AUC, see above.
Western Blotting
To investigate activation of S6K, S6 and 4E-BP1 in the spinal cord and dorsal root ganglia (DRGs), rats were injected with 2% carrageenan in the plantar (University of California, San Diego) or dorsal (Karolinska Institutet) side of the left hind paw. No difference in phosphorylation pattern of S6K, S6 or 4E-BP1 was observed between the different sites of carrageenan injection (data not shown). Spinal cords and DRGs were harvested in naïve animals and at 1, 2, 4, 8, 12, 18 and 24 hours after injection of carrageenan. To collect the tissue the animals were deeply anesthetized with isoflurane, decapitated and the ipsilateral lumbar spinal cord and DRGs quickly dissected and transferred into eppendorf tubes containing iced 50 mM Tris buffer (pH 8.0, 0.5% Triton X-100, 150 mM NaCl, 1 mM EDTA, protease inhibitor and phosphatase inhibitor cocktail 1 and 2) (1:100, Sigma) and homogenized by sonication. Tissue extracts were denatured and subjected to NuPAGE 4–12% Bis-Tris gel electrophoresis and then electrophoretically transferred to nitrocellulose membranes (Osmonics, Inc., Minnetonka, MN, USA). After blocking nonspecific binding sites with 5% non-fat milk in tris-buffered saline (TBS; pH 7.4; Sigma) containing 0.1% Tween 20 for 1 hour at room temperature, membranes were incubated with primary antibody overnight at 4° C. Following 3 consecutive 5 minute washes, the antibody protein complexes were probed with secondary antibody conjugated to horseradish peroxidase for 1 hour at room temperature and detected with chemiluminescent reagents (Supersignal, Pierce Biotechnology, Inc., Rockford, IL, USA). The nitrocellulose membranes were stripped with the Re-Blot Western blot recycling kits (Chemicon, Temecula, CA, USA) and re-blotted with other primary antibodies. Primary antibodies were directed against phosphorylated mouse S6K (thr-389) (1:2000, cat. no. 9234), rabbit S6 (ser-235/236) (1:500, cat. no. 2211), rabbit 4E-BP1 (thr-37/46) (1:1000, cat. no. 2855), total rabbit S6K (thr-389) (1:2000, cat. no. 2708), total mouse S6 (1:1000, cat. no. 2317) and total rabbit 4E-BP1 (1:1000, cat. no. 9452) (Cell Signaling Technology, Beverly, MA, USA), as well as against mouse β-actin (1: 4000; Sigma, cat. no. A5441 or 1:5000, Cell Signaling Technology, cat. no. 3700) as loading control. Immunopositive bands were quantified using Quantity One software (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Data for p-S6K, p-S6 and p-4E-BP1 are normalized to their individual β-actin and compared to tissue samples from naïve control animals.
Immunohistochemistry
To assess localization of p-S6 and Rheb, animals were perfused and spinal cords harvested 4 hours after intraplantar injection of carrageenan in the left hind paw of the rat. The animals were deeply anesthetized with isofluorane and perfused intracardially with saline followed by freshly prepared 4% paraformaldehyde in 0.1 M PBS. The lumbar spinal cord was removed, post-fixed in the same fixative for 6 hours (20 minutes for OX-42 staining) at 4° C and transferred to PBS containing 20% sucrose for 48 hours followed by 24 hours in 30% sucrose. The lumbar segments L4-6 were dissected and frozen in isopentane and cut into 14 μm or 30 μm thick sections using a cryostat. The 30 μm thick sections were transferred to PBS and the 14 μm thick sections were thaw-mounted onto glass slides. After permeabilizing the tissue in PBS containing 0.2% Triton X-100 for 10 minutes, the sections were blocked in PBS containing 0.2% Triton X-100 and 5% normal goat serum for 1 hour at room temperature. Sections were then incubated with primary antibody for 1 or 2 days at 4° C. Primary antibodies were rabbit anti-p-S6 (Ser 235/236) (1:100, Cell Signaling Technology, cat. no. 4857), rabbit anti-Rheb (1:1000, Cell Signaling Technology, cat. no. 4935), mouse anti-GFAP (1:1000, Chemicon, cat. no. MAB360), mouse anti-NeuN (1:1000, Chemicon, cat. no. MAB377) and mouse anti-OX-42 (1:200, Serotec, Raleigh, NC, USA, cat. no. MCA275GA). Tyramide signaling amplification (TSA) (Invitrogen) was used to amplify the Rheb signal. After washing, the sections were probed with secondary antibody conjugated to horseradish peroxidase for 1 hour at room temperature and washed with PBS. The sections were then re-probed with primary antibody to determine cellular co-localization. Following antibody incubation, sections were dried and mounted on glass slides using cover slip mounting medium containing DAPI (Prolong Gold with DAPI, Invitrogen). Confocal images were captured using a confocal microscope system (Zeiss, LSM 710, Munich, Germany) operated by LSM software ZEN 2008.
Quantitative real-time PCR
mRNA from the ipsilateral lumbar spinal cord was isolated using RNA Stat (Tel-Test, Friendswood, TX, USA), as described previously (Boyle et al., 2003). Complementary DNA was prepared and quantitative real-time PCR performed with TaqMan Gene Expression Assays (both according to the manufacturer’s instructions, Applied Biosystems, Foster City, CA, USA) to determine relative mRNA levels, using the GeneAmp 7000 Sequence Detection system (Applied Biosystems). Pre-developed specific primers were used to detect Rheb (Assay ID Rn00788207) and HPRT1 (Assay ID Rn01527838) (Applied Biosystems). Sample threshold cycle (Ct) values in standard curve samples (C6 cells stimulated with 20% serum for 2 hours) containing Rheb and HPRT1 mRNA were used to calculate the cDNA concentration equivalents in the test samples. The Rheb data were then normalized to HPRT gene expression to obtain relative concentration and are presented as relative units.
Cell culture experiments
To investigate the effect of rapamycin on neurons, a rat neuronal cell line (PC12 cells) (ATCC, Manassas, VA, USA) was used. Neurons were cultured in Dulbecco’s modified eagle medium (DMEM) (Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS) (Sigma), penicillin and streptomycine (cDMEM) and serum starved for 24 hours (DMEM containing 0.1 % FBS) prior to the experiment. Cells were pre-treated with rapamycin (100 nM in 0.05% ethanol in saline) for 30 minutes and then stimulated with 20% FBS for 20 minutes. After washing with ice-cold PBS, cells were lysed in ice-cold lysis buffer (50 nM Tris, 150 mM NaCl, 1 mM EDTA, 0.5% Triton X-100 and 1% SDS, protease inhibitor, phosphatase inhibitor 1 and 2, pH 7.4) and homogenized by sonication. Western blots were performed as described above.
Data analysis
Western blots and behavioral data were expressed as mean ± SEM. Western blot and behavioral AUC data were analyzed by one-way ANOVA followed by Newman-Keul’s multiple comparison test. p values <0.05 were considered significant.
RESULTS
Spinal activation of the mTOR pathway following induction of peripheral inflammation
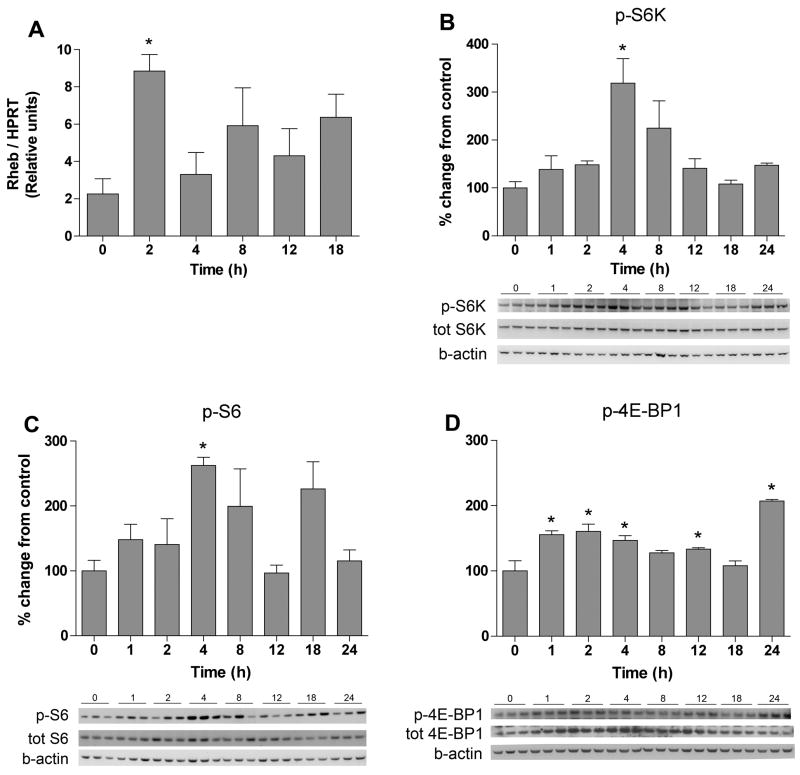
Injection of carrageenan results in a transient inflammation, apparent as an increase in paw volume, reddening of the skin and hyperalgesia. The inflammation is visible approximately 2 hours post injection and is resolved after 36 hours. In the first part of the study we collected the ipsilateral lumbar portion of the spinal cord in order to examine if peripheral inflammation alters signaling through the mTOR pathway in the spinal cord. Previous studies have shown that the small GTPase Rheb increases mTOR activity by sequestering the mTOR inhibitor FKBP-38 (Bai et al., 2007; Codeluppi et al., 2009). Hence, we examined Rheb gene expression in the ipsilateral lumbar spinal cords of naïve rats and rats subjected to 2, 4, 8 12 and 18 hours of paw inflammation. A significant increase in Rheb gene expression was observed in the 2-hour samples compared to samples from naïve animals (8.9±0.9 vs 2.3±0.8 relative expression units, n=4, p<0.05) (Fig. 1A), suggesting that peripheral inflammation drives induction of this factor, potentially regulating mTOR activity in the spinal cord.
Figure 1.
Peripheral inflammation activates spinal Rheb, S6 kinase, S6 and 4E-BP1. Bar graphs display % change from naïve control (t = 0h) for Rheb (A), phoshpo (p)-S6 kinase (B), p-S6 (C) and p-4E-BP1 (D) in the spinal cord at different time points following carrageenan-induced inflammation. Representative Western blots of p-S6K, p-S6 and p-4E-BP1 are shown below each bar graph. Data are reported as mean ± SEM, n = 4. * represents p < 0.05 compared to naïve control.
Phosphorylation of mTOR at serine-2448 may be used to monitor general mTOR activity. However, because it is not completely clear if phosphorylation of mTOR at the ser-2448 is directly correlated with mTOR activity (mutated mTOR, which can not be phosphorylated at mTOR ser-2448, still retains the ability to phosphorylate S6K) (Chiang et al., 2005) we instead assessed activation of the mTOR pathway by measuring phosphorylation of downstream targets. Threonine-389 on S6K is an mTOR-specific phosphorylation site and widely used to monitor mTOR activation (Sabatini, 2006). Basal levels of phosphorylated S6K (thr-389) were observed and induction of peripheral inflammation led to an increase in p-S6K (thr-389) at 4 hours (318.9±50.8% vs 100±12.7%, compared to naïve animal, n=4–8, p<0.05) (Fig. 1B). No change in total S6K protein level was observed at any time point (p>0.05).
We also measured phosphorylation of the S6K target S6 at ser-235/236 (S6K-dependent phosphorylation site) as well as the mTOR substrate 4E-BP1 at thr-37/46, as indicators of mTOR activation. Phosphorylation of S6 significantly increased at 4 hours as compared with naïve animals (262.6±21.8% vs 100±21.8%, n=3, p<0.05) (Fig. 1C). There was no difference in total S6 protein expression (p>0.05) following carrageenan injection. Phosphorylation levels of 4E-BP1 (thr-37/46) were also significantly increased at the 4-hour time point compared to naïve animals (146.5±7.6% vs 100±15.6%, n=3, p<0.05) (Fig. 1D). In contrast to S6K and S6, a significant increase in total 4E-BP1 protein levels were detected 2 and 4 hours subsequent to carrageenan injection, as compared with naïve animals (100±10% vs 2h: 303± 46% and 4h: 358±26% n=3, p<0.05).
Effect of blocking spinal mTOR on inflammation-induced hypersensitivity
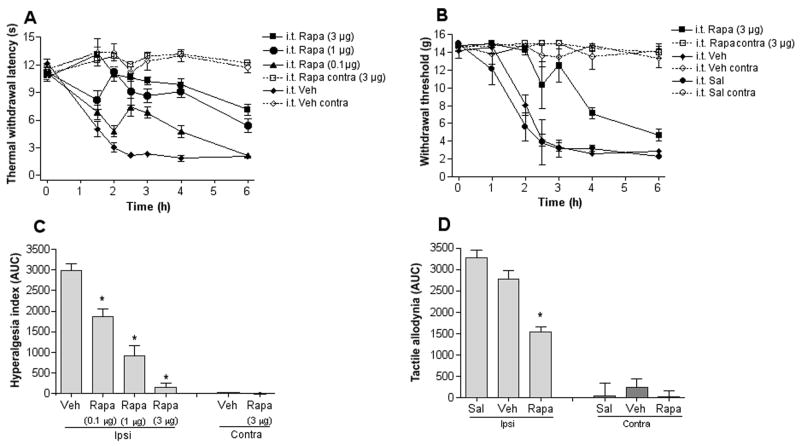
As mTOR activity is increased in the spinal cord following peripheral inflammation, we next examined if spinal inhibition of mTOR activity, by i.t. injection of rapamycin, attenuates inflammation-induced hypersensitivity. Hypersensitivity was assessed by measuring the response latency to a thermal stimulus and withdrawal threshold to application of von Frey filaments. Both the thermal withdrawal latency and tactile threshold were higher for rapamycin pre-treated animals (0.1–3μg (0.1–3.2 nmol), 15 min) compared to control vehicle treated animals (Fig. 2A, B). Calculation of the hyperalgesic index (AUC) showed that i.t. rapamycin caused a dose-dependent and significant decrease in the thermal hypersensitivity (3 μg,142±98.7; 1 μg, 907±25.9; 0.3μg 1872±184.5; vs vehicle control, 2984±162.9, n= 6–8, p<0.05) (Fig. 2C) and tactile allodynia (3 μg,1541±128 vs vehicle control, 2769±217, n= 5–6, p<0.05) (Fig. 2D) induced by intraplantar injection of carrageenan, indicating that mTOR is involved in the regulation of inflammation-induced spinal sensitization. We observed no difference in carrageenan-induced hypersensitivity in animals that received i.t. injection of the rapamycin vehicle (0.5% ethanol in saline) compared to animals that received i.t. injection of saline (Fig. 2B, D). Thermal withdrawal latency was not altered in the contralateral paw following i.t. injection of rapamycin and ipsilateral injection of carrageenan, indicating that rapamycin did not have an impact on normal thermal thresholds (Fig. 2A, C). No significant effect on tactile allodynia was observed after i.t. injection of rapamycin in absence of carrageenan compared to naïve animals (3 μg, AUC: 338±70 vs naïve control, 219±91).
Figure 2.
Intrathecal (i.t.) injection of rapamycin attenuates thermal hyperalgesia and tactile allodynia induced by peripheral inflammation. Graphs display (A) thermal withdrawal thresholds in seconds (s) and (B) tactile thresholds in grams (g) before (t = 0h) and after injection of carrageenan to the paw and i.t. injection of vehicle or different doses of rapamycin (15 min pretreatment). The histograms represent the hyperalgesic index calculated as area under the curve (AUC, 0–240 min) for thermal hyperalgesia (C) and tactile allodynia (D) for each group shown in panel A and B. Data are reported as mean ± SEM, n = 6–7 rats per group. * represents p < 0.05 compared to vehicle control.
Peripheral inflammation does not activate mTOR in lumbar dorsal root ganglia
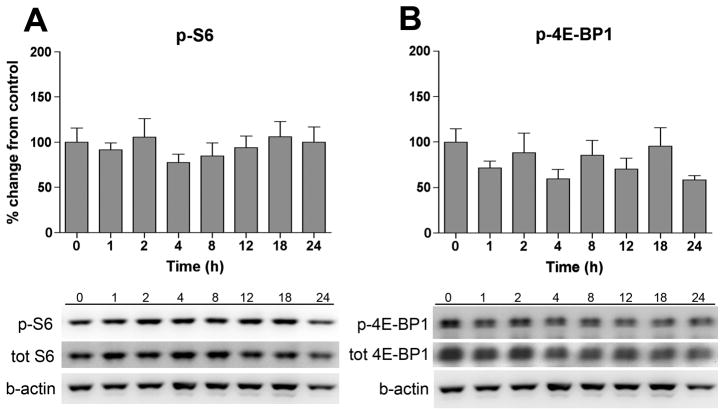
DRGs were harvested at the same time points as the spinal cord samples and phosphorylation of S6 and 4E-BP1 was measured in the ipsilateral DRGs. However, no change in phosphorylation was observed for any of the proteins measured subsequent to carrageenan-induced peripheral inflammation (Fig. 3A, B).
Figure 3.
Intraplantar injection of carrageenan had no effect on S6 or 4E-BP1 phosphorylation in the DRGs. Bar graphs display % change from naïve controls (t = 0h) for (A) phospho (p)-S6 and (B) p-4E-BP1 in the ipsilateral DRGs (L4-L6) at different time points following carrageenan-induced inflammation. Representative Western blots of p-S6 and p-4E-BP1 are shown below each bar graphs. Data is shown as % change from naïve control and expressed as means ± SEM, n = 4–6.
Spinal localization of Rheb and S6 phosphorylation
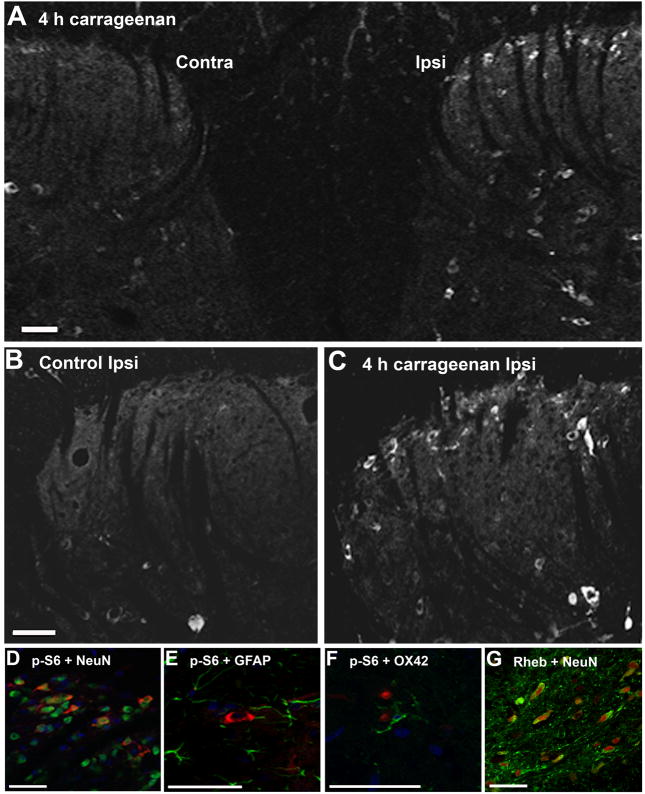
In order to determine the cellular location of mTOR activation in the spinal cord, immunohistochemistry was undertaken comparing spinal cord sections from naïve animals with animals sacrificed 4 hours after injection of carrageenan. As there are no commercially available antibodies recommended for p-S6K immunohistochemistry, our work centered on detection of S6 phosphorylation in the spinal cord. Immunohistochemistry confirmed our Western blot data showing that phosphorylation of S6 is elevated in the ipsilateral dorsal horn (Fig. 4A, C) as compared to naïve spinal cord (Fig. 4B). While a marked increase in p-S6 was observed in the superficial laminae and the deep dorsal horn of the ipsilateral side of the dorsal horn 4 hours after carrageenan injection (Fig. 4A, C), only few cells showed p-S6 immunoreactivity on the contralateral side (Fig. 4A), indicating that mTOR activation was primarily unilateral. Basal S6 phosphorylation was observed in the larger motor neurons in the ventral horn of the spinal cord, however, this activity did not appear to change subsequent to carrageenan-induced inflammation (data not shown). Double labeling with the neuronal marker NeuN showed that p-S6 co-localized predominantly with neurons in the gray matter (Fig. 4D) but not with the astrocyte marker GFAP (Fig. 4E) or microglia marker OX-42 (Fig. 4F). Some immunoreactivity was observed in radial glia-like cells in the white matter (Fig. 5), though this cellular localization was not further examined. Rheb immunoreactivity was detected in the dorsal horn 4 hours after intraplantar carrageenan injection (Fig. 4G) and double labeling with NeuN showed that the Rheb was expressed in neurons (Fig. 4G).
Figure 4.
Peripheral inflammation induces phosphorylation of S6 in dorsal horn neurons. (A) Representative confocal microscopy images depicting S6 phosphorylation in the contralateral (contra) and ipsilateral (ipsi) superfical laminae and deep dorsal horn 4 hours after injection of carrageenan to the paw. p-S6 immunoreactivity in the naïve dorsal horn (B) compared to 4 hours after carreageenan injection (C) demonstrates an increase in phospho (p)-S6 following induction of inflammation. The induced p-S6 (red) co-localized with NeuN, a marker for neurons (D), but not with GFAP (E) or OX-42 (F) markers for astrocytes and microglia, respectively. Rheb immunoreactivity colocalized with NeuN (G). DAPI was used as nuclear stain in panels D-F. The scale bar represents 50 μm.
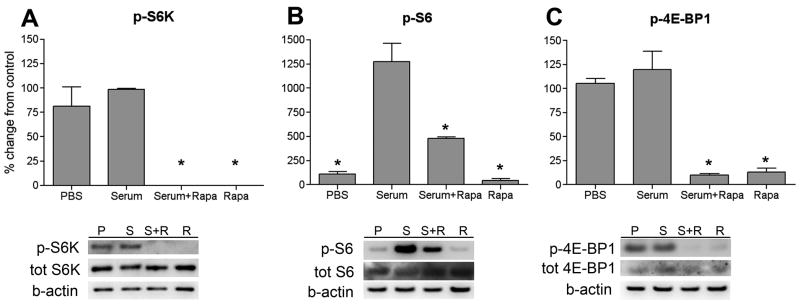
Fig 5.
Rapamycin treatment of PC12 cells attenuates serum-induced phosphorylation of S6 kinase, S6 and 4E-BP1. Bar graphs display % change from serum for phospho (p)-S6K (A), p-S6 (B) and p-4E-BP1 (C). Representative blots of p-S6K, p-S6 and p-4E-BP1 are shown below each bar graph. Data are reported as mean ± SEM, n = 3. * represents p < 0.05 compared to serum.
In order to confirm that rapamycin inhibits activation of mTOR in neurons, PC12 cells were pre-treated with rapamycin (100nM) and stimulated with 20% FBS. FBS was used to induce mTOR activation because it has been shown previously that growth factors such as epidermal growth factor and brain-derived neurotrophic factor (BDNF) activate mTOR and its downstream targets in cortical neurons and spinal astrocytes (Codeluppi et al., 2009; Takei et al., 2004). The PC12 cells were subjected to serum starvation with media containing 0.1% FBS for 24 hours prior to the experiment. FBS stimulation for 20 minutes induced a small increase in p-S6K, p-S6 and p-4E-BP1 levels, though this increase did not reach statistical significance. Importantly, pre-treatment with rapamycin abolished phosphorylation of S6K, S6 and 4E-BP1 in both control cells as well as in cells subjected to 20% FBS (Fig. 5, A–C).
DISCUSSION
The present study shows that there is a time-dependent increase in mRNA levels of the mTORC1 activator Rheb, as well as an increased phosphorylation of S6K, S6 and 4E-BP1, downstream targets of mTOR, in the ipsilateral dorsal horn subsequent to carrageenan-induced inflammation. This implicates that peripheral inflammation leads to an increased signaling through the mTORC1 pathway. Intrathecal injection of the mTORC1 selective inhibitor rapamycin attenuated both thermal and tactile allodynia, further supporting a role for spinal mTORC1 in the regulation of spinal sensitization and hypersensitivity. Following carrageenan-induced hypersensitivity, a clear increase in phosphorylated S6 was observed in the ipsilateral superficial and deep dorsal horn neurons but not in microglia or astrocytes, indicating that mTORC1-mediated signaling is important in dorsal horn neurons during inflammatory pain. Activation of mTORC2 was not assessed in present work, hence the role of mTORC2 in pain processing warrants further studies.
The number of reports examining the role of mTOR in pain processing is increasing and it is becoming clear that mTORC1 is important in the regulation of nociception both in the peripheral and the central nervous system. Previous work has shown that there is constitutive mTORC1 activity in myelinated fibers in the peripheral nerve and that local injection of rapamycin into the paw reduces the basal phosphorylation of mTOR in the peripheral nerve and prevents capsaicin-induced secondary mechanical hyperalgesia but not thermal hyperalgesia (Jimenéz-Diaz et al., 2008). Other studies point to a regulatory function of neuronal mTOR in pain processing in the dorsal horn of the spinal cord. It has been demonstrated that i.t. injection of rapamycin attenuates not only tactile allodynia and thermal hyperalgesia in the carrageenan model, tactile allodynia in the spared nerve injury model (Géranton et al., 2009) and pain behavior in the formalin model (Price et al., 2007; Asante et al., 2009) but also nociceptive transmission onto spinal wide dynamic range neurons and formalin-induced spinal neuronal hyperexcitability assessed by electrophysiology (Asante et al., 2009). In most studies, i.t. injection of rapamycin has no effect on basal nociceptive thresholds (Price et al., 2007; Géranton et al., 2009, but see Asante et al., 2009).
mTOR activation leads to phosphorylation of S6K and 4E-BP1. In our behavioral experiments a significant reduction in response thresholds for both thermal and mechanical hypersensitivity started around 1 hour after carrageenan injection. Noteworthy, reduction in nociceptive thresholds correlated with increases in 4E-BP1 phosphorylation, which were observed from 1–4 hours after carrageenan injection. This may imply that 4E-BP1 is involved in the initiation of hypersensitivity, and that S6K and S6, which showed elevated phospohorylation at 4 hours, contribute to spinal sensitization at later time points.
To our surprise, i.t. injection of rapamycin attenuated not only tactile allodynia but also thermal hyperalgesia in the carrageenan model. This was not observed after local or i.t. delivery of rapamycin in the capsaicin model (Jimenéz-Diaz et al., 2008; Géranton et al., 2009). Though the discrepancy in rapamycin’s effect on thermal hyperalgesia warrants further studies, one may speculate that this in part depends on differences between the two experimental models. In the capsaicin model, activation of TRPV1 receptors lead to a fast onset of hypersensitivity, lasting approximately two hours. The inflammatory component is mild, though peripheral release of pro-inflammatory cytokines, such as IL-1β, IL-6 and TNF has been reported (Saadé et al., 2002). Carrageenan-induced hypersensitivity is mediated by local release of a number of pro-inflammatory factors, including prostaglandins and cytokines, causing a pronounced inflammation with reddening and swelling of the entire paw (including the digits, dorsal and plantar side of the paw as well as the heel) and sensitization and activation of the peripheral sensory neurons through multiple receptors. This model has a significantly longer-lasting allodynia and hyperalgesia (up to 36 hours) and there are several reports indicating release of inflammatory mediators not only peripherally but also at the spinal level (Ianaro et al., 1994; Beloeil et al., 2006; Choi et al., 2010). A link between pro-inflammatory cytokines, such as TNF, and activation of mTORC1 has been proposed (Lee et al., 2007). This positive relationship between inflammation and mTORC1 activation may turn out to be important in pain processing, in particular considering the proposed role of cytokines in regulation of synaptic and neuronal activity in the superficial spinal cord (Kawasaki et al., 2008). Thus, differences in the mechanisms driving induction and maintenance of hypersensitivity between the capsaicin and carrageenan models could account for the different effects of rapamycin on thermal hyperalgesia. Further, in our study chronic intrathecal catheters were used for drug delivery, while temporary or direct lumbar injection have been used in previous studies investigating the antinociceptive effect of rapamycin (Price et al., 2007; Asante et al., 2009; Géranton et al., 2009). While the catheterized animals used in our study showed similar baseline nociceptive thresholds as naïve animals, one cannot exclude the possibility that chronic intrathecal catheters have an effect on the spinal environment, which leads to changes in pain processing. Another noteworthy difference between the study by Géranton et al. and current work is the baseline phosphorylation of S6 in glia. Though the same strain of rats and antibodies directed towards the same S6 phosphorylation site (ser-235/236) were used, we could only detect p-S6 in spinal neuronal cell bodies and not in glia. While the difference in immunoreactivity may due to p-S6 signal amplification, which was used in the Geranton study but not in the current study, it is also possible that differences in baseline cell specific mTOR activity alters nociceptive processing and the effect of i.t. rapamycin.
Our work, in agreement with the report by Géranton and colleagues (Géranton et al., 2009), shows that mTOR is constitutively activated in DRGs. As no increased signaling through the mTOR pathway following carrageenan-induced inflammation was noted and i.t. injection of rapamycin failed to reduce constitutive mTOR activity in DRGs (Géranton et al., 2009), the antihyperalgesic effect of i.t. delivered rapamycin that was found in current work is not likely to be mediated at the level of the DRGs. Noteworthy, mTOR, S6 and 4E-PB1 are constitutively activated in the dorsal root, predominantly in myelinated A-fibers. I.t. delivery of rapamycin reduces phosphorylation of S6, downstream of mTOR, in the dorsal roots (Géranton et al., 2009) implying that the anti-allodynic effect observed by i.t. rapamycin may be mediated by an inhibition of mTOR activity in the roots. As i.t. rapamycin reduces mTOR activity in the naïve spinal cord and also prevents capsaicin-induced increases in the number of cells that express phosphorylated S6 (Géranton et al., 2009), rapamycin may attenuate hypersensitivity through a combined peripheral and a central action. As we did not assess mTOR activity in the dorsal root prior to or following carrageenan-injection, comparison of dorsal root mTOR activity between the two studies cannot be made.
Immunohistochemistry showed that activation of the mTOR pathway following peripheral inflammation, as measured by p-S6 immunoreactivity, was localized to the ipsilateral superficial lamina in the dorsal horn, supporting a role for increased mTOR activity in pain signaling and confirming the unilateral characteristic of the carrageenan model. Double labeling revealed that S6 was phosphorylated in neurons, as S6 immunoreactivity co-localized with a neuronal marker, but not with markers for astrocytes or microglia. Of interest, mTOR is thought to play a key role in neuronal plasticity through regulation of protein synthesis (Kelleher et al., 2003). In support of this concept, Rheb has been proposed to regulate mTOR-dependent protein translation in neuronal dendrites during modulation of synaptic plasticity (Takei et al., 2004). Our finding that Rheb is expressed in spinal neurons in the superficial dorsal horn is in line a potential role of mTOR in spinal pain processing. Recent work suggests that spinal mRNA translation is an important component both in spinal neuronal hyperexcitability and behavioral hypersensitivity induced by injection of formalin to the hind paw (Price et al., 2007; Asante et al., 2009). Hence, it is possible that mTOR-regulated protein translation in spinal neurons also plays an important role in spinal sensitization following carrageenan-induced inflammation. However, it is important to note that mTOR is implicated in multiple cellular functions, including regulation of phosphatase activity, transcription, ubiquitin-dependent proteolysis and microtubule and actin stability, all of which are crucial for modification of synaptic strength (Jaworski and Sheng, 2006). On this notion, the serine/threonine-specific protein phosphatase PP2A, which is activated by rapamycin in an mTOR-dependent fashion, could potentially be linked to rapamycins anti-nociceptive effect. PP2A deactivates S6K and 4E-BP1 through dephosphorylation of sites critical for S6K and 4E-BP1 activity (Peterson et al., 1999). A role for phosphatases in inflammatory pain transmission is suggested by studies showing that PP2A inhibition potentiates central sensitization of nociceptive dorsal horn neurons (Zhang et al., 2006) and increases mechanical and thermal hypersensitivity after peripheral capsaicin injection (Zhang et al., 2003). While the most likely effect of rapamycin in the context of our work is inhibition of mTOR-dependent protein translation in spinal neurons, one must also consider the link between PP2A and dephosphorylation of other proteins associated with spinal sensitization. For example, PP2A dephophorylates p38, c-Jun N-terminal kinase and ERK (Prickett and Brautigan, 2007; Shanley et al., 2001; Van Kanegan et al., 2005), all thought to play important roles in pain processing.
The carrageenan model used in this study is a model with a modest activation of microglia and astrocytes, and while biochemical changes may be detected in these cells, the morphological signs of activation are not present (Schreiber et al., 2008; Svensson et al., 2005; Hua et al., 2005). Since glial activation has been demonstrated to be important in the regulation of pain processing (Watkins and Maier, 2003; Sweitzer et al., 1999) and mTOR was recently reported to regulate astrocyte and microglia activity and proliferation (Codeluppi et al., 2009; Dello Russo et al., 2009) one may speculate that activation of mTOR in these cells could be of importance in experimental models of pain with a more pronounced glial involvement. However, recent work does not support this hypothesis. Although p-mTOR, p-S6 and p-4E-BP1 were observed in spinal glia in naïve tissue and following spared nerve injury, no increase in phosphorylation was detected at the time points investigated (Géranton et al., 2009). Our results from the neuronal cell line experiments show a clear inhibitory effect of rapamycin on the level of phosphorylation of p-S6K, p-S6 and p-4E-BP1, supporting an inhibitory action of rapamycin on the mTOR pathway in neurons. However, stimulation with 20 % FBS failed to induce an increase of phosphorylation of mTOR related targets. This may be due to the presence of the low levels of FBS in the serum starvation media (0.1%) being sufficient to generate maximal mTOR activation. Alternatively, there may be a general and strong basal activation of mTOR in this type of neuronal cell line, giving little room for further activation following stimulation.
Rapamycin is used clinically and was the first mTORC1 inhibitor to be approved in the US for cancer treatment (Guertin and Sabatini, 2009). It is also used in angioplasty stents (Marx et al., 2001) and to prevent organ rejection following transplantations (Alamo et al., 2009). The immunosuppressive properties of rapamycin are due to inhibition of mTORC1, which affects both the innate and adaptive immune response (Thomson et al., 2009). While rapamycin is generally considered a specific inhibitor of mTOR, it should be noted that rapamycin also act on the ryanodine receptor and alter calcium release from the sarcoplastic reticulum (Brillantes et al., 1994). If this effect is coupled to the antinociceptive effect of rapamycin warrants further studies. Of importance, rapamycin has low organ toxicity compared to similar immunosuppressants, making it a suitable drug for long-term use (Sehgal, 1998; Alamo et al., 2009; Caixeta et al., 2009). Though not assessed clinically for treatment of chronic pain, the relatively mild side-effect profile of rapamycin during long-term treatment is an advantage, in particular if the analgesic effect would be seen at lower doses than what is used for current approved indications. Ongoing efforts are aimed at developing new ATP-competitive inhibitors that would block both mTORC1 and mTORC2 and therefore have a wider spectrum of action compared to rapamycin (Guertin and Sabatini, 2009).
In conclusion, the current work demonstrates a role for mTOR in spinal modulation of inflammatory pain. Intrathecal rapamycin blocks carrageenan-induced tactile and thermal hypersensitivity through inhibition of the mTOR pathway in the spinal cord. Thus, the mTOR pathway may represent a potential novel target for pain treatment.
Acknowledgments
This work was supported by the NIH: DA21654 (CIS), Swedish Research Council (CIS), Marie Curie International Reintegration grant (CIS), International Association for the Study of Pain (CIS), Karolinska Institutet (ENG) and the Wenner-Gren foundation (SC, JG).
Abbreviations
- AUC
area under the curve
- BDNF
brain-derived neurotrophic factor
- DRG
dorsal root ganglion
- DMEM
Dulbecco’s modified eagle medium
- EDTA
ethylenediaminetetraacetic acid
- 4E-BP1
eukaryotic initiation factor 4E-binding protein
- ERK
extracellular-regulating kinase
- FBS
fetal bovine serum
- GFAP
glial fibrillary acidic protein
- 1IL-1β
interleukin 1 beta
- i.t
intrathecal
- mTOR
mammalian target of rapamycin
- PBS
phosphate-buffered saline
- PP2A
protein phosphatase 2A
- NeuN
neuronal N
- Rheb
Ras homolog enriched in brain
- S6K
S6 kinase
- S6
ribosomal protein S6
- SEM
standard error of the mean
- TNF
tumor necrosis factor
- TOP
5′ oligopyrimidic tract
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alamo JM, Barrera L, Casado MD, Bernal C, Marin LM, Suarez G, Sanchez-Moreno L, Jimenez R, Suarez-Grau JM, Sousa JM, Cordero E, Gomez-Bravo MA. Efficacy, tolerance, and safety of mammalian target of rapamycin inhibitors as rescue immunosuppressants in liver transplantation. Transplant Proc. 2009;41:2181–2183. doi: 10.1016/j.transproceed.2009.06.083. [DOI] [PubMed] [Google Scholar]
- Asante CO, Wallace VC, Dickenson AH. Formalin-induced behavioral hypersensitivity and neuronal hyperexcitability are mediated by rapid protein synthesis at the spinal level. Mol Pain. 2009;5:27. doi: 10.1186/1744-8069-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X, Ma D, Liu A, Shen X, Wang QJ, Liu Y, Jiang Y. Rheb activates mTOR by antagonizing its endogenous inhibitor, FKBP38. Science. 2007;318:977–978. doi: 10.1126/science.1147379. [DOI] [PubMed] [Google Scholar]
- Beloeil H, Ji RR, Berde CB. Effects of buivacaine and tetrodotoxin on carrageenan-induced hind paw inflammation in rats (Part 2): cytokines and p38 mitogen-activated protein kinases in dorsal root ganglia and spinal cord. Anesthesiology. 2006;105:139–145. doi: 10.1097/00000542-200607000-00023. [DOI] [PubMed] [Google Scholar]
- Boyle DL, Rosengren S, Bugbee W, Kavanaugh A, Firestein GS. Quantitative biomarker analysis of synovial gene expression by real-time PCR. Arthritis Res Ther. 2003;5:R352–360. doi: 10.1186/ar1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brillantes AB, Ondrias K, Scott A, Kobrinsky E, Ondriasová E, Moschella MC, Jayaraman T, Landers M, Ehrlich BE, Marks AR. Stabilization of calcium release channel (ryanodine receptor) function by FK506-binding protein. Cell. 1994;774:513–23. doi: 10.1016/0092-8674(94)90214-3. [DOI] [PubMed] [Google Scholar]
- Caixeta A, Leon MB, Lansky AJ, Nikolsky E, Aoki J, Moses JW, Schofer J, Morice MC, Schampaert E, Kirtane AJ, Popma JJ, Parise H, Fahy M, Mehran R. 5-year clinical outcomes after sirolimus-eluting stent implantation insights from a patient-level pooled analysis of 4 randomized trial comparing sirolimus-eluting stents with bare-metal stents. J Am Coll Cardiol. 2009;54:894–902. doi: 10.1016/j.jacc.2009.04.077. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chiang GC, Abraham RT. Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J Biol Chem. 2005;280:25485–25490. doi: 10.1074/jbc.M501707200. [DOI] [PubMed] [Google Scholar]
- Choi J, Svensson CI, Koehrn FJ, Bhuskute A, Sorkin LS. Peripheral inflammation induces tumor necrosis factor dependent AMPA receptor trafficking and Akt phosphorylation in spinal cord in addition to pain behavior. Pain. 2010 doi: 10.1016/j.pain.2010.02.008. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codeluppi S, Svensson CI, Hefferan MP, Valencia F, Silldorff MD, Oshiro M, Marsala M, Pasquale EB. The Rheb-mTOR pathway is upregulated in reactive astrocytes of the injured spinal cord. J Neurosci. 2009;29:1093–1104. doi: 10.1523/JNEUROSCI.4103-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dello Russo C, Lisi L, Tringali G, Navarra P. Involvement of mTOR kinase in cytokine-dependent microglial activation and cell proliferation. Biochem Pharmacol. 2009;78:1242–1251. doi: 10.1016/j.bcp.2009.06.097. [DOI] [PubMed] [Google Scholar]
- Géranton SM, Jiménez-Diaz L, Torsney C, Tochiki KK, Stuart SA, Leith JL, Lumb BM, Hunt SP. A rapamycin-sensitive signaling pathway is essential for the full expression of persistent pain states. J Neurosci. 2009;29:15017–15027. doi: 10.1523/JNEUROSCI.3451-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras AC, Raught B, Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem. 1999;68:913–963. doi: 10.1146/annurev.biochem.68.1.913. [DOI] [PubMed] [Google Scholar]
- Groth R, Aanonsen L. Spinal brain-derived neurotrophic factor (BDNF) produces hyperalgesia in normal mice while antisense directed against either BDNF or trkB, prevent inflammation-induced hyperalgesia. Pain. 2002;100:171–81. doi: 10.1016/s0304-3959(02)00264-6. [DOI] [PubMed] [Google Scholar]
- Guertin DA, Sabatini DM. The pharmacology of mTOR inhibition. Sci Signal. 2009;2:pe24. doi: 10.1126/scisignal.267pe24. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Hua XY, Svensson CI, Matsui T, Fitzsimmons B, Yaksh TL, Webb M. Intrathecal minocycline attenuates peripheral inflammation-induced hyperalgesia by inhibiting p38 MAPK in spinal microglia. Eur J Neurosci. 2005;22:2431–2440. doi: 10.1111/j.1460-9568.2005.04451.x. [DOI] [PubMed] [Google Scholar]
- Hunt SP, Mantyh PW. The molecular dynamics of pain control. Nat Rev Neurosci. 2001;2:83–91. doi: 10.1038/35053509. [DOI] [PubMed] [Google Scholar]
- Ianaro A, O’Donnell CA, Di Rosa M, Liew FY. A nitric oxide synthase inhibitor reduces inflammation, down-regulates inflammatory cytokines and enhances interleukin-10 production in carrageenin-induced oedema in mice. Immunology. 1994;82:370–375. [PMC free article] [PubMed] [Google Scholar]
- Jaworski J, Sheng M. The growing role of mTOR in neuronal development and plasticity. Mol Neurobiol. 2006;34:205–219. doi: 10.1385/MN:34:3:205. [DOI] [PubMed] [Google Scholar]
- Jimenez-Diaz L, Géranton SM, Passmore GM, Leith JL, Fisher AS, Berliocchi L, Sivasubramaniam AK, Sheasby A, Lumb BM, Hunt SP. Local translation in primary afferent fibers regulates nociception. PLoS One. 2008;3:e1961. doi: 10.1371/journal.pone.0001961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28:5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Govindarajan A, Tonegawa S. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron. 2004;44:59–73. doi: 10.1016/j.neuron.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DF, Kuo HP, Chen CT, Hsu JM, Chou CK, Wei Y, Sun HL, Li LY, Ping B, Huang WC, He X, Hung JY, Lai CC, Ding Q, Su JL, Yang JY, Sahin AA, Hortobagyi GN, Tsai FJ, Tsai CH, Hung MC. IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130:440–455. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- Lenz G, Avruch J. Glutamatergic regulation of the p70S6 kinase in primary mouse neurons. J Biol Chem. 2005;280:38121–38124. doi: 10.1074/jbc.C500363200. [DOI] [PubMed] [Google Scholar]
- Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- Marx SO, Marks AR. Bench to bedside: the development of rapamycin and its application to stent restenosis. Circulation. 2001;104:852–855. doi: 10.1161/01.cir.104.8.852. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Cafferty WB, Marchand F. Immune and glial cell factors as pain mediators and modulators. Exp Neurol. 2005;192:444–462. doi: 10.1016/j.expneurol.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson TR, Desai BN, Hardwick JS, Schreiber SL. Protein phosphatase 2A interacts with the 70-kDa S6 kinase and is activated by inhibition of FKBP12-rapamycinassociated protein. Proc Natl Acad Sci U S A. 1999;96:4438–4442. doi: 10.1073/pnas.96.8.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, Rashid MH, Millecamps M, Sanoja R, Entrena JM, Cervero F. Decreased nociceptive sensitization in mice lacking the fragile X mental retardation protein: role of mGluR1/5 and mTOR. J Neurosci. 2007;27:13958–13967. doi: 10.1523/JNEUROSCI.4383-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prickett TD, Brautigan DL. Cytokine activation of p38 mitogen-activated protein kinase and apoptosis is opposed by alpha-4 targeting of protein phosphatase 2A for site-specific dephosphorylation of MEK3. Mol Cell Biol. 2007;27:4217–4227. doi: 10.1128/MCB.00067-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadé NE, Massaad CA, Ochoa-Chaar CI, Jabbur SJ, Safieh-Garabedian B, Atweh SF. Upregulation of proinflammatory cytokines and nerve growth factor by intraplantar injection of capsaicin in rats. J Physiol. 2002;545:241–253. doi: 10.1113/jphysiol.2002.028233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- Schreiber KL, Beitz AJ, Wilcox GL. Activation of spinal microglia in a murine model of peripheral inflammation-induced, long-lasting contralateral allodynia. Neurosci Lett. 2008;440:63–67. doi: 10.1016/j.neulet.2008.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal SN. Rapamune (RAPA, rapamycin, sirolimus): mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. Clin Biochem. 1998;31:335–340. doi: 10.1016/s0009-9120(98)00045-9. [DOI] [PubMed] [Google Scholar]
- Shanley TP, Vasi N, Denenberg A, Wong HR. The serine/threonine phosphatase PP2A: endogenous regulator of inflammatory cell signaling. J Immunol. 2001;166:966–972. doi: 10.4049/jimmunol.166.2.966. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Fitzsimmons B, Azizi S, Powell HC, Hua XY, Yaksh TL. Spinal p38beta isoform mediates tissue injury-induced hyperalgesia and spinal sensitization. J Neurochem. 2005;92:1508–1520. doi: 10.1111/j.1471-4159.2004.02996.x. [DOI] [PubMed] [Google Scholar]
- Sweitzer SM, Colburn RW, Rutkowski M, DeLeo JA. Actute peripheral inflammation induces moderate glial activation and spinal IL-1beta expression that correlates with pain behavior in the rat. Brain Res. 1999;829:209–221. doi: 10.1016/s0006-8993(99)01326-8. [DOI] [PubMed] [Google Scholar]
- Takei N, Kawamura M, Hara K, Yonezawa K, Nawa H. Brain-derived neurotrophic factor enhances neuronal translation by activating multiple initiation processes: comparison with the effects of insulin. J Biol Chem. 2001;276:42818–42825. doi: 10.1074/jbc.M103237200. [DOI] [PubMed] [Google Scholar]
- Takei N, Inamura N, Kawamura M, Namba H, Hara K, Yonezawa K, Nawa H. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J Neurosci. 2004;24:9760–9769. doi: 10.1523/JNEUROSCI.1427-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci U S A. 2002;99:467–472. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlmann EJ, Li W, Scheidenhelm DK, Gau CL, Tamanoi F, Gutmann DH. Loss of tuberous sclerosis complex 1 (Tsc1) expression results in increased Rheb/S6K pathway signaling important for astrocyte cell size regulation. Glia. 2004;47:180–188. doi: 10.1002/glia.20036. [DOI] [PubMed] [Google Scholar]
- Van Kanegan MJ, Adams DG, Wadzinski BE, Strack S. Distinct protein phosphatase 2A heterotrimers modulate growth factor signaling to extracellular signal-regulated kinases and Akt. J Biol Chem. 2005;280:36029–36036. doi: 10.1074/jbc.M506986200. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. Glia: a novel drug discovery target for clinical pain. Nat Rev Drug Discov. 2003;2:973–985. doi: 10.1038/nrd1251. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. Central sensitization: uncovering the relation between pain and plasticity. Anesthesiology. 2007;106:864–867. doi: 10.1097/01.anes.0000264769.87038.55. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TS. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wu J, Fang L, Willis WD. The effects of protein phosphatase inhibitors on nociceptive behavioral responses of rats following intradermal injection of capsaicin. Pain. 2003;106:443–451. doi: 10.1016/j.pain.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wu J, Fang L, Willis WD. The effects of protein phosphatase inhibitors on the duration of central sensitization of rat dorsal horn neurons following injection of capsaicin. Mol Pain. 2006;2:23. doi: 10.1186/1744-8069-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]