Abstract
We have developed microarrays with all eight proteins encoded by 13 different human papillomavirus types associated with anogenital cancer (HPV-16, 18, 31, 33, 35, 45, 53), genital warts (HPV-6, 11), or skin lesions (HPV-1, 2, 4, 5). We analyzed the seroprevalence of antibodies in 546 patients, which had either cervical carcinomas, or precursor lesions, or which were asymptomatic. All patient groups contained sera ranging from high reactivity against multiple HPV proteins to low or no reactivity. Computational analyses showed the E7 proteins of carcinogenic HPV types as significantly more reactive in cancer patients compared to asymptomatic individuals and discriminating between cancer and HSIL or LSIL patients. Antibodies against E4 and E5 had the highest seroprevalence, but did not exhibit differential reactivity relative to pathology. Our study introduces a new approach to future evaluation of the overall antigenicity of HPV proteins and cross-reaction between homologous proteins.
INTRODUCTION
Human papillomaviruses (HPVs) infect the skin and anogenital and oropharyngeal mucosal epithelia. More than 100 HPV types have been described (Bernard et al., 2010), and each of these viruses shows type-specific tropism for cutaneous or mucosal epithelia as well as type-specific histological characteristics of the lesion. Many HPV infections are subclinical, while other infections become clinically apparent in the form of benign neoplastic growth. A subset of lesions, associated normally with so-called “high-risk” HPV types (Munoz et al., 2003), evolve into malignancies. This causal association between high-risk HPV types and anogenital cancers, most notably cancer of the cervix uteri, has attracted by far the largest share of research activities in HPV biology and pathogenesis (zur Hausen, 2002).
Research of humoral immune responses against HPV proteins is highly developed (Egelkrout and Galloway, 2007; Reuschenbach et al., 2008), but its success differs between different research specialties. On the one side, assembly of virus-like particles based on the capsid L1 protein has led to the introduction of anti-HPV vaccines, whose success is based on stimulating an anti-L1 humoral immune response and immunoglobulin G secretion into the cervical mucus at concentrations exceeding those measured in response to natural infections (Villa et al., 2006). On the other side, independent serological studies of natural HPV infections have not led to the development of a serology-based diagnosis of HPV infections or the use of serology as a predictive marker of HPV disease progression. Many discrepancies that were encountered by this research are still poorly understood. For example, some cervical cancer patients, although expressing the oncoproteins E6 and E7 throughout the tumor, do not show immune responses against these proteins, although increases of immune reactivity with disease progression have been observed (Lehtinen et al., 2003; Meschede et al., 1998; Reuschenbach et al., 2008; Stanley, 2003). Patients without detectable HPV associated lesions, but diagnosed being infected with specific HPV types by DNA testing, often lack serological responses against the incident infection (Rosales et al., 2001). And patients with documented serological responses against specific HPV proteins often do not exhibit neoplastic HPV infections, particularly in the case of HPV types specific for the skin (Steger et al., 1990; Waterboer et al., 2009). One may suspect that some of these discrepancies stem from histological idiosyncrasies of HPV infections. Regulatory HPV proteins as well as viral particles are expressed in suprabasal layers of squamous epithelia and subsequently shed at mucosal or cutaneous surfaces rather than spread systemically as the viral gene products in many other virus infections. On the other side, the serological literature also suffers from technical limitations, as most studies targeted single or few HPV proteins of one or few HPV types, and there are no studies yet that measured the immune responses against all eight proteins of a single HPV type or even of numerous HPV types.
We describe here a high-throughput approach that allows examining the sera of hundreds of patients for reactivity against all eight proteins encoded by a large number of different HPV types. In this study we examined the humoral immuneresponse against the proteomes of thirteen of the most common HPV types associated with cervical cancer, genital and laryngeal warts, common warts, and epidermodysplasia verruciformis. The approach is based on PCR amplification of genes, in vivo recombination cloning and in vitro expression of these proteins, and printing onto microarray chips. This technology has been used to characterize the humoral immune response profile qualitatively and quantitatively in tiny amounts of serum in studies of the proteomes of large DNA viruses, bacteria, and protozoa (Barbour et al., 2008; Beare et al., 2008; Davies et al., 2008; Doolan et al., 2008). Our project aimed to generate global insight into the humoral immune response against HPV proteins, and asked the particular question of whether a comparison of the seroresponses against all proteins of numerous HPV types would reveal preferential immune reactions in relationship to pathology, e.g. to progression of anogenital cancer. We are presenting selected data sets generated with a technological platform that offers broad approaches for future research as it overcomes restriction to only small sets of HPV proteins and HPV types.
RESULTS
Overall strategy
All previously published investigations of humoral immune responses against HPV proteins addressed individual proteins or small sets of proteins of one or few HPV types, respectively. We undertook the research reported here to simultaneously evaluate serological responses against the proteins derived from all eight open reading frames from a set of the most common and most often studied HPV types. For this purpose, we selected seven HPV types of the alpha-PV genus that are considered carcinogenic and frequently found in cervical cancer (HPV-16, 18, 31, 33, 35, 45, and 53), two HPV types, also of the alpha-PV genus, which are the cause of genital and laryngeal warts (HPV-6 and 11), and one more alpha HPV type, HPV-2, that causes common warts. We also included three additional HPV types found in cutaneous lesions and that belong to three unrelated genera, namely HPV-1, 4, and 5 (de Villiers et al., 2004).
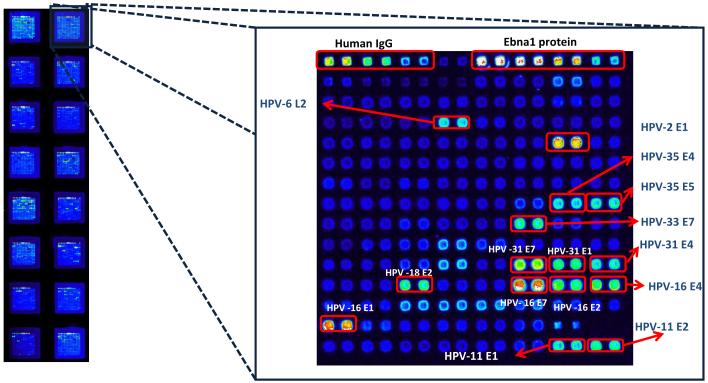
The laboratory of one of us (P.L.F.) has developed a strategy to clone all open reading frames of any organism into a phage-T7 promoter based E. coli vector by homologous recombination, followed by expression of the whole proteome of the organism by in vitro transcription and translation. Aliquots of each protein are printed on slides in a microarray format for quantitative serological evaluation of patient sera using fluorescent anti-human secondary IgG antibodies (Barbour et al., 2008; Beare et al., 2008; Davies et al., 2005a and b; Davies et al., 2008). For this, a restriction linearized and PCR amplified T7 expression vector, pXi, was used encoding an N-terminal His- and a C-terminal HA tag spanning the cloning site. We inserted all eight genes from the genomes of the 13 HPV types into this vector with gene-specific primers containing 20 nucleotide extensions complementary to HPV gene sequences and 33 nucleotide extensions complementary to the ends of the linear T7 vector. The two components were linked in vivo by transformation of mixtures of HPV genes and linear vector into E. coli, where high-efficiency homologous recombination led to expression vectors for all 104 HPV proteins. Recombinants were grown under antibiotic resistance without colony selection and minipreparations of each were sequenced in order to confirm the expected presence of the HPV genes. HPV proteins were expressed by in vitro transcription and translation in an E. coli based cell-free system and spotted in a microarray format. Expression of all cloned proteins was confirmed by reaction with anti-his and anti-HA antibodies. Fig. 1 shows the data output and demonstrates a typical slide with 16 microarrays, each containing duplicates of 104 HPV proteins and additional controls.
Fig. 1. Representative example of the raw data generated by processing HPV protein microarrays with a patient serum.
The figure shows a slide with 16 microarrays, each containing duplicates of 104 HPV proteins and additional controls. Brightness of spots correlates with increased sero-reactivity. The upper line of white spots indicating maximal reactivity contains all positive controls. This particular microarray has been probed with serum from a cervical cancer patient. The strongest reactions, indicated, are 15 different proteins from eight HPV types. The uppermost line includes human IgGs and EBNA1 protein as controls.
Validation of the microarray with anti-HPV monoclonal antibodies
As initial validation of the array we investigated whether commercial monoclonal antibodies raised against specific proteins of various HPV types would detect the homologous target protein. We also looked for cross-reactivity with homologous proteins of other HPV types and non-specific cross-reactivity with heterologous proteins. Altogether, we analyzed twelve different monoclonal antibodies, which are listed in the Materials and Methods section. Fig. S1A shows the reaction of an anti-HPV-16 E7 antibody against HPV-16 E7 and cross-reaction with the E7 proteins of HPV-31, 33, and 35, the three closest relatives of HPV-16, indicating conservation of the epitope. Fig. S1B shows a similar example, the reaction of an anti-HPV-11 E7 antibody with the HPV-11 E7 protein. There is cross-reaction with the E7 protein of HPV-6, the closest relative of HPV-11, and with the remotely related HPV-16 and HPV-31 E7 proteins. Fig. S1C shows an example of strict specificity. An anti-HPV-16 E1/E4 antibody correctly recognizes the HPV-16 E4 protein, and no other protein. A very complex outcome is shown in Fig. S1D. An anti-HPV-16 L1 monoclonal antibody recognizes the HPV-16 L1 protein, and the homologous L1 proteins of HPV-1, 2, 6, 11, 18, 31, 33 and 53. In contrast, HPV-4 and 5 L1 were not recognized by this antibody even though controls showed high expression of the HPV-4 and 5 L1 proteins. The possible cross-reaction with HPV-45 L1 could not be examined, as this protein was not present on this particular chip. Surprisingly, this antibody also shows that our test system is occasionally not sufficiently specific, as there was non-specific cross-reactivity with the E1 proteins of HPV-31, 35, and 53, and the L2 protein of HPV-18 in spite of lack of sequence homologies. The arrays also contained HPV-16 virus like particles obtained from an anti-HPV-16 vaccine. This positive control was included since HPV-16 L1 gave in this experiment a weaker signal with the anti-HPV-16 L1 monoclonal than with the L1 proteins of some related HPV types either due to suboptimal folding or suboptimal expression of HPV-16 L1. A quantitative comparison of signal intensities between these two L1 proteins may not be reliable due to the non-uniform amount of proteins spotted in this type of array.
The other eight monoclonal antibodies showed specific reactivity with the appropriate target, and no cross-reactivity with homologous proteins of other HPV types or non-specific reactions with heterologous proteins was observed (data not shown).
Low, or lack of, anti-HPV protein reactivity of sera from children without known exposure to sexually transmitted HPV types
Since studies by others as cited above have found extensive anti-HPV immunoreactivity of sera of adult and sexually active individuals, we chose to include a negative control in our study and analyzed the sera of ten children aged two to three that were presumed not to have been exposed to sexually transmitted HPV types (Fig. S2). Eight of these children, represented by the individuals K27 and K39, showed no anti-HPV protein reactivity whatsoever. Individual K73 had antibodies against HPV-5 L2, and K66 antibodies against HPV-35 E4 and E5. These sera, which were obtained from a region of Africa (Mali) with endemic malaria, showed strong reactivity to antigens from Plasmodium falciparum (Doolan et al., 2008) indicating these sera were in good condition. However, we are cognizant that immunosuppression, often associated with malaria, may influence the antibody profile. With this caveat in mind, we conclude that the sera sampled from children before puberty in most cases do not show extensive reactivity against HPV proteins.
Complex multi-target serological reactivity in patients with HPV associated cancers, in HPV infected asymptomatic patients, and in HPV negative patients
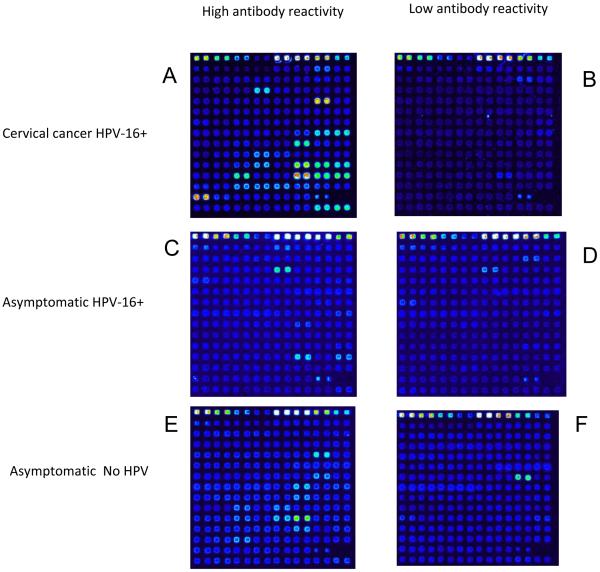
We asked whether our approach may confirm observations by others (de Gruijl et al., 1997; Meschede et al., 1998; Ravaggi et al., 2006) that patients may have strong reactivity against HPV proteins only if they are afflicted with HPV associated neoplasia and that the majority of healthy subjects without cervical cancer would be seronegative. Fig. 2 shows examples that visualize the raw data obtained with six patient sera that represent the range of responses throughout the whole group. Surprisingly, our results show that independent of disease status, subjects may or may not have strong reactivity against HPV proteins. Patients with an HPV-16 cervical carcinoma can have extensive reactivity against HPV proteins (with four of 21 signals derived from HPV-16 E1, E2, E4, and E7) as shown in Fig. 2A, or nearly no reactivity as shown in Fig. 2B. Patients with an HPV-16 positive smear can have anti-HPV reactivity as shown in Fig. 2C, while others with this diagnosis may lack such antibodies (Fig. 2D). Patients without present HPV infections may show extensive or no anti-HPV protein reactivity (Figs 2E and 2F, respectively). The six panels of Fig. 2 document individual data, but a representation of all 113,568 data points (duplicates of 104 HPV proteins targeted by 546 sera) requires a heatmap.
Fig 2. Complex multi-target as well as low serological activity in patients with HPV associated cancers, in HPV infected asymptomatic patients, and in HPV negative patients.
Documentation of the diversity of individual data that led to the heatmap in Fig. 3 and the analyses in Fig. 4 and 5. This figure represents the only data in this paper that compare HPV positive and HPV negative asymptomatic patients, as analyses not discussed here did not reveal obvious serological differences between the two groups.
Heatmaps of the reaction of 546 sera with the eight proteins of 13 HPV types
Fig. 3 shows all data of this study in the form of a heatmap, the 546 sera aligned along the x-axis, and the 104 HPV proteins along the y-axis. This particular alignment presents all data after sorting the samples into cancer patients, or individuals with HSIL, LSIL, or asymptomatic infection. Mere inspection of this crude and unedited presentation of the raw data of our study does not reveal obvious differences between these groupings of patients.
Fig. 3. Heatmap of 113,568 microarray signals generated by 546 sera targeted at all 104 proteins encoded by 13 HPV types.
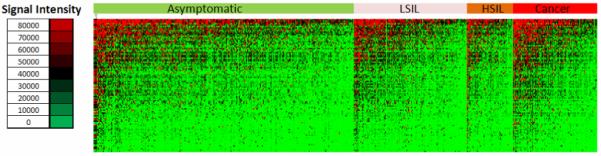
A color scale identifies signal intensities ranging from strong (red), moderate (black) to weak (green). Columns correlate to samples sorted by subjects, lines correlate to the different proteins. The whole collection of sera is grouped into patients with cervical cancer, HSIL, LSIL and asymptomatic infection.
Seroreactivity in correlation with disease state or HPV infection
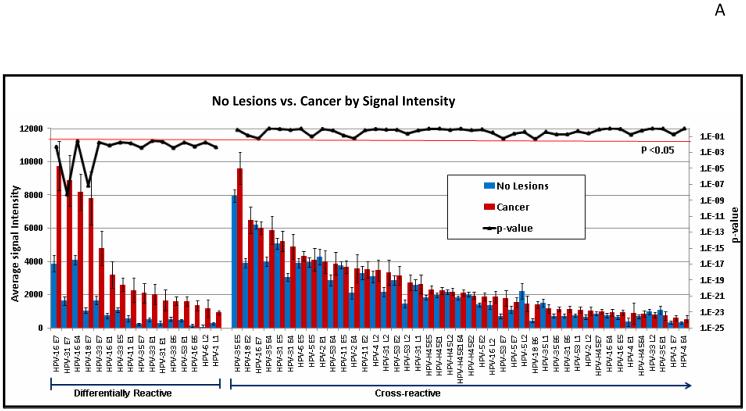
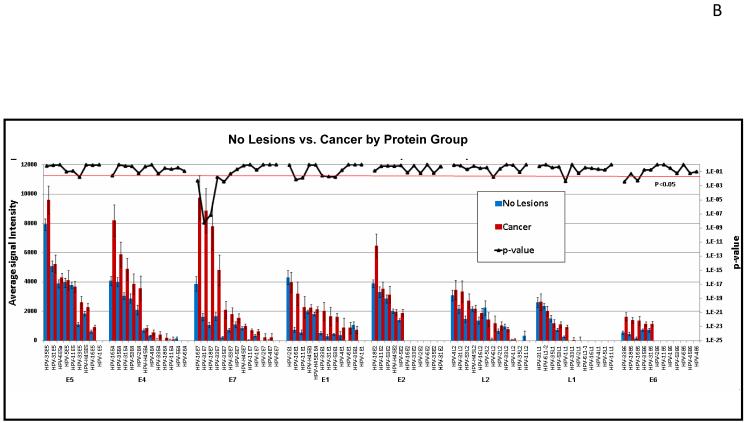
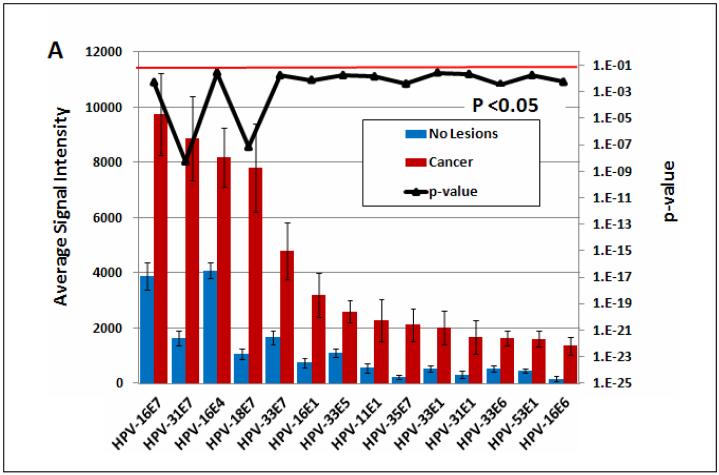
Fig. 4 shows one approach to edit the data of Fig. 3. Here, we have used T-tests to identify differentially reactive antigens between two different patient groups. In Fig. 4A, the data are organized showing 16 significantly differentially reactive antigens (14 of these early proteins) (left) and 43 cross-reactive antigens (right). Red and blue bars are based on the reactivity of sera from cancer patients (93 individuals) and asymptomatic subjects (280 individuals), respectively. The p-values comparing the two groups are plotted in the upper part of the figures as a dark black line, whose exponential scale is indicated along the right margin of the figure. The threshold for significance was set at 0.05 (light red line). It is apparent that the p values of all antigens on the left side of Fig. 4A are smaller than this threshold, and that the five proteins with the highest differential reactivity of all 104 proteins include four E7 proteins of cancer-associated HPV types, namely those of HPV-16, 31, 18, and 33, with E7 of HPV-35 following in ninth position. In Fig. 6B, the data are organized according to the genetic identity of the antigen. The most significantly differentially reactive antigens are the E7 proteins, followed by E1 and E4. Fig. 4B also gives an impression of the overall seroreactivity of all proteins, which, however, could not be precisely quantified, since the exact quantity of each protein spotted in the microarray was not constant. It is apparent, however, that E5, E4, and E7 were the most highly reactive proteins, while E6 showed least reactivity. The dichotomy between the reactivity of the two oncoproteins is not surprising, as it is generally recognized that E6 is only marginally detectable in cancer and cancer cell lines, while E7 is highly expressed in all lesions (McLaughlin-Drubin and Münger, 2009).
Fig. 4. Comparison of the seroreactivity of HPV proteins in cancer and asymptomatic patients.
The mean and standard error of each antigen on the microarray was determined for 280 healthy subjects (blue bars) and 93 cervical cancer patients (red bars), and the p-value (black line) comparing the two groups was determined. Multiple comparison corrected p-values less than 0.05 are considered significant (below the red horizontal line). A. The antigens were sorted by decreasing average signal intensity and organized to show 16 significantly differentially reactive antigens (left) and 43 cross-reactive antigens (p-value >0.05, right). Proteins with average signal intensity lower than no DNA controls are not shown. B. The antigens were organized according to annotated gene ID. All HPV proteins are shown. One third of the significant proteins (p<0.05) are E7 proteins.
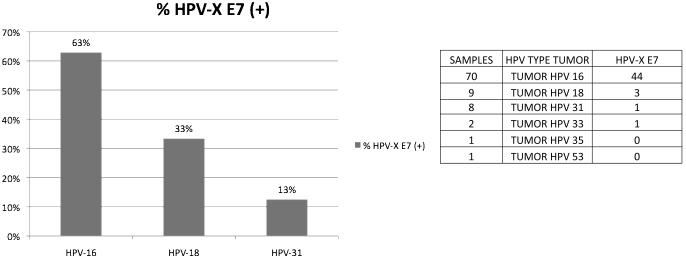
Fig. 6. Anti-E7 immune responses exist only in a subset of cancer patients, whose HPV type status was known.
The data document that the preferential reactivity of E7 in cancer patients does not mean that the presence of this antigen in all patients induces consistently an anti-E7 immune response.
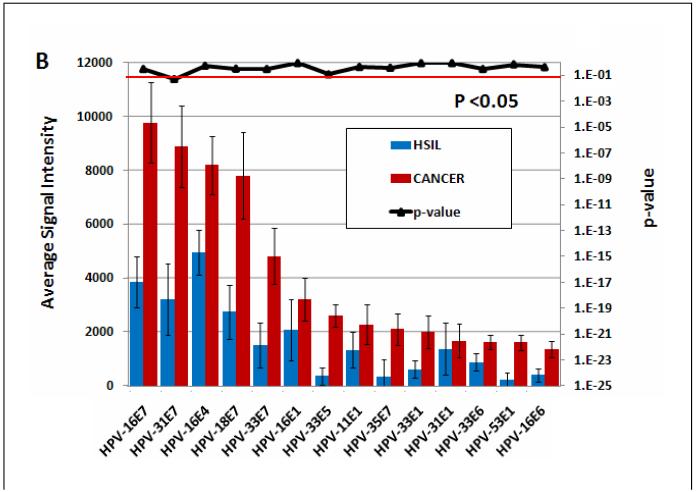
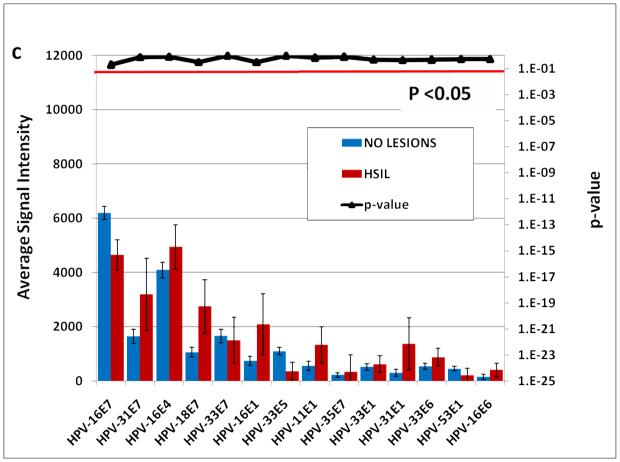
The 14 E antigens that are differentially reactive between cancer patients and asymptomatic subjects are further analyzed in Figure 5A. HPV-31 E7 and HPV-18 E7 are the most significant differentially reactive antigens with p values <1 E6. . Differential reactivity is still evident between cancer patients and HSIL subjects although with reduced significance (Fig. 5B). However, there is no significant difference in reactivity of these antigens between HSIL patients and asymptomatic subjects (Fig. 5C). All individual p values for the comparison of fourteen E proteins among all groups of subjects are listed in Table 1.
Fig. 5. Differential seroreactivity against HPVs early proteins.
A. The differentially reactive E antigens comparing 280 subjects without lesions and 93 cervical cancer patients. B. The same antigens were plotted comparing 93 cancer patients with 50 HSIL patients. C. The same antigens were plotted comparing 280 subjects without Lesions with 50 HSIL patients. Multiple comparison corrected p-values less than 0.05 are considered significant (signal extending below the red horizontal line).
Table 1. Individual p values comparing the seroresponse against listed individual HPV early proteins in the comparison of individuals with cancer, high- and low-grade lesions and asymptomatic smears.
Significantly differentially reactive E proteins (p <0.05) are highlighted with bold fonts. The antigens are ranked by p-value for discriminating individuals with cancer (column 2).
| Comparison (Number of Patients) |
No Lesions (280) vs. Cancer (93) |
Low Grade Lesions (LSIL)(123) vs. Cancer (93) |
High Grade Lesions (HSIL) (50) vs. Cancer (93) |
No Lesions (280) vs. Low Grade Lesions (LSIL) (123) |
No Lesions (280) vs. High Grade Lesions (HSIL)(50) |
Low Grade Lesions (LSIL) (123) VS High Grade Lesions (HSIL)(50) |
|---|---|---|---|---|---|---|
| HPV-31 E7 | 5.87E-09 | 3.87E-05 | 0.0500 | 0.3900 | 0.7441 | 0.9359 |
| HPV-18 E7 | 7.33E-08 | 0.0003 | 0.3283 | 0.5208 | 0.3101 | 0.9359 |
| HPV-33 E6 | 0.0037 | 0.0417 | 0.3045 | 0.4123 | 0.4606 | 0.9359 |
| HPV-35 E7 | 0.0040 | 0.0228 | 0.3796 | 0.9866 | 0.7929 | 0.9359 |
| HPV-16 E7 | 0.0052 | 0.0050 | 0.3045 | 0.3900 | 0.1890 | 0.9359 |
| HPV-16 E6 | 0.0055 | 0.2773 | 0.4246 | 0.3845 | 0.5425 | 0.9862 |
| HPV-16 E1 | 0.0077 | 0.1926 | 0.8683 | 0.3845 | 0.3101 | 0.9359 |
| HPV-11 E1 | 0.0139 | 0.4976 | 0.4427 | 0.1939 | 0.6589 | 0.9359 |
| HPV-33 E7 | 0.0171 | 0.0050 | 0.3045 | 0.7503 | 0.9685 | 0.9359 |
| HPV-33 E5 | 0.0174 | 0.0417 | 0.1198 | 0.8140 | 0.9646 | 0.9862 |
| HPV-53 E1 | 0.0176 | 0.0968 | 0.6998 | 0.8140 | 0.5425 | 0.9359 |
| HPV-31 E1 | 0.0212 | 0.3483 | 0.8561 | 0.4123 | 0.4456 | 0.9970 |
| HPV-33 E1 | 0.0275 | 0.5247 | 0.8529 | 0.5208 | 0.4817 | 0.9862 |
| HPV-16 E4 | 0.0280 | 0.2272 | 0.5426 | 0.8140 | 0.8443 | 0.9640 |
| Number of Differentially Reactive E Proteins |
14 | 7 | 1 | 0 | 0 | 0 |
As the E7 protein is consistently and strongly expressed in cervical carcinomas, we asked whether in cancer patients with known HPV DNA diagnosis, there are consistent serological responses against the homologous E7 proteins. As shown in Fig. 6 there was an anti-E7 response in 44 (63%), three (33%) and one serum (13%) of 70, nine and eight patients afflicted with HPV-16, 18 and 31 tumors, respectively. As a group cervical cancer patients react preferentially to E7 antigens, some cancer patients fail to react.
We also asked whether it is possible that many signals evaluated in this study do not reflect HPV type specific seroreactivity but rather cross-reactivity with homologous proteins of related HPV types, as suggested by the cross-reactivity of monoclonal antibodies that had been raised against HPV type specific E7 and L1 proteins (Fig. S1). A precise evaluation of this possibility would only be possible if one would observe frequently simple patterns of seroreactivity against single HPV proteins, which unfortunately were very rarely encountered. In order to get an estimate whether cross-reactivities created a major bias in this study, we asked how frequently one obtains reactions with multiple homologous proteins. As an example, we chose E7, based on its strong differential seroreactivity between cancer and asymptomatic patients, and since E7 is continuously expressed in HPV associated cancers. Fig. S3 documents that while 55% of all samples showed reaction against any HPV E7 protein, only 24%, 10%, 5% and 1% of all samples reacted with two or more, three or more, four or more, or five or more different E7 proteins. We conclude that for proteins with limited sequence identity such as E7, there is only a moderate bias due to serological cross-reactions.
We also sought to identify a set of antigens able to accurately distinguish HPV infected patients from healthy subjects. As such, we studied the discriminatory power of different sets of antigens using receiver operating characteristic (ROC) curves. We used kernel methods and support vector machines to build linear and nonlinear classifiers. Table 1 shows the top 14 antigens that best discriminate between cancer and asymptomatic subjects. Thirteen of the top 14 discriminatory antigens were early gene products from ‘high-risk’ HPV types.
DISCUSSION
Automated micro-deposition technologies have become powerful tools for molecular studies as they allow screening high-density protein arrays for enzyme-substrate, DNA-protein and protein-protein interactions and are ideally suited for comprehensive investigation of humoral immune responses to infections due to their high-throughput format and miniaturization. Protein microarrays can be used to interrogate the entire proteome of infectious microorganisms consisting of hundreds to thousands of potential antigens while consuming only small quantities of individual sera (a few microliter of sera per patient). This approach permits investigators to perform large-scale sero-epidemiological, longitudinal and sero-surveillance analyses and immunoreactive responses at various stages of the infectious process in a manner not possible with other technologies.
Protein microarray technologies have limitations, though, as protein purification in combination with high-throughput gene expression systems is difficult. One problem is the complexity of protein folding and post-translational modification, which are difficult to recreate on a microarray platform. On the other side, standard criteria for array production and data normalization with noise models, variance estimation, and differential expression analysis techniques have become powerful for the interpretation of results. Based on this progress, successful attempts have been made using protein microarrays for profiling the humoral immune response to numerous infectious agents (Barbour et al., 2008; Davies et al., 2005a and b; Davies et al., 2008; Doolan et al., 2008; Felgner et al., 2009; Sundaresh et al., 2006).
Our study demonstrates the power of a microarray-based approach to scan the humoral immune responses against antigens of the most prevalent HPV types. Our data confirm generalizations that emerged from previous studies of individual or few HPV proteins, namely unsystematic seroresponses to HPV-associated lesions and in the absence of symptoms. Apparently, long-term expression of HPV proteins in patients with cancers or precancerous lesions does not necessarily lead to a strong antibody response against all HPV proteins that are expressed at some stage of the infectious process. This lack of consistent humoral immune response even applies to the E7 protein whose continued expression is a molecular hallmark of HPV associated cancers. However, our research identified antibodies against E7 proteins (but not against the second oncoprotein E6), as the most prominent response in cancer patients, as our analyses confirmed E7 as the most consistent differentially reactive antigen, when sera from cancer patients were compared with those of asymptomatic subjects. Our research has to be compared with – and largely confirms - previous studies of anti-HPV seroresponses in cervical cancer and control patients (DiBonito et al., 2006; Lehtinen et al., 2003; Meschede et al., 1998; Reuschenbach et al, 2009; Smith et al., 2007; Suchánková et al 1991; Viscidi et al 1993; Waterboer et al., 2005). These publications reported immune responses against E6, E7, and L1 as more frequent in patients with invasive cancer than in asymptomatic controls based on enzyme-linked immunosorbent assays (ELISAs) of individual or few HPV proteins. Only one of these studies developed a multiplex approach with glutathione S-transferase fusion E6 proteins (Waterboer et al., 2005) of HPV-16, 18, 52, and 58.
In our analysis of cancer patients, we detected anti-E7 and E1 antibodies most frequently. While significant, these values are still sub-optimal for clinical diagnosis. While measurements of anti-E7 immune responses are at this point not useful as a primary diagnosis to detect HPV associated cancer, they nevertheless raise the issue of medical applications of this finding. Could monitoring of anti-E7 immune response become more useful in the context of cellular tumor specific antigens? Does anti-E7 seroreactivity define a subset of cervical cancers with a unique treatment profile? Do anti-E7 antibodies affect HPV associated tumors, and if yes, would this notion support the case for therapeutic vaccination?
Unfortunately, in contrast to the differences between cancer and asymptomatic patients, we could not confirm anti-E7 seroresponses as useful biomarkers of early progression, as the differences between asymptomatic subjects and patients with precursor lesions were not significant. This is disappointing as serology often has the power to detect latent viral and bacterial infections such as those leading to AIDS and syphilis. A large and long-term prospective Scandinavian study has come to the same conclusion as our research, namely that anti-HPV oncoprotein seropositivity is not induced before invasion and may not be a powerful early diagnostic marker or marker of occult disease (Lehtinen et al., 2003; Stanley, 2003) nor did the presence of antibodies associate significantly with disease prognosis (Silins et al., 2002).
Antibodies against L1 proteins, of central interest in the era of anti-HPV vaccination, were not detected as frequently as those against several early HPV proteins. We did not systematically investigate whether potentially inappropriate folding of L1 affected this part of our data set. However, while anti-L1 immune responses clearly identify HPV infected patients, it was not discriminatory of cancers. This may be because L1 proteins are often not produced in cancers due to interruption of the HPV genomes. However, ELISA assays using VLPs indicate the prevalence of antibodies against L1 may be higher than we find here (for example, Jeong et al 2009, Wang et al 2004) which is likely owing to the native configuration of the L1 presented by the VLP.
Our approach can obviously be used to address many questions that we did not yet address. For example, microarrays could be powerful to better understand the humoral immune response against those HPVs specifically infecting the skin. Our microarrays included HPV-1, 2, 4, and 5, four remotely related HPV types that are normally found in lesions of the skin. Previous serological studies indicate that infections with cutaneous HPVs are much more frequent than reflected by the relatively rare occurrence of skin lesions (Boxman et al., 1999; Steger et al., 1990; Waterboer et al., 2009). Data included in the figures, but not explicitly discussed, showed relatively frequent immuneresponses only against some few proteins of these types, notably HPV-2 E1, HPV-2 E4, HPV-4 L2, HPV-5 E5, and HPV-5 L2. Additional studies could be used to gain a more detailed understanding of the serology of these and other cutaneous HPV types.
MATERIALS AND METHODS
Cloning of HPV genes
The genome clones of HPV types 1, 2, 4, 5, 6, 11, 16, 18, 31, 33, 35, 45, and 53 have been maintained in our (M.K., H.U.B.) labs for more than twenty years and were originally received from the reference center for human papillomaviruses at the German Cancer Research Center, Heidelberg. DNA segments with all the specific open reading frames were generated based on Genbank entries and with reference to the original compilation of the HPV sequence database (Baker and Calef, 1995; Myers et al., 1994). We isolated the open reading frames E6, E7, E1, E2, E4, E5, L2 and L1 of each HPV type listed above in the form of PCR amplicons with primer pairs that corresponded to the first and last 20 nucleotides of each gene. The primer 5′-CATATCGACGACGACGACAAGCATATGCTCGAGN5′-20-3′ contained the first 20 nucleotides of each HPV gene (coded N5′-20), and the primer 5′-N3′20ATCTTAAGCGTAATCCGGAACATCGTATGGGTA-3′ the last 20 nucleotides of the respective gene (N3′-20). The listed nucleotides sequences are complimentary to those used for vector linearization (see following paragraph) and are targets of homologous recombination between HPV amplicons and the vector. In 50 μl PCR reactions, we used 0.02 units/μl Taq DNA polymerase (buffer A, Fisher Scientific), 0.1 mg/ml gelatin (Bloom 300, Porcine; G-1890, Sigma), and a 0.2 mM concentration of each dNTP. Conditions were as follows: Initial denaturation of 95°C for 5 min; 30 cycles of 20 sec at 95°C, 30 sec at 50°C, and 60 sec/kb at 72°C. The PCR product was visualized by agarose gel electrophoresis of 3 μl of the reaction mix.
PCR amplification of the acceptor vector
The details of this protocol have been published (Davies et al., 2005a and b). Linear acceptor vector was generated by PCR amplifying 30 pg plasmid pXT7-DEST with the Expand Long Template PCR system buffer 1 (Roche, Switzerland) containing 0.2 mM dNTPs and 0.8 u Taq polymerase and the two primers (0.5 μM each): 5′-TACCCATACGATGTTCCGGATTAC-3′ (facing from the end of the linear PCR product toward the T7 promoter and recipient of the 5′ end of the HPV insert) and 5′-CTCGAGCATATGCTTGTCGTCGTCG-3′ (recipient of the 3′ end of the HPV insert) with the following conditions: initial denaturation of 94°C for 5 min, followed by a 35 fold repetition of 95°C for 30 sec, 50°C for 30 sec, and 68°C for 3 min, and a final extension at 68°C for 10 min.
In vivo recombination cloning method
10 μl of competent E. coli DH-alpha were transformed with 40 ng of PCR generated linear vector and 10 ng of PCR generated gene fragment (molar ratio approximately 1:1) following the typical temperature shift and growth steps. Prior purification of the PCR products was not necessary. The transformed E. coli preparations were grown overnight in LB medium under kanamycin selection. Plasmid isolation and purification from these cultures did not require prior colony selection as described (Davies et al., 2005a and b).
HPV protein expression and microarray chip printing
Plasmid templates for in vitro transcription/translation were prepared by using QIAprep Spin Miniprep kits (Qiagen, Venlo, Netherlands). In vitro transcription/translation reactions (RTS 100 Escherichia coli HY kits; Roche, Switzerland) were set up in 0.2 ml PCR 12-well strip tubes and incubated for 5 h at 30 °C, according to the manufacturer’s instruction. For microarrays, 32.5 μl of 0.2% Tween-20 was mixed with 100 μl of RTS reaction (to a final concentration of 0.05% Tween-20), and 15 μl volumes were transferred to 384-well plates for printing. The plates were centrifuged at 1600 x g to pellet any precipitate, and the supernatant was printed without further purification onto NC coated FAST glass slides (Schleicher and Schuell Bioscience, Keen, NH, USA) with an OmniGrid 100 microarray printer (Genomic Solutions, Ann Arbor, MI, USA). The proteins from all genes were spotted in duplicate, and all data values were used as the average of pairs. In addition, each chip contained an area printed with controls consisting of RTS reaction without template DNA or with an empty T7 vector control. Expression of complete proteins was monitored with monoclonal anti-polyhistidine (clone His-1, Sigma-Aldrich, St. Louis, USA) and anti-hemagglutinin (clone 3F10; Roche, Switzerland).
Antibody assays and reading of microarrays
Prior to array probing, sera were diluted to 1/100 in Protein Array Blocking Buffer (Whatman, GE Healthcare) containing E. coli lysate at a final concentration of 10 mg/ml and incubated at room temperature for 1 h with constant mixing. The arrays were rehydrated and blocked in blocking buffer for 30 min and then probed with the pretreated sera for 24 h at 4°C with constant agitation. The slides were washed five times in 10 mM Tris (pH 8.0)-150 mM NaCl containing 0.05% Tween 20 buffer, and bound human antibodies were detected by 1 h incubation in Biotin-sp conjugated Affini Pure Goat anti-human IgG Fc (Fc-γ fragment specific) secondary antibody (Jackson Immunoresearch, West Grove, PA) diluted 1/1000 in blocking buffer. The slides were washed three times in 10 mM Tris buffer (pH 8.0)-150 mM NaCl containing 0.05% Tween 20. Bound antibodies were detected by 1 h incubation with streptavidin-conjugated SureLight® P-3 tertiary reagent (Columbia Biosciences, Columbia, MD) diluted 1/200 in blocking buffer. After being washed five times, the slides were air dried under brief centrifugation and stored at 18°C in a desiccator. The arrays were examined with a Perkin Elmer ScanArray Express HT confocal laser scanner at a wavelength of 670 nm and intensities were quantified using ProScanArray Express software (Perkin Elmer, Waltham, MA). All signal intensities were corrected for spot-specific background.
Origin of clinical samples
All sera for this project came from three archival collections, i.e. they were aliquots of samples that had been collected for other research than our study. Altogether, 546 samples entered this project. Among these, 427 samples were obtained from patients treated in Monterrey, Mexico, between the years 2002 and 2008. Among these samples from Monterrey, 280 subjects did not contain any detectable cervical lesions. From these aymptomatic individuals we obtained smears that were presently HPV positive and negative as judged by DNA testing. HPV status in these asymptomatic patients did not enter this study as a separate variable, as pilot data did not show any serological differences between the two groups. 94 patients from Monterrey had low-grade (LSIL) and 39 patients high-grade squamous intraepithelial lesions (HSIL), 13 patients had squamous cervical carcinomas and one patient had an adenocarcinoma. Sera of 61 patients with squamous cell carcinomas and 18 with adenocarcinomas were obtained from Mexico City, Mexico. Squamous cell carcinomas and adenocarcinomas were treated as one category in this study. The sera from 29 patients with LSIL and 11 patients with HSIL were obtained from Sao Paulo, Brazil. As a control for patients without a history of sexual activity, we used ten sera of two- to three-year-old children from Kenya that had been sampled during malaria related research (Doolan et al., 2008). The original studies had been done with permission of the respective Institutional Review Boards (IRB). For our research, the IRB of the University of California Irvine classified this project as exempt as all our samples were archival and did not contain any patient identifiers.
Monoclonal antibodies
The following 12 monoclonal antibodies were used: from Santa Cruz Biotechnology, Inc., Santa Cruz, California: HPV16 E6 (SC-1583); HPV-16 E7 (SC-264); HPV-16 E7 (SC-6981); HPV-16 E1/E4 (SC-53324); HPV-16 L2 (SC-65708); HPV-18 E7 (SC-51954); HPV-18 E2 (SC-26938); HPV-11 E7 (SC-66145); from Chemicon, Temecula, California: HPV-18,16 E6 (MAB874); HPV-16 E2 (MAB8678); HPV-16 E2 (MAB8679); HPV-16 L1 (MAB885).
Data Analysis
Data were analyzed with the R (http://www.r-project.org) and SAS (http://www.sas.com/) statistical software. Previous literature has shown that data derived from microarray platforms are heteroskedatic (Baldi and Hatfield, 2002; Sundaresh et al., 2006 and 2007). In order to stabilize the variance (Durbin et al., 2002; Huber et al., 2002), the vsn normalization method implemented as part of the Bioconductor suite (www.bioconductor.org) is applied to the quantified array intensities. In addition to removing heteroskedacity, this procedure corrects for non-specific noise effects by finding maximum likelihood shifting and scaling parameters for each array such that control probe variance is minimized. This calibration has been shown to be effective on a number of platforms (Krell et al., 2004).
Differentially reactive proteins between groups were determined using a Bayes regularized t-test adapted from Cyber-T for protein arrays (Baldi and Hatfield, 2002; Baldi and Long, 2001), which has been shown to be more effective than other differential expression techniques (Long et al., 2001). To account for multiple comparison conditions, the Benjamini and Hochberg (BH) method was used to control the false discovery rate (Benjamini and Hochbert, 1995). After Benjamini and Hochberg correction, p-values smaller than 0.05 are considered significant and the corresponding protein is considered differentially reactive. Multiplex classifiers were constructed using linear and non-linear Support Vector Machines (SVMs) using the “e1071” R package. Plots of receiver operating characteristic (ROC) curves were made with the “ROCR” R package. Sensitivity and specificity were determined from the resulting ROC curves.
Supplementary Material
Supplementary Fig 1. Processing of microarrays containing 104 proteins from 13 HPV types with four monoclonal antibodies. A: Reaction of an anti-HPV-16 E7 antibody (SC-6981) with the homologous HPV-16 E7 and cross-reactivity with the E7 proteins of the three closest relatives of HPV-16, HPV-31, 33, and 35, apparently due to a conserved epitope. B: Reaction of an anti-HPV-11 E7 antibody (SC-66145) with the HPV-11 E7 protein, cross-reaction with the E7 protein of HPV-6, the closest relative of HPV-11, and cross-reaction with the E7 proteins of the remotely related types HPV-16 and HPV-31. C: Selective recognition of the HPV-16 E4 protein by an anti-HPV-16 E1/E4 antibody (SC-53324). This type of result was typical for the experiments with those ten monoclonal antibodies not shown in this paper. D: An anti-HPV-16 L1 antibody (MAB885) recognizes the homologous HPV-16 L1 protein, and the L1 proteins of HPV-1, 2 (spotted twice), 6 (spotted twice), 11, 18, 31, and 33. This particular antibody (CamVir-1) was raised to non-native HPV16 L1 fusion protein (McLean et al., 1990). It is known to react well with denatured L1 (such as in Western blots), and it also shows cross-reactivity with the L1 antigen of other HPV types. Unexpectedly, this antibody also reacted non-specifically with the E1 proteins of HPV-31, 35, and 53, and the L2 protein of HPV-18 on they array.
Supplementary Fig 2. Lack of anti-HPV protein reactivity of sera from children without known exposure to sexually transmitted HPV types. Lack of serological activity of the individuals K27 and K39 was typical for eight out of ten children, while individual K73 had antibodies against HPV-5 L2, and K66 antibodies against HPV-35 E4 and E5.
Supplementary Fig 3. Low reactivity against the E7 proteins of multiple HPV types excludes type-independent cross-reactivity as a major source of generation of false positive signals. This analysis shows that the cross-reactivity of monoclonal antibodies as documented in Fig. S1 does not lead systematically to multiple anti-E7 scores.
ACKNOWLEDGEMENTS
Our research was supported by funds from the Chao Family Comprehensive Cancer Center of the University of California Irvine to H.U.B., by the NIH grant U01AI078213 to P.L.F., by the NIH grant K23CA087558 to B.M., and by four grants from UC Mexus to M.L., H.B., A.G.C., and H.U.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Baker CC, Calef C. Maps of papillomavirus transcripts. In: Myers G, Bernard HU, Delius H, Baker CC, Icenogle J, Halpern A, Wheeler C, editors. Human papillomaviruses 1995 compendium. C. Los Alamos National Laboratory; Los Alamos, New Mexico: 1995. pp. 3–19. part III-A. [Google Scholar]
- Baldi P, Hatfield GW. Experiments to Data Analysis and Modeling. Cambridge University Press; 2002. DNA Microarrays and Gene Expression. [Google Scholar]
- Baldi P, Long AD. A Bayesian framework for the analysis of microarray expression data: regularized t-test and statistical inferences of gene changes. Bioinformatics. 2001;17:509–519. doi: 10.1093/bioinformatics/17.6.509. [DOI] [PubMed] [Google Scholar]
- Barbour AG, Jasinskas A, Kayala MA, Davies DH, Steere AC, Baldi P, Felgner PL. A genome-wide proteome array reveals a limited set of immunogens in natural infections of humans and white-footed mice with Borrelia burgdorferi. Infect Immun. 2008;76:3374–3389. doi: 10.1128/IAI.00048-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beare PA, Chen C, Bouman T, Pablo J, Unal B, Cockrell DC, Brown WC, Barbian KD, Porcella SF, Samuel JE, Felgner PL, Heinzen RA. Candidate antigens for Q fever serodiagnosis revealed by immunoscreening of a Coxiella burnetii protein microarray. Clin. Vaccine Immunol. 2008;15:1771–1779. doi: 10.1128/CVI.00300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Roy. Statist. Soc. Ser. B. 1995;57:289–300. [Google Scholar]
- Bernard HU, Burk RD, Chen Z, van Doorslaer K, zur Hausen H, de Villiers EM. Classification of papillomaviruses based on 189 PV types and proposal of taxonomic amendments. Virology. 2010 doi: 10.1016/j.virol.2010.02.002. publ. by epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxman IL, Mulder LH, Russell A, Bouwes-Bavinck JN, Green A, Ter Schegget J. Human papillomavirus type 5 is commonly present in immunosuppressed and immunocompetent individuals. Br. J. Dermatol. 1999;141:246–249. doi: 10.1046/j.1365-2133.1999.02972.x. [DOI] [PubMed] [Google Scholar]
- Davies DH, Liang X, Hernandez JE, Randall A, Hirst S, Mu Y, Romero KM, Nguyen TT, Kalantari-Dehaghi M, Crotty S, Baldi P, Villarreal LP, Felgner PL. Profiling the humoral immune response to infection by using proteome microarrays: high-throughput vaccine and diagnostic antigen discovery. Proc. Natl. Acad. Sci. USA. 2005a;102:547–552. doi: 10.1073/pnas.0408782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DH, McCausland MM, Valdez C, Huynh D, Hernandez JE, Mu Y, Hirst S, Villarreal L, Felgner PL, Crotty S. Vaccinia virus H3L envelope protein is a major target of neutralizing antibodies in humans and elicits protection against lethal challenge in mice. J. Virol. 2005b;79:11724–11733. doi: 10.1128/JVI.79.18.11724-11733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DH, Wyatt LS, Newman FK, Earl PL, Chun W, Hernandez JE, Molina DM, Hirst S, Moss B, Frey SE, Felgner PL. Antibody profiling by proteome microarray reveals the immunogenicity of the attenuated smallpox vaccine modified vaccinia virus ankara is comparable to that of Dryvax. J. Virol. 2008;82:652–663. doi: 10.1128/JVI.01706-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gruijl TD, Bontkes HJ, Walboomers JM, Schille JT, Stukart MJ, Groot BS, Chabaut MM, Remmink AJ, Verheijen RH, Helmerhorst TJ, Meijer CJ, Scheper RJ. Immunoglobulin G responses against human papillomavirus type 16 virus-like particles in a prospective nonintervention cohort study of women with cervical intraepithelial neoplasia. J. Natl. Cancer Inst. 1997;89:630–638. doi: 10.1093/jnci/89.9.630. [DOI] [PubMed] [Google Scholar]
- de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324:17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- DiBonito P, Grasso F, Mochi S, Accardi L, Dona MG, Branca M, Costa S, Mariani L, Agarossi A, Ciotti M, Syrjänen K, Giorgi C. Serum antibody response to Human papillomavirus (HPV) infections detected by a novel ELISA technique based on denatured recombinant HPV16 L1, L2, E4, E6 and E7 proteins. Infect. Agent. Cancer. 2006;8:1–6. doi: 10.1186/1750-9378-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolan DL, Mu Y, Unal B, Sundaresh S, Hirst S, Valdez C, Randall A, Molina D, Liang X, Freilich DA, Oloo JA, Blair PL, Aguiar JC, Baldi P, Davies DH, Felgner PL. Profiling humoral immune responses to P. falciparum infection with protein microarrays. Proteomics. 2008;8:4680–4694. doi: 10.1002/pmic.200800194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorbar J, Ely S, Sterling J, McLean C, Crawford L. Specific interaction between HPV-16 E1-E4 and cytokeratins results in collapse of the epithelial cell intermediate filament network. Nature. 1991;352:824–827. doi: 10.1038/352824a0. [DOI] [PubMed] [Google Scholar]
- Durbin BP, Hardin JS, Hawkins DM, Rocke DM. A variance-stabilizing transformation for gene-expression microarray data. Bioinformatics. 2002;18(Suppl 1):S105–110. doi: 10.1093/bioinformatics/18.suppl_1.s105. [DOI] [PubMed] [Google Scholar]
- Egelkrout EM, Galloway DA. The humoral immune response to human papillomavirus. In: Garcea RL, DiMaio D, editors. The Papillomaviruses. Springer Science; New York: 2007. pp. 277–312. [Google Scholar]
- Felgner PL, Kayala MA, Vigil A, Burk C, Nakajima-Sasaki R, Pablo J, Molina DM, Hirst S, Chew JS, Wang D, Tan G, Duffield M, Yang R, Neel J, Chantratita N, Bancroft G, Lertmemongkolchai G, Davies DH, Baldi P, Peacock S, Titball RW. A Burkholderia pseudomallei protein microarray reveals serodiagnostic and cross-reactive antigens. Proc. Natl. Acad. Sci. USA. 2009;106:13499–13504. doi: 10.1073/pnas.0812080106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber W, von Heydebreck A, Sültmann H, Poustka A, Vingron M. Variance stabilization applied to microarray data calibration and to the quantification of differential expression. Bioinformatics. 2002;18(Suppl 1):S96–104. doi: 10.1093/bioinformatics/18.suppl_1.s96. [DOI] [PubMed] [Google Scholar]
- Jeong NH, Woo MK, Lee NW, Hur SJ, Choi KS, Kim HJ. Human papillomavirus 16 and 18 L1 serology in Korean women with high-grade cervical intraepithelial neoplasia and cervical cancer. Arch. Pharm. Res. 2009;32:1013–1018. doi: 10.1007/s12272-009-1706-z. [DOI] [PubMed] [Google Scholar]
- Kreil DP, Karp NA, Lilley KS. DNA microarray normalization methods can remove bias from differential protein expression analysis of 2D difference gel electrophoresis results. Bioinformatics. 2004;20:2026–2034. doi: 10.1093/bioinformatics/bth193. [DOI] [PubMed] [Google Scholar]
- Lehtinen M, Pawlita M, Zumbach K, Lie K, Hakama M, Jellum E, Koskela P, Luostarinen T, Paavonen J, Pukkala E, Sigstad E, Thoresen S, Dillner J. Evaluation of antibody response to human papillomavirus early proteins in women in whom cervical cancer developed 1 to 20 years later. Am. J. Obstet. Gynecol. 2003;188:49–55. doi: 10.1067/mob.2003.98. [DOI] [PubMed] [Google Scholar]
- Long AD, Mangalam HJ, Chan BY, Tolleri L, Hatfield GW, Baldi P. Improved statistical inference from DNA microarray data using analysis of variance and a Bayesian statistical framework. Analysis of global gene expression in Escherichia coli K12. J. Biol. Chem. 2001;276:19937–19944. doi: 10.1074/jbc.M010192200. [DOI] [PubMed] [Google Scholar]
- McLaughlin-Drubin ME, Münger K. The human papillomavirus E7 oncoprotein. Virology. 2009;384:335–344. doi: 10.1016/j.virol.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CS, Churcher MJ, Meinke J, Smith GL, Higgins G, Stanley M, Minson AC. Production and characterisation of a monoclonal antibody to human papillomavirus type 16 using recombinant vaccinia virus. J. Clin. Pathol. 1990;43:488–492. doi: 10.1136/jcp.43.6.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meschede W, Zumbach K, Braspenning J, Scheffner M, Benitez-Bribiesca L, Luande J, Gissmann L, Pawlita M. Antibodies against early proteins of human papillomaviruses as diagnostic markers for invasive cervical cancer. J. Clin. Microbiol. 1998;36:475–480. doi: 10.1128/jcm.36.2.475-480.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, Snijders PJF, Meijer CJLM. Epidemiological classification of human papillomavirus types associated with cervical cancer. New Engl. J. Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- Myers G, Bernard HU, Delius H, Favre M, Icenogel J, van Ranst M, Wheeler C, editors. Human papillomaviruses 1994. A compilation and analysis of nucleic acid and amino acid sequences. Los Alamos National Laboratory; Los Alamos, New Mexico, USA: [Google Scholar]
- Ravaggi A, Romani C, Pasinetti B, Tassi R, Bignotti E, Bandiera E, Odicino FE, Ragnoli M, Donzelli C, Falchetti M, Calza S, Santin AD, Pecorelli S. Correlation between serological immune response analyzed by a new ELISA for HPV-16/18 E7 oncoprotein and clinical characteristics of cervical cancer patients. Arch. Virol. 2006;151:1899–1916. doi: 10.1007/s00705-006-0787-y. [DOI] [PubMed] [Google Scholar]
- Reuschenbach M, Waterboer T, Wallin KL, Einenkel J, Dillner J, Hamsikova E, Eschenbach D, Zimmer H, Heilig B, Kopitz J, Pawlita M, Knebel Doeberitz M, Wentzensen N. Characterization of humoral immune responses against p16, p53, HPV16 E6 and HPV16 E7 in patients with HPV-associated cancers. Int. J. Cancer. 2008;123:2626–2631. doi: 10.1002/ijc.23837. [DOI] [PubMed] [Google Scholar]
- Reuschenbach M, von Knebel Doeberitz M, Wentzensen N. A systematic review of humoral immune responses against tumor antigens. Cancer Immunol. Immunother. 2009;58:1535–1544. doi: 10.1007/s00262-009-0733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosales R, López-Contreras M, Cortes RR. Antibodies against human papillomavirus (HPV) type 16 and 18 E2, E6 and E7 proteins in sera: correlation with presence of papillomavirus DNA. J. Med. Virol. 2001;65:736–644. doi: 10.1002/jmv.2098. [DOI] [PubMed] [Google Scholar]
- Silins I, Avall-Lundqvist E, Tadesse A, Jansen KU, Stendahl U, Lenner P, Zumbach K, Pawlita M, Dillner J, Frankendal B. Evaluation of antibodies to human papillomavirus as prognostic markers in cervical cancer patients. Gynecol. Oncol. 2002;85:333–338. doi: 10.1006/gyno.2002.6628. [DOI] [PubMed] [Google Scholar]
- Smith EM, Ritchie JM, Pawlita M, Rubenstein LM, Haugen TH, Turek LP, Hamsikova E. Human papillomavirus seropositivity and risks of head and neck cancer. Int. J. Cancer. 2007;120:825–832. doi: 10.1002/ijc.22330. [DOI] [PubMed] [Google Scholar]
- Stanley M. Antibody reactivity to HPV E6 and E7 oncoproteins and early diagnosis of invasive cervical cancer. Am. J. Obstet. Gynecol. 2003;188:3–4. doi: 10.1067/mob.2003.97. [DOI] [PubMed] [Google Scholar]
- Steger G, Olszewsky M, Stockfleth E, Pfister H. Prevalence of antibodies to human papillomavirus type 8 in human sera. J. Virol. 1990;64:4399–4406. doi: 10.1128/jvi.64.9.4399-4406.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchánková A, Ritterová L, Krcmár M, Krchnák V, Vágner J, Jochmus I, Gissmann L, Kanka J, Vonka V. Comparison of ELISA and western blotting for human papillomavirus type 16 E7 antibody determination. J. Gen. Virol. 1991;10:2577–2581. doi: 10.1099/0022-1317-72-10-2577. [DOI] [PubMed] [Google Scholar]
- Sundaresh S, Doolan DL, Hirst S, Mu Y, Unal B, Davies DH, Felgner PL, Baldi P. Identification of humoral immune responses in protein microarrays using DNA microarray data analysis techniques. Bioinformatics. 2006;22:1760–1766. doi: 10.1093/bioinformatics/btl162. [DOI] [PubMed] [Google Scholar]
- Sundaresh S, Randall A, Unal B, Petersen JM, Belisle JT, Hartley MG, Duffield M, Titball RW, Davies DH, Felgner PL, Baldi P. From protein microarrays to diagnostic antigen discovery: a study of the pathogen Francisella tularensis. Bioinformatics. 2007;23:i508–518. doi: 10.1093/bioinformatics/btm207. [DOI] [PubMed] [Google Scholar]
- Villa LL, Ault KA, Giuliano AR, Costa RL, Petta CA, Andrade RP, Brown DR, Ferenczy A, Harper DM, Koutsky LA, Kurman RJ, Lehtinen M, Malm C, Olsson SE, Ronnett BM, Skjeldestad FE, Steinwall M, Stoler MH, Wheeler CM, Taddeo FJ, Yu J, Lupinacci L, Railkar R, Marchese R, Esser MT, Bryan J, Jansen KU, Sings HL, Tamms GM, Saah AJ, Barr E. Immunologic responses following administration of a vaccine targeting human papillomavirus Types 6, 11, 16, and 18. Vaccine. 2006;24:5571–5583. doi: 10.1016/j.vaccine.2006.04.068. [DOI] [PubMed] [Google Scholar]
- Viscidi RP, Sun Y, Tsuzaki B, Bosch FX, Muñoz N, Shah KV. Serologic response in human papillomavirus-associated invasive cervical cancer. Int. J. Cancer. 1993;55:780–784. doi: 10.1002/ijc.2910550515. [DOI] [PubMed] [Google Scholar]
- Wang SS, Schiffman M, Herrero R, Carreon J, Hildesheim A, Rodriguez AC, Bratti MC, Sherman ME, Morales J, Guillen D, Alfaro M, Clayman B, Burk RD, Viscidi RP. Determinants of human papillomavirus 16 serological conversion and persistence in a population-based cohort of 10 000 women in Costa Rica. Br.J. Cancer. 2004;91:1269–74. doi: 10.1038/sj.bjc.6602088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterboer T, Sehr P, Michael KM, Franceschi S, Nieland JD, Joos TO, Templin MF, Pawlita M. Multiplex human papillomavirus serology based on in situ-purified glutathione s-transferase fusion proteins. Clin. Chem. 2005;51:1845–1853. doi: 10.1373/clinchem.2005.052381. [DOI] [PubMed] [Google Scholar]
- Waterboer T, Neale R, Michael KM, Sehr P, de Koning MN, Weissenborn SJ, Sampogna F, Abeni D, Green AC, Bouwes Bavinck JN, Pawlita M. Antibody responses to 26 skin human papillomavirus types in the Netherlands, Italy and Australia. J. Gen. Virol. 2009;90:1986–1998. doi: 10.1099/vir.0.010637-0. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat. Rev. Cancer. 2002;2:342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig 1. Processing of microarrays containing 104 proteins from 13 HPV types with four monoclonal antibodies. A: Reaction of an anti-HPV-16 E7 antibody (SC-6981) with the homologous HPV-16 E7 and cross-reactivity with the E7 proteins of the three closest relatives of HPV-16, HPV-31, 33, and 35, apparently due to a conserved epitope. B: Reaction of an anti-HPV-11 E7 antibody (SC-66145) with the HPV-11 E7 protein, cross-reaction with the E7 protein of HPV-6, the closest relative of HPV-11, and cross-reaction with the E7 proteins of the remotely related types HPV-16 and HPV-31. C: Selective recognition of the HPV-16 E4 protein by an anti-HPV-16 E1/E4 antibody (SC-53324). This type of result was typical for the experiments with those ten monoclonal antibodies not shown in this paper. D: An anti-HPV-16 L1 antibody (MAB885) recognizes the homologous HPV-16 L1 protein, and the L1 proteins of HPV-1, 2 (spotted twice), 6 (spotted twice), 11, 18, 31, and 33. This particular antibody (CamVir-1) was raised to non-native HPV16 L1 fusion protein (McLean et al., 1990). It is known to react well with denatured L1 (such as in Western blots), and it also shows cross-reactivity with the L1 antigen of other HPV types. Unexpectedly, this antibody also reacted non-specifically with the E1 proteins of HPV-31, 35, and 53, and the L2 protein of HPV-18 on they array.
Supplementary Fig 2. Lack of anti-HPV protein reactivity of sera from children without known exposure to sexually transmitted HPV types. Lack of serological activity of the individuals K27 and K39 was typical for eight out of ten children, while individual K73 had antibodies against HPV-5 L2, and K66 antibodies against HPV-35 E4 and E5.
Supplementary Fig 3. Low reactivity against the E7 proteins of multiple HPV types excludes type-independent cross-reactivity as a major source of generation of false positive signals. This analysis shows that the cross-reactivity of monoclonal antibodies as documented in Fig. S1 does not lead systematically to multiple anti-E7 scores.