In 1954 Rudolf Altschul wrote “One is as old as one’s endothelium;”1 a visionary statement in a time when the endothelial layer was viewed as a passive sheet of cells lining the vasculature. It is now clear that the endothelium is a dynamic structure, actively involved in numerous arterial functions, from regulation of permeability to modulation of vascular responses to hemodynamic forces. As such, slight disruptions in the physiology of the endothelium can lead to pathophysiological consequences, with endothelial dysfunction considered to be a key initial event for the development of atherosclerosis2. In this regard, aging is associated with impairments in endothelial function and an increased prevalence of atherosclerosis3, 4. Even in atherosclerotic-free normotensive individuals, endothelial dependent dilation is impaired in older populations5, 6. In addition, with age, the endothelium favors constrictive properties7, expresses pro-inflammatory markers8, and exhibits enhanced production of reactive oxygen species9; factors conducive to the development and progression of atherosclerosis. However, the mechanism(s) contributing to a pro-atherogenic endothelial cell environment with advanced age remain incompletely defined.
Interestingly, atherosclerotic lesions are preferentially located in regions distinguished by oscillatory (bidirectional blood flow) and low mean shear stress; whereas areas exposed to unidirectional and moderate shear are protected10-12. In this context, in vitro studies using cell culture and isolated perfused arteries have demonstrated that unidirectional high shear stress (within physiological limits) induces expression of anti-atherogenic genes (i.e. eNOS, SOD) and inhibits expression of atherogenic genes (VCAM-1, ICAM-1, E-selectin, MCP-1), while exposure of endothelial cells to periods of disturbed flow (increased oscillatory shear) and/or low mean shear promotes a pro-atherogenic phenotype13-17. In short, a growing body of evidence indicates that favorable hemodynamic forces are crucial for endothelial regulation and conditions that result in decreased or disturbed shear may precipitate the development of atherosclerosis. Therefore, we sought to characterize vascular shear rate patterns in young and older adults in order to better understand the enhanced susceptibility to atherosclerosis with aging.
Shear rate magnitudes and patterns were examined in the common femoral artery; a vessel highly prone to atherosclerosis18-20. Twenty young (24 ± 1 yr; 14 men) and eighteen older (60 ± 1 yr; 12 men) healthy adults volunteered for participation in this study. All subjects were recreationally active, free of known cardiovascular, pulmonary, metabolic, or neurological disease and none were using prescribed or over the counter medications. Fasting blood chemistry screening indicated that triglycerides, cholesterol, lipoproteins, and glucose concentrations in the older subjects were within the normal range for healthy adults. There were no significant age-group differences in resting heart rate, systolic, diastolic or mean arterial blood pressures (P>0.05). Experimental procedures were approved by the University of Missouri Health Sciences Institutional Review Board and all subjects provided written informed consent prior to participation
Under supine resting conditions, the common femoral artery was imaged ~2 cm proximal to the bifurcation of the superficial and deep branches using a duplex Doppler ultrasound unit (Logiq 7, GE Medical Systems, Milwaukee, WI) equipped with a linear array transducer operating at a frequency of 10 MHz. Femoral blood velocity was obtained using the same probe in pulsed-wave mode, operating at a linear frequency of 5 Hz and at an insonation angle of 60°. Recordings of femoral artery blood velocity were obtained using a large sample volume to encompass the entire vessel lumen without extending outside of it. Therefore, the measurements of blood velocity represent a mean of the entire cross-sectional area of the vessel. Using femoral artery diameter and velocity, antegrade, retrograde and mean shear rates were calculated. Antegrade shear represents forward flow through the femoral artery, whereas retrograde shear results from a reversal of flow. In addition oscillatory shear, representative of bidirectional flow, was evaluated by calculating the oscillatory shear index: |retrograde shear|/(|retrograde + antegrade shear|).
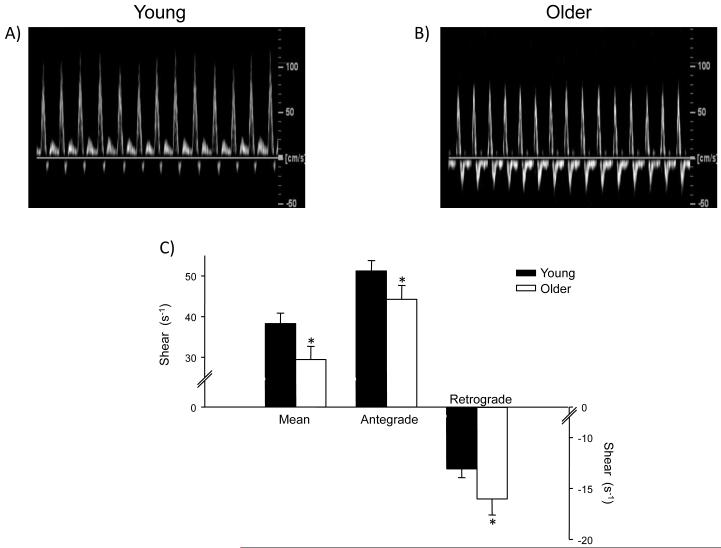
Femoral artery antegrade shear was lower, whereas retrograde shear was significantly enhanced (i.e. more negative) in the older group; thus both contributing to an overall low mean shear (Figure 1). Moreover, oscillatory shear was 30% greater in the older adults, when compared to the younger subjects, indicating that the femoral artery is exposed to greater bidirectional shear throughout the cardiac cycle. Similar results were found when variables were normalized to lean leg muscle mass obtained from a dual-energy X-ray absorptometry scan (data not shown). Importantly, measurements made on multiple days in a subset of subjects (6 young and 6 older) indicated that these findings are highly reproducibile, with intraclass correlation coefficients of 0.99, 0.98 and 0.98 for mean, antegrade and retrograde shear, respectively. In addition, common femoral artery diameter was similar between the young (0.87 ± 0.03 cm) and older (0.86 ± 0.03 cm) groups.
Figure 1.
Original common femoral artery velocity tracings from a young (panel A) and older (panel B) subject. Panel C illustrates the group summary data for mean, antegrade and retrograde shear.
These findings clearly indicate that shear patterns within the common femoral artery are altered with healthy aging. Given that disturbed shear forces have been proposed to be involved in the pathogenesis of atherosclerosis13-17, these data lend support to a potential mechanism contributing to atherosclerotic development with aging. Interestingly, recent data in young healthy humans has demonstrated that exposing the brachial artery to an acute period of enhanced retrograde and oscillatory shear negatively impacts flow-mediated dilation21. In this regard, even in the absence of overt disease, a chronic decrease in antegrade shear, along with enhanced retrograde and oscillatory shear with age (as shown in the current findings), may promote a pro-atherogenic environment and place older adults at an increased risk for the development of atherosclerosis.
Considering the potential detrimental impact of age-dependent alterations in vascular shear patterns, an understanding of the possible contributing mechanisms is necessary. In the present study, femoral vascular resistance was higher in the older group (P<0.05), with femoral vascular resistance being positively correlated to oscillatory shear in the older (R2=0.353, P=0.009), but not younger subjects (R2=0.011, P=0.661). As such, any factor that could increase femoral vascular resistance (i.e. increased sympathetic nerve activity, or increased levels of circulating constrictors such as AngII, and ET-1) may contribute to age-related alterations in shear profiles4. In addition, age-dependent changes in the structure of the vasculature, such as increased collagen, calcification or decreased elastin content of the arteries, could enhance peripheral vascular resistance and negatively impact shear patterns4.
Overall, these novel observations demonstrate for the first time that healthy aging is associated with alterations in femoral artery shear patterns. A shift to pro-atherogenic shear patterns was noted, including a decreased overall mean and antegrade shear, and an enhanced retrograde and oscillatory shear. Considering the potential detrimental impact of these hemodynamic patterns, future studies are warranted to determine the underlying mechanisms, as well as potential interventions (e.g. exercise training) that may offset and/or improve shear patterns with aging.
Abbreviations
- Ang II
angiotensin II
- eNOS
endothelial nitric oxide synthase
- ET-1
endothelin 1
- ICAM-1
inter-cellular adhesion molecule 1
- MCP-1
monocyte chemotactic protein 1
- SOD
superoxide dismutase
- VCAM-1
vascular cell adhesion molecule 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Altschul R. Endothelium, Its Development, Morphology, Function and Pathology. MacMillan; New York: 1954. [Google Scholar]
- 2.De Meyer GR, Herman AG. Vascular endothelial dysfunction. Progress in cardiovascular diseases. 1997;39:325–342. doi: 10.1016/s0033-0620(97)80031-x. [DOI] [PubMed] [Google Scholar]
- 3.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–497. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 4.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 5.Gerhard M, Roddy MA, Creager SJ, Creager MA. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension. 1996;27:849–853. doi: 10.1161/01.hyp.27.4.849. [DOI] [PubMed] [Google Scholar]
- 6.Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- 7.Yasue H, Matsuyama K, Matsuyama K, Okumura K, Morikami Y, Ogawa H. Responses of angiographically normal human coronary arteries to intracoronary injection of acetylcholine by age and segment. Possible role of early coronary atherosclerosis. Circulation. 1990;81:482–490. doi: 10.1161/01.cir.81.2.482. [DOI] [PubMed] [Google Scholar]
- 8.Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR. Aging is associated with greater nuclear NF kappa B, reduced I kappa B alpha, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging cell. 2008;7:805–812. doi: 10.1111/j.1474-9726.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circulation research. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- 10.Cornhill JF, Roach MR. A quantitative study of the localization of atherosclerotic lesions in the rabbit aorta. Atherosclerosis. 1976;23:489–501. doi: 10.1016/0021-9150(76)90009-5. [DOI] [PubMed] [Google Scholar]
- 11.Glagov S, Zarins C, Giddens DP, Ku DN. Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Archives of pathology & laboratory medicine. 1988;112:1018–1031. [PubMed] [Google Scholar]
- 12.Stone PH, Coskun AU, Yeghiazarians Y, Kinlay S, Popma JJ, Kuntz RE, Feldman CL. Prediction of sites of coronary atherosclerosis progression: In vivo profiling of endothelial shear stress, lumen, and outer vessel wall characteristics to predict vascular behavior. Current opinion in cardiology. 2003;18:458–470. doi: 10.1097/00001573-200311000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Conway DE, Williams MR, Eskin SG, McIntire LV. Endothelial cell responses to atheroprone flow are driven by two separate flow components: low time-average shear stress and fluid flow reversal. American journal of physiology. 298:H367–374. doi: 10.1152/ajpheart.00565.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies PF. Flow-mediated endothelial mechanotransduction. Physiological reviews. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nature clinical practice. 2009;6:16–26. doi: 10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Keulenaer GW, Chappell DC, Ishizaka N, Nerem RM, Alexander RW, Griendling KK. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: role of a superoxide-producing NADH oxidase. Circulation research. 1998;82:1094–1101. doi: 10.1161/01.res.82.10.1094. [DOI] [PubMed] [Google Scholar]
- 17.Ziegler T, Bouzourene K, Harrison VJ, Brunner HR, Hayoz D. Influence of oscillatory and unidirectional flow environments on the expression of endothelin and nitric oxide synthase in cultured endothelial cells. Arteriosclerosis, thrombosis, and vascular biology. 1998;18:686–692. doi: 10.1161/01.atv.18.5.686. [DOI] [PubMed] [Google Scholar]
- 18.Kroger K, Kucharczik A, Hirche H, Rudofsky G. Atherosclerotic lesions are more frequent in femoral arteries than in carotid arteries independent of increasing number of risk factors. Angiology. 1999;50:649–654. doi: 10.1177/000331979905000805. [DOI] [PubMed] [Google Scholar]
- 19.Moore W. Vascular Surgery: A Comprehensive Review. Saunders; Philadelphia: 2002. [Google Scholar]
- 20.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr., Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis. American Heart Association, Arteriosclerosis, thrombosis, and vascular biology. 1995;15:1512–1531. doi: 10.1161/01.atv.15.9.1512. [DOI] [PubMed] [Google Scholar]
- 21.Thijssen DH, Dawson EA, Tinken TM, Cable NT, Green DJ. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension. 2009;53:986–992. doi: 10.1161/HYPERTENSIONAHA.109.131508. [DOI] [PubMed] [Google Scholar]