Abstract
Replication of avian influenza viruses (AIVs) in dogs may facilitate their adaptation in humans; however, the data to date on H5N1 influenza virus infection in dogs are conflicting. To elucidate the susceptibility of dogs to this pathogen, we infected two groups of 6 beagles with 106 50% egg-infectious dose of H5N1 AIV A/bar-headed goose/Qinghai/3/05 (BHG/QH/3/05) intranasally (i.n.) and intratracheally (i.t.), respectively. The dogs showed disease symptoms, including anorexic, fever, conjunctivitis, labored breathing and cough, and one i.t. inoculated animal died on day 4 post-infection. Virus shedding was detected from all 6 animals inoculated i.n. and one inoculated i.t. Virus replication was detected in all animals that were euthanized on day 3 or 5 post-infection and in the animal that died on day 4 post-infection. Our results demonstrate that dogs are highly susceptible to H5N1 AIV and may serve as an intermediate host to transfer this virus to humans.
Introduction
Replication of avian influenza viruses in mammals may facilitate their adaptation in humans. The H5N1 influenza viruses have been isolated from several mammalian hosts (Amonsin et al., 2006; Keawcharoen et al., 2004; Zhu et al., 2008). Pigs, for example, are regarded as a natural influenza virus host that could be infected by an H5N1 influenza virus in the field or an experimental setting (Lipatov et al., 2008; Zhu et al., 2008), possibly leading to the generation of an H5N1 pandemic strain. Cats and dogs, which come into close contact with humans, may also play a role in the interspecies transmission of influenza viruses. Cats are highly susceptible to highly pathogenic avian influenza viruses (Klopfleisch et al., 2007; Kuiken et al., 2004; Nakamura and Iwasa, 1942; Rimmelzwaan et al., 2006; Songserm et al., 2006a; Yingst, Saad, and Felt, 2006); however the data are conflicting on dogs with H5N1 influenza virus infection (Giese et al., 2008; Maas et al., 2007; Songserm et al., 2006b). Songserm et al. (2006b) reported that a dog was killed by natural infection of H5N1 influenza virus and found systemic replication of the virus in the animal. Giese et al. (2008) and Maas et al. (2007), however, reported that experimentally infected dogs only developed mild disease including conjunctivitis and elevated body temperatures, and infectious virus was not detected from any organs, although all of the inoculated animals seroconverted. Further study is, therefore, required to clarify the susceptibility of dogs to H5N1 influenza virus infection.
Results
We used 45-day-old specific-pathogen free Beagles to evaluate the replication and virulence of H5N1 influenza virus in dogs. Two groups of six dogs lightly anesthetized with ketamine were inoculated intranasally (i.n.) (animals B1-B6) or intratracheally (i.t.) (animals B7-B12) with 1 ml of 106 50% egg-infectious dose (EID50) of H5N1 avian influenza virus A/bar-headed goose/Qinghai/3/05 (BHG/QH/3/05), which is a clade 2.2 virus that was isolated from a wild bird in 2005 (Chen et al., 2006). Nasal and rectal swabs were collected from infected dogs everyday from days 2 to 6 post-inoculation (p.i.) for virus titration in chicken embryos. Two animals in each group were euthanized on days 3 and 5 p.i., and organs were collected for assessment of viral replication and pathology. The two remaining animals in each group were observed and euthanized on day 14 p.i.
The animals in both groups showed disease symptoms after challenge. All twelve animals became anorexic on day 1 p.i. and completely lost their appetites for two days, the remaining four animals recovered by days 6 and 7 p.i. (Table 1). All animals developed fever on day 2 p.i. and temperature declined on day 5 p.i. (Figure 1A, B). Conjunctivitis was observed in two of the six animals inoculated i.n. and all six animals inoculated i.t.; diarrhea was detected in two animals of each group (Table 1). In the i.t. inoculated group, two animals had cough and all six animals had labored breathing, one animal in this group died on day 4 p.i. (Table 1).
Table 1.
Virus shedding and appearance of dogs infected with H5N1 influenza virus A/bar-headed goose/Qinghai/3/05a
| Animal | Inoculate route |
Day euthanized post-infection (p.i.) |
Virus titer in nasal swabs at indicated day p.i. (Log10EID50) |
Clinical symptoms | Seroconversionb | ||
|---|---|---|---|---|---|---|---|
| Day 2 p.i | Day 3 p.i | Day 4 p.i | |||||
| B1 | Intranasal | 3 | 3.3 | 0.8 | / | Fever, anorexia, decreased activity | / |
| B2 | Intranasal | 3 | 2.8 | 1.8 | / | Fever, anorexia, decreased activity | / |
| B3 | Intranasal | 5 | 2.5 | 0.8 | 0.8 | Fever, anorexia, decreased activity | / |
| B4 | Intranasal | 5 | 2.8 | 4.3 | 1.3 | Fever, anorexia, decreased activity | / |
| B5 | Intranasal | 14 | 2.3 | 2.3 | 1.8 | Fever, anorexia, conjunctivitis, diarrhea | 160 |
| B6 | Intranasal | 14 | 2.5 | 1.3 | <0.5 | Fever, anorexia, conjunctivitis, diarrhea | 160 |
| B7 | Intratracheal | 3 | < | < | / | Fever, anorexia, conjunctivitis, labored breathing | / |
| B8 | Intratracheal | 3 | < | < | / | Fever, anorexia, conjunctivitis, labored breathing | / |
| B9 | Intratracheal | 5 | < | < | < | Fever, anorexia, conjunctivitis, labored breathing | / |
| B10 | Intratracheal | Died on day 4 p.i. | 1.8 | < | / | Fever, anorexia, conjunctivitis, labored breathing, cough, diarrhea |
/ |
| B11 | Intratracheal | 14 | < | < | < | Fever, anorexia, conjunctivitis, labored breathing, cough |
160 |
| B12 | Intratracheal | 14 | < | < | < | Fever, anorexia, conjunctivitis, labored breathing, diarrhea |
80 |
Dogs were inoculated with 1 ml of 106EID50 of the indicated H5N1 influenza virus intranasally or intratracheally. Nasal and rectal swabs were collected from day 2 to day 6 p.i. and titrated in 10-day-old chicken embryos. Virus was not detected from nasal swabs collected on days 5 and 6 p.i. or from any of the rectal swabs; these results are not included in the Table. /, animals were euthanized or died when the tests were performed. <, no virus was isolated from the sample.
Sera collected on day 14 p.i. were treated with Vibrio cholerae receptor destroying enzyme and tested for the presence of HI antibody with 0.5% (V/V) chicken erythrocytes
Figure 1. Body temperature of and virus replication in beagles after inoculation with H5N1 influenza virus.
Body temperature in beagles after inoculation with BHG/QH/3/05 intranasally (A) or intratracheally (B). Virus titers were determined in embryonated eggs injected with tissue homogenates from the animals that were euthanized on day 3 (C, E) and day 5 (D,E) or that died on day 4 (G) post-challenge with BHG/QH/3/05 intranasally (C, D) or intratracheally (E, F, G). Titers are reported for tissues from individual animals, as log10EID50/g tissue. The dashed blue lines indicate the lower limit of detection.
To investigate whether the inoculated animals shed virus, nasal and rectal swabs were collected from days 2 to 6 p.i. and titrated in 10-day-old chicken embryos. As shown in Table 1, virus was detected from the nasal swabs of all six animals in the i.n. inoculated group, but was detected from only one animal of the i.t. inoculated group (Table 1). Virus was not detected from any rectal swabs (data not shown). These results indicate that the virus replicated better in the upper respiratory tract of the i.n. inoculated animals than in that of the i.t. inoculated animals.
To investigate virus replication in the inoculated animals, two animals in each group were scheduled to be euthanized on days 3 and day 5 p.i.; however, since one of the animal in the i.t. inoculated group died on day 4 p.i., we euthanized only one animal in this group on day 5 p.i. (Table 1). As shown in Figure 1, high titers of virus were found in the tonsil of both animals (B1 and B2) that were inoculated i.n. and euthanized on day 3, and low titers were detected in the trachea (B2) and lung (B1) (Figure 1C). In the animals of the i.n. inoculated group that were euthanized on day 5 p.i. (B3 and B4), virus was detected in the tonsil, tracheobronchial lymph node (B4), trachea (B3) and lung (Figure 1D), and the virus titers in lung were clearly higher than those of the animals inoculated i.n. and euthanized on day 3 p.i.. In the i.t. inoculated animals that were euthanized on day 3 p.i. (B7 and B8), virus was detected in the tonsil, tracheobronchial lymph node and lung of both animals and also in the trachea of B7 (Figure 1E). Virus was also detected in the trachea and lung of the animal that was inoculated i.t. and euthanized on day 5 (B9) (Figure 1F), and in the tonsil, trachea and lung of the animal that inoculated i.t. and died on day 4 p.i.(B10) (Figure 1G). Virus was not detected from any other organs collected (data not shown). Of the four animals that were euthanized on day 14, virus was not detected from any organs collected (data not shown), but all four animals seroconverted on the base of a hemagglutinin inhibition (HI) test (Table 1). These results indicate that the BHG/QH/05 virus could replicate efficiently in dogs, but the replication was limited to the tonsil and respiratory system.
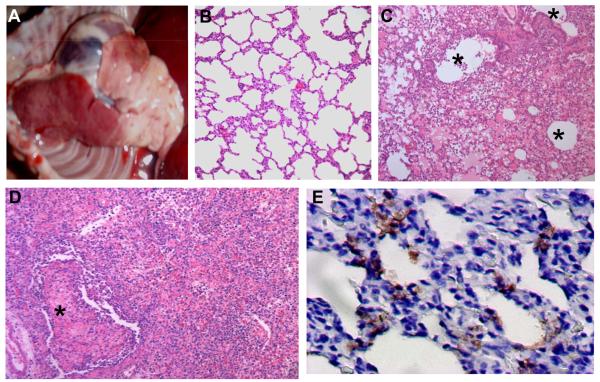
Macroscopic lesions in the lungs were observed in all 7 animals euthanized on days 3 and 5 p.i. and in the animal that died on day 4 p.i. Emphysema and focal discoloration of the lobes were mainly seen with two animals (B1 and B2) that were i.n. inoculated and euthanized on day 3 p.i. A spectrum of macroscopic lesions—including congestion, exudation, and consolidation—were observed in the lung lobes of the other six animals (B3, B4, B7-B10) (Figure 2A). Histologically, in comparison to the lung of the control animals (Figure 2B), the consolidated area in the lungs of the virus infected animals was consistent with bronchointerstitial pneumonia with massive recruitment of lymphocytes (Figure 2C, D). In extrapulmonary organs, prominent swelling and hyperaemia of tonsil were observed in several animals. Exudation of proteinous fluid and neutrophilic infiltration were common seen in these tonsils histologically. Viral antigen expression was detected in most of the areas of tonsil and lung with severe lesions (Figure 2E, Table 2).
Figure 2. Macroscopic lesions and histological findings of beagles infected with H5N1 virus BHG/QH/3/05.
A) Macroscopic lung lesion of a beagle infected with virus. Day 3 post-intratracheal infection (animal B8). B) A lung section from the control animal that was mock-inoculated with 1 ml PBS i.t. and euthanized on day 5 p. i., HE stain. C) A section from a consolidated area of the lung shows bronchointerstitial pneumonia with significant infiltration of inflammatory cells. The lung lesions were distributed around the bronchioli. Day 3 post-intratracheal infection (HE stain, animal B8). D) Severe alveolar damage within and along the periphery of the consolidated area. Day 5 post-intranasal infection (HE stain, animal B4). Severe proliferative and reactive hyperplasia of alveolar cells with massive recruitment of lymphocytes, fibrin exudates, and alveolar edema are shown. E) Viral antigens in the lung on day 5 post-intranasal infection (brown). IHC. Asterisks indicate lumen of bronchioli (Animal B4).
Table 2.
Pathological lesions and antigen distribution in tissues of dogs infected with H5N1 virus A/bar-headed goose/Qinghai/3/05a.
| Animal | Administration route | Day euthanized post-infection (p.i.) |
Tonsil | Trachea | TBLN | Left lungs | Middle lung | Right lungs | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Upper | Lower | Upper | Middle | Lower | |||||||
| B1 | Intranasal | 3 | +/++ b | −/− | −/− | +/+ | −/− | −/− | −/− | −/− | −/− |
| B2 | Intranasal | 3 | +/++ | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− |
| B3 | Intranasal | 5 | +/+ | −/− | −/− | −/− | −/− | ++/++ | −/− | −/− | −/− |
| B4 | Intranasal | 5 | +/+ | −/− | −/− | ++/+ | +/− | +++/++ | +/− | +/− | ++/+ |
| B7 | Intratracheal | 3 | −/− | −/− | −/− | +/− | +/− | −/− | +++/+++ | +/− | +/− |
| B8 | Intratracheal | 3 | +/++ | −/− | −/− | ++/+ | −/− | −/− | +++/++ | +/− | +/+ |
| B9 | Intratracheal | 5 | −/− | −/− | −/− | +++/++ | +++/++ | +++/++ | +++/++ | ++/++ | ++/+ |
| B10 | Intratracheal | 4c | −/− | −/− | −/− | ++/− | ++/− | +++/++ | +++/+ | +++/+ | +++/+ |
Beagles (45 days old) were inoculated intranasally (i.n.) or intrachacheally (i.t.) with 1 ml of 106EID50 of the BHG/QH/3/05 virus. Two animals from each group were euthanized on days 3 and 5 p.i., respectively, and their organs were collected for assessment of pathological lesions and antigen distribution.
Pathological lesions/viral antigens. −, no pathological change/antigen. +, limited pathological change/antigen. ++, moderate pathological change/antigen. +++, severe pathological change/abundant antigen. TBLN: tracheobronchial lymph node.
This animal died on day 4 p.i.
Discussion
Here, we evaluated the susceptibility of dogs to H5N1 influenza virus infection in beagles. We found that the H5N1 influenza virus could replicate efficiently in the respiratory tract following i.n. or i.t. inoculation, and that, upon infection, the animals showed severe disease symptoms, including fever, anorexia, conjunctivitis, and labored breathing. One dog died on day 4 p.i. Though susceptibility in other canine breeds remains to be tested, our results in beagles demonstrate that dogs are highly susceptible to H5N1 influenza virus and may serve as a potentially intermediate host to transfer this virus to humans.
Susceptibility to virus is determined by both host and virus factors. The expression of α-2,3 or α-2,6 glycans in the respiratory tract is a major determinant for host sensitivity to avian or human influenza viruses. The cells of upper respiratory tract of humans mainly express the α-2,6 glycan (Nicholls et al., 2007; Shinya et al., 2006; van Riel et al., 2006), an environment that favors the replication and transmission of human influenza viruses, which preferentially bind to α-2,6 glycans (Rogers and Paulson, 1983). The avian influenza viruses primarily bind to α-2,3 glycans (Rogers and Paulson, 1983; Matrosovich et al., 2000), which are mainly expressed in the cells of the intestinal tract of avian species (Ito et al., 2000, Pillai and Lee, 2010). A previous study indicated that the BHG/QH/3/05 virus can bind to both the α-2,3 and α-2,6 glycans (Gao et al., 2009), and this property may enable the virus to replicate in all parts of the respiratory tract of beagles as we observed in this study, although the actual distribution of these two different receptors in the respiratory tract of beagles is unknown.
Previous studies documented limited virus shedding and a lack of virus re-isolation from the organs of dogs experimentally inoculated with H5N1 viruses (Giese et al., 2008; Maas et al., 2007). Songserm et al. (2006b) reported that an H5N1 influenza virus was detected from multiple organs of a dog that died from eating duck carcasses infected with H5N1 virus. However, in our study, efficient virus replication was detected from the respiratory system of all animals and virus shedding was detected from all of the intranasally inoculated animals. Virus replication in dogs, even the dead animal, was restricted to the respiratory system, which is similar to what has been observed in many humans who have died from H5N1 influenza infection (Uiprasertkul et al., 2005) and in nonhuman primates experimentally infected with H5N1 influenza virus (Baskin et al., 2009; Fan et al., 2009; Ruat et al., 2008). Therefore, the replication of H5N1 influenza viruses in dogs may vary from different strains, as seen previously in mice (Chen et al., 2004; Gao et al., 1999; Lu et al., 1999).
H5N1 influenza viruses have been catagorized into ten distinct HA phylogenetic clades (0–9), and the clade 2 viruses have been further classified into different subclades (Abdel-Ghafar et al., 2008). The clade 2.2 viruses, represented by BHG/QH/3/05, were first detected in wild birds in Qinghai Lake, western China, and continues to infect wild birds, poultry, and humans in many countries (Abdel-Ghafar et al., 2008). These viruses have two known genetic markers, the amino acid lysine at position 627 of PB2 and the absence of a potential N-linked glycosylation site at HA amino acid positions 158–160, that favor their replication and transmission of H5N1 influenza viruses in mammalian hosts (Gao et al., 2009; Hatta et al., 2001; Steel et al., 2009). Our study demonstrates that the clade 2.2 virus BHG/QH/3/05 can replicate in dogs much more efficient than the strains tested by others (Giese et al., 2008; Maas et al., 2007). Therefore, effort should be made to prevent dogs from being infected by H5N1 influenza viruses to avoid the generation of a virus with pandemic potential in this host.
Materials and Methods
Facility
Studies with highly pathogenic H5N1 avian influenza viruses were conducted in a biosafety level 3+ laboratory approved by the Chinese Ministry of Agriculture. All animal studies were approved by the Review Board of Harbin Veterinary Research Institute, Chinese Academy of Agricultural Sciences.
Virus
The H5N1 avian influenza virus, BHG/QH/3/05, used in this study was isolated from a wild bird at Qinghai Lake in northwest China as described previously (Chen et al., 2006). The virus was propagated in 10-day-old specific-pathogen free (SPF) embryonated chicken eggs and stored at −70°C before use.
Animal studies
Twelve beagles (45-day-old) were divided into two groups of six animals each; one group (B1–B6) was inoculated intranasally with 1 ml of 106EID50 of the BHG/QH/3/05 virus, while the other (B7–B12) received the same amount of virus by intratracheal inoculation. Two animals from each group were euthanized on days 3 and 5 post-inoculation (p.i.), respectively, and organs including brain, spleen, kidney, liver, duodenum and different parts of their respiratory system were collected for virus titration and/or histologic and immunohistochemical studies; the remaining animals were observed and euthanized on day 14 post-inoculation and serum samples were collected for HI antibody testing. Nasal and rectal swabs were collected from all available dogs everyday from days 2 to 6 p.i. for virus titration in chicken embryos, as described previously (Chen et al., 2004). One beagle was mock-inoculated with 1ml PBS i.t. and euthanized on day 5p.i., lung sample was collected for the histological study. Temperature of all animals were monitored three days before inoculation and 14 days after the inoculation. Amount of food and water consumed by the animals were monitored everyday during the experiment period.
Pathologic examination
Tissues fixed in 10% phosphate-buffered formalin were dehydrated, embedded in paraffin, cut into 5-μm thick sections, and stained with standard hematoxylin-and-eosin. Immunohistochemistry was performed with a monoclonal antibody against the HA of A/goose/Guangdong/1/96 by using the Dako Envision system (Dako, www.dako.com).
Antibody detection
Sera were treated with Vibrio cholerae (Denka-Seiken, www.denka-seiken.co.jp) receptor destroying enzyme before being tested for the presence of HI antibody with 0.5% (V/V) chicken erythrocytes.
Acknowledgements
We thank Susan Watson for editing the manuscript. This work was supported by Chinese National Natural Science Foundation 30825032, by the Chinese National Key Basic Research Program (973) (2010CB534000, 2005CB523005 and 2005CB523200), by the Chinese National S&T Plan Grant 2006BAD06A05 and 2009ZX10004-214, by a grant-in-aid for Specially Promoted Research, by a contract research fund for the Program of Funding Research Centers for Emerging and Reemerging Infectious Diseases from the Ministry of Education, Culture, Sports, Science and Technology of Japan, by the ERATO (Exploratory Research for Advanced Technology) grant from the Japan Science and Technology Agency and by Public Health Service research grants from the National Institute of Allergy and Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-Ghafar AN, Chotpitayasunondh T, Gao Z, Hayden FG, Nguyen DH, de Jong MD, Naghdaliyev A, Peiris JS, Shindo N, Soeroso S, Uyeki TM. Update on avian influenza A (H5N1) virus infection in humans. N Engl J Med. 2008;358(3):261–73. doi: 10.1056/NEJMra0707279. [DOI] [PubMed] [Google Scholar]
- Amonsin A, Payungporn S, Theamboonlers A, Thanawongnuwech R, Suradhat S, Pariyothorn N, Tantilertcharoen R, Damrongwantanapokin S, Buranathai C, Chaisingh A, Songserm T, Poovorawan Y. Genetic characterization of H5N1 influenza A viruses isolated from zoo tigers in Thailand. Virology. 2006;344(2):480–91. doi: 10.1016/j.virol.2005.08.032. [DOI] [PubMed] [Google Scholar]
- Baskin CR, Bielefeldt-Ohmann H, Tumpey TM, Sabourin PJ, Long JP, Garcia-Sastre A, Tolnay AE, Albrecht R, Pyles JA, Olson PH, Aicher LD, Rosenzweig ER, Murali-Krishna K, Clark EA, Kotur MS, Fornek JL, Proll S, Palermo RE, Sabourin CL, Katze MG. Early and sustained innate immune response defines pathology and death in nonhuman primates infected by highly pathogenic influenza virus. Proc Natl Acad Sci U S A. 2009;106(9):3455–60. doi: 10.1073/pnas.0813234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Deng G, Li Z, Tian G, Li Y, Jiao P, Zhang L, Liu Z, Webster RG, Yu K. The evolution of H5N1 influenza viruses in ducks in southern China. Proc Natl Acad Sci U S A. 2004;101(28):10452–7. doi: 10.1073/pnas.0403212101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Li Y, Li Z, Shi J, Shinya K, Deng G, Qi Q, Tian G, Fan S, Zhao H, Sun Y, Kawaoka Y. Properties and dissemination of H5N1 viruses isolated during an influenza outbreak in migratory waterfowl in western China. J Virol. 2006;80(12):5976–83. doi: 10.1128/JVI.00110-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan S, Gao Y, Shinya K, Li CK, Li Y, Shi J, Jiang Y, Suo Y, Tong T, Zhong G, Song J, Zhang Y, Tian G, Guan Y, Xu XN, Bu Z, Kawaoka Y, Chen H. Immunogenicity and protective efficacy of a live attenuated H5N1 vaccine in nonhuman primates. PLoS Pathog. 2009;5(5):e1000409. doi: 10.1371/journal.ppat.1000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Watanabe S, Ito T, Goto H, Wells K, McGregor M, Cooley AJ, Kawaoka Y. Biological heterogeneity, including systemic replication in mice, of H5N1 influenza A virus isolates from humans in Hong Kong. J Virol. 1999;73(4):3184–9. doi: 10.1128/jvi.73.4.3184-3189.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Zhang Y, Shinya K, Deng G, Jiang Y, Li Z, Guan Y, Tian G, Li Y, Shi J, Liu L, Zeng X, Bu Z, Xia X, Kawaoka Y, Chen H. Identification of amino acids in HA and PB2 critical for the transmission of H5N1 avian influenza viruses in a mammalian host. PLoS Pathog. 2009;5(12):e1000709. doi: 10.1371/journal.ppat.1000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese M, Harder TC, Teifke JP, Klopfleisch R, Breithaupt A, Mettenleiter TC, Vahlenkamp TW. Experimental infection and natural contact exposure of dogs with avian influenza virus (H5N1) Emerg Infect Dis. 2008;14(2):308–10. doi: 10.3201/eid1402.070864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatta M, Gao P, Halfmann P, Kawaoka Y. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science. 2001;293(5536):1840–2. doi: 10.1126/science.1062882. [DOI] [PubMed] [Google Scholar]
- Ito T, Suzuki Y, Suzuki T, Takada A, Horimoto T, Wells K, Kida H, Otsuki K, Kiso M, Ishida H, Kawaoka Y. Recognition of N-glycolylneuraminic acid linked to galactose by the alpha2,3 linkage is associated with intestinal replication of influenza A virus in ducks. J Virol. 2000;74(19):9300–5. doi: 10.1128/jvi.74.19.9300-9305.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keawcharoen J, Oraveerakul K, Kuiken T, Fouchier RA, Amonsin A, Payungporn S, Noppornpanth S, Wattanodorn S, Theambooniers A, Tantilertcharoen R, Pattanarangsan R, Arya N, Ratanakorn P, Osterhaus DM, Poovorawan Y. Avian influenza H5N1 in tigers and leopards. Emerg Infect Dis. 2004;10(12):2189–91. doi: 10.3201/eid1012.040759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfleisch R, Wolf PU, Uhl W, Gerst S, Harder T, Starick E, Vahlenkamp TW, Mettenleiter TC, Teifke JP. Distribution of lesions and antigen of highly pathogenic avian influenza virus A/Swan/Germany/R65/06 (H5N1) in domestic cats after presumptive infection by wild birds. Vet Pathol. 2007;44(3):261–8. doi: 10.1354/vp.44-3-261. [DOI] [PubMed] [Google Scholar]
- Kuiken T, Rimmelzwaan G, van Riel D, van Amerongen G, Baars M, Fouchier R, Osterhaus A. Avian H5N1 influenza in cats. Science. 2004;306(5694):241. doi: 10.1126/science.1102287. [DOI] [PubMed] [Google Scholar]
- Lipatov AS, Kwon YK, Sarmento LV, Lager KM, Spackman E, Suarez DL, Swayne DE. Domestic pigs have low susceptibility to H5N1 highly pathogenic avian influenza viruses. PLoS Pathog. 2008;4(7):e1000102. doi: 10.1371/journal.ppat.1000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Tumpey TM, Morken T, Zaki SR, Cox NJ, Katz JM. A mouse model for the evaluation of pathogenesis and immunity to influenza A (H5N1) viruses isolated from humans. J Virol. 1999;73(7):5903–11. doi: 10.1128/jvi.73.7.5903-5911.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas R, Tacken M, Ruuls L, Koch G, van Rooij E, Stockhofe-Zurwieden N. Avian influenza (H5N1) susceptibility and receptors in dogs. Emerg Infect Dis. 2007;13(8):1219–21. doi: 10.3201/eid1308.070393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrosovich M, Tuzikov A, Bovin N, Gambaryan A, Klimov A, Castrucci MR, Donatelli I, Kawaoka Y. Early alterations of the receptor-binding properties of H1, H2, and H3 avian influenza virus hemagglutinins after their introduction into mammals. J Virol. 2000;74(18):8502–12. doi: 10.1128/jvi.74.18.8502-8512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura J, Iwasa T. On the fowl-pest infection in cat. The Japanese journal of veterinary science. 1942;4:511–523. [Google Scholar]
- Nicholls JM, Chan MC, Chan WY, Wong HK, Cheung CY, Kwong DL, Wong MP, Chui WH, Poon LL, Tsao SW, Guan Y, Peiris JS. Tropism of avian influenza A (H5N1) in the upper and lower respiratory tract. Nat Med. 2007;13(2):147–9. doi: 10.1038/nm1529. [DOI] [PubMed] [Google Scholar]
- Pillai SP, Lee CW. Species and age related differences in the type and distribution of influenza virus receptors in different tissues of chickens, ducks and turkeys. Virology Journal. 2010;7:5. doi: 10.1186/1743-422X-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmelzwaan GF, van Riel D, Baars M, Bestebroer TM, van Amerongen G, Fouchier RA, Osterhaus AD, Kuiken T. Influenza A virus (H5N1) infection in cats causes systemic disease with potential novel routes of virus spread within and between hosts. Am J Pathol. 2006;168(1):176–83. doi: 10.2353/ajpath.2006.050466. quiz 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers GN, Paulson JC. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology. 1983;127(2):361–73. doi: 10.1016/0042-6822(83)90150-2. [DOI] [PubMed] [Google Scholar]
- Ruat C, Caillet C, Bidaut A, Simon J, Osterhaus AD. Vaccination of macaques with adjuvanted formalin-inactivated influenza A virus (H5N1) vaccines: protection against H5N1 challenge without disease enhancement. J Virol. 2008;82(5):2565–9. doi: 10.1128/JVI.01928-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinya K, Ebina M, Yamada S, Ono M, Kasai N, Kawaoka Y. Avian flu: influenza virus receptors in the human airway. Nature. 2006;440(7083):435–6. doi: 10.1038/440435a. [DOI] [PubMed] [Google Scholar]
- Songserm T, Amonsin A, Jam-on R, Sae-Heng N, Meemak N, Pariyothorn N, Payungporn S, Theamboonlers A, Poovorawan Y. Avian influenza H5N1 in naturally infected domestic cat. Emerg Infect Dis. 2006a;12(4):681–3. doi: 10.3201/eid1204.051396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songserm T, Amonsin A, Jam-on R, Sae-Heng N, Pariyothorn N, Payungporn S, Theamboonlers A, Chutinimitkul S, Thanawongnuwech R, Poovorawan Y. Fatal avian influenza A H5N1 in a dog. Emerg Infect Dis. 2006b;12(11):1744–7. doi: 10.3201/eid1211.060542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel J, Lowen AC, Mubareka S, Palese P. Transmission of influenza virus in a mammalian host is increased by PB2 amino acids 627K or 627E/701N. PLoS Pathog. 2009;5(1):e1000252. doi: 10.1371/journal.ppat.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uiprasertkul M, Puthavathana P, Sangsiriwut K, Pooruk P, Srisook K, Peiris M, Nicholls JM, Chokephaibulkit K, Vanprapar N, Auewarakul P. Influenza A H5N1 replication sites in humans. Emerg Infect Dis. 2005;11(7):1036–41. doi: 10.3201/eid1107.041313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Riel D, Munster VJ, de Wit E, Rimmelzwaan GF, Fouchier RA, Osterhaus AD, Kuiken T. H5N1 Virus Attachment to Lower Respiratory Tract. Science. 2006;312(5772):399. doi: 10.1126/science.1125548. [DOI] [PubMed] [Google Scholar]
- Yingst SL, Saad MD, Felt SA. Qinghai-like H5N1 from domestic cats, northern Iraq. Emerg Infect Dis. 2006;12(8):1295–7. doi: 10.3201/eid1208.060264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Yang H, Chen W, Cao W, Zhong G, Jiao P, Deng G, Yu K, Yang C, Bu Z, Kawaoka Y, Chen H. A naturally occurring deletion in its NS gene contributes to the attenuation of an H5N1 swine influenza virus in chickens. J Virol. 2008;82(1):220–8. doi: 10.1128/JVI.00978-07. [DOI] [PMC free article] [PubMed] [Google Scholar]