Abstract
The intracellular restriction factor TRIM5α, inhibits infection by numerous retroviruses in a species-specific manner. The best characterized example of this restriction is the TRIM5α protein from rhesus macaques (rhTRIM5α), which potently inhibits HIV-1 infection. TRIM5α localizes to cytoplasmic assemblies of protein referred to as cytoplasmic bodies, though the role that these bodies play in retroviral restriction is unclear. We employed a series of truncation mutants to identify a discrete region, located within the Linker2 region connecting the coiled-coil and B30.2/PRYSPRY domains of TRIM5α, which is required for cytoplasmic body localization. Deletion of this region in the context of full-length rhTRIM5α abrogates cytoplasmic body localization. Alanine mutagenesis of the residues in this region identifies two stretches of amino acids that are required for both cytoplasmic body localization and retroviral restriction. This work suggests that the determinants that mediate TRIM5α localization to cytoplasmic bodies play a requisite role in retroviral restriction.
Keywords: TRIM5α, multimerization, TRIM, HIV-1, restriction factor
Introduction
The species-specific tropism of numerous retroviruses is determined by host cell proteins, termed restriction factors, which inhibit viral replication at numerous stages of the viral life cycle. The TRIM family of proteins represents one such class of restriction factors, of which the best characterized example is the ability of TRIM5α from rhesus macaques (rhTRIM5α) to inhibit human immunodeficiency virus type-1 (HIV-1) (Stremlau et al., 2004).
TRIM proteins, including TRIM5α, are defined by a TRIpartite Motif, which includes an N-terminal RING domain, B-Box/B-Box2 domain and coiled-coil domains (Reymond et al., 2001). The RING domain contains a putative E3 ubiquitin ligase activity, and the established biological activity of many TRIM proteins has been shown to require the E3 ligase activity mediated by this domain (Balastik et al., 2008; Barr, Smiley, and Bushman, 2008; Gack et al., 2007; Meroni and Diez-Roux, 2005; Ozato et al., 2008). The B-Box 2 domain has been shown to be important in mediating the cooperative, higher-order multimerization of TRIM5α during restriction (Diaz-Griffero et al., 2007; Diaz-Griffero et al., 2009; Diaz-Griffero et al., 2006). The coiled-coil domain has been shown to mediate the low-order multimerization of many proteins, including TRIM family proteins. In the case of TRIM5α, this is currently thought to facilitate protein dimerization (Kar et al., 2008; Langelier et al., 2008). In addition to these domains, TRIM5α also contains a C- terminal B30.2/PRYSPRY (hereafter SPRY) domain, which has been shown to dictate the species-specific antiviral activity of TRIM5α proteins (Sawyer et al., 2005; Song et al., 2005b; Yap, Nisole, and Stoye, 2005). In some cases, most notably that of owl monkeys, the SPRY domain has been functionally replaced by the retrotransposition of cyclophilin A into the TRIM5 locus (Brennan, Kozyrev, and Hu, 2008; Newman et al., 2008; Sayah et al., 2004; Wilson et al., 2008). The resulting TRIM-Cyp protein also exhibits antiviral activity against various retroviruses.
Like most other TRIM family proteins (Reymond et al., 2001), TRIM5α is observed to localize to cytoplasmic accumulations of protein termed cytoplasmic bodies (Campbell et al., 2007; Stremlau et al., 2004). However, the role that these cytoplasmic bodies play in the process of restriction remains unclear. Two studies have found that preexisting cytoplasmic bodies are not required for restriction (Perez-Caballero et al., 2005; Song et al., 2005a). In one of these studies, the authors observe that a cell line stably expressing TRIM-Cyp can potently restrict HIV-1 infection in the absence of notable cytoplasmic bodies in these cells (Perez-Caballero et al., 2005). In the second study, Song and colleagues observed that treatment of cells stably expressing rhTRIM5α with the heat shock protein inhibitor Geldanamycin, reduced or eliminated the localization of TRIM5α to cytoplasmic bodies without affecting the ability of rhTRIM5α to restrict HIV-1 infection (Song et al., 2005a). These studies collectively suggest that cytoplasmic body localization is not required for retroviral restriction.
Alternatively, our previous studies have observed that cytoplasmic bodies are dynamic structures which turnover rapidly and can traffic through the cell utilizing the microtubule network (Campbell et al., 2007). This study also demonstrated that TRIM5α exists in two populations in cells, including a population localized to cytoplasmic bodies and a more diffuse cytoplasmic population (Campbell et al., 2007). We have also utilized fluorescently labeled HIV-1 virions to observe virions associating with rhTRIM5α cytoplasmic bodies in restricted cells. In this study, live cell imaging was able to visualize the de novo formation of cytoplasmic bodies around individual, fluorescently labeled HIV-1 virions (Campbell et al., 2008). These studies suggest that cytoplasmic bodies might play an important role in the interactions occurring between TRIM5α and incoming retroviral capsids during restriction.
We reasoned that if we could identify TRIM5α mutants that fail to localize to cytoplasmic bodies, we could determine if the ability to restrict retroviral infection is genetically separable from the ability to localize to cytoplasmic bodies. In this study, we utilized a series of truncation mutants to identify regions of TRIM5α that are required for cytoplasmic body localization of TRIM5α. These truncation mutants identified the region between the coiled-coil and the SPRY domains as having determinants that mediate the accumulation of TRIM5α in cytoplasmic bodies, previously described as the linker 2 region (Javanbakht et al., 2006). We find that deletion of this region in the context of a full length rhTRIM5α abrogates the ability of rhTRIM5α to localize to cytoplasmic bodies and restrict HIV-1 infection. Alanine scanning mutagenesis of this region identified two distinct regions of the L2 domain that are required for cytoplasmic body localization. Disruption of either of these regions also generated TRIM5α proteins that lost the ability to restrict infection. Alternatively, similar mutations within this region that did not disrupt rhTRIM5α localization to cytoplasmic bodies did not impair the ability of rhTRIM5α to restrict retroviral infection. This suggests that the L2 region of TRIM5α is relevant to the ability of TRIM5α to self-associate, allowing TRIM5α proteins to form multimeric assemblies around a virion, which is likely to be critical during the restriction process.
Materials and Methods
Recombinant DNA Constructs
Wild type rhTRIM5α plasmid was a kind gift from Dr. Joseph Sodroski (Harvard School of Public Health). HA-tagged rhTRIM5α and variants were created by inserting SmaI and EcoRI sites flanking rhTRIM5α using the primers GCCTGGCATTATGCCCAG and AGCTTGCCAAACCTAC. This PCR product was then digested with SmaI and EcoRI and inserted into the EXN retroviral vector, which was generously provided by the lab of Dr. Greg Towers (Royal Free and University College, London). The EXN construct was used to derive the YXN retroviral vector, which was generated by PCR amplification of the YFP coding region of YFP-N1 (Clontech) using the primers TGGATGAACTATACAAGTGGATCCGGCCG and CGGCCGGATCCACTTGTATAGTTCATCCA. This fragment was then digested with AgeI and BsrGI and inserted into the similarly digested EXN parental plasmid. To facilitate easier subsequent steps, the BamHI site of wt rhTRIM5α was disrupted by SOEing PCR using the interior primers CCCCAGTATCCAAGCACTTTT and AGTGCTTGGATACTGGGGGTATGT and exterior primers GCGGCGGGATCCATGGCTTCTGGAATCCT and CGGCCGGCTCGAGTCAAGAGCTTGGTGAGC. These primers introduced silent mutations eliminating the BamHI site present in wt rhTRIM5α. This PCR product was then inserted into YXN between the BamHI and XhoI sites of YXN. Alanine mutations were introduced into wt rhTRIM5α or rhTRIM5α lacking a BamHI site using SOEing PCR. The primers used in these reactions are provided in supplementary data table 1.
Cell culture, transfection and virus production
HeLa and 293T cells were cultured in complete DMEM containing 10% fetal bovine serum, penicillin (final concentration 100 U/ml), and streptomycin (final concentration 100 μg/ml).
HIV reporter virus was produced by Polyethylenimine (PEI) transfection (Durocher, Perret, and Kamen, 2002) of 293T cells with 8 μg of pVSV-G and 12 μg of the proviral construct R7Δ EnvGFP in which the Nef gene was replaced with GFP. Virus and vector were harvested identically as previously described (Campbell, Nunez, and Hope, 2004). To assess virus infectivity, equivalent numbers of cells in a 24 well plate were infected for 14 h, after which virus was removed, normal medium added, and GFP expression was determined 48–72 h after infection using a FACS Canto II flow cytometer (Becton Dickinson).
Stable cell lines
Hela cells were transduced with retroviral vector overnight and G418 (400μg/mL) was used to generate cell lines expressing the indicated, epitope tagged rhTRIM5α variants. The expression of polyclonal or single colony clones were screened by immunofluorescence to ensure all cells expressed the transduced protein. Such cell lines were then analyzed by western blot analysis, and the clones expressing comparable amounts of protein were chosen for subsequent analysis.
Image Analysis
20 Z-stack images of the indicated cell lines were acquired using identical acquisition parameters. Individual coverslips were coded such that the individual acquiring the images did not know the identity of the cell lines. Deconvolved images were analyzed for cytoplasmic bodies using the Surface Finder function of the Imaris software package (Bitplane). Surfaces for cytoplasmic bodies in all samples analyzed were identified using defined fluorescence intensity and size criteria (Volume = above 0.066 μm3). The number of cytoplasmic bodies/cell was obtained for each cell line. The data were plotted in Prism (Graphpad Software Inc) for statistical analysis. Dunnett’s Multiple Comparison test was used to determine the statistical significance between samples (P<0.05). Unpaired t-Test with Welch’s correction was used to determine the statistical significance between the KPK266–268AAA and RRV275–277AAA mutants (p<0.0001).
Western Blotting and Glutaraldehyde Crosslinking Assay
Whole cell lysates were prepared by treating 1 × 105 cells with NP-40 lysis buffer (100 mM Tris pH 8.0, 1% NP-40, 150 mM NaCl) containing protease inhibitor cocktail (Roche) for 15 minutes on ice. Coomassie Plus Bradford Assay (Thermo scientific) was used to determine total protein concentration. 2X SDS sample buffer was added to the cell lysates and the samples were boiled for 5 minutes at 100°C. Equal amount of protein was loaded into a 10% polyacrylamide gel for SDS-polyacrylamide gel electrophoresis (SDS-PAGE). After separation, the proteins were transferred to nitrocellulose membrane and detected by incubation with anti-GFP (Covance) or anti-HA (clone 3F10) conjugated to Horseradish Peroxidase (HRP) (Roche). Secondary antibodies conjugated to HRP (Thermo Scientific) were used where necessary and antibody complexes were detected using SuperSignal™ West Femto Chemilluminescent Substrate (Thermo Scientific). Chemiluminescence was detected using the UVP EC3™ Imaging System (UVP LLC). Glutaraldehyde crosslinking assays were performed as previously described (Javanbakht et al., 2005). Briefly, the cell lysates were incubated on ice for 30 minutes and centrifuged at 3000 rpm for 1 minute to remove the cell debris. The supernatant was divided into 20μL aliquots and incubated with 0, 1, 2 and 4mM glutaraldehyde for 5 minutes at room temperature. The glutaraldehyde was saturated by adding 1M glycine. 2X SDS sample buffer was added and the mixture was boiled for 5 minutes at 100°C. The samples were then subjected to SDS-PAGE using 4%–15% Tris-HCl gradient gels (Ready Gels, BioRad) and subsequent Western Blot analysis.
Protein Turnover Assay
Cell lines stable expressing the indicated TRIM5α variant were treated with cyclohexamide (20 μg/ml) and cells were harvested at the indicated time following cytohexamide addition. Coomassie Plus Bradford Assay (Thermo scientific) was used to determine total protein concentration. Equivalent amounts of protein from individual samples was subjected to SDS-PAGE and the YFP-TRIM5α protein was detected by western blot.
Infectivity Assay
Equivalent numbers of cells (0.75 × 105) in a 24-well plate were infected with VSV-g pseudotyped GFP reporter HIV-1 (R7ΔEnvGFP) for 14 hours, after which the virus was removed and normal DMEM was added. Percentage of GFP positive cells was determined 48 hpi using a FACS Canto II flow cytometer (Becton Dickinson).
Results
Identification of a region of the Linker2 region of rhTRIM5α required for cytoplasmic body localization
To understand the determinants that influence the cellular localization of rhTRIM5α, we generated a series of GFP fusion proteins to regions of rhTRIM5α that have been implicated in facilitating the multimerization or high-order multimerization of rhTRIM5α (Fig 1A). GFP fusions to the coiled-coil domain alone (data not shown) and to the B-Box2 and coiled coil domains (amino acids 84–236) exhibited a diffuse localization in both the cytoplasm and the nucleus. In contrast, GFP fusions including the B-Box2, coiled coil domain and L2 region (84–300) formed discrete accumulations in the cytoplasm and the nucleus (Data not shown), as did a similar construct including the SPRY domain (84-end). This suggests that the L2 region of rhTRIM5α possesses determinants required for cytoplasmic body formation.
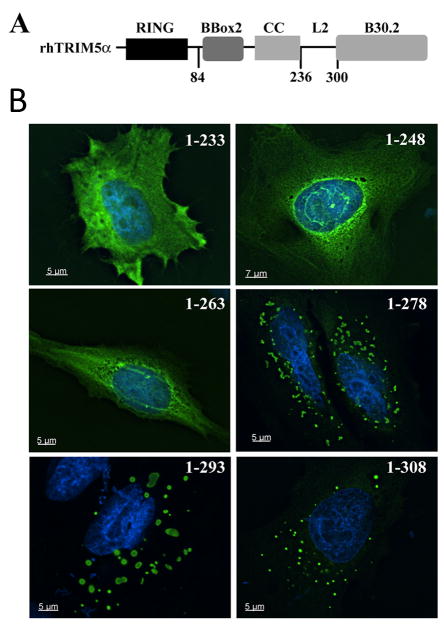
Fig 1. Cytopasmic body determinants are present in the L2 region of TRIM5α.
A. Domain structure of rhesus TRIM5α, including the residues demarcating the L2 region of the protein. B. HeLa cells were transiently transfected with the indicated GFP-rhTRIM5α truncation mutant. Images provided show an individual focal plane of collected, deconvolved Z-stack images.
To further our understanding of which residues in this region are required for cytoplasmic body localization of rhTRIM5α, we generated a series of C-terminal truncations within the L2 region (Fig 1B). All C-terminal truncations that included amino acids 263–278 prominently localized to discrete, cytoplasmic accumulations that resembled the cytoplasmic bodies formed by full-length rhTRIM5α (Fig 1B). In contrast, truncations including amino acids 1–233 or 1–248, and 1–263 exhibited a diffuse localization in transfected cells. This result suggests that the amino acids 263–278 include determinants required for rhTRIM5α cytoplasmic body formation.
Two discrete regions of L2 are required for rhTRIM5α cytoplasmic body localization
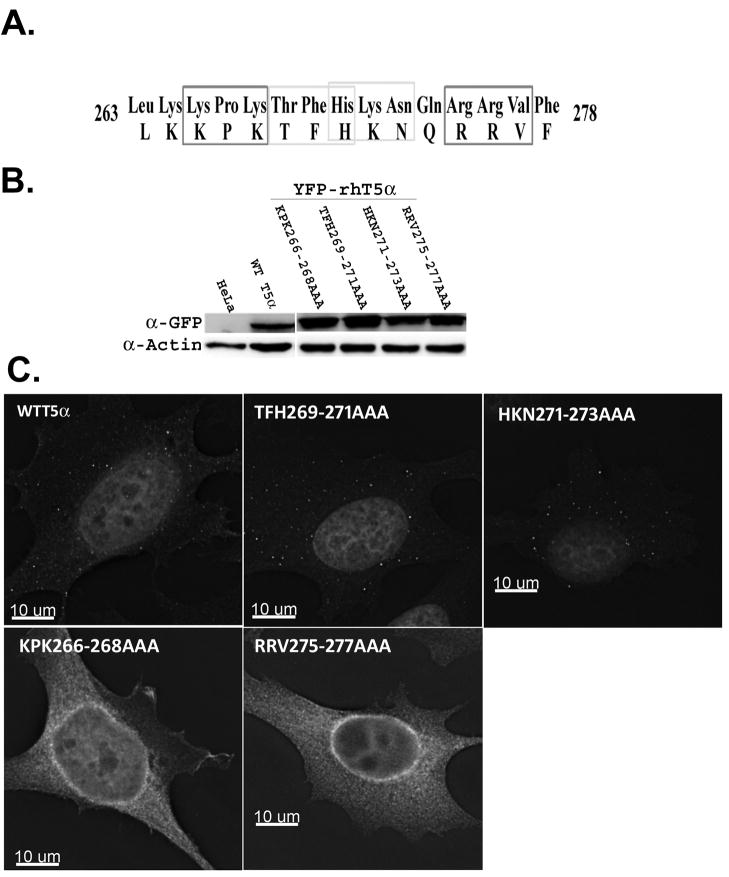
We next sought to identify which specific residues within amino acids 263–278 are required for cytoplasmic body localization. We reasoned that charged residues were more likely to contribute to or facilitate protein-protein interactions that may be required for cytoplasmic body formation. We therefore performed charge-cluster-to-alanine scanning mutagenesis of the region (Bass, Mulkerrin, and Wells, 1991; Cunningham and Wells, 1989; Sebastian et al., 2009). Clusters of 2 charged amino acids within a 3 residue stretch were converted to alanine, as shown in figure 2. Cell lines stably expressing YFP- tagged forms of these proteins were generated and cell lines that expressed comparable amounts of protein were chosen for subsequent analysis (Fig 2B). The localization of these YFP-TRIM5α fusions was examined by fluorescence microscopy. As previously described, cytoplasmic bodies were observable in HeLa cells expressing wild type YFP-rhTRIM5α (Campbell et al., 2007) (Fig 2C). Alanine mutagenesis of amino acids 263–278 revealed that there are two discrete stretches of the L2 region that are required for cytoplasmic body localization. The first includes the residues 266–268, as rhTRIM5α variant in which these residues were converted to alanine (KPK266–268AAA) assumed a diffuse, cytoplasmic localization (Fig 2C). The second includes residues 275–277, as the RRV275–277AAA mutant exhibited a diffuse localization (Fig 2C). In contrast, alanine mutagenesis of the amino acids between these two patches, specifically TFH269–271AAA and HKN271–273AAA, result in cytoplasmic body localization of these rhTRIM5a mutants (Fig 2C). Similar results were obtained with TRIM mutants possessing an HA-epitope tag (data not shown).
Fig. 2. Subcellular localization of YFP-tagged WT and mutant rhTRIM5 in HeLa cells.
(A) Residues 263–278 within the L2 region of rhTRIM5α are shown. Charged residues within this stretch that were converted to Alanine are indicated by light or dark boxes. (b) TRIM5α protein levels were analysed by Western Blot analysis. HeLa cells stably expressing YFP-WT or YFP- rhTRIM5α variants were lysed and the cell lysates were separated by SDS-PAGE. WT and mutant TRIM5α proteins were detected by immunoblotting with α-GFP antibody. Actin was used as a loading control. (c) These stable cells were also fixed and examined for the subcellular localization of the YFP-WT and mutant TRIM5α proteins. Results are representative of 3 independent experiments.
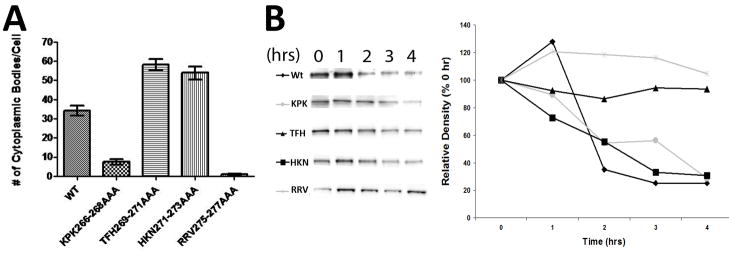
In our analysis, we observed that TRIM5α localization was not completely homogeneous in all cells. To quantitatively compare the localizations exhibited by these various mutants, we collected 20 or more Z-stack images of each cell line using deconvolution microscopy. We then performed quantitative image analysis on our images to identify cytoplasmic bodies in cells using fixed fluorescence and size definitions (Supplemental Movie 1; Fig 3A). This allowed for the unbiased, automated quantification of the number of cytoplasmic bodies per cell for each cell line. As shown in figure 3, automated image analysis of these cell lines recapitulated the phenotypes identified by visual inspection. Cytoplasmic bodies were readily detected in HeLa cells expressing wild type rhTRIM5α, as well as the cell lines expressing TRIM proteins harboring the TFH269–271AAA and HKN271–273AAA mutations (Fig 3B). Conversely, virtually no cytoplasmic bodies were identified in the RRV275–277AAA variant, and very few cytoplasmic bodies were identified in the KPK266–268AAA mutant, though this analysis observed a statistically significant increase relative to the RRV275–277AAA mutant (p<0.0001). This indicates that, while the KPK266–268AAA mutant exhibits a reduced ability to localize to cytoplasmic bodies, it appears to be slightly less diminished in this regard than the RRV275–277AAA mutant.
Fig 3. Quantification of TRIM5α localization and turnover.
A. 20 individual Z-stack images of HeLa cells stably expressing the indicated YFP-rhTRIM5α protein were collected, deconvolved and analyzed using Imaris image analysis software. The number of cytoplasmic bodies per cell was determined using fixed size and intensity criteria for all images. The average number of cytoplasmic bodies identified per cell in all 20 images is plotted. Error bars represent the SEM. B. HeLa cells stably expressing the indicated YFP-TRIM5α variant were treated with cyclohexamide for the indicated time period and YFP-protein expression was analyzed by western blot. Samples were normalized to include an identical amount of total protein. The graph on the right is a densitometric analysis of the samples shown on the left pane. Results are representative of 3 independent experiments.
Cytoplasmic body localization does not correlate to TRIM5α protein turnover
To assess if the localization of our individual TRIM5α variants to cytoplasmic bodies correlated to their relative stability in cells, we analyzed protein turnover in cells following cytohexamide treatment. As shown in figure 3B, we observed that the rate of turnover of two of our mutants, RRV275–277AAA and HKN271–273AAA, was decreased relative to the turnover of wild type rhTRIM5α and the other mutants in our study. If cytoplasmic body localization were affecting the turnover of TRIM5α, this would predict that mutants that do not localize to cytoplasmic bodies exhibit aberrant turnover compared to the wild type protein. However, while one of our diffuse mutants exhibits a decreased rate of turnover (RRV275–277AAA), the other diffuse mutant (KPK266–268) appears to be degraded with wild type kinetics. Conversely, one mutant that localized normally to cytoplasmic body localization (TFH269–271AAA) exhibited increased stability relative to wild type, while another mutant localizing to cytoplasmic bodies (HKN271–273AAA) was degraded with kinetics similar to the wild type protein. Thus, while two mutants in our panel exhibit a longer half-life, this did not correlate to cytoplasmic body localization.
rhTRIM5α variants that do not localize to cytoplasmic bodies retain the ability to multimerize
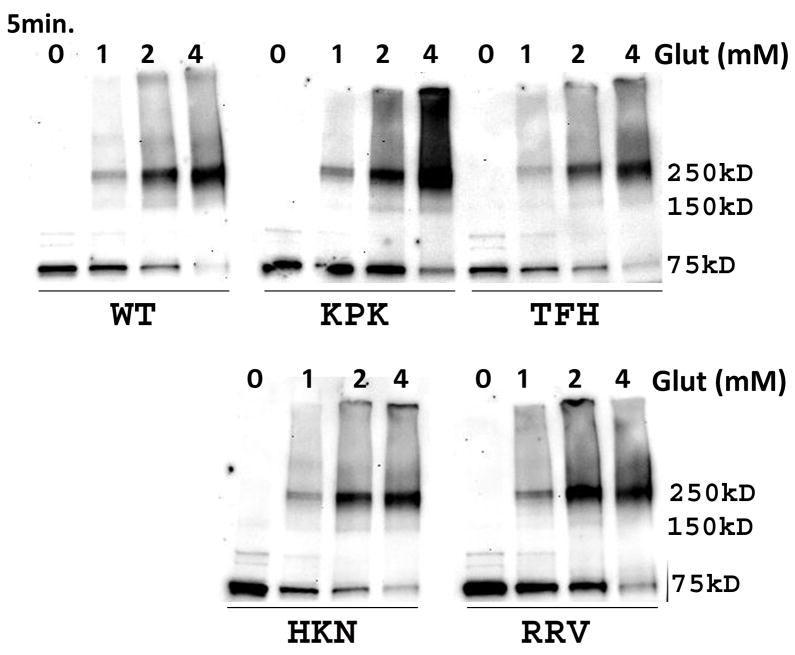
We next sought to assess if the mutations we had introduced into the L2 region were grossly affecting the conformation or folding of TRIM5α. Previous biochemical characterization of TRIM5α, and a related variant possessing the RING domain of TRIM21 have identified low order multimers of TRIM5α following biochemical crosslinking with EGS or glutaraldehyde (Javanbakht et al., 2005; Kar et al., 2008; Langelier et al., 2008; Mische et al., 2005). To assess if our mutations were affecting the ability of the protein to multimerize, we performed biochemical crosslinking of our panel of mutant TRIM proteins. Glutaraldehyde crosslinking of YFP-rhTRIM5α with increasing concentrations of glutaraldehyde revealed multimeric forms of the protein at higher glutaraldehyde concentrations (Fig 4). The lowest molecular weight at which crosslinked protein could be observed was approximately 150 kDa, which likely represents dimeric forms of TRIM5α. However, the predominant low order multimer detected following crosslinking was approximately 250 kDa. While some previous studies originally identified this band as a trimer (Javanbakht et al., 2006; Mische et al., 2005), subsequent studies have determined that this complex is likely to be a dimer exhibiting anomalously slow electrophoretic mobility (Kar et al., 2008; Langelier et al., 2008). These results are consistent with those studies. Similar results were observed in the cases of the TFH269–271AAA and HKN271–273AAA mutants, which localized to cytoplasmic bodies, as well as for the RRV275–277AAA and KPK266–268AAA mutants which do not localize to cytoplasmic bodies (Fig 4). All of the proteins in this study were also able to form higher-order multimers wit increasing concentrations of glutaraldehyde (Fig 4). Similar results were observed with HA-tagged forms of these mutants (data not shown). These data suggest that the reduced cytoplasmic body localization observed for the RRV275–277AAA and KPK266–268AAA mutants is not due to these mutants being grossly misfolded or unable to form low order or higher-order multimers.
Fig 4. Alanine substitution of residues in the L2 region of rhTRIM5α does not affect protein multimerization.
YFP-WT and the alanine mutants of rhTRIM5α were analyzed for their ability to form low and higher-order multimers. HeLa cells stably expressing these proteins were lysed and the cell lysates were treated with increasing concentration of glutaraldehyde (0, 1, 2, 4 mM) for 5 min at RT. 1M glycine was added to saturate the glutaraldehyde, and the protein samples were subjected by SDS-PAGE. Monomeric and multimeric forms of the protein were identified by immunoblotting with a mouse anti-GFP antibody. Results are representative of 3 independent experiments.
rhTRIM5α variants that do not localize to cytoplasmic bodies do not restrict HIV-1 infection
To examine the correlation between cellular localization and restriction activity, we assessed the ability of our rhTRIM5α variants to restrict HIV-1 infection. Cell lines expressing the indicated HA- or YFP-tagged rhTRIM5α variants were infected with serial dilutions of HIV-1 virus expressing GFP following infection (Campbell et al., 2007; Stremlau et al., 2004). In our cell lines expressing YFP-rhTRIM5α, both diffuse mutants KPK266–268AAA and RRV275–277AAA were infected as readily as control HeLa cells (Fig 5). In contrast, cell lines expressing the TFH269–271AAA or HKN271–273AAA variants, both of which localize to cytoplasmic bodies, restricted HIV-1 infection to a similar degree as cells expressing wild type rhTRIM5α (Fig 5). Thus, the ability to restrict infection correlates with the ability of the protein to localize into cytoplasmic bodies in these cell lines.
Fig 5. rhTRIM5α mutants that do not localize to cytoplasmic bodies no longer restrict HIV-1 infection.
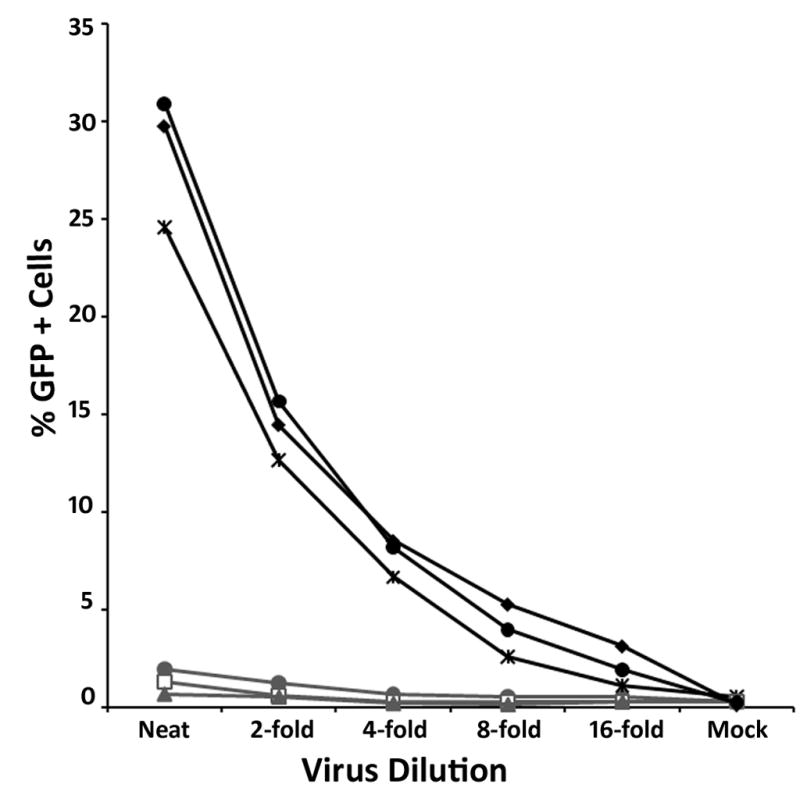
HeLa cells stably expressing YFP-WT rhTRIM5α or rhTRIM5α variants and control HeLa cells were mock infected (DMEM) or infected with serial dilutions of VSV-g psuedotyped GFP reporter HIV-1. 48 hours pi the infected cells were harvested, fixed and the percentage of GFP positive cells was determined by flow cytometry. HeLa (◆), WTrhTRIM5α ( ), KPK266–268AAA (
), KPK266–268AAA ( ), RRV275–277AAA (●), TFH269–271AAA (
), RRV275–277AAA (●), TFH269–271AAA ( ), HKN271–273AAA (
), HKN271–273AAA ( ). Results are representative of 3 independent experiments.
). Results are representative of 3 independent experiments.
A similar pattern of restriction was observed when we infected our cell lines expressing HA-tagged rhTRIM5α variants (data not shown). Collectively, these data suggest that mutations that disrupt the ability of rhTRIM5α to localize to cytoplasmic bodies also disrupt the ability of the protein to restrict retroviral infection.
Discussion
In this study, we identify the Linker2, or L2 region of rhTRIM5α as possessing determinants required for the localization of the protein to cytoplasmic bodies. Using scanning alanine mutagenesis, we identify two stretches of amino acids within this region that are required for cytoplasmic body localization. Mutations within these regions induce a diffuse, cytoplasmic localization of protein (Figure 2) while not affecting the ability of the protein to dimerize (Figure 4), demonstrating that these mutations do not induce a global defect in protein folding. In all cases tested, the ability to restrict retroviral infection was abrogated in mutants that exhibited a diffuse, cytoplasmic localization. Conversely, mutations that did not disrupt protein localization to cytoplasmic bodies did not affect the ability to restrict HIV-1 infection.
While cytoplasmic body localization of our epitope tagged proteins correlates well with the ability to restrict HIV-1 infection, we do not believe it is likely that these bodies themselves, as they exist in cells in the absence of virus, play a role in restriction. Two studies have clearly demonstrated that pre-existing cytoplasmic bodies are not required for retroviral restriction (Perez-Caballero et al., 2005; Song et al., 2005a). Using live cell microscopy, we have previously visualized the de novo formation of rhTRIM5α cytoplasmic bodies around individual, fluorescently labeled HIV-1 virions (Campbell et al., 2008). Taken together with the data presented here, it seems reasonable to speculate that the residues in the L2 region identified in this study facilitate the self-association of rhTRIM5α. Studies of the BBox2 domain have found that this domain also mediates TRIM5α self-association, and that this ability to self-associate is critical to the ability of TRIM5α to restrict infection (Diaz-Griffero et al., 2009). Therefore, while the localization of our variants to cytoplasmic bodies correlates perfectly to the ability to restrict, we favor the hypothesis that this localization is a reflection of this ability to rapidly form a multimeric assembly around a virus. Self-associative activities, mediated by the BBox2 domain and L2 region, likely enhance the rate at which TRIM5α cytoplasmic assemblies form around restriction sensitive virions. However, the data presented here cannot specifically exclude the possibility that the pre-existing bodies themselves play some role in restriction. It is also possible that the L2 region mediates an interaction with another cellular protein that may be important in cytoplasmic body formation.
Previous studies have found that the L2 region can affect the ability of rhTRIM5α to form low and high-order multimers when analyzed by biochemical crosslinking (Javanbakht et al., 2005; Javanbakht et al., 2007; Javanbakht et al., 2006; Mische et al., 2005). Using this technique, we can observe that our L2 variants still form multimers when crosslinked, regardless of their cellular localization. Glutaraldehyde crosslinking also showed that our mutants were able to form higher-order multimers of protein, as indicated by the higher molecular weight species visible following treatment with increasing glutaraldehyde concentrations (Figure 4). This suggests that other domains of TRIM5α, notably the BBox2 domain (Diaz-Griffero et al., 2009; Li and Sodroski, 2008), may mediate the formation of higher-order multimers detected using this method. Other studies examining large deletions of amino acids in the L2 region show that these proteins do not form higher-order multimers, as detected by glutaraldehyde crosslinking, nor do they form cytoplasmic bodies (Javanbakht et al., 2007; Javanbakht et al., 2006). Our mutants retain the ability to form higher-order multimers following glutaraldehyde crosslinking while losing their cytoplasmic body localization. This suggests that cytoplasmic body localization may not be simply reflective of the ability of the protein to multimerize. It is therefore tempting to speculate that the L2 region may facilitate the interaction with a cofactor required for restriction.
The L2 region has not been thoroughly studied, primarily because this region of the protein is not predicted to form any notable secondary structure. Because of this fact, it was assumed that this stretch of amino acids served to functionally tether adjacent domains of the protein. However, the data presented here suggest a more important, functional role for this region. This idea is also supported by the independent evolution of TRIM-Cyp proteins, in which retrotransposition of the Cylophilin A gene into the TRIM5α ORF has functionally replaced the SPRY domain responsible for capsid binding. It seems noteworthy that all of the transpositions identified to date, have retained the regions identified in this study as contributing to cytoplasmic body formation and restriction (Brennan, Kozyrev, and Hu, 2008; Newman et al., 2008; Sayah et al., 2004; Wilson et al., 2008).
It is striking that we observe two distinct regions required for cytoplasmic body localization interceded by a stretch of residues that are dispensable for cytoplasmic body localization. A similar pattern of activity was observed in another study in which cyclophilin A was fused to the TRIM motif of human TRIM5α (Neagu et al., 2009). This study found that ability of these engineered proteins to restrict HIV-1 depended on the residues in the L2 region to which cyclophilin A was genetically tethered. These authors did not observe that specific residues within the L2 region were required, but rather observed that restriction capacity of their fusion proteins exhibited an apparent periodicity across the L2 region, with alternating regions of L2 mediating restricting or non-restricting fusion proteins.
Collectively, the data presented here, as well as the works discussed above, indicate that the Linker2 region of TRIM5α plays a previously unappreciated role in mediating interactions that are critical during retroviral restriction. Further studies are required to determine if this region of TRIM5α mediates the higher-order multimerization of TRIM5α or is required to mediate an interaction with other cellular factors involved in the restriction process.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balastik M, Ferraguti F, Pires-da Silva A, Lee TH, Alvarez-Bolado G, Lu KP, Gruss P. Deficiency in ubiquitin ligase TRIM2 causes accumulation of neurofilament light chain and neurodegeneration. Proc Natl Acad Sci U S A. 2008;105(33):12016–21. doi: 10.1073/pnas.0802261105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr SD, Smiley JR, Bushman FD. The interferon response inhibits HIV particle production by induction of TRIM22. PLoS Pathog. 2008;4(2):e1000007. doi: 10.1371/journal.ppat.1000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass SH, Mulkerrin MG, Wells JA. A systematic mutational analysis of hormone-binding determinants in the human growth hormone receptor. Proc Natl Acad Sci U S A. 1991;88(10):4498–502. doi: 10.1073/pnas.88.10.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan G, Kozyrev Y, Hu SL. TRIMCyp expression in Old World primates Macaca nemestrina and Macaca fascicularis. Proc Natl Acad Sci U S A. 2008;105(9):3569–74. doi: 10.1073/pnas.0709511105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EM, Dodding MP, Yap MW, Wu X, Gallois-Montbrun S, Malim MH, Stoye JP, Hope TJ. TRIM5 alpha cytoplasmic bodies are highly dynamic structures. Mol Biol Cell. 2007;18(6):2102–11. doi: 10.1091/mbc.E06-12-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EM, Nunez R, Hope TJ. Disruption of the actin cytoskeleton can complement the ability of Nef to enhance human immunodeficiency virus type 1 infectivity. J Virol. 2004;78(11):5745–55. doi: 10.1128/JVI.78.11.5745-5755.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EM, Perez O, Anderson JL, Hope TJ. Visualization of a proteasome-independent intermediate during restriction of HIV-1 by rhesus TRIM5alpha. J Cell Biol. 2008;180(3):549–61. doi: 10.1083/jcb.200706154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham BC, Wells JA. High-resolution epitope mapping of hGH-receptor interactions by alanine-scanning mutagenesis. Science. 1989;244(4908):1081–5. doi: 10.1126/science.2471267. [DOI] [PubMed] [Google Scholar]
- Diaz-Griffero F, Kar A, Perron M, Xiang SH, Javanbakht H, Li X, Sodroski J. Modulation of Retroviral Restriction and Proteasome Inhibitor-resistant Turnover by Changes in the TRIM5{alpha} B-box 2 Domain. J Virol. 2007 doi: 10.1128/JVI.00703-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Griffero F, Qin XR, Hayashi F, Kigawa T, Finzi A, Sarnak Z, Lienlaf M, Yokoyama S, Sodroski J. A B-box 2 surface patch important for TRIM5alpha self-association, capsid binding avidity, and retrovirus restriction. J Virol. 2009;83(20):10737–51. doi: 10.1128/JVI.01307-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Griffero F, Vandegraaff N, Li Y, McGee-Estrada K, Stremlau M, Welikala S, Si Z, Engelman A, Sodroski J. Requirements for capsid-binding and an effector function in TRIMCyp-mediated restriction of HIV-1. Virology. 2006;351(2):404–19. doi: 10.1016/j.virol.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Durocher Y, Perret S, Kamen A. High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-EBNA1 cells. Nucleic Acids Res. 2002;30(2):E9. doi: 10.1093/nar/30.2.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gack MU, Shin YC, Joo CH, Urano T, Liang C, Sun L, Takeuchi O, Akira S, Chen Z, Inoue S, Jung JU. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature. 2007;446(7138):916–920. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]
- Javanbakht H, Diaz-Griffero F, Stremlau M, Si Z, Sodroski J. The contribution of RING and B-box 2 domains to retroviral restriction mediated by monkey TRIM5alpha. J Biol Chem. 2005;280(29):26933–40. doi: 10.1074/jbc.M502145200. [DOI] [PubMed] [Google Scholar]
- Javanbakht H, Diaz-Griffero F, Yuan W, Yeung DF, Li X, Song B, Sodroski J. The ability of multimerized cyclophilin A to restrict retrovirus infection. Virology. 2007;367(1):19–29. doi: 10.1016/j.virol.2007.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javanbakht H, Yuan W, Yeung DF, Song B, Diaz-Griffero F, Li Y, Li X, Stremlau M, Sodroski J. Characterization of TRIM5alpha trimerization and its contribution to human immunodeficiency virus capsid binding. Virology. 2006;353(1):234–46. doi: 10.1016/j.virol.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Kar AK, Diaz-Griffero F, Li Y, Li X, Sodroski J. Biochemical and biophysical characterization of a chimeric TRIM21-TRIM5alpha protein. J Virol. 2008;82(23):11669–81. doi: 10.1128/JVI.01559-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langelier CR, Sandrin V, Eckert DM, Christensen DE, Chandrasekaran V, Alam SL, Aiken C, Olsen JC, Kar AK, Sodroski JG, Sundquist WI. Biochemical characterization of a recombinant TRIM5alpha protein that restricts human immunodeficiency virus type 1 replication. J Virol. 2008;82(23):11682–94. doi: 10.1128/JVI.01562-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Sodroski J. The TRIM5alpha B-box 2 domain promotes cooperative binding to the retroviral capsid by mediating higher-order self-association. J Virol. 2008;82(23):11495–502. doi: 10.1128/JVI.01548-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meroni G, Diez-Roux G. TRIM/RBCC, a novel class of ‘single protein RING finger’ E3 ubiquitin ligases. Bioessays. 2005;27(11):1147–57. doi: 10.1002/bies.20304. [DOI] [PubMed] [Google Scholar]
- Mische CC, Javanbakht H, Song B, Diaz-Griffero F, Stremlau M, Strack B, Si Z, Sodroski J. Retroviral restriction factor TRIM5alpha is a trimer. J Virol. 2005;79(22):14446–50. doi: 10.1128/JVI.79.22.14446-14450.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neagu MR, Ziegler P, Pertel T, Strambio-De-Castillia C, Grutter C, Martinetti G, Mazzucchelli L, Grutter M, Manz MG, Luban J. Potent inhibition of HIV-1 by TRIM5-cyclophilin fusion proteins engineered from human components. J Clin Invest. 2009;119(10):3035–47. doi: 10.1172/JCI39354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman RM, Hall L, Kirmaier A, Pozzi LA, Pery E, Farzan M, O’Neil SP, Johnson W. Evolution of a TRIM5-CypA splice isoform in old world monkeys. PLoS Pathog. 2008;4(2):e1000003. doi: 10.1371/journal.ppat.1000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozato K, Shin DM, Chang TH, Morse HC., 3rd TRIM family proteins and their emerging roles in innate immunity. Nat Rev Immunol. 2008;8(11):849–60. doi: 10.1038/nri2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Caballero D, Hatziioannou T, Zhang F, Cowan S, Bieniasz PD. Restriction of Human Immunodeficiency Virus Type 1 by TRIM-CypA Occurs with Rapid Kinetics and Independently of Cytoplasmic Bodies, Ubiquitin, and Proteasome Activity. J Virol. 2005;79(24):15567–72. doi: 10.1128/JVI.79.24.15567-15572.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond A, Meroni G, Fantozzi A, Merla G, Cairo S, Luzi L, Riganelli D, Zanaria E, Messali S, Cainarca S, Guffanti A, Minucci S, Pelicci PG, Ballabio A. The tripartite motif family identifies cell compartments. Embo J. 2001;20(9):2140–51. doi: 10.1093/emboj/20.9.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer SL, Wu LI, Emerman M, Malik HS. Positive selection of primate TRIM5alpha identifies a critical species-specific retroviral restriction domain. Proc Natl Acad Sci U S A. 2005;102(8):2832–7. doi: 10.1073/pnas.0409853102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayah DM, Sokolskaja E, Berthoux L, Luban J. Cyclophilin A retrotransposition into TRIM5 explains owl monkey resistance to HIV-1. Nature. 2004;430(6999):569–73. doi: 10.1038/nature02777. [DOI] [PubMed] [Google Scholar]
- Sebastian S, Grutter C, de Castillia CS, Pertel T, Olivari S, Grutter MG, Luban J. An invariant surface patch on the TRIM5alpha PRYSPRY domain is required for retroviral restriction but dispensable for capsid binding. J Virol. 2009;83(7):3365–73. doi: 10.1128/JVI.00432-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Diaz-Griffero F, Park DH, Rogers T, Stremlau M, Sodroski J. TRIM5alpha association with cytoplasmic bodies is not required for antiretroviral activity. Virology. 2005a doi: 10.1016/j.virol.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Song B, Gold B, O’Huigin C, Javanbakht H, Li X, Stremlau M, Winkler C, Dean M, Sodroski J. The B30.2(SPRY) domain of the retroviral restriction factor TRIM5alpha exhibits lineage-specific length and sequence variation in primates. J Virol. 2005b;79(10):6111–21. doi: 10.1128/JVI.79.10.6111-6121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004;427(6977):848–53. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Webb BL, Ylinen LM, Verschoor E, Heeney JL, Towers GJ. Independent evolution of an antiviral TRIMCyp in rhesus macaques. Proc Natl Acad Sci U S A. 2008;105(9):3557–62. doi: 10.1073/pnas.0709003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap MW, Nisole S, Stoye JP. A single amino acid change in the SPRY domain of human Trim5alpha leads to HIV-1 restriction. Curr Biol. 2005;15(1):73–8. doi: 10.1016/j.cub.2004.12.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.