Abstract
Transcriptional co-activators, co-repressors and chromatin remodeling machines are essential elements in the transcriptional programs directed by the master adipogenic transcription factor PPARγ. Many of these components have orthologs in other organisms, where they play roles in development and pattern formation, suggesting new links between cell fate decision-making and adipogenesis. This review focuses on bromodomain-containing protein complexes recently shown to play a critical role in adipogenesis. Deeper understanding of these pathways is likely to have major impact on treatment of obesity-associated diseases, including metabolic syndrome, cardiovascular disease and Type 2 diabetes. The research effort is urgent because the obesity epidemic is serious; the medical community is ill prepared to cope with the anticipated excess morbidity and mortality associated with diet-induced obesity.
Keywords: Brd2, SWI/SNF, PPARgamma, mouse models, obesity
A newly described gene that influences obesity
Worldwide, 1.7 billion people are classified as overweight [1]. Excess consumption of calories leads to human obesity, which is one of the major health crises of this century. The World Health Organization estimates that 171 million people worldwide have diabetes, due primarily to obesity. This figure is expected to at least double by 2030. The US Centers for Disease Control reports that six US states currently have a prevalence of obesity of ≥30% and only one state (Colorado) has a prevalence of obesity of <20%. Obesity is characterized by dysregulated metabolism, dyslipidemia, insulin resistance, metabolic syndrome, non-alcoholic fatty liver disease, hyperglycemia, hypertension, some forms of cancer and increased risk for development of Type 2 diabetes (T2D) and its co-morbidities, the most serious of which is cardiovascular disease (CVD). About 90% of T2D is attributable to excess weight [2]. Unless reversed, the deepening problem of obesity predicts an epidemic of these co-morbidities that will strain or break many health care delivery systems. Thus, obesity poses a formidable challenge of overarching importance for public health. However, the obesogenic genes, transcriptional processes and chromatin regulation that control weight gain remain incompletely understood.
The in vivo mechanisms that regulate adipogenic transcription are crucial for cell fate decisions, the formation of adipose tissue from progenitors and the response of adipocytes to over-nutrition. Recent work showing that mice with reduced whole-body expression of the ubiquitously expressed, dual bromodomain protein Brd2 (‘Bromodomain-containing 2’) have dramatically expanded adipose tissue [3], has refocused attention on the role of bromodomain-containing transcriptional co-activators/co-repressors in adipogenesis. Specifically, Brd2 hypomorphic mice, which harbor a lacZ disruption of the gene that encodes this transcriptional co-activator/co-repressor, showed severe adipogenesis and obesity. These ‘brd2 lo’ animals gained fat on an ad libitum diet of regular rodent chow to weights approaching 100g by 12 months of age. At all ages, brd2 lo mice accumulate about twice the fat of matched control mice. For example, epididymal adipocytes of male brd2 lo mice on chow diet were significantly larger than age-matched wild type controls on chow diet: 62.9% of brd2 lo adipocytes were larger than 10,000 µm2, compared to only 1.1% of wild type adipocytes (P < 0.001) [3]. Interestingly, all adipose depots were healthy; severe obesity was observed without concomitant insulin resistance or T2D. Until this report, Brd2 function had not been linked to obesity or glucose homeostasis.
Significantly, shRNA knockdown in vitro of Brd2 in 3T3-L1 pre-adipocytes strongly promotes adipogenic differentiation. Pre-adipocytes with Brd2 stably knocked down show about 50% more Oil Red O staining of adipocytes upon insulin/ dexamethasone/ isobutylmethylxanthine differentiation than control adipocytes. Brd2 and PPARγ are each detectable by co-immunoprecipitation of the other, and shRNA knockdown in vitro of Brd2 in 3T3-L1 cells approximately doubles the signal from a PPRE-regulated transcriptional reporter. Taken together, these results suggest a mechanism that works through alleviated Brd2 co-repression of PPARγ-directed transcription of adipogenic genes [3]. Previous work has identified histone modification enzymes and nucleosome remodeling proteins associated with Brd2-containing multiprotein complexes [4,5]. These new results offer an opportunity to revisit what is known about the role of chromatin in adipogenic transcription and to develop hypotheses that will channel effort into a deeper excavation of the relevant mechanisms.
The Bromodomain and Extraterminal domain (BET) family of regulators
The metazoic members of the Brd2 family, the best known subgroup of BET proteins, possess dual, mutually-related bromodomains in the amino-terminal region of the protein that bind to acetylated chromatin, and protein-protein interaction domains for association with transcription machinery in the carboxyl-terminal region. The bromodomains account for the reported co-localization with chromosomes of this protein family (Fig. 1). Specifically, the bromodomains bind to acetylated lysine 12 of histone H4 in nucleosomal promoters [6], a chromatin-binding function first illustrated for the single-bromodomain histone acetyltransferase (HAT) Gcn5 [7] and p/CAF, (a p300/CREB binding protein-associated factor) [8]. Structural requirements for chromatin interaction have been established in detail for Brd2 [5,6,9,10]. Similar interactions have been reported for other dual bromodomain proteins such as Brd3 [11,12], Brd4 [13,14,15], Brd6 [Brdt; 16], the basal transcription factor TAFII250 [17,18] and Brd2 gene orthologs: Saccharomyces BDF1 [19–21]; Arabidopsis GTE4 [22], Drosophila female sterile (1) homeotic (fs(1)h) [23–25], Caenorhabditis bet-1 [26], and Danio and Xenopus brd4 [27]. Dual bromodomain proteins thereby couple histone acetylation to transcription in a wide variety of organisms and transcriptional contexts.
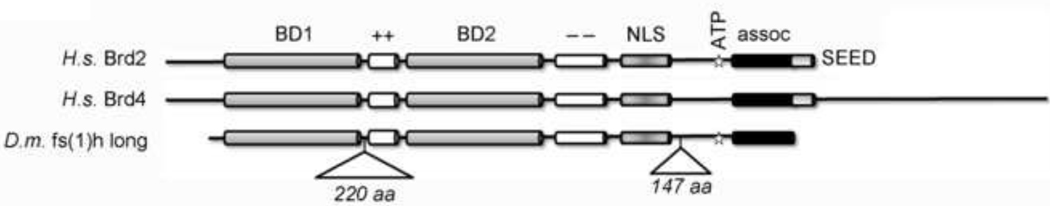
Figure 1. Major forms of BET proteins.
The structure of human Brd2 is compared to human Brd4 and the long form of Drosophila fs(1)h. Both Brd2 bromodomains (BD) are about 100 amino acids in length and are highly homologous to each other. They are separated by a basic domain (++), followed by an acidic domain (−−), a short nuclear localization sequence (NLS), an ATP binding/kinase domain (ATP), a multiprotein complex association domain (assoc) 164 amino acids in length and an acidic polyserine (SEED) domain. Brd2 BD1 is located between amino acid positions 75 and 175, and BD2 is located between 350 and 450. Brd4 possesses a long carboxyl-terminal region of unknown function that lacks association motifs; fs(1)h possesses a number of insertions, also of unknown function. The two largest insertions are shown. Mammalian BET proteins exhibit alternative start sites and splice sites, but tend to cluster into one of two subtypes: either a short form with dual bromodomains and an association domain (Brd2, Brd3, Brd6), or a long form with an unstructured carboxyl terminal tail (Brd4). [See ref. 66 for more detailed discussion].
This highly conserved family of transcriptional co-regulators is primarily known for function in cell fate during development, in cancer and the cell cycle. Dual bromodomain proteins have crucial functions in pattern formation in Drosophila [28–32] and mice [13,33,34]. Mutation of fs(1)h causes severe defects in differentiation and cell fate; fs(1)hnull is lethal [23,29,35]. The fs(1)h locus is an upstream activator of trithorax in Drosophila [30,36], an important, homeotic control gene that positively regulates Hox-controlled differentiation in mice, countering repression by the Polycomb group (PcG) proteins. Disruption of human BRD4 through t(15;19) chromosomal translocation generates aggressive midline carcinomas [37; reviewed in 38]. Brd2 and Brd4 control cell cycle in mice [13,39,40] and in cultured cells [5,41]. In the mouse, Brd4 is necessary for the G2-to-M transition of the cell cycle, and brd4(+/−) mice show severe defects in differentiation and organogenesis. In mice, brd4null is lethal [13,42,43]. The TAFII250 subunit of the TFIID basal transcription factor complex [44] is also crucial for cell cycle control through its regulation of cyclin A, which is a critical driver of S phase [45]. Brd2 transduces mitogenic signals [46,47], leading to increased proliferation [48]. Forced expression of Brd2 transcriptionally co-activates cyclin A, causing earlier S phase entry during cell cycle progression [5]. Brd2 constitutive expression in B cell progenitors causes a B cell malignancy in mouse models [40] that is most similar to human diffuse large B cell lymphoma [49,50]. Thus, the dual bromodomain proteins exert non-redundant, chromatin-based activities that are essential for growth, development, differentiation and cell cycle progression.
These proteins use a structural component, the bromodomain, to bring transcriptional functions to chromatin that has already been identified for transcriptional regulation through histone hyperacetylation or sequence-specific DNA-binding transcription factors. Virtually all of the nuclear HATs contain bromodomains [51,52], but not all bromodomain proteins are HATs. Instead, the enzymatic activities (HAT or ATP-dependent chromatin remodelase) are either encoded within the same polypeptide chain or are recruited to a multiprotein complex, including bromodomain proteins resident at the promoter, thereby coupling structure to function. Chromosomal translocation can decouple this system, targeting HAT activity to the wrong promoter [53], a genetic abnormality frequently associated with cancer [38,54–57].
The bromodomain motif
In 1992, the bromodomain was first noticed as a primary amino acid sequence present in certain proteins that have chromatin or transcription functions [58]. Many bromodomain-containing proteins are found in transcription complexes [51,52], where they perform scaffolding functions [59]. The bromodomain takes its name from Drosophila brahma, an important chromatin modifying factor discovered by Tamkun and colleagues [60] and discussed in a prescient 1994 paper of Randazzo and colleagues [61], who noted that brahma (brm, Snf2a, SMARCA2) likely assists trithorax to overcome Polycomb repression of chromatin. Given the crucial role played by the human homolog of trithorax (MLL) in mixed lineage leukemias derived from 11q23 chromosomal translocations [62,63], they speculated that the brahma-related bromodomain protein BRG1 (Snf2β, SMARCA4), which is an essential catalytic component of the SWI/SNF complex (discussed in detail below), would be implicated in mammalian malignancy, as later work verified [64,65]. The conserved, 110 amino acid bromodomain motif is comprised of four left-handed α helices bundled together and connected with two segments, the so-called ZA and BC loops. The structure was solved first by analysis of nuclear Overhauser effects in the p/CAF single bromodomain [8]. The field has enjoyed a number of excellent reviews that discuss the relationship between bromodomain protein structure and transcriptional co-activation or co-repression function [53,66–68].
Transcriptional co-activation and co-repression by bromodomain proteins
Bromodomain proteins that encode HAT activity, or recruit HAT enzymes to chromatin, establish a paradigm for transcriptional co-activation. The model also implies that a basal level of histone acetylation of nucleosomes is required to catalyze the initial association with bromodomain proteins. It is widely appreciated that histone hyperacetylation is a mark of transcription activation of promoter chromatin. Brd2 binds endogenous cyclin A promoter chromatin, but mutants of Brd2 with deleted bromodomains or carboxyl-terminal protein association domains do not transactivate [5]. Functional cis-acting E2F binding sites are required for Brd2-dependent transcriptional function [48], and overexpressed RB, which halts E2F-dependent cell cycle progression [69], ablates Brd2-driven transactivation [48]. E2F1 and E2F2 are present in Brd2 multiprotein complexes purified from nuclear extracts [48] and Brd2 works with E2Fs to recruit HAT activity and other epigenetic regulators, including Mediator complex proteins, and the hSWI/SNF components BRG1 and BAF155, to chromatin [4,5] thus to transactivate cyclin A. Dysregulation of this process leads to the classic disease of uncontrolled proliferation: cancer [40].
Transcriptional programs that promote cellular proliferation/cell cycle progression function in balance with programs that promote cellular differentiation/cell cycle exit. The balance between the two lies at the heart of cell decisions to grow, specialize or undergo apoptosis. Imbalances are pathogenic. For example, diverse leukemias arise in the bone marrow through defective differentiation closely coupled to abnormal proliferation [70–72]. The resultant leukemic blasts are often blocked at an early stage of differentiation, consistent with their continued active proliferation at the expense of normal differentiation. Important and effective therapies for certain leukemias take advantage of this transcriptional switch as a rationale to treat leukemic patients with differentiation-promoting agents, such as retinoic acid derivatives that force cell cycle exit and block proliferation [73,74].
Bromodomain proteins also play important roles in transcriptional co-repression, as first identified in studies showing that the bromodomain protein BRM contacts the retinoblastoma protein (RB) [75,76]. RB and its family members p107 and p130 bind to E2F proteins and block their transcription activation function to oppose cell cycle progression. RB also recruits a histone deacetylase (HDAC), as do p107 and p130, through contact with BRM and other proteins in the SWI/SNF complex [77–80]. Not all SWI/SNF complexes contribute to this repressive function, however. Indeed, recent studies indicate that a specific variant SWI/SNF complex (the ARID1A BAF complex) is important for repression of E2F activated cell cycle control genes, whereas another variant (the ARID1B BAF complex) contributes to the activation of these genes [81,82]. Until recently [3], there was no evidence of a role for Brd2 in mammalian transcriptional co-repression, although clues from studies of Drosophila development identified in fs(1)h, the homolog of Brd2, transcriptional repression functions that are essential for proper differentiation in the early embryo [38,83].
The SWI/SNF complex
As discussed above, local modification of histones on enhancers and promoters is required to activate gene expression [84,85]. Transcription factors that bind to nucleosome-free regions of DNA or to DNA within nucleosomes recruit enzymatic activities that also modify the surrounding chromatin architecture. These ATP-dependent remodeling complexes may contribute to gene regulation through a variety of mechanisms, including movement in cis of nucleosomes away from or over regulatory elements, removal or deposition of nucleosomes in conjunction with cellular chaperones, changes in the histone composition of nucleosomes, regulation of covalent histone modifications, alteration of nucleosome structure, and/or changes in higher order chromatin folding. While these models mostly derive from in vitro biochemical studies, examples of many of these effects in gene regulation are beginning to accumulate [86].
The SWI/SNF complex offers an important example of an evolutionarily conserved, bromodomain-containing, ATP-dependent, chromatin remodeling machine, with roles in both transcriptional activation and repression [65,87,88]. Mammalian SWI/SNF comprises a 2 MDa subunit complex that possess the essential, catalytic proteins BRG1 or BRM, and an additional 9–12 proteins called BRM/BRG1 Associated Factors (BAFs) [89; for review, see ref. 90]. The function of SWI/SNF complexes can vary depending on the complex components. Two major classes of SWI/SNF complexes have been identified. The BAF complexes (most similar to Saccharomyces SWI/SNF) contain either the BRG1 or BRM ATPase subunit together with one of two variant BAF250/OSA/ARID1 subunits. These complexes contain a single bromodomain in their ATPase subunit. The choice of BAF250 subunit can dramatically alter complex function, such as the opposing effects of ARID1A versus ARID1B complexes in cell cycle control [81,82] and the specific function of ARID1A in stem cell renewal [91]. The choice of ATPase may also be critical, since BRG1 tends to be highly expressed in proliferating cells, whereas BRM is preferentially expressed in terminally differentiated tissues [92], and because the regulation of specific target genes is sometimes affected by only one ATPase or the other [93,94]. Furthermore, Brg1−/− mice are embryonic lethal [95], whereas Brm knockout shows a relatively mild phenotype [96]. Studies have shown that these variant SWI/SNF complexes have distinct, but often overlapping functions [90]. Variant forms of other subunits also exist, and show differential cell-type distributions and functions (such as the presence of BAF60a), but not BAF60c in the esBAF complex, that are critical for stem cell renewal [97]. The emerging model is that SWI/SNF complex composition varies by tissue and cell type [89,95,98], and that the distinct combinations of subunits enable these variant complexes to interact with distinct DNA binding transcription factors and co-regulators, or to interact with histones that bear specific modifications, to carry out tissue-specific, divergent functions.
The bromodomains in SWI/SNF complexes appear to play a critical role in maintaining the stable association of the complex with chromatin. This interaction was shown, for Saccharomyces SWI/SNF, in an elegant set of in vitro experiments [99]. This mechanism is also evidenced, in mammalian cells, by the requirement of p/CAF-mediated acetylation to support SWI/SNF recruitment to the myogenin promoter [100]. However, relatively little is known about potential differential functions of the bromodomains in BRG1, BRM or Polybromo.
Determination of genes that require SWI/SNF enzymes for proper regulation has been accomplished in part by use of antibodies that function in co-immunoprecipitation (co-IP) and chromatin immunoprecipitation (ChIP) assays. These experiments show SWI/SNF components localized with specific activators and/or at specific gene sequences, indicating that the role of SWI/SNF in co-activation and co-repression is mediated by direct recruitment of the complex to target promoters [101–104]. SWI/SNF complexes bind to a wide variety of transcription factors, acting either as coactivators or co-repressors [for a recent review, see ref. 105]. Of particular relevance to adipogenic differentiation, hSWI/SNF complexes bind to and serve as coactivators for many nuclear hormone receptors, including estrogen, glucocorticoid, retinoic acid receptor (RAR) families and PPARγ [102,103,106–109]. SWI/SNF and PPARγ are crucial for adipogenesis, as discussed below. Sequence-specific DNA binding transcription factors are required to target individual adipogenic genes and marshal the transcriptional program, but the general transcriptional factors, and non-sequence specific complexes, such as the bromodomain-containing chromatin remodeling factors and co-activators are also critical. It is not well understood how these specific and general factors work together with chromatin remodeling enzymes on the promoters of adipogenic genes.
Functions of PPARγ and its transcriptional co-activators in adipogenesis
Not only is transcriptional control of proliferation subject to tight control, but differentiation must also be carefully regulated. Adipocyte differentiation from fibroblast-like progenitors, for example, is regulated by two well-studied families of transcriptional regulatory proteins: C/EBPs (CCAAT/enhancer binding protein) and PPARs (peroxisome proliferator-activated receptor), especially PPARγ, a master regulator of differentiation of white adipose tissue (WAT) and brown adipose tissue (BAT) [110–113]. To act as a transcription factor, PPARγ forms a complex with the retinoid X receptor (RXR) transcription factor [114–116]. Improper or deficient activation of PPARγ is associated with insulin resistance and T2D [117,118]. The transcriptional programs of adipogenesis have been effectively reviewed [119].
Nuclear receptors like PPARγ are Cys4-type Zn2+-finger transcription factors. It has been proposed that this class prefers to interact with BRG1 subunits of SWI/SNF [104,120]. Seminal studies from the Imbalzano group [121] showed that the catalytic subunits of the SWI/SNF complex, BRG1 and BRM, are required for induction of adipogenic transcription programs. Specifically, they established that general transcription factors assemble at the promoter of the PPARγ2 gene. Upon subsequent association of SWI/SNF and TFIIH with the promoter, a pre-initiation complex forms and is capable of transcription. This topic has been recently reviewed [122,123]. It is now clear that SWI/SNF and associated bromodomain-containing co-activator complexes are crucial for PPARγ function. Interestingly, expression of dominant negative PPARγ is capable of partially reversing terminal adipogenesis [124], suggesting that some basal form of ongoing chromatin maintenance or nucleosomal remodeling is required to maintain an adipogenic pattern of gene expression, but this would come at high energetic cost to the adipocyte.
PPARγ co-activators, including members of the p160 family [125,126] must be regulated in their association with the chromatin-bound transcription complex. It is apparent that adipogenesis or differentiation of adipose tissue from progenitors during development could be severely affected by loss or dysregulation of this association. We speculate that these associated co-activator and SWI/SNF complexes localized on the chromatin of adipogenic genes are partially disassembled upon cessation of the adipogenic program. However, how this is achieved, to what extent, and the signal transduction events that prompt complex disassembly, are obscure. For a model of this process, we have begun to analyze the stoichiometry and kinetics of Brd2-dependent transcriptional control of the cyclin A promoter [4], which requires complexes that must be activated and inactivated each time the cell traverses the cell cycle.
The dramatic adiposity of brd2 lo mice was completely unexpected. However, in retrospect the co-activator/co-repressor functions of bromodomain proteins make sense as a mechanism for regulating the adipogenic phenotype. The increased adipogenesis of 3T3-L1 pre-adipocytes in which Brd2 was knocked down [3] suggests PPARγ interactions with Brd2 are crucial. In addition, two important transcriptional targets of PPARγ and its co-activator PGC-1α are the genes that encode mitochondrial uncoupling protein-1 and −2 (ucp1, ucp2), which have been linked to obesity [127] in mice [128] and humans [129] and are important for thermogenesis in BAT. We noted that both ucp1 and 2 were dramatically elevated in brd2 lo mice [3]. Intriguingly, PCG-1α binds a transcriptional co-activator/co-repressor complex [130,131] that contains the Mediator complex [132] and Brd2 [4,133–135]. These observations reinforce the hypothesis that Brd2 levels regulate the transcription of genes that are targets of the PPARγ/PCG-1 family.
Certain crucial transcription co-factors are shared between Brd2 transcription complexes [4,5,48] (Fig. 1) and PPARγ-containing complexes (Table 1; common factors shown in bold [123]. Net co-activation/co-repression depends on the relative abundance, targeting and activity of these associated factors [59] and their ability to switch the chromatin status of key metabolic genes. This insight suggested two easily testable hypotheses: that (1) Brd2 and PPARγ interact, either directly through protein-protein association, or indirectly through association in a ternary complex and that (2) a drop in Brd2 levels in certain cell types, such as the pre-adipocyte, derepresses PPARγ-regulated transcription. Co-immunoprecipitation experiments showed that indeed, Brd2 and PPARγ associate, and Brd2 opposes the action of PPARγ on PPAR-responsive transcriptional elements in DNA [3]. Interestingly, mice harboring a knock-in mutation of ‘silencing mediator of retinoid and thyroid hormone receptors’ (SMRT), a nuclear co-repressor, thought normally to antagonize PPARγ-directed transcription, exhibit a pro-adipogenic phenotype [136], as do mice harboring a knockout of estrogen receptor β [137]. This phenotype shares certain features with Brd2 knockdown, particularly the lower threshold for a PPARγ-directed program of transcription. This pattern also resembles the insulin sensitizing action of glitazones and thiazolidinediones (TZDs) [138]. Indeed, the observations suggest Brd2 might be a novel, useful, ‘druggable’ therapeutic target for insulin resistance. In addition, the thyroid hormone receptor-associated protein (TRAP)220 component of the Mediator complex (encoded by MED1) is important for PPARγ-directed adipogenesis [139]. The observation that Brd2 associates with a number of components of the Mediator complex [4] suggests that PPARγ and Brd2 may be functionally linked through Mediator. Thus, it will be important to verify the presence on chromatin of the Brd2 complex factors shared with PPARγ-associated complexes, and then test their function individually, to understand the combined functions of Brd2 and PPARγ in transcriptional regulation of adipogenesis. Given the failure of intensive effort to identify an obvious endogenous ligand for PPARγ, we can reasonably speculate that specific post-translational modification in response to nutritional signal transduction, such as phosphorylation of co-repressor proteins or acetylation/ubiquitylation of histones, might behave as a ‘pseudo-ligand’ for shifting co-repressor complex function and enable PPARγ-directed adipogenesis. If so, Brd2 may be poised to respond to these signals either as a target for modification or as a ‘reader’ of the resulting modifications, especially histone acetylation.
Table 1.
Transcriptional co-factors that interact with PPARγ
| Co-repressors | Co-activators |
|---|---|
| Mediator | SWI/SNF |
| HDACs | p300/CBP |
| RB | CAF |
| NCoR | PPAR-binding protein (PBP) |
| SMRT | PPAR-interacting protein (PRIP) |
| Sirt 1 | PGC-1,2 |
Factors shared between Brd2 complexes and PPARγ complexes are shown in boldface.
A model for the role of bromodomain proteins in adipocyte differentiation
Recent studies have shown that Brd2 cooperates with E2F1, stabilizing a transcriptional activation complex on acetylated chromatin at cyclin A. This complex also contains SWI/SNF, the association of which will be stabilized both through interaction with Brd2 and via binding of the bromodomain in its ATPase to acetylated chromatin. By contrast, a combination of RB binding to E2F1 (that potentially recruits the inactivating ARID1A form of SWI/SNF), loss of cyclin A promoter acetylation and loss of Brd2 would function to silence cyclin A, and slow growth of pre-adipocytes. In addition to the slowing of growth, adipocyte differentiation requires the upregulation of PPARγ (which requires SWI/SNF for increased transcription). PPARγ activation of its target genes is also likely to require SWI/SNF (although the specific variant complex involved has not been identified). Importantly, however, Brd2 can inhibit transactivation by PPARγ [3]. Thus, Brd2 is required both to activate genes that enable growth and to repress differentiation-specific genes in pre-adipocytes. Accordingly, it is not surprising that deletion of Brd2 leads to a near-complete elimination of mature adipocytes.
Important outstanding issues
1. Signal transduction and specificity
The mechanisms by which signal transduction pathways instruct the chromatin remodeling machinery to conduct an adipogenic program are very poorly understood. The notion that chromatin remodeling machines can function as effectors of signal transduction, particularly of mitogenic signals, has been discussed with respect to Mediator [134] and hSWI/SNF [140,141]. For example, mitogenic signals through the ras pathway [142] or inflammatory signals through the TLR pathway [143,144] convey information to chromatin to create a coherent transcriptional state that is also reversible. Conversely, it is reasonable to hypothesize that in response to an adipogenic differentiation signal, a specialized cell mobilizes a MDa transcriptional apparatus at a limited number of genes. This restricted response – only a few ‘immediate early’ adipogenic promoters – could explain why the global disruption of so fundamental a transcriptional cofactor as Brd2 generates a coherent response on PPARγ-responsive promoters and a clear, adipogenic transcriptional program in a pre-adipocyte. Most progenitor cells, such as pre-adipocytes, are already primed for a specific fate, thus, manipulation of global transcriptional and chromatin programs does not create transcriptional confusion, because the map of cell fate is restricted. It will be important to learn how, upon cell cycle exit and induction of differentiation, chromatin in the adipocyte resolves the differential responses to a combination of mitogenic and differentiation–promoting signal transduction pathways. It also remains to be explored whether epigenetic predetermination of adipogenic promoters is a major mechanism that defines the cell fate of the pre-adipocyte. More generally, a more comprehensive knowledge of the signal transduction-mediated mechanisms of priming in progenitor cells will be critical if we wish to understand how lineage-specific transcription factors establish cell fate.
2. Functional shifts in chromatin remodeling and histone modification complex composition
Biochemical studies of SWI/SNF complexes sometimes identify BRG1 and BRM subunits associated with the same locus [121], reflecting the view that these subunits identify complexes that exhibit a combination of overlapping and specific functions. Differential recruitment of SWI/SNF subunits BAF155 and BAF170 to the same promoter in response to estrogen determines subsequent recruitment of a co-activator HAT or a co-repressor HDAC [145], suggesting that different mechanisms of PPARγ activation (which may include signal transduction pathways or as-yet unidentified endogenous ligands) could differentially regulate transcription factor/chromatin complexes formed during adipogenesis.
The dramatic adipogenic phenotype of Brd2 deficiency strongly suggests that Brd2 and its associated bromodomain-containing transcriptional co-regulators (including SWI/SNF) are central to the decision to undergo adipogenic differentiation. During this process, the chromatin- associated SWI/SNF complexes likely change character in a coordinated fashion. These shifts will be most directly measurable with analysis of chromatin-modifying activities, along with DNA accessibility, associated with proliferative and adipogenic genes during adipogenesis (i.e. at the end of the clonal expansion) in 3T3-L1 adipocytes that have been induced to undergo adipogenic differentiation.
It appears that E2Fs govern a link between proliferative signaling pathways and terminal adipocyte differentiation. E2Fs trigger clonal expansion, then, through RB-mediated repression and replacement of pro-proliferative E2Fs with pro-differentiation E2Fs, coherently switch a variety of promoters to the new program. Apart from the proposed role of E2F-1 in PPARγ1 transcription, to switch between proliferative, clonal expansion and terminal adipocyte differentiation through control of PPARγ levels [146], reviewed in [119], it is reasonable to hypothesize that reduced levels of Brd2 or a related bromodomain protein reprograms a panel of target genes, analogous to the result of swi/snf mutation in Saccharomyces [88], transcriptionally repressing the proliferative genes [5] and activating the adipogenic genes [3]. Likewise, transcriptionally activating SWI/SNF complexes may need to shift character to transcriptionally repressing complexes on the relevant promoters. It is unclear whether this switch would occur by swapping out subunits on chromatin-bound SWI/SNF or by exchanging one entire complex for another. However, given the rapid exchange seen for most transcription factors on and off chromatin [for review, see ref. 147], together with the strong association of SWI/SNF complex subunits in biochemical studies, the latter possibility seems most likely [148]. As discussed above, experiments in the 3T3-L1 model will be useful to define these mechanisms. It will be expected that patterns of histone and DNA methylation and acetylation, DNA accessibility, DNAse hypersensitivity and transcript levels will follow suit and reflect the differential functions of the variant chromatin-bound complexes.
3. Cell fate and development
Interesting recent work on adipose cell fate used an RNAi screen in Drosophila to identify candidate obesity genes and discovered an important, previously unappreciated role for hedgehog signal transduction [149]. Significantly, reported activators of the fat-specific obesity pathway included Nejire (a fly homolog of the well known HAT p300/CBP); and repressors included trr (trithorax-related histone methyltransferase), CG3075 (histone H2A), Su(fu) (an mSin3 co-repressor) and slmb (required for E2F function). These factors implicate chromatin modification in adipogenic transcriptional programs and should be studied in detail in mouse models. In this regard, developmental regulators such as the bone morphogenetic proteins (BMPs) [150] with morphogen roles first identified in Drosophila [151] and transcriptional coactivators such as PRDM16 [152] and PGC-1 [153] have newfound significance in adipogenic transcriptional programs and cell fate, particularly the crucial function of BMP-7 in BAT adipogenesis [154]. However, very little is known about how these developmental factors communicate with nucleosomes and chromatin remodeling machinery during an adipogenic program in adult progenitor cells.
4. Maternal effect on adipogenesis
The Drosophila homolog of Brd2, fs(1)h, is a maternal effect gene [23,30,31], which suggests the possibility that adipogenic transcriptional programs in humans are influenced by maternal effect inheritance of BRD2. Brd2 remains mitotically associated with chromatin [6], and Brd4 tethers virus episomes to host mitotic chromatin across cell divisions [155]. This behavior suggests a role for dual bromodomain proteins in inheritance, not only of specific histone modifications from one cell generation to the next, but also of chromatin-bound complexes, which likely has significance for epigenetic inheritance of predisposition to adiposity. Convincing evidence from epidemiological study of the Dutch ‘Hunger Winter’ of 1944 – 1945 establishes an environmental maternal effect of starvation during gestation. Specifically, maternal hunger promotes insulin insensitivity, obesity, an atherogenic lipid profile and elevates CVD risk in the surviving children as they age [156]. A number of animal models explore the effect of gestational stress on obesity, hypertension, insulin resistance and hyperinsulinemia in progeny [recently reviewed in 157]. However, there has been insufficient study of genetic maternal effect on obesity. It is likely that alleles of chromatin modification genes will be found to play a role in maternally inherited patterns of human adipogenesis and insulin sensitivity, independent of environment and nutrition status.
Future Directions
Deficiency of Brd2, a gene that encodes a dual bromodomain protein in mice, generates an unexpected and dramatic adipogenic phenotype, revealing a pathway of transcriptional co-repression and chromatin modification that normally opposes the action of PPARγ. Obese brd2 lo mice develop severe obesity but, surprisingly, completely avoid insulin resistance. These mice may provide a useful model for decoupling these two aspects of metabolic syndrome. The Drosophila homolog of Brd2, called female sterile (1) homeotic, is a maternal effect, developmental gene and upstream activator of the trithorax complex, which opposes Polycomb action. These surprising connections suggest that research effort in humans that focuses on the adipocyte-specific functions of developmental and patterning genes will be fruitful, because the size and health of adipose tissue depots, body mass index, insulin sensitivity and WAT/BAT specification from progenitors are all likely to be affected by this pathway. This area of investigation is surprisingly underdeveloped, yet is of great medical significance because of the potential for new mechanistic insight into the ‘metabolically healthy but obese’ (MHO) human phenotype [158], which exhibits a reduced CVD risk and a diminished inflammatory profile [159]. Novel developmental pathways could be exploited to design a next generation of insulin-sensitizing drugs to treat obesity and its co-morbidities, or re-direct energy storage from undesirable, central obesity to peripheral, subcutaneous depots of adipose tissue. In addition, this work highlights the connections between chromatin status, nucleosome positioning and histone modification and adipogenic transcription programs. Particularly, research effort should focus on the critical role of bromodomain-containing protein complexes, such as Brd2, SWI/SNF and their associated co-activator/co-repressor factors, in transcriptional reprogramming from proliferation in the pre-adipocyte to differentiation in the adipocyte. These epigenetic mechanisms have an importance at least equal to lineage-specific transcription factors in the determination of cell fate.
Acknowledgements
This work is supported by grants from the National Institutes of Health (NCI and NIDDK), the American Cancer Society and the Leukemia and Lymphoma Society. We thank our colleagues for their elegant and detailed work that explores the transcriptional programs of adipogenesis; space constraints do not permit comprehensive citation. Any omissions and errors are of course our own.
Abbreviations
- BAF
BRM/BRG1-associated factors
- BAT
brown adipose tissue
- BET
bromodomain and extraterminal domain
- BMP
bone morphogenetic proteins
- BRG1
brahma related gene 1
- BRM
brahma
- CBP
CREB (cyclic AMP-responsive element binding) binding protein
- C/EBP
CCAAT/enhancer binding protein
- ChIP
chromatin immunoprecipitation
- co-IP
co-immunoprecipitation
- CVD
cardiovascular disease
- HAT
histone acetyltransferase
- HDAC
histone deacetylase
- MHO
metabolically healthy obese
- p/CAF
p300/CBP-associated factor
- PPARγ
peroxisome proliferator-activated receptor γ
- PPRE
PPAR responsive element
- RB
retinoblastoma protein
- RXR
retinoid X receptor
- SMRT
silencing mediator of retinoid and thyroid hormone receptors
- SWI/SNF
switch mating type/sucrose non-fermenting
- TAF
TBP (TATA box binding protein)-associated factors
- TRAP
thyroid hormone receptor-associated protein
- TZD
thiazolidinedione
- T2D
Type 2 diabetes
- WAT
white adipose tissue
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Haslam DW, James WP. Obesity. Lancet. 2009;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 2.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world--a growing challenge. N. Engl. J. Med. 2007;356:213–215. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- 3.Wang F, Liu H, Blanton WP, Belkina A, LeBrasseur NK, Denis GV. Brd2 disruption in mice causes severe obesity without type 2 diabetes. Biochem J. 2010;425:71–83. doi: 10.1042/BJ20090928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denis GV, McComb ME, Faller DV, Sinha A, Romesser PB, Costello CE. Identification of transcription complexes that contain the dual bromodomain protein Brd2 and chromatin remodeling machines. J. Proteome Res. 2006;5:502–511. doi: 10.1021/pr050430u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinha A, Faller DV, Denis GV. Bromodomain analysis of Brd2-dependent transcriptional activation of cyclin A. Biochem. J. 2005;387:257–269. doi: 10.1042/BJ20041793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanno T, Kanno Y, Siegel RM, Jang MK, Lenardo MJ, Ozato K. Selective recognition of acetylated histones by bromodomain proteins visualized in living cells. Mol. Cell. 2004;13:33–43. doi: 10.1016/s1097-2765(03)00482-9. [DOI] [PubMed] [Google Scholar]
- 7.Ornaghi P, Ballario P, Lena AM, González A, Filetici P. The bromodomain of Gcn5p interacts in vitro with specific residues in the N terminus of histone H4. J. Mol. Biol. 1999;287:1–7. doi: 10.1006/jmbi.1999.2577. [DOI] [PubMed] [Google Scholar]
- 8.Dhalluin C, Carlson JE, Zeng L, He C, Aggarwal AK, Zhou MM. Structure and ligand of a histone acetyltransferase bromodomain. Nature. 1999;399:491–496. doi: 10.1038/20974. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura Y, Umehara T, Nakano K, Jang MK, Shirouzu M, Morita S, Uda-Tochio H, Hamana H, Terada T, Adachi N, Matsumoto T, Tanaka A, Horikoshi M, Ozato K, Padmanabhan B, Yokoyama S. Crystal structure of the human BRD2 bromodomain: insights into dimerization and recognition of acetylated histone H4. J. Biol. Chem. 2007;282:4193–4201. doi: 10.1074/jbc.M605971200. [DOI] [PubMed] [Google Scholar]
- 10.Umehara T, Nakamura Y, Jang MK, Nakano K, Tanaka A, Ozato K, Padmanabhan B, Yokoyama S. Structural basis for acetylated histone H4 recognition by the human BRD2 bromodomain. J. Biol. Chem. 2010;285:7610–7618. doi: 10.1074/jbc.M109.062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thorpe KL, Gorman P, Thomas C, Sheer D, Trowsdale J, Beck S. Chromosomal localization, gene structure and transcription pattern of the ORFX gene, a homologue of the MHC-linked RING3 gene. Gene. 1997;200:177–183. doi: 10.1016/s0378-1119(97)00415-0. [DOI] [PubMed] [Google Scholar]
- 12.LeRoy G, Rickards B, Flint SJ. The double bromodomain proteins Brd2 and Brd3 couple histone acetylation to transcription. Mol. Cell. 2008;30:51–60. doi: 10.1016/j.molcel.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dey A, Chitsaz F, Abbasi A, Misteli T, Ozato K. The double bromodomain protein Brd4 binds to acetylated chromatin during interphase and mitosis. Proc. Nat’l. Acad. Sci. USA. 2003;100:8758–8763. doi: 10.1073/pnas.1433065100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shang E, Salazar G, Crowley TE, Wang X, Lopez RA, Wang X, Wolgemuth DJ. Identification of unique, differentiation stage-specific patterns of expression of the bromodomain-containing genes Brd2, Brd3, Brd4, and Brdt in the mouse testis. Gene Expr. Patterns. 2004;4:513–519. doi: 10.1016/j.modgep.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Lee AY, Chiang CM. Chromatin adaptor Brd4 modulates E2 transcription activity and protein stability. J. Biol. Chem. 2009;284:2778–2786. doi: 10.1074/jbc.M805835200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones MH, Numata M, Shimane M. Identification and characterization of BRDT: A testis-specific gene related to the bromodomain genes RING3 and Drosophila fsh. Genomics. 1997;45:529–534. doi: 10.1006/geno.1997.5000. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson RH, Ladurner AG, King DS, Tjian R. Structure and function of a human TAFII250 double bromodomain module. Science. 2000;288:1422–1425. doi: 10.1126/science.288.5470.1422. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Wang X, Zhang J, Huang H, Ding B, Wu J, Shi Y. Structural basis and binding properties of the second bromodomain of Brd4 with acetylated histone tails. Biochemistry. 2008;47:6403–6417. doi: 10.1021/bi8001659. [DOI] [PubMed] [Google Scholar]
- 19.Lygerou Z, Conesa C, Lesage P, Swanson RN, Ruet A, Carlson M, Sentenac A, Séraphin B. The yeast BDF1 gene encodes a transcription factor involved in the expression of a broad class of genes including snRNAs. Nucl. Acids Res. 1994;22:5332–5340. doi: 10.1093/nar/22.24.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chua P, Roeder GS. Bdf1, a yeast chromosomal protein required for sporulation. Mol. Cell. Biol. 1995;15:3685–3696. doi: 10.1128/mcb.15.7.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ladurner AG, Inouye C, Jain R, Tjian R. Bromodomains mediate an acetyl-histone encoded antisilencing function at heterochromatin boundaries. Mol. Cell. 2003;11:365–376. doi: 10.1016/s1097-2765(03)00035-2. [DOI] [PubMed] [Google Scholar]
- 22.Airoldi CA, Rovere FD, Falasca G, Marino G, Kooiker M, Altamura MM, Citterio S, Kater MM. The Arabidopsis BET bromodomain factor GTE4 is involved in maintenance of the mitotic cell cycle during plant development. Plant Physiol. 2010;152:1320–1334. doi: 10.1104/pp.109.150631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Digan ME, Haynes SR, Mozer BA, Dawid IB, Forquignon F, Gans M. Genetic and molecular analysis of fs(1)h, a maternal effect homeotic gene in Drosophila. Dev. Biol. 1986;114:161–169. doi: 10.1016/0012-1606(86)90392-1. [DOI] [PubMed] [Google Scholar]
- 24.Haynes SR, Mozer BA, Bhatia-Dey N, Dawid IB. The Drosophila fsh locus, a maternal effect gene, encodes apparent transmembrane proteins. Dev. Biol. 1989;134:246–257. doi: 10.1016/0012-1606(89)90094-8. [DOI] [PubMed] [Google Scholar]
- 25.Chang YL, King B, Lin SC, Kennison JA, Huang DH. A double-bromodomain protein, FSH-S, activates the homeotic gene ultrabithorax through a critical promoter-proximal region. Mol. Cell. Biol. 2007;27:5486–5498. doi: 10.1128/MCB.00692-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shibata Y, Takeshita H, Sasakawa N, Sawa H. Double bromodomain protein BET-1 and MYST HATs establish and maintain stable cell fates in C. elegans. Development. 2010;137:1045–1053. doi: 10.1242/dev.042812. [DOI] [PubMed] [Google Scholar]
- 27.Toyama R, Rebbert ML, Dey A, Ozato K, Dawid IB. Brd4 associates with mitotic chromosomes throughout early zebrafish embryogenesis. Dev. Dyn. 2008;237:1636–1644. doi: 10.1002/dvdy.21576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forquignon F. A maternal effect mutation leading to deficiencies of organs and homeotic transformations in the adults of Drosophila. Wilhelm Roux’s Arch. Dev. Biol. 1981;190:132–138. doi: 10.1007/BF00867798. [DOI] [PubMed] [Google Scholar]
- 29.Gans M, Forquignon F, Masson M. The role of dosage in the region 7D1-7D5-6 of the X chromosome in the production of homeotic transformations in Drosophila melanogaster. Genetics. 1980;96:887–902. doi: 10.1093/genetics/96.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mozer BA, Dawid IB. Cloning and molecular characterization of the trithorax locus of Drosophila melanogaster. Proc. Nat’l. Acad. Sci. USA. 1989;86:3738–3742. doi: 10.1073/pnas.86.10.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang DH, Dawid IB. The maternal-effect gene fsh is essential for the specification of the central region of the Drosophila embryo. New Biol. 1990;2:163–170. [PubMed] [Google Scholar]
- 32.D’Costa A, Reifegerste R, Sierra S, Moses K. The Drosophila ramshackle gene encodes a chromatin-associated protein required for cell morphology in the developing eye. Mech. Dev. 2006;123:591–604. doi: 10.1016/j.mod.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 33.Shang E, Nickerson HD, Wen D, Wang X, Wolgemuth DJ. The first bromodomain of Brdt, a testis-specific member of the BET sub-family of double-bromodomain- containing proteins, is essential for male germ cell differentiation. Development. 2007;134:3507–3715. doi: 10.1242/dev.004481. [DOI] [PubMed] [Google Scholar]
- 34.Shang E, Wang X, Wen D, Greenberg DA, Wolgemuth DJ. Double bromodomain-containing gene Brd2 is essential for embryonic development in mouse. Dev. Dyn. 2009;238:908–917. doi: 10.1002/dvdy.21911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gans M, Audit C, Masson M. Isolation and characterization of sex-linked female-sterile mutants in Drosophila melanogaster. Genetics. 1975;81:683–704. doi: 10.1093/genetics/81.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazo AM, Huang DH, Mozer BA, Dawid IB. The trithorax gene, a trans-acting regulator of the bithorax complex in Drosophila, encodes a protein with zinc-binding domains. Proc. Nat’l. Acad. Sci. USA. 1990;87:2112–2116. doi: 10.1073/pnas.87.6.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.French CA, Miyoshi I, Aster JC, Kubonishi I, Kroll TG, Dal Cin P, Vargas SO, Perez-Atayde AR, Fletcher JA. BRD4 bromodomain gene rearrangement in aggressive carcinoma with translocation t(15;19) Am. J. Pathol. 2001;159:1987–1992. doi: 10.1016/S0002-9440(10)63049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu SY, Chiang CM. The double bromodomain-containing chromatin adaptor Brd4 and transcriptional regulation. J. Biol. Chem. 2007;282:13141–13145. doi: 10.1074/jbc.R700001200. [DOI] [PubMed] [Google Scholar]
- 39.Farina A, Hattori M, Qin J, Nakatani Y, Minato N, Ozato K. Bromodomain protein Brd4 binds to GTPase-activating SPA-1, modulating its activity and subcellular localization. Mol. Cell. Biol. 2004;24:9059–9069. doi: 10.1128/MCB.24.20.9059-9069.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greenwald R, Tumang JR, Sinha A, Currier N, Cardiff RD, Rothstein TL, Faller DV, Denis GV. E µ-BRD2 transgenic mice develop B cell lymphoma and leukemia. Blood. 2004;103:1475–1484. doi: 10.1182/blood-2003-06-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dey A, Nishiyama A, Karpova T, McNally J, Ozato K. Brd4 marks select genes on mitotic chromatin and directs postmitotic transcription. Mol. Biol. Cell. 2009;20:4899–4909. doi: 10.1091/mbc.E09-05-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dey A, Ellenberg J, Farina A, Coleman AE, Maruyama T, Sciortino S, Lippincott-Schwartz J, Ozato K. A bromodomain protein, MCAP, associates with mitotic chromosomes and affects G(2)-to-M transition. Mol. Cell. Biol. 2000;20:6537–6549. doi: 10.1128/mcb.20.17.6537-6549.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Houzelstein D, Bullock SL, Lynch DE, Grigorieva EF, Wilson VA, Beddington RSP. Growth and early postimplantation defects in mice deficient for the bromodomain-containing protein Brd4. Mol. Cell. Biol. 2002;22:3794–3802. doi: 10.1128/MCB.22.11.3794-3802.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruppert S, Wang EH, Tjian R. Cloning and expression of human TAFII250: a TBP-associated factor implicated in cell-cycle regulation. Nature. 1993;362:175–179. doi: 10.1038/362175a0. [DOI] [PubMed] [Google Scholar]
- 45.Wang EH, Zou S, Tjian R. TAFII250-dependent transcription of cyclin A is directed by ATF activator proteins. Genes Dev. 1997;11:2658–2669. doi: 10.1101/gad.11.20.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Denis GV, Green MR. A novel, mitogen-activated nuclear kinase is related to a Drosophila developmental regulator. Genes Dev. 1996;10:261–271. doi: 10.1101/gad.10.3.261. [DOI] [PubMed] [Google Scholar]
- 47.Ostrowski J, Florio SK, Denis GV, Suzuki H, Bomsztyk K. Stimulation of p85/RING3 kinase in multiple organs after systemic administration of mitogens into mice. Oncogene. 1998;16:1223–1227. doi: 10.1038/sj.onc.1201624. [DOI] [PubMed] [Google Scholar]
- 48.Denis GV, Vaziri C, Guo N, Faller DV. RING3 kinase transactivates promoters of cell cycle regulatory genes through E2F. Cell Growth Diff. 2000;11:417–424. [PMC free article] [PubMed] [Google Scholar]
- 49.Lenburg M, Sinha A, Faller DV, Denis GV. Tumor-specific and proliferation-specific gene expression typifies murine transgenic B cell lymphomagenesis. J. Biol. Chem. 2007;282:4803–4811. doi: 10.1074/jbc.M605870200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Romesser PB, Perlman DH, Faller DV, Costello CE, McComb ME, Denis GV. Development of a malignancy-associated proteomic signature for diffuse large B cell lymphoma. Am. J. Pathol. 2009;175:25–35. doi: 10.2353/ajpath.2009.080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jeanmougin F, Wurtz J-M, Le Douarin B, Chambon P, Losson R. The bromodomain revisited. Trends Biochem. Sci. 1997;22:151–153. doi: 10.1016/s0968-0004(97)01042-6. [DOI] [PubMed] [Google Scholar]
- 52.Winston F, Allis CD. The bromodomain: a chromatin-targeting module? Nature Struct. Biol. 1999;6:601–604. doi: 10.1038/10640. [DOI] [PubMed] [Google Scholar]
- 53.Filetici P, Ornaghi P, Ballario P. The bromodomain: a chromatin browser? Front. Biosci. 2001;6:D866–D876. doi: 10.2741/filetici. [DOI] [PubMed] [Google Scholar]
- 54.Sobulo OM, Borrow J, Tomek R, Reshmi S, Harden A, Schlegelberger B, Housman D, Doggett NA, Rowley JD, Zeleznik-Le NJ. MLL is fused to CBP, a histone acetyltransferase, in therapy-related acute myeloid leukemia with a t(11;16)(q23;p13.3) Proc. Nat’l. Acad. Sci. USA. 1997;94:8732–8737. doi: 10.1073/pnas.94.16.8732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lavau C, Du C, Thirman M, Zeleznik-Le N. Chromatin-related properties of CBP fused to MLL generate a myelodysplastic-like syndrome that evolves into myeloid leukemia. EMBO J. 2000;19:4655–4664. doi: 10.1093/emboj/19.17.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Panagopoulos I, Fioretos T, Isaksson M, Samuelsson U, Billström R, Strömbeck B, Mitelman F, Johansson B. Fusion of the MORF and CBP genes in acute myeloid leukemia with the t(10;16)(q22;p13) Hum. Mol. Genet. 2001;10:395–404. doi: 10.1093/hmg/10.4.395. [DOI] [PubMed] [Google Scholar]
- 57.French CA, Miyoshi I, Kubonishi I, Grier HE, Perez-Atayde AR, Fletcher JA. BRD4-NUT fusion oncogene: a novel mechanism in aggressive carcinoma. Cancer Res. 2003;63:304–307. [PubMed] [Google Scholar]
- 58.Haynes SR, Dollard C, Winston F, Beck S, Trowsdale J, Dawid IB. The bromodomain: a conserved sequence found in human, Drosophila and yeast proteins. Nucl. Acids Res. 1992;20:2603. doi: 10.1093/nar/20.10.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Denis GV. Bromodomain motifs and “scaffolding”? Front. Biosci. 2001;6:D1065–D1068. doi: 10.2741/a668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tamkun JW, Deuring R, Scott MP, Kissinger M, Pattatucci AM, Kaufman TC, Kennison JA. brahma: a regulator of Drosophila homeotic genes structurally related to the yeast transcriptional activator SNF2/SWI2. Cell. 1992;68:561–572. doi: 10.1016/0092-8674(92)90191-e. [DOI] [PubMed] [Google Scholar]
- 61.Randazzo FM, Khavari P, Crabtree G, Tamkun J, Rossant J. brg1: a putative murine homologue of the Drosophila brahma gene, a homeotic gene regulator. Dev. Biol. 1994;161:229–242. doi: 10.1006/dbio.1994.1023. [DOI] [PubMed] [Google Scholar]
- 62.Gu Y, Nakamura T, Alder H, Prasad R, Canaani O, Cimino G, Croce CM, Canaani E. The t(4;11) chromosomal translocation of human acute leukemias fuses the ALL-1 gene, related to Drosophila trithorax, to the AF-4 gene. Cell. 1992;71:701–708. doi: 10.1016/0092-8674(92)90603-a. [DOI] [PubMed] [Google Scholar]
- 63.Tkachuk DC, Kohler S, Cleary ML. Involvement of a homolog of Drosophila trithorax by 11q23 chromosomal translocations in acute leukemias. Cell. 1992;71:691–700. doi: 10.1016/0092-8674(92)90602-9. [DOI] [PubMed] [Google Scholar]
- 64.Reisman DN, Strobeck MW, Betz BL, Sciariotta J, Funkhouser W, Jr, Murchardt C, Yaniv M, Sherman LS, Knudsen ES, Weissman BE. Concomitant down-regulation of BRM and BRG1 in human tumor cell lines: differential effects on RB-mediated growth arrest vs CD44 expression. Oncogene. 2002;21:1196–1207. doi: 10.1038/sj.onc.1205188. [DOI] [PubMed] [Google Scholar]
- 65.Hendricks KB, Shanahan F, Lees E. Role for BRG1 in cell cycle control and tumor suppression. Mol. Cell. Biol. 2004;24:362–376. doi: 10.1128/MCB.24.1.362-376.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Florence B, Faller DV. You bet-cha: a novel family of transcriptional regulators. Front. Biosci. 2001;6:D1008–D1018. doi: 10.2741/florence. [DOI] [PubMed] [Google Scholar]
- 67.de la Cruz X, Lois S, Sánchez-Molina S, Martínez-Balbás MA. Do protein motifs read the histone code? Bioessays. 2005;27:164–175. doi: 10.1002/bies.20176. [DOI] [PubMed] [Google Scholar]
- 68.Mujtaba S, Zeng L, Zhou MM. Structure and acetyl-lysine recognition of the bromodomain. Oncogene. 2007;26:5521–5527. doi: 10.1038/sj.onc.1210618. [DOI] [PubMed] [Google Scholar]
- 69.Angus SP, Fribourg AF, Markey MP, Williams SL, Horn HF, DeGregori J, Kowalik TF, Fukasawa K, Knudsen ES. Active RB elicits late G1/S inhibition. Exp. Cell Res. 2002;276:201–213. doi: 10.1006/excr.2002.5510. [DOI] [PubMed] [Google Scholar]
- 70.Frankfurt O, Tallman MS. Growth factors in leukemia. J. Nat’l. Compr. Canc. Netw. 2007;5:203–215. doi: 10.6004/jnccn.2007.0020. [DOI] [PubMed] [Google Scholar]
- 71.Brandts CH, Berdel WE, Serve H. Oncogenic signaling in acute myeloid leukemia. Curr. Drug Targets. 2007;8:237–246. doi: 10.2174/138945007779940197. [DOI] [PubMed] [Google Scholar]
- 72.Moore MA. Converging pathways in leukemogenesis and stem cell self-renewal. Exp. Hematol. 2005;33:719–737. doi: 10.1016/j.exphem.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 73.Mongan NP, Gudas LJ. Diverse actions of retinoid receptors in cancer prevention and treatment. Differentiation. 2007;75:853–870. doi: 10.1111/j.1432-0436.2007.00206.x. [DOI] [PubMed] [Google Scholar]
- 74.Berman JN, Look AT. Targeting transcription factors in acute leukemia in children. Curr. Drug Targets. 2007;8:727–737. doi: 10.2174/138945007780830818. [DOI] [PubMed] [Google Scholar]
- 75.Trouche D, Le Chalony C, Muchardt C, Yaniv M, Kouzarides T. RB and hbrm cooperate to repress the activation functions of E2F1. Proc. Nat’l. Acad. Sci. USA. 1997;94:11268–11273. doi: 10.1073/pnas.94.21.11268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang HS, Gavin M, Dahiya A, Postigo AA, Ma D, Luo RX, Harbour JW, Dean DC. Exit from G1 and S phase of the cell cycle is regulated by repressor complexes containing HDAC-Rb-hSWI/SNF and Rb-hSWI/SNF. Cell. 2000;101:79–89. doi: 10.1016/S0092-8674(00)80625-X. [DOI] [PubMed] [Google Scholar]
- 77.Stiegler P, De Luca A, Bagella L, Giordano A. The COOH-terminal region of pRb2/p130 binds to histone deacetylase 1 (HDAC1), enhancing transcriptional repression of the E2F-dependent cyclin A promoter. Cancer Res. 1998;58:5049–5052. [PubMed] [Google Scholar]
- 78.Brehm A, Miska EA, McCance DJ, Reid JL, Bannister AJ, Kouzarides T. Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature. 1998;391:597–601. doi: 10.1038/35404. [DOI] [PubMed] [Google Scholar]
- 79.Magnaghi-Jaulin L, Groisman R, Naguibneva I, Robin P, Lorain S, Le Villain JP, Troalen F, Trouche D, Harel-Bellan A. Retinoblastoma protein represses transcription by recruiting a histone deacetylase. Nature. 1998;391:601–605. doi: 10.1038/35410. [DOI] [PubMed] [Google Scholar]
- 80.Ferreira R, Jr, Magnaghi-Jaulin L, Robin P, Harel-Bellan A, Trouche D. The three members of the pocket proteins family share the ability to repress E2F activity through recruitment of a histone deacetylase. Proc. Nat’l. Acad. Sci. USA. 1998;95:10493–10498. doi: 10.1073/pnas.95.18.10493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nagl NG, Jr, Zweitzig DR, Thimmapaya B, Beck GR, Jr, Moran E. The c-myc gene is a direct target of mammalian SWI/SNF-related complexes during differentiation-associated cell cycle arrest. Cancer Res. 2006;66:1289–1293. doi: 10.1158/0008-5472.CAN-05-3427. [DOI] [PubMed] [Google Scholar]
- 82.Nagl NG, Jr, Wang X, Patsialou A, Van Scoy M, Moran E. Distinct mammalian SWI/SNF chromatin remodeling complexes with opposing roles in cell-cycle control. EMBO J. 2007;26:752–763. doi: 10.1038/sj.emboj.7601541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Florence BL, Faller DV. Drosophila female sterile (1) homeotic is a multifunctional transcriptional regulator that is modulated by Ras signaling. Dev. Dyn. 2008;237:554–564. doi: 10.1002/dvdy.21432. [DOI] [PubMed] [Google Scholar]
- 84.Peterson CL, Workman JL. Promoter targeting and chromatin remodeling by the SWI/SNF complex. Curr. Opin. Genet. Dev. 2000;10:187–192. doi: 10.1016/s0959-437x(00)00068-x. [DOI] [PubMed] [Google Scholar]
- 85.Hassan AH, Neely KE, Vignali M, Reese JC, Workman JL. Promoter targeting of chromatin-modifying complexes. Front. Biosci. 2001;6:1054–1064. doi: 10.2741/hassan. [DOI] [PubMed] [Google Scholar]
- 86.Schnitzler GR. Control of nucleosome positions by DNA sequence and remodeling machines. Cell. Biochem. Biophys. 2008;51:67–80. doi: 10.1007/s12013-008-9015-6. [DOI] [PubMed] [Google Scholar]
- 87.Burns LG, Peterson CL. The yeast SWI-SNF complex facilitates binding of a transcriptional activator to nucleosomal sites in vivo. Mol. Cell. Biol. 1997;17:4811–4819. doi: 10.1128/mcb.17.8.4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Holstege FC, Jennings EG, Wyrick JJ, Lee TI, Hengartner CJ, Green MR, Golub TR, Lander ES, Young RA. Dissecting the regulatory circuitry of a eukaryotic genome. Cell. 1998;95:717–728. doi: 10.1016/s0092-8674(00)81641-4. [DOI] [PubMed] [Google Scholar]
- 89.Wang W, Côte J, Xue Y, Zhou S, Khavari PA, Biggar SR, Muchardt C, Kalpana GV, Goff SP, Yaniv M, Workman JL, Crabtree GR. Purification and biochemical heterogeneity of the mammalian SWI-SNF complex. EMBO J. 1996;15:5370–5382. [PMC free article] [PubMed] [Google Scholar]
- 90.Mohrmann L, Verrijzer CP. Composition and functional specificity of SWI2/SNF2 class chromatin remodeling complexes. Biochim. Biophys. Acta. 2005;1681:59–73. doi: 10.1016/j.bbaexp.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 91.Gao X, Tate P, Hu P, Tjian R, Skarnes WC, Wang Z. ES cell pluripotency and germ-layer formation require the SWI/SNF chromatin remodeling component BAF250a. Proc. Nat’l. Acad. Sci. USA. 2008;105:6656–6661. doi: 10.1073/pnas.0801802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reisman DN, Sciarrotta J, Bouldin TW, Weissman BE, Funkhouser WK. The expression of the SWI/SNF ATPase subunits BRG1 and BRM in normal human tissues. Appl. Immunohistochem. Mol. Morphol. 2005;13:66–74. doi: 10.1097/00129039-200503000-00011. [DOI] [PubMed] [Google Scholar]
- 93.Lemon B, Inouye C, King DS, Tjian R. Selectivity of chromatin-remodelling cofactors for ligand-activated transcription. Nature. 2001;414:924–928. doi: 10.1038/414924a. [DOI] [PubMed] [Google Scholar]
- 94.Mizutani T, Ito T, Nishina M, Yamamichi N, Watanabe A, Iba H. Maintenance of integrated proviral gene expression requires Brm, a catalytic subunit of SWI/SNF complex. J. Biol. Chem. 2002;277:15859–15864. doi: 10.1074/jbc.M112421200. [DOI] [PubMed] [Google Scholar]
- 95.Bultman S, Gebuhr T, Yee D, La Mantia C, Nicholson J, Gilliam A, Randazzo F, Metzger D, Chambon P, Crabtree G, Magnuson T. A Brg1 null mutation in the mouse reveals functional differences among mammalian SWI/SNF complexes. Mol. Cell. 2000;6:1287–1295. doi: 10.1016/s1097-2765(00)00127-1. [DOI] [PubMed] [Google Scholar]
- 96.Reyes JC, Barra J, Muchardt C, Camus A, Babinet C, Yaniv M. Altered control of cellular proliferation the absence of mammalian brahma (SNF2alpha) EMBO J. 1998;17:6979–6991. doi: 10.1093/emboj/17.23.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ho L, Ronan JL, Wu J, Staahl BT, Chen L, Kuo A, Lessard J, Nesvizhskii AI, Ranish J, Crabtree GR. An embryonic stem cell chromatin remodeling complex, esBAF, is essential for embryonic stem cell self-renewal and pluripotency. Proc. Nat’l. Acad. Sci. USA. 2009;106:5181–5186. doi: 10.1073/pnas.0812889106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Olave I, Wang W, Xue Y, Kuo A, Crabtree GR. Identification of a polymorphic, neuron-specific chromatin remodeling complex. Genes Dev. 2002;16:2509–2517. doi: 10.1101/gad.992102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hassan AH, Prochasson P, Neely KE, Galasinski SC, Chandy M, Carrozza MJ, Workman JL. Function and selectivity of bromodomains in anchoring chromatin-modifying complexes to promoter nucleosomes. Cell. 2002;111:369–379. doi: 10.1016/s0092-8674(02)01005-x. [DOI] [PubMed] [Google Scholar]
- 100.Li ZY, Yang J, Gao X, Lu JY, Zhang Y, Wang K, Cheng MB, Wu NH, Zhang Y, Wu Z, Shen YF. Sequential recruitment of PCAF and BRG1 contributes to myogenin activation in 12-O-tetradecanoylphorbol-13-acetate-induced early differentiation of rhabdomyosarcoma-derived cells. J. Biol. Chem. 2007;282:18872–18878. doi: 10.1074/jbc.M609448200. [DOI] [PubMed] [Google Scholar]
- 101.Armstrong JA, Bieker JJ, Emerson BM. A SWI/SNF-related chromatin remodeling complex, E-RC1, is required for tissue-specific transcriptional regulation by EKLF in vitro. Cell. 1998;95:93–104. doi: 10.1016/s0092-8674(00)81785-7. [DOI] [PubMed] [Google Scholar]
- 102.Fryer CJ, Archer TK. Chromatin remodelling by the glucocorticoid receptor requires the BRG1 complex. Nature. 1998;393:88–91. doi: 10.1038/30032. [DOI] [PubMed] [Google Scholar]
- 103.DiRenzo J, Shang Y, Phelan M, Sif S, Myers M, Kingston R, Brown M. BRG-1 is recruited to estrogen-responsive promoters and cooperates with factors involved in histone acetylation. Mol. Cell. Biol. 2000;20:7541–7549. doi: 10.1128/mcb.20.20.7541-7549.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kadam S, Emerson BM. Transcriptional specificity of human SWI/SNF BRG1 and BRM chromatin remodeling complexes. Mol. Cell. 2003;11:377–389. doi: 10.1016/s1097-2765(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 105.de la Serna IL, Ohkawa Y, Imbalzano AN. Chromatin remodelling in mammalian differentiation: lessons from ATP-dependent remodellers. Nat. Rev. Genet. 2006;7:461–473. doi: 10.1038/nrg1882. [DOI] [PubMed] [Google Scholar]
- 106.Chiba H, Muramatsu M, Nomoto A, Kato H. Two human homologues of Saccharomyces cerevisiae SWI2/SNF2 and Drosophila brahma are transcriptional coactivators cooperating with the estrogen receptor and the retinoic acid receptor. Nucl. Acids Res. 1994;22:1815–1820. doi: 10.1093/nar/22.10.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Muchardt C, Yaniv M. A human homologue of Saccharomyces cerevisiae SNF2/SWI2 and Drosophila brm genes potentiates transcriptional activation by the glucocorticoid receptor. EMBO J. 1993;12:4279–4290. doi: 10.1002/j.1460-2075.1993.tb06112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wallberg AE, Neely KE, Hassan AH, Gustafsson JA, Workman JL, Wright AP. Recruitment of the SWI-SNF chromatin remodeling complex as a mechanism of gene activation by the glucocorticoid receptor tau1 activation domain. Mol. Cell. Biol. 2000;20:2004–2013. doi: 10.1128/mcb.20.6.2004-2013.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Debril MB, Gelman L, Fayard E, Annicotte JS, Rocchi S, Auwerx J. Transcription factors and nuclear receptors interact with the SWI/SNF complex through the BAF60c subunit. J. Biol. Chem. 2004;279:16677–16686. doi: 10.1074/jbc.M312288200. [DOI] [PubMed] [Google Scholar]
- 110.Rangwala SM, Lazar MA. Transcriptional control of adipogenesis. Ann. Rev. Nutr. 2000;20:535–559. doi: 10.1146/annurev.nutr.20.1.535. [DOI] [PubMed] [Google Scholar]
- 111.Rosen ED, Spiegelman BM. Molecular regulation of adipogenesis. Ann. Rev. Cell Dev. Biol. 2000;16:145–171. doi: 10.1146/annurev.cellbio.16.1.145. [DOI] [PubMed] [Google Scholar]
- 112.Debril MB, Renaud JP, Fajas L, Auwerx J. The pleiotropic functions of peroxisome proliferator-activated receptor gamma. J. Mol. Med. 2001;79:30–47. doi: 10.1007/s001090000145. [DOI] [PubMed] [Google Scholar]
- 113.Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr. Rev. 2006;27:728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- 114.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 115.Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPARgamma2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- 116.Qi C, Zhu Y, Reddy JK. Peroxisome proliferator-activated receptors, coactivators, and downstream targets. Cell. Biochem. Biophys. 2000;32:187–204. doi: 10.1385/cbb:32:1-3:187. [DOI] [PubMed] [Google Scholar]
- 117.Gurnell M. PPARgamma and metabolism: insights from the study of human genetic variants. Clin. Endocrinol. (Oxf) 2003;59:267–277. doi: 10.1046/j.1365-2265.2003.01767.x. [DOI] [PubMed] [Google Scholar]
- 118.Meirhaeghe A, Amouyel P. Impact of genetic variation of PPARgamma in humans. Mol. Genet. Metab. 2004;83:93–102. doi: 10.1016/j.ymgme.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 119.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dahiya A, Wong S, Gonzalo S, Gavin M, Dean DC. Linking the Rb and polycomb pathways. Mol. Cell. 2001;8:557–569. doi: 10.1016/s1097-2765(01)00346-x. [DOI] [PubMed] [Google Scholar]
- 121.Salma N, Xiao H, Mueller E, Imbalzano AN. Temporal recruitment of transcription factors and SWI/SNF chromatin-remodeling enzymes during adipogenic induction of the Peroxisome Proliferator-Activated Receptor-gamma nuclear hormone receptor. Mol. Cell. Biol. 2004;24:4651–4663. doi: 10.1128/MCB.24.11.4651-4663.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Musri MM, Gomis R, Párrizas M. Chromatin and chromatin-modifying proteins in adipogenesis. Biochem. Cell. Biol. 2007;85:397–410. doi: 10.1139/O07-068. [DOI] [PubMed] [Google Scholar]
- 123.Powell E, Kuhn P, Xu W. Nuclear receptor cofactors in PPARgamma-mediated adipogenesis and adipocyte energy metabolism. PPAR Res. 2007;2007:53843–53854. doi: 10.1155/2007/53843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tamori Y, Masugi J, Nishino N, Kasuga M. Role of peroxisome proliferator-activated receptor-gamma in maintenance of the characteristics of mature 3T3-L1 adipocytes. Diabetes. 2002;51:2045–2055. doi: 10.2337/diabetes.51.7.2045. [DOI] [PubMed] [Google Scholar]
- 125.Wang Z, Qi C, Krones A, Woodring P, Zhu X, Reddy JK, Evans RM, Rosenfeld MG, Hunter T. Critical roles of the p160 transcriptional coactivators p/CIP and SRC-1 in energy balance. Cell Metab. 2006;3:111–122. doi: 10.1016/j.cmet.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 126.Louet JF, O’Malley BW. Coregulators in adipogenesis: what could we learn from the SRC (p160) coactivator family? Cell Cycle. 2007;6:2448–2452. doi: 10.4161/cc.6.20.4777. [DOI] [PubMed] [Google Scholar]
- 127.Villarroya F, Iglesias R, Giralt M. PPARs in the control of uncoupling proteins gene expression. PPAR Res. 2007;2007:74364–74376. doi: 10.1155/2007/74364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kontani Y, Wang Y, Kimura K, Inokuma KI, Saito M, Suzuki-Miura T, Wang Z, Sato Y, Mori N, Yamashita H. UCP1 deficiency increases susceptibility to diet-induced obesity with age. Aging Cell. 2005;4:147–155. doi: 10.1111/j.1474-9726.2005.00157.x. [DOI] [PubMed] [Google Scholar]
- 129.Salopuro T, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Tuomilehto J, Laakso M, Uusitupa M. Common variants in beta2- and beta3-adrenergic receptor genes and uncoupling protein 1 as predictors of the risk for type 2 diabetes and body weight changes. The Finnish Diabetes Prevention Study. Clin Genet. 2004;66:365–367. doi: 10.1111/j.1399-0004.2004.00313.x. [DOI] [PubMed] [Google Scholar]
- 130.Malik S, Wallberg AE, Kang YK, Roeder RG. TRAP/SMCC/ mediator-dependent transcriptional activation from DNA and chromatin templates by orphan nuclear receptor hepatocyte nuclear factor 4. Mol. Cell. Biol. 2002;22:5626–5637. doi: 10.1128/MCB.22.15.5626-5637.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wallberg AE, Yamamura S, Malik S, Spiegelman BM, Roeder RG. Coordination of p300-mediated chromatin remodeling and TRAP/mediator function through coactivator PGC-1alpha. Mol. Cell. 2003;12:1137–1149. doi: 10.1016/s1097-2765(03)00391-5. [DOI] [PubMed] [Google Scholar]
- 132.Chen W, Yang Q, Roeder RG. Dynamic interactions and cooperative functions of PGC-1alpha and MED1 in TRalpha-mediated activation of the brown-fat-specific UCP-1 gene. Mol. Cell. 2009;35:755–768. doi: 10.1016/j.molcel.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Myers LC, Gustafsson CM, Bushnell DA, Lui M, Erdjument-Bromage H, Tempst P, Kornberg RD. The Med proteins of yeast and their function through the RNA polymerase II carboxy-terminal domain. Genes Dev. 1998;12:45–54. doi: 10.1101/gad.12.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jiang YW, Veschambre P, Erdjument-Bromage H, Tempst P, Conaway JW, Conaway RC, Kornberg RD. Mammalian mediator of transcriptional regulation and its possible role as an end-point of signal transduction pathways. Proc. Nat’l. Acad. Sci. USA. 1998;95:8538–8543. doi: 10.1073/pnas.95.15.8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kuras L, Borggrefe T, Kornberg RD. Association of the Mediator complex with enhancers of active genes. Proc. Nat’l. Acad. Sci. USA. 2003;100:13887–13891. doi: 10.1073/pnas.2036346100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nofsinger RR, Li P, Hong SH, Jonker JW, Barish GD, Ying H, Cheng SY, Leblanc M, Xu W, Pei L, Kang YJ, Nelson M, Downes M, Yu RT, Olefsky JM, Lee CH, Evans RM. SMRT repression of nuclear receptors controls the adipogenic set point and metabolic homeostasis. Proc. Nat’l. Acad. Sci. USA. 2008;105:20021–20026. doi: 10.1073/pnas.0811012105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Foryst-Ludwig A, Clemenz M, Hohmann S, Hartge M, Sprang C, Frost N, Krikov M, Bhanot S, Barros R, Morani A, Gustafsson JA, Unger T, Kintscher U. Metabolic actions of estrogen receptor beta (ERbeta) are mediated by a negative crosstalk with PPARgamma. PLoS Genet. 2008;4:e1000108–e1000124. doi: 10.1371/journal.pgen.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Samarasinghe SP, Sutanto MM, Danos AM, Johnson DN, Brady MJ, Cohen RN. Altering PPARgamma ligand selectivity impairs adipogenesis by thiazolidinediones but not hormonal inducers. Obesity (Silver Spring) 2009;17:965–972. doi: 10.1038/oby.2008.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ge K, Guermah M, Yuan CX, Ito M, Wallberg AE, Spiegelman BM, Roeder RG. Transcription coactivator TRAP220 is required for PPAR gamma 2-stimulated adipogenesis. Nature. 2002;417:563–567. doi: 10.1038/417563a. [DOI] [PubMed] [Google Scholar]
- 140.Rando OJ, Zhao K, Janmey P, Crabtree GR. Phosphatidylinositol-dependent actin filament binding by the SWI/SNF-like BAF chromatin remodeling complex. Proc. Nat’l. Acad. Sci. USA. 2002;99:2824–2829. doi: 10.1073/pnas.032662899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Serra C, Palacios D, Mozzetta C, Forcales SV, Morantte I, Ripani M, Jones DR, Du K, Jhala US, Simone C, Puri PL. Functional interdependence at the chromatin level between the MKK6/p38 and IGF1/PI3K/AKT pathways during muscle differentiation. Mol. Cell. 2007;28:200–213. doi: 10.1016/j.molcel.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dunn KL, Espino PS, Drobic B, He S, Davie JR. The Ras-MAPK signal transduction pathway, cancer and chromatin remodeling. Biochem. Cell. Biol. 2005;83:1–14. doi: 10.1139/o04-121. [DOI] [PubMed] [Google Scholar]
- 143.Medzhitov R, Horng T. Transcriptional control of the inflammatory response. Nat. Rev. Immunol. 2009;9:692–703. doi: 10.1038/nri2634. [DOI] [PubMed] [Google Scholar]
- 144.Jagannathan M, Hasturk H, Liang Y, Shin H, Hetzel JT, Kantarci A, Rubin D, McDonnell ME, Van Dyke TE, Ganley-Leal LM, Nikolajczyk BS. TLR crosstalk specifically regulates cytokine production by B cells from chronic inflammatory disease patients. J. Immunol. 2009;183:7461–7470. doi: 10.4049/jimmunol.0901517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang B, Chambers KJ, Faller DV, Wang S. Reprogramming of the SWI/SNF complex for co-activation or co-repression in prohibitin-mediated estrogen receptor regulation. Oncogene. 2007;26:7153–7157. doi: 10.1038/sj.onc.1210509. [DOI] [PubMed] [Google Scholar]
- 146.Fajas L, Landsberg RL, Huss-Garcia Y, Sardet C, Lees JA, Auwerx J. E2Fs regulate adipocyte differentiation. Dev. Cell. 2002;3:39–49. doi: 10.1016/s1534-5807(02)00190-9. [DOI] [PubMed] [Google Scholar]
- 147.Mellor J. The dynamics of chromatin remodeling at promoters. Mol Cell. 2005;19:147–157. doi: 10.1016/j.molcel.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 148.Schnitzler G, Sif S, Kingston RE. Human SWI/SNF interconverts a nucleosome between its base state and a stable remodeled state. Cell. 1998;94:17–27. doi: 10.1016/s0092-8674(00)81217-9. [DOI] [PubMed] [Google Scholar]
- 149.Pospisilik JA, Schramek D, Schnidar H, Cronin SJ, Nehme NT, Zhang X, Knauf C, Cani PD, Aumayr K, Todoric J, Bayer M, Haschemi A, Puviindran V, Tar K, Orthofer M, Neely GG, Dietzl G, Manoukian A, Funovics M, Prager G, Wagner O, Ferrandon D, Aberger F, Hui CC, Esterbauer H, Penninger JM. Drosophila genome-wide obesity screen reveals hedgehog as a determinant of brown versus white adipose cell fate. Cell. 2010;140:148–160. doi: 10.1016/j.cell.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 150.Tseng YH, He TC. Bone morphogenetic proteins and adipocyte differentiation. Cell Sci. Rev. 2007;3:342–360. [Google Scholar]
- 151.Graff JM. Embryonic patterning: to BMP or not to BMP, that is the question. Cell. 1997;89:171–174. doi: 10.1016/s0092-8674(00)80196-8. [DOI] [PubMed] [Google Scholar]
- 152.Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6:38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 154.Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, Ahrens MJ, Dudley AT, Norris AW, Kulkarni RN, Kahn CR. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454:1000–1004. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.You J, Srinivasan V, Denis GV, Harrington WJ, Jr, Ballestas ME, Kaye KM, Howley PM. Kaposi’s Sarcoma-associated herpesvirus latency-associated nuclear antigen interacts with bromodomain protein Brd4 on host mitotic chromosomes. J. Virol. 2006;80:8909–8919. doi: 10.1128/JVI.00502-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kyle UG, Pichard C. The Dutch Famine of 1944–1945: a pathophysiological model of long-term consequences of wasting disease. Curr. Opin. Clin. Nutr. Metab. Care. 2006;9:388–394. doi: 10.1097/01.mco.0000232898.74415.42. [DOI] [PubMed] [Google Scholar]
- 157.Bocock PN, Aagaard-Tillery KM. Animal models of epigenetic inheritance. Semin. Reprod. Med. 2009;27:369–379. doi: 10.1055/s-0029-1237425. [DOI] [PubMed] [Google Scholar]
- 158.Ruderman NB, Schneider SH, Berchtold P. The “metabolically-obese,” normal-weight individual. Am. J. Clin. Nutr. 1981;34:1617–1621. doi: 10.1093/ajcn/34.8.1617. [DOI] [PubMed] [Google Scholar]
- 159.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J. Clin. Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]