Abstract
Estrogen and its receptors are implicated in the promotion and prevention of various cancers. While the uterine cervix is highly responsive to estrogen, the role of estrogen in cervical cancer, which is strongly associated with human papillomavirus (HPV) infections, is poorly understood. Recent studies in HPV transgenic mouse models provide evidence that estrogen and its nuclear receptor promote cervical cancer in combination with HPV oncogenes. While epidemiological studies further support this hypothesis, there is little experimental data assessing the hormonal responsiveness of human cervical cancers. If these cancers are dependent upon estrogen, then drugs targeting estrogen and its receptors may be effective in treating and/or preventing cervical cancer, the second leading cause of death by cancer amongst women worldwide.
Estrogen and cancer
Estrogen, through its nuclear receptors ERα and ERβ, and membrane receptor GPR30, influences physiological processes in various tissues/systems including but not limited to the female reproductive tract, breast, colon, brain, bone, cardiovascular and immune systems (Figure 1a). Not surprisingly, estrogen is implicated in various human diseases including cancer (e.g. breast, endometrium, and colon) wherein it can either promote or suppress tumor development [1]. The uterine cervix is a part of the female reproductive tract that is highly responsive to estrogen. During the menstrual cycle, cervical epithelial cells proliferate and differentiate as estrogen levels increase, resulting in hyperplastic epithelium without pathological changes. Estrogen and ERα, the major ER expressed in the cervix, are necessary for this dynamic change in cervical epithelium. In this article we discuss laboratory and epidemiological studies that provide insight into the roles of estrogen and its receptor ERα in cervical cancer.
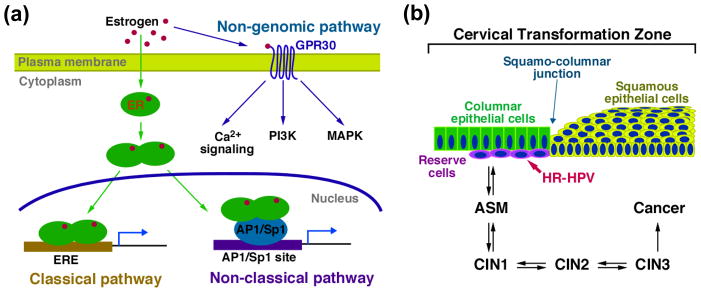
Figure 1.
Estrogen pathway and cervical carcinogenesis. (a) Estrogen pathway. Estrogen binds to its cytosolic/nuclear receptors (ERα and ERβ) and membrane receptor GPR30 to exert its functions. Estrogen binds to ERs in the cytoplasm and induces ER homo- or hetero-dimerization. Estrogen-bound ERs then translocate to the nucleus, where they activate or repress target genes by two different mechanisms: classical pathway: ER binds to ERE and modulates target genes, and non-classical pathway: ER binds to AP1 or Sp1 transcription factors associated with their recognition sites in enhancer elements and modifies their function. Estrogen also binds to the membraneous receptor GPR30, a member of the G-protein coupled receptor family, and rapidly transduces various signaling pathways including but not limited to phosphatidyl inositol 3-kinase (PI3K), mitogen-activated protein kinase (MAPK), and Ca2+ signaling (non-genomic pathway). (b) Progressive disease leading to cervical cancer. It is believed that the multipotent reserve cells present in the transformation zone of the cervix are the progenitor cell type for cervical cancer. High-risk HPVs (HR-HPVs), such as HPV-16, infect and persist in these cells, and promote their aberrant squamous differentiation, which leads to atypical squamous metaplasia (ASM). ASM can progress to cervical intraepithelial neoplasia (CIN) grade 1–3 and eventually cervical cancer. This progressive neoplastic disease process normally takes over a decade following initial HPV infection before culminating in frank cancer. In most women, cervical disease spontaneously resolves prior to the development of cervical cancer.
Cervical cancer
Cervical cancer is the second most frequent cancer and the second leading cause of cancer death in women worldwide, with approximately 470,000 new cases and 233,000 deaths per year [2]. The high mortality rate is largely due to the lack of effective therapies for eliminating disease in women with high-grade cervical cancer and the lack of response to chemotherapy of inoperable disease. A major causal factor for cervical cancer is the high-risk human papillomaviruses (HPVs), which are also associated with other anogenital cancers as well as a small fraction of head & neck cancer (Box 1) [3]. Over 99% of human cervical cancers are positive for these sexually transmitted HPVs [4]. Currently available prophylactic vaccines that inhibit infection by a subset of high-risk HPVs hold promise for reducing cervical cancer incidence in future generations of women [5]. These vaccines, however, do not protect women who are already infected or afflicted by the cancer. The fact that E6 and E7 are always expressed in HPV+ cervical cancer has led to great efforts in developing therapeutic vaccines against these viral antigens. Although such therapeutic vaccines induce viral antigen-specific cytotoxic T cells in human, they have largely proven ineffective in treating HPV-induced cervical neoplasia in women [5].
Box 1. HPV and cancer.
HPV is one of the most common sexually transmitted pathogen. It is estimated that ~75% of sexually active individuals are infected by this virus. Over 100 different types of HPV are identified and classified in two groups depending on their tissue tropism [62, 63]. Cutaneous types (e.g. HPV-5 & -8) infect the skin and cause warts and sometimes non-melanoma skin cancer. Mucosal types infect epithelial lining of anogenital tracts and oral cavity. They are further divided to low-risk and high-risk types based on their propensity to induce malignancies [62, 63]. Low-risk HPVs (e.g. HPV-6 & -11) are associated only with benign lesions such as genital warts and laryngeal papillomas. On the contrary, high-risk HPVs (e.g. HPV-16 & -18) elicit various cancers including but not limited to those in uterine cervix, vagina, anus, and oral cavity. Specifically, HPV-16 and -18 are responsible for 60% and 20% of cervical cancer, respectively. Thus current prophylactic vaccines commonly target these two major high-risk HPV types [64].
HPV virions are nonenveloped icosahedral particles that are 55 nm in diameter and enclose 7.9 kb-long double-stranded DNA genome [62, 63]. HPV encodes eight genes, among which E5, E6, and E7 possess oncogenic activity. Two of these viral oncogenes, E6 and E7, are invariably expressed in human cervical cancer, and their continued expression is required for maintenance of the cancerous state. The strong tumorigenic potential of high-risk HPVs stems, in part, from the ability of their E6 and E7 oncoproteins to inactivate the potent cellular tumor suppressor proteins p53 and pRb, respectively.
Transgenic mice expressing high-risk HPV E6 and E7 individually or in combination have been generated and extensively characterized for their cancer phenotypes. Although these transgenic mice develop spontaneous skin tumors, they rarely develop cancers in mucosal epithelia of anogenital tracts or in the oral cavity, indicating that HPV oncogenes are not sufficient for promoting tumorigenesis in those relevant tissues. Consistently, the majority of these mice succumb to cervical/vaginal cancer with treatment with physiologic levels of exogenous estrogen [9], head & neck cancers with a low dose of chemical carcinogen, 4-nitroquinoline 1-oxide (4-NQO) [65].
Cervical cancer develops slowly, is often established over a decade after initial infection with high-risk HPVs, and only arises in those women whose infections do not resolve spontaneously. Such persistent infections can lead to the development of severe cervical intraepithelial neoplasia (CIN3) (Figure 1b) with a 3-year probability that varies from approximately 14% for any high-risk HPVs (any) to 40% for HPV 16 [6]. Cytological screening (i.e. the Pap smear) to detect CIN lesions in the cervix has greatly reduced cervical cancer incidence in those countries with well-developed medical care systems. If not appropriately treated, however, CIN3 can progress to cervical cancer in at least 30% of women, while in the rest, CIN3 lesions are thought to regress spontaneously [7]. The high probability of spontaneous elimination of HPV infection and the relatively low frequency of cervical cancer in women with persistent HPV infection pointed to the existence of non-viral factors other than the virus that contribute to disease onset. A growing body of evidence supports our hypothesis that estrogen is one such factor.
Estrogen synergizes with HPV oncogenes to cause cervical cancer in mice
The strongest evidence that estrogen contributes to cervical carcinogenesis comes from laboratory studies on HPV transgenic mice. In these mice, multiple or individual HPV oncogenes (i.e. E6 and E7) are placed under the transcriptional control of the human keratin 14 promoter, which directs their expression to stratified squamous epithelia including that of the skin, oral cavity, anus, vagina and cervix. While these HPV transgenic mice can develop spontaneous tumors, primarily in the skin, they rarely develop cervical cancers spontaneously [8]. However, if HPV transgenic mice are treated with exogenous 17β-estradiol, they efficiently develop cervical cancers [9–11]. Whereas HPV oncogenes have an impact on normal cellular processes first at adolescent or adulthood when women are infected by the virus for the first time, these transgenic mice start to express HPV oncogenes from the early stages of embryonic development. In addition, whole epithelium in the cervix is under the influence of HPV oncogenes when tumorigenic events are initiated, which is different from the human situation where HPV oncogene expression is restricted to a limited area of epithelium. Therefore, cancer initiation events and the cancer microenvironment in these mouse models may be different from those in women. Nonetheless, these mice recapitulated key aspects of cervical cancer in women in that (i) the progressive neoplastic disease leading to frank cancer is highly similar to that observed in women; (ii) cancers frequently arise in the transformation zone of the cervix where columnar epithelium converts to squamous epithelium (Figure 1b); and (iii) cervical cancers arising in these mice express similar biomarkers as found in human cervical cancer [12, 13]. Only physiological levels of estrogen sufficient to induce continuous estrus are necessary to efficiently promote cervical carcinogenesis in HPV-transgenic mice [13]. These experimental conditions mimic that seen in premenopausal women exposed to the continuous estrogenic stimulation such as from oral contraceptives or as a consequence of pregnancy. In this mouse model, removal of exogenous estrogen led to a diminishment in the progression of cervical disease and partial regression of pre-existing neoplasia [14]. These mouse studies together provide compelling evidence that estrogen contributes to cervical carcinogenesis.
Role of the estrogen receptor α in cervical carcinogenesis
Despite intrinsic caveats of most genetically modified mouse models including available HPV transgenic mice [15], HPV transgenic mice have provided a powerful experimental platform for dissecting the roles of viral and host genes in cervical cancer [16–20]. Given the potency of estrogen in inducing cervical cancer in these mice, the roles of its nuclear receptor ERα (ERβ was undetectable in the mouse cervix, as is the case in the human cervix), was investigated [21]. In contrast to ERα-sufficient HPV transgenic mice, ERα-deficient HPV transgenic mice failed to develop cervical cancer when treated with estrogen (Figure 2a). In fact, estrogen-treated ERα-null HPV transgenic mice did not develop any aspect of the progressive disease leading to cervical cancer, including the most cancer-prone epithelium, atypical squamous metaplasia (ASM, Figure 1b). In addition, these mice failed to display cervical hyperplasia, the normal acute response to estrogen stimulation. These studies demonstrate an absolute requirement for ERα in mediating estrogen’s carcinogenic properties in the cervix.
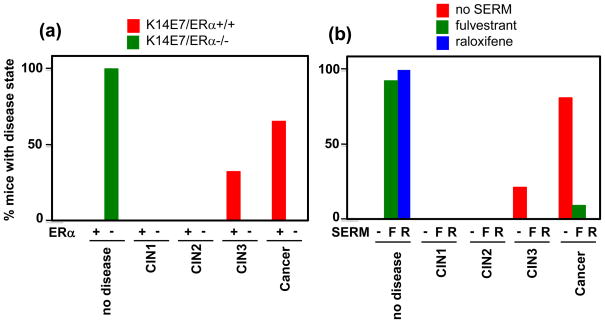
Figure 2.
Requirement of ERα for genesis and persistence of cervical cancer. (a) Influence of ERα status on cervical disease in HPV transgenic mice. Shown is the worst disease state in the cervices of K14E7/ERα+/+ (red; +) or K14E7/ERα−/− (green; −) mice treated for 6 months with 17β-estradiol. Note the complete absence of disease in mice deficient for ERα as compared to the presence of high-grade dysplasia, CIN3 (33%), or cervical cancer (66%) in ERα-sufficient mice. Data taken from [21]. (b) Effectiveness of SERMs in treating cervical neoplasia in HPV transgenic mice. Shown is the worst disease state in the cervices of K14E6E7 mice treated with 17β-estradiol for 6 months to induce cervical cancer, then either left untreated (red; −) or treated with fulvestrant (green; F) or raloxifene (blue; R) for 1 month. Note the complete (100%) or near complete (92%) loss of disease in mice treated with raloxifene or flulvestrant, respectively, compared to the retention of CIN3 (21%) or cervical cancer (79%) in those mice not treated with a SERM. Data taken from [22].
Targeting ERα: a new mode of treating and/or preventing cervical cancer
Knowing that ERα is required for estrogen’s carcinogenic activities in the cervix [21] and that estrogen contributes not only to the genesis but also to the maintenance of cervical neoplastic disease [14], the utility of ERα antagonistic drugs in treating or preventing cervical cancer was evaluated using this HPV transgenic mouse model. Two ERα antagonists were evaluated, raloxifene and fulvestrant [22]. These are FDA-approved drugs used in the treatment/prevention of human breast cancer, and are members of a class of drugs referred to as selective estrogen receptor modulators (SERMs). Both raloxifene and fulvestrant were highly effective in eliminating pre-existing cervical cancers with only a month-long treatment period (Figure 2b). They also eliminated pre-existing CIN lesions, which led to the hypothesis that ERα antagonists could also prevent cervical cancers from arising. Indeed, treatment of CIN-bearing mice with fulvestrant during estrogen treatment prevented CIN lesions from progressing to cancer [22]. Thus, in a preclinical model for HPV-associated cervical cancer, inhibiting ERα function leads to the elimination and prevention of cervical neoplastic disease, providing further evidence for the role of estrogen and its receptor in cervical cancer. If this is translatable to human cervical cancer, SERMs that inhibit ERα in the cervix will be effective in controlling this gynecological disease.
The interplay between estrogen and HPV
That there is such a strong synergy between estrogen and HPV oncogenes in the development of cervical cancers in mice begs the question – what is responsible for the high degree of interaction? Both estrogen and HPV E7 can independently induce cervical epithelial hyperplasia [21]. In addition, estrogen and HPV oncoproteins regulate Eag1 potassium channel, of which inhibition leads to apoptosis of human cervical cancer cells [23]. Chronic exposure to estrogen induces overexpression of Aurora-A, a centrosome kinase, and centrosome amplification, leading to chromosomal instability in mammary gland of ACI rats [24]. E6 and E7 each can inhibit cellular DNA damage responses, which in part may contribute to their promoting genomic instability [25, 26]. However, there is also data indicating that estrogen and HPV affect each other’s activities. Estrogen and other ER agonists have been shown by multiple investigators to induce the expression of HPV oncogenes when they are under the control of the endogenous viral promoter [27–30]. This could contribute to a synergy between estrogen and HPV in human cervical cancers, which are ERα-positive. However, the mechanism by which estrogen increases HPV gene expression remains unclear, as some of the studies demonstrating the effect of estrogen on HPV gene expression were carried out using human cervical cancer cell lines that have lost expression of ERα protein [27, 28, 30]. It is also important to note that this level of interplay does not contribute to cervical carcinogenesis in the context of the above-described HPV transgenic mice using K14 promoter-driven transgenes, as this promoter is not estrogen-responsive [11]. However in a different HPV transgenic mouse model, in which the high-risk HPV18 E6 and E7 oncogenes were kept under the control of the endogenous viral promoter, estrogen induced expression of E6 and E7, and this correlated with increased severity of cervical dysplasia [31]. Other hormone receptors, particularly glucocorticoid and progesterone receptors, also have been implicated in modulating HPV gene expression by activating the endogenous viral promoter and cooperating with high-risk HPV for cellular transformation [32–34]. These observations support the hypothesis that cervical cancer depends on estrogen. They also suggest that steroid hormones other than estrogen may be implicated in cervical cancer.
In addition to estrogen having a potential effect on HPV gene expression and activity, the converse may also be true. For example, HPV transcription factor E2 can cooperate with nuclear receptor co-activators to increase the estrogen response element (ERE)-dependent transcriptional activity of ERα [35]. This could be relevant in the context of progressive disease leading to the development of human cervical cancers. E2 is predicted to be expressed in precancerous lesions, as well as in the subset of cervical cancers in which the E2 gene is not disrupted by integration of the viral genome into the host genome. Both E6 and E7 have been argued also to enhance ERE-dependent activity [36], though other investigators have claimed the opposite [37, 38]. Contradictory results may stem from differences in estrogen concentration and cell lines used in each study. This controversy can be resolved with the use of HPV-negative normal cervical keratinocytes. Nonetheless, these studies support a model for the role of estrogen and HPV in cervical cancer in which estrogen and HPV have the capacity to influence each other’s activities (Figure 3).
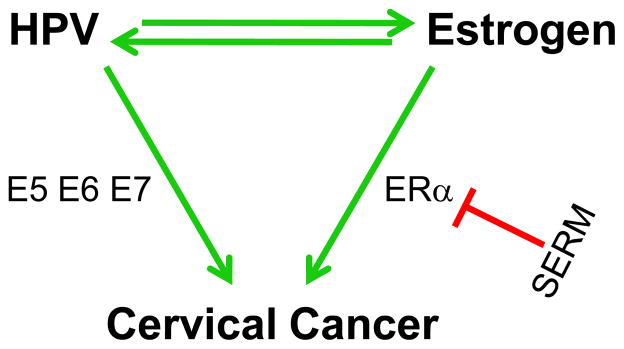
Figure 3.
Model for synergy between HPV and estrogen in cervical cancer. The three HPV oncogenes E5, E6, and E7 contribute to varying degrees to the development of cervical cancer through their abilities to induce cell proliferation, inhibit cell death, abrogate cellular DNA damage responses, and induce genomic instability. Estrogen contributes to cervical cancer at least in part through its ERα-dependent induction of cell proliferation. HPV gene products can either enhance or suppress estrogen’s stimulation of ERα transcriptional activity. Estrogen can in turn activate expression of HPV oncogenes potentially through ERα or perhaps other means. The interplay between HPV and estrogen may contribute to the synergistic activities of these viral and cellular factors in causing cervical cancer. Disrupting the estrogen/ERα pathway provides a novel approach to preventing or treating cervical cancer, for example, through SERMs.
Epidemiological data on the role of sex hormones in human cervical cancer
Although incidence for most epithelial cancers increase with age, estrogen-dependent cancers do not share this age pattern [39]. Instead, after menopause they either increase more slowly, or cease to increase, depending on the level of circulating and/or local estrogens. Breast cancer is the best-studied example of estrogen-dependent epithelial cancer [39, 40], and knowledge gained from such studies has led to the vast use of SERMs in breast cancer treatment [41], as well as the consideration of their use for breast cancer prevention in high-risk women [42]. In unscreened populations, incidence rates of cervical cancer by age behave in a way that closely resembles those of breast cancer, i.e. age-specific incidence curves for cervical cancer in unscreened populations in Africa, Asia and Latin America show a steep rise up to age 45 years and then a plateau [43]. In well-screened populations, the age pattern of cervical cancer is different, i.e., incidence stops increasing at age 25-to-30 years when regularly scheduled cytological screening has been initiated and early detection of precancerous cervical lesions reduces the risk of cervical cancer.
On account of the strong link between cervical cancer and HPV infection, which typically first occurs soon after becoming sexually active, the age-related behavior of cervical cancer may be driven by the natural history of HPV infections, i.e. the frequency of HPV infections in a woman will correlate with the number of sexual partners she has at any given time in life. A large bulk of epidemiological evidence suggests, however, that endogenous and exogenous sex hormones also affect a woman’s risk of developing the disease, in combination with HPV infection. In a collaborative re-analysis of over 16,000 women with cervical cancer and twice as many cancer-free women from 25 different studies, it was found that the number of full-term pregnancies (FTPs) and age at first FTP were associated with cervical cancer risk after careful adjustment for lifetime number of sexual partners and age at first FTP [44]. For example, Figure 4a shows that a woman who started bearing children at age 17 years or younger and had seven FTPs or more had a relative risk (RR) of 3.3 of developing cervical cancer (95% confidence interval (CI): 2.7–4.0) compared to nulliparous women. This trend was similar for squamous-cell carcinoma and adenocarcinoma of the cervix [44]. Multiparity, however, showed no clear association with CIN3/carcinoma in situ, whereas age at first FTP showed an inverse association that was similar, but weaker than for cervical cancer. The increased risk of cervical cancer among women with high parity has been hypothesized to be a consequence of alterations in female hormones arising during pregnancy. Alternatively, immune suppression that is linked with pregnancy could increase the risk of new HPV infections, and/or reduce the body’s ability to eliminate pre-existing infections, thereby increasing the risk of neoplastic progression.
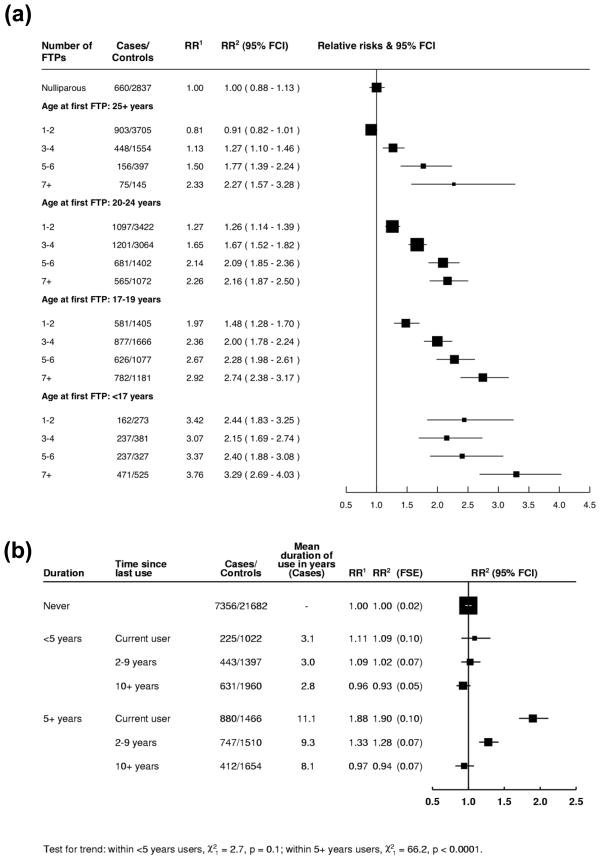
Figure 4.
Meta-analyses of risks of cervical cancer related to parity and oral contraceptive use. (a) Relative risks (RRs) of cervical cancer and corresponding 95% floating confidence intervals (FCIs) by number of full-term pregnancies (FTPs) stratified by age at first FTP [40]. Among parous women, there was an increased risk of cervical cancer with number of FTPs within each stratum of age at first FTP and vice versa. Compared to nulliparae, the risk for parity increased across strata of younger age at first FTP. 1Conditioned on age and study or study centre. 2As in1, and conditioned on age at first sexual intercourse and lifetime number of sexual partners. (b) Relative risk (RR) of cervical cancer, floating standard errors (FSEs) and corresponding floating confidence intervals (FCIs) in relation to time since last use within categories of duration of use of combined oral contraceptives [41]. For duration of use less than 5 years, there was no significant increase of cervical cancer risk, compared to never users, in any category of time since last use. For women who had used combined oral contraceptives for 5 or more years, the RR in current use was almost twice that of never users. By 10 or more years since last use, the risk was not significantly different from that in never users. 1Conditioned on age and study centre. 2As in1, and conditioned on age at first sexual intercourse, lifetime number of sexual partners, number of full-term pregnancies, smoking and screening status.
The use of combined oral contraceptives was also shown to increase cervical cancer risk after careful adjustment for sexual and reproductive factors and screening history in the aforementioned large collaborative reanalysis [45]. Among women still using oral contraceptives, the risk of cervical cancer increased with increasing duration of use (RR for ≥5 years of use versus never-users: 1.9; 95% CI: 1.7–2.1) (Figure 4b). The risk declined, however, after this use ceased, and by 10 or more years had returned to that of never-users. A similar pattern of risk was seen for squamous cell carcinoma and adenocarcinoma, and for CIN3/carcinoma in situ. Among the relatively few users of progestogen-only injectable contraceptives, the RRs found for cervical cancer were somewhat weaker, but not incompatible, with the RRs found among oral contraceptive users [45]. These studies provide further support that female hormones can increase risk of cervical cancer.
The relationship between events surrounding and following menopause (i.e. age at menopause, use of hormone replacement therapy (HRT), and overweight/obesity, as the strongest determinants of circulating estrogen levels in post-menopausal women) is still not well understood. The International Collaboration of Epidemiological Studies of Cervical Cancer has not reported pooled estimates of RRs for any of these potential risk factors, and individual studies of cervical cancer do not show conclusive results. An association between overweight/obesity and cervical cancer that is as strong as that for endometrial cancer, or even breast cancer in post-menopausal women, can be excluded [46]. In a case-control study from the United States, body mass index and waist-to-hip ratios were found to be directly associated with risk of adenocarcinoma but not with squamous cell carcinoma of the cervix [47]. Clearly further study is needed in this area of research.
Investigating the correlation between HRT and cervical cancer risk has been hampered to a much greater extent than the assessment of hormonal contraceptives by two main problems: i) the predominant use of HRT in rich countries where cervical cancer risk has been greatly reduced by cytological screening; and ii) the additional tendency of HRT users to be screened more intensively than non-users. The only relatively unbiased data on HRT and cervical cancer and precancerous lesions derives, therefore, from the few randomized studies of HRT. In the Women’s Health Initiative (WHI) trial, 15,733 women (aged 50–79 years) with an intact uterus were randomized to receive either placebo or 0.625 mg/day of conjugated equine estrogen plus 2.5 mg/day of medroxyprogesterone acetate, with cytological findings assessed during a 6-year follow-up period [48]. The annual incidence rate of any cytological abnormality in the HRT group was significantly higher than in the placebo group (hazard ratio: 1.4; 95% CI: 1.2–1.6) but no difference was found in incidence rates of high-grade squamous intraepithelial lesions (HSIL, comparable to CIN2/CIN3) and cervical cancer (found in only 54 and 10 women, respectively). A non-significantly higher incidence of cytological abnormalities (hazard ratio: 1.4; 95% CI; 0.9–2.0) was also reported among women in the HRT group in a smaller trial (2,561 randomized women, same formulation as in the WHI trial) [49]. The excess was due to higher incidence of atypical squamous cells of undetermined significance (hazard ratio: 1.6; 95% CI: 1.0–2.5) [49].
Randomized trials of HRT were clearly underpowered to estimate the difference in HSIL and cervical cancer by hormone use, due to the extreme rarity of these conditions in the well-screened middle-aged and elderly women included [48]. With respect to the excess of mild cytological abnormalities in users, HRT has been reported to decrease the number of false negative cytological results due to improved cellular maturation and less drying artifact compared to untreated women of the same age [48].
A large prospective study on the use of SERMs in the prevention of breast cancer recurrence is evaluating the effects of tamoxifen, a weak ERα agonist in the human cervix, and raloxifene on breast cancer incidence and other disease outcomes, including other cancers [50]. Unfortunately, it is unclear whether this study will shed light on the influence of SERMs on risk of cervical cancer. First off, HPV infection and preexisting cervical lesions were not evaluated. Second, as with HRT studies, the study population is primarily postmenopausal (mean age 58.5, only 7% under 50 years of age) and from countries with screening programs that reduce the incidence of cervical cancer. Consequently the frequency of cervical cancer is extremely low. There were no reported cases of cervical cancer in the raloxifene treatment group (group size = 9745), and 1 case reported in the tamoxifen arm (group size = 9726). Furthermore, there was no control arm (no drug) to this study.
Endogenous hormone levels can differ in women and, consequently, one group looked at whether this can be a risk factor in cervical cancer. Levels of sex hormone-binding globulin, estradiol, estone, estrone sulphate, dehydroepiandrosterone sulphate, and progesterone were similar in 110 women, with CIN2/3 and cervical cancer, and 440 control women matched by age and menopausal status [51]. The small study size and the reliance on a single measure of hormone levels taken at the time of diagnosis were, however, important limitations of the only study available on levels of endogenous hormones and cervical cancer [51].
To summarize the epidemiological studies, the plateau in cervical cancer incidence after age 45 years in unscreened populations and the increased risk among multiparous women and long-term users of hormonal contraceptives are consistent with the hypothesis that female hormones contribute to the risk of cervical cancer. Both FTPs and hormonal contraceptives involve, however, exposure to high levels of both estrogen and progesterone. Lack of data on the influence of age at menopause, different formulations of HRT and overweight/obesity do not allow one to draw conclusions on which sex hormone(s) may be involved in promoting cervical cancer in humans.
SERMs and cervical cancer
Because the cervix is highly responsive to estrogen, it has long been suspected that estrogen may contribute to cervical cancer. Based on this, several clinical trials have been carried out with tamoxifen that provided inconclusive results [52, 53]. Unfortunately tomoxifen was a poor choice of SERM to use as it acts as an ER agonist rather than ER antagonist in human cervix and vagina [54]. Tamoxifen has also been evaluated for its effect on proliferation of human cervical cancer cell lines; however, it is difficult to interpret these data because the cells used are apparently ERα-negative, and tamoxifen had differential effects depending on its concentration [28, 55, 56].
Indole-3-carbinol (I3C), a natural compound enriched in cruciferous vegetables, possesses anti-estrogenic activity by modulating estrogen metabolism in a way that favors the production of anti-estrogenic 2-hydroxyestrone [57]. It was also shown that I3C binds weakly to ERα. Interestingly, this phytochemical and its metabolites have been shown to be effective in preventing cervical disease in HPV transgenic mice [58, 59] and increasing regression of the disease in women [60]. It is, however, difficult to ascribe the anti-cervical cancer activity of I3C to its anti-estrogenic function because of its multifunctional nature. For instance, I3C prevents loss of PTEN in HPV transgenic mouse model and is efficient in treating tumors that do not depend on estrogen [61].
Concluding Remarks
Several lines of evidence support a role for estrogen and ERα in human cervical cancer. First, long-term use of oral contraceptives and/or multiple pregnancies increases the risk for cervical cancer, and cervical cancer incidence plateaus after menopause. Second, estrogen and ERα are required for cervical carcinogenesis, and SERMs are effective in controlling cervical cancer in well-validated mouse models for HPV-associated cervical cancer. Third, cervical cancer is often positive for ERα, although its functionality in such cancer has yet to be demonstrated. Fourth, estrogen increases expression of E6 and E7 oncogenes, which are the major driving force for cervical cancer. Further investigation of the role of estrogen and its receptors in human cervical cancer is indicated (Box 2). Such studies could lead to the development of new effective therapies in the treatment or prevention of cervical cancer.
Box 2. Outstanding questions.
Despite the long suspicion that estrogen may play a role in human cervical cancer, and strong evidence that this is the case in mouse models for this cancer, the role of estrogen and its receptors in human cervical cancer remains unclear. New research focusing on this topic is needed. Critical to this area of research will be the availability of cell lines derived from human cervical cancers in which the pattern of expression of estrogen receptors reflects that seen in the cancers themselves. In the absence of ERα-positive cell lines, alternative means (e.g. primary human cervical cancer xenografts) may have to be employed.
From the clinical perspective, new therapies are most urgently needed for patients with recurrent cervical cancer. Determining the dependence of these cancers on estrogen, and their responsiveness to SERMs should be of utmost priority. The potential value of SERMs in preventing cervical cancer in women with pre-existing CIN lesions is also indicated from mouse model studies [22]. But because of SERMs’ unwanted side effects such as induction of menopausal symptoms, particularly in premenopausal women, novel drugs or delivery approaches may be needed to allow ERα antagonists to be useful in this context.
Abbreviations
- HPV
human papillomavirus
- HR
high-risk
- CIN
cervical intraepithelial neoplasia
- ASM
atypical squamous metaplasia
- RR
relative risk
- ER
estrogen receptor
- ERα
estrogen receptor α
- ERβ
estrogen receptor β
- GPR30
G protein-coupled estrogen receptor 1
- FDA
Federal Drug Administration
- SERMs
selective estrogen receptor modulators
- ERE
estrogen response element
- FTP
full-term pregnancies
- CI
confidence interval
- HRT
hormone replacement therapy
- WHI
Women’s Health Initiative
- HSIL
high-grade squamous intraepithelial lesion
- PI3K
phophatidyl inositol 3-kinase
- MAPK
mitogen-activated protein kinase
- FCIs
floating confidence intervals
- FSEs
floating standard errors
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006;116:561–570. doi: 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO/IARC. Biennial report 2000–2001. 2001. [Google Scholar]
- 3.zur Hausen H. Papillomaviruses in the causation of human cancers - a brief historical account. Virology. 2009;384:260–265. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 4.Walboomers JM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 5.Frazer IH. Interaction of human papillomaviruses with the host immune system: a well evolved relationship. Virology. 2009;384:410–414. doi: 10.1016/j.virol.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Castle PE, et al. Short term persistence of human papillomavirus and risk of cervical precancer and cancer: population based cohort study. BMJ. 2009;339:b2569. doi: 10.1136/bmj.b2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCredie MR, et al. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008;9:425–434. doi: 10.1016/S1470-2045(08)70103-7. [DOI] [PubMed] [Google Scholar]
- 8.Lambert PF, Griep AE. In Vivo Models for the Study of Animal and Human Papillomaviruses. In: Garcea R, DiMaio D, editors. The Papillomaviruses. Springer Scientific (Kluwer Academic); 2005. [Google Scholar]
- 9.Riley RR, et al. Dissection of human papillomavirus E6 and E7 function in transgenic mouse models of cervical carcinogenesis. Cancer Res. 2003;63:4862–4871. [PubMed] [Google Scholar]
- 10.Shai A, et al. The human papillomavirus E6 oncogene dysregulates the cell cycle and contributes to cervical carcinogenesis through two independent activities. Cancer Res. 2007;67:1626–1635. doi: 10.1158/0008-5472.CAN-06-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arbeit JM, et al. Chronic estrogen-induced cervical and vaginal squamous carcinogenesis in human papillomavirus type 16 transgenic mice. Proc Natl Acad Sci U S A. 1996;93:2930–2935. doi: 10.1073/pnas.93.7.2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brake T, et al. Comparative analysis of cervical cancer in women and in a human papillomavirus-transgenic mouse model: identification of minichromosome maintenance protein 7 as an informative biomarker for human cervical cancer. Cancer Res. 2003;63:8173–8180. [PubMed] [Google Scholar]
- 13.Elson DA, et al. Sensitivity of the cervical transformation zone to estrogen-induced squamous carcinogenesis. Cancer Res. 2000;60:1267–1275. [PubMed] [Google Scholar]
- 14.Brake T, Lambert PF. Estrogen contributes to the onset, persistence, and malignant progression of cervical cancer in a human papillomavirus-transgenic mouse model. Proc Natl Acad Sci U S A. 2005;102:2490–2495. doi: 10.1073/pnas.0409883102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rangarajan A, Weinberg RA. Opinion: Comparative biology of mouse versus human cells: modelling human cancer in mice. Nat Rev Cancer. 2003;3:952–959. doi: 10.1038/nrc1235. [DOI] [PubMed] [Google Scholar]
- 16.Jabbar SF, et al. Persistence of high-grade cervical dysplasia and cervical cancer requires the continuous expression of the human papillomavirus type 16 E7 oncogene. Cancer Res. 2009;69:4407–4414. doi: 10.1158/0008-5472.CAN-09-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shai A, et al. p53 Loss synergizes with estrogen and papillomaviral oncogenes to induce cervical and breast cancers. Cancer Res. 2008;68:2622–2631. doi: 10.1158/0008-5472.CAN-07-5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin MK, et al. Human papillomavirus E7 oncoprotein overrides the tumor suppressor activity of p21Cip1 in cervical carcinogenesis. Cancer Res. 2009;69:5656–5663. doi: 10.1158/0008-5472.CAN-08-3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diaz-Chavez J, et al. Down-regulation of transforming growth factor-beta type II receptor (TGF-betaRII) protein and mRNA expression in cervical cancer. Mol Cancer. 2008;7:3. doi: 10.1186/1476-4598-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balsitis S, et al. Critical roles for non-pRb targets of human papillomavirus type 16 E7 in cervical carcinogenesis. Cancer Res. 2006;66:9393–9400. doi: 10.1158/0008-5472.CAN-06-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung SH, et al. Requirement for estrogen receptor alpha in a mouse model for human papillomavirus-associated cervical cancer. Cancer Res. 2008;68:9928–9934. doi: 10.1158/0008-5472.CAN-08-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung SH, Lambert PF. Prevention and treatment of cervical cancer in mice using estrogen receptor antagonists. Proc Natl Acad Sci U S A. 2009;106:19467–19472. doi: 10.1073/pnas.0911436106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diaz L, et al. Estrogens and human papilloma virus oncogenes regulate human ether-a-go-go-1 potassium channel expression. Cancer Res. 2009;69:3300–3307. doi: 10.1158/0008-5472.CAN-08-2036. [DOI] [PubMed] [Google Scholar]
- 24.Li JJ, et al. Estrogen mediates Aurora-A overexpression, centrosome amplification, chromosomal instability, and breast cancer in female ACI rats. Proc Natl Acad Sci U S A. 2004;101:18123–18128. doi: 10.1073/pnas.0408273101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howie HL, et al. Papillomavirus E6 proteins. Virology. 2009;384:324–334. doi: 10.1016/j.virol.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLaughlin-Drubin ME, Munger K. The human papillomavirus E7 oncoprotein. Virology. 2009;384:335–344. doi: 10.1016/j.virol.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitrani-Rosenbaum S, et al. Oestrogen stimulates differential transcription of human papillomavirus type 16 in SiHa cervical carcinoma cells. J Gen Virol. 1989;70 (Pt 8):2227–2232. doi: 10.1099/0022-1317-70-8-2227. [DOI] [PubMed] [Google Scholar]
- 28.Hwang JY, et al. Tamoxifen stimulates human papillomavirus type 16 gene expression and cell proliferation in a cervical cancer cell line. Cancer Res. 1992;52:6848–6852. [PubMed] [Google Scholar]
- 29.Chen YH, et al. Differential effects of progestins and estrogens on long control regions of human papillomavirus types 16 and 18. Biochem Biophys Res Comm. 1996;224:651–659. doi: 10.1006/bbrc.1996.1080. [DOI] [PubMed] [Google Scholar]
- 30.Kim CJ, et al. Regulation of cell growth and HPV genes by exogenous estrogen in cervical cancer cells. Int J Gynecol Cancer. 2000;10:157–164. doi: 10.1046/j.1525-1438.2000.00016.x. [DOI] [PubMed] [Google Scholar]
- 31.Park JS, et al. Neoplastic change of squamo-columnar junction in uterine cervix and vaginal epithelium by exogenous estrogen in hpv-18 URR E6/E7 transgenic mice. Gynecol Oncol. 2003;89:360–368. doi: 10.1016/s0090-8258(02)00106-3. [DOI] [PubMed] [Google Scholar]
- 32.Gloss B, et al. The upstream regulatory region of the human papilloma virus-16 contains an E2 protein-independent enhancer which is specific for cervical carcinoma cells and regulated by glucocorticoid hormones. EMBO J. 1987;6:3735–3743. doi: 10.1002/j.1460-2075.1987.tb02708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pater MM, et al. Glucocorticoid-dependent oncogenic transformation by type 16 but not type 11 human papilloma virus DNA. Nature. 1988;335:832–835. doi: 10.1038/335832a0. [DOI] [PubMed] [Google Scholar]
- 34.Pater MM, et al. Role of steroid hormones in potentiating transformation of cervical cells by human papillomaviruses. Trends Microbiol. 1994;2:229–234. doi: 10.1016/0966-842x(94)90627-0. [DOI] [PubMed] [Google Scholar]
- 35.Wu MH, et al. Human papillomavirus E2 protein associates with nuclear receptors to stimulate nuclear receptor- and E2-dependent transcriptional activations in human cervical carcinoma cells. Int J Biochem Cell Biol. 2007;39:413–425. doi: 10.1016/j.biocel.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 36.Wang WM, et al. Regulation of nuclear receptor activities by two human papillomavirus type 18 oncoproteins, E6 and E7. Biochem Biophys Res Comm. 2003;303:932–939. doi: 10.1016/s0006-291x(03)00444-3. [DOI] [PubMed] [Google Scholar]
- 37.Meng G, et al. Human ADA3 binds to estrogen receptor (ER) and functions as a coactivator for ER-mediated transactivation. J Biol Chem. 2004;279:54230–54240. doi: 10.1074/jbc.M404482200. [DOI] [PubMed] [Google Scholar]
- 38.Baldwin A, et al. Human papillomavirus E7 oncoprotein dysregulates steroid receptor coactivator 1 localization and function. J Virol. 2006;80:6669–6677. doi: 10.1128/JVI.02497-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pike MC, et al. ‘Hormonal’ risk factors, ‘breast tissue age’ and the age-incidence of breast cancer. Nature. 1983;303:767–770. doi: 10.1038/303767a0. [DOI] [PubMed] [Google Scholar]
- 40.Santen RJ, et al. Critical assessment of new risk factors for breast cancer: considerations for development of an improved risk prediction model. Endocr Relat Cancer. 2007;14:169–187. doi: 10.1677/ERC-06-0045. [DOI] [PubMed] [Google Scholar]
- 41.Dowsett M, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010;28:509–518. doi: 10.1200/JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
- 42.Sasieni P, et al. Benefit of cervical screening at different ages: evidence from the UK audit of screening histories. Br J Cancer. 2003;89:88–93. doi: 10.1038/sj.bjc.6600974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Curado MP, et al. IARC Scientific Publications No. 160. IX. International Agency for Research on Cancer; 2007. Cancer Incidence in Five Continents. [Google Scholar]
- 44.Cancer I.C.o.E.S.o.C. Cervical carcinoma and reproductive factors: collaborative reanalysis of individual data on 16,563 women with cervical carcinoma and 33,542 women without cervical carcinoma from 25 epidemiological studies. Int J Cancer. 2006;119:1108–1124. doi: 10.1002/ijc.21953. [DOI] [PubMed] [Google Scholar]
- 45.Appleby P, et al. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16,573 women with cervical cancer and 35,509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370:1609–1621. doi: 10.1016/S0140-6736(07)61684-5. [DOI] [PubMed] [Google Scholar]
- 46.IARC. IARC Handbooks of Cancer Prevention Volume 6: Weight Control and Physical Activity. International Agency for Research on Cancer; 2002. [Google Scholar]
- 47.Lacey JV, Jr, et al. Obesity as a potential risk factor for adenocarcinomas and squamous cell carcinomas of the uterine cervix. Cancer. 2003;98:814–821. doi: 10.1002/cncr.11567. [DOI] [PubMed] [Google Scholar]
- 48.Yasmeen S, et al. Incidence of cervical cytological abnormalities with aging in the women’s health initiative: a randomized controlled trial. Obstet Gynecol. 2006;108:410–419. doi: 10.1097/01.AOG.0000225976.69396.fb. [DOI] [PubMed] [Google Scholar]
- 49.Sawaya GF, et al. The positive predictive value of cervical smears in previously screened postmenopausal women: the Heart and Estrogen/progestin Replacement Study (HERS) Ann Intern Med. 2000;133:942–950. doi: 10.7326/0003-4819-133-12-200012190-00009. [DOI] [PubMed] [Google Scholar]
- 50.Vogel VG, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 51.Shields TS, et al. A case-control study of endogenous hormones and cervical cancer. Br J Cancer. 2004;90:146–152. doi: 10.1038/sj.bjc.6601514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vargas Roig LM, et al. Effects of short-term tamoxifen administration in patients with invasive cervical carcinoma. Anticancer Res. 1993;13:2457–2463. [PubMed] [Google Scholar]
- 53.Bigler LR, et al. Evaluation of tamoxifen in persistent or recurrent nonsquamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Int J Gynecol Cancer. 2004;14:871–874. doi: 10.1111/j.1048-891X.2004.14523.x. [DOI] [PubMed] [Google Scholar]
- 54.Friedrich M, et al. Tamoxifen and proliferation of vaginal and cervical epithelium in postmenopausal women with breast cancer. Eur J Obstet Gynecol Reprod Biol. 1998;80:221–225. doi: 10.1016/s0301-2115(98)00117-1. [DOI] [PubMed] [Google Scholar]
- 55.Grenman S, et al. In vitro response of cervical cancer cell lines CaSki, HeLa, and ME-180 to the antiestrogen tamoxifen. Gynecol Oncol. 1988;30:228–238. doi: 10.1016/0090-8258(88)90029-7. [DOI] [PubMed] [Google Scholar]
- 56.Tsai LC, et al. Effects of tamoxifen and retinoic acid on cell growth and c-myc gene expression in human breast and cervical cancer cells. Anticancer Res. 1997;17:4557–4562. [PubMed] [Google Scholar]
- 57.Rieck GC, Fiander AN. Human papillomavirus, cervical carcinogenesis and chemoprevention with Indole derivates - a review of pathomechanisms. Mol Nutr Food Res. 2008;52:105–113. doi: 10.1002/mnfr.200700138. [DOI] [PubMed] [Google Scholar]
- 58.Sepkovic DW, et al. Diindolylmethane inhibits cervical dysplasia, alters estrogen metabolism, and enhances immune response in the K14-HPV16 transgenic mouse model. Cancer Epidemiol Biomarkers Prev. 2009;18:2957–2964. doi: 10.1158/1055-9965.EPI-09-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jin L, et al. Indole-3-carbinol prevents cervical cancer in human papilloma virus type 16 (HPV16) transgenic mice. Cancer Res. 1999;59:3991–3997. [PubMed] [Google Scholar]
- 60.Bell MC, et al. Placebo-controlled trial of indole-3-carbinol in the treatment of CIN. Gynecol Oncol. 2000;78:123–129. doi: 10.1006/gyno.2000.5847. [DOI] [PubMed] [Google Scholar]
- 61.Aggarwal BB, Ichikawa H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle. 2005;4:1201–1215. doi: 10.4161/cc.4.9.1993. [DOI] [PubMed] [Google Scholar]
- 62.Woodman CB, et al. The natural history of cervical HPV infection: unresolved issues. Nat Rev Cancer. 2007;7:11–22. doi: 10.1038/nrc2050. [DOI] [PubMed] [Google Scholar]
- 63.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 64.Roden R, Wu TC. How will HPV vaccines affect cervical cancer? Nat Rev Cancer. 2006;6:753–763. doi: 10.1038/nrc1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Strati K, et al. Identification of biomarkers that distinguish human papillomavirus (HPV)-positive versus HPV-negative head and neck cancers in a mouse model. Proc Natl Acad Sci U S A. 2006;103:14152–14157. doi: 10.1073/pnas.0606698103. [DOI] [PMC free article] [PubMed] [Google Scholar]