Abstract
Pathological movement patterns like crouch gait are characterized by abnormal kinematics and muscle activations that alter how muscles support the body weight during walking. Individual muscles are often the target of interventions to improve crouch gait, yet the roles of individual muscles during crouch gait remain unknown. The goal of this study was to examine how muscles contribute to mass center accelerations and joint angular accelerations during single-limb stance in crouch gait and compare these contributions to unimpaired gait. Subject-specific dynamic simulations were created for ten children who walked in a mild crouch gait and had no previous surgeries. The simulations were analyzed to determine the acceleration of the mass center and angular accelerations of the hip, knee, and ankle generated by individual muscles. The results of this analysis indicate that children walking in crouch gait have less passive skeletal support of body weight and utilize substantially higher muscle forces to walk than unimpaired individuals. Crouch gait relies on the same muscles as unimpaired gait to accelerate the mass center upward, including the soleus, vasti, gastrocnemius, gluteus medius, rectus femoris, and gluteus maximus. However, during crouch gait these muscles are active throughout single-limb stance, in contrast to the modulation of muscle forces seen during single-limb stance in unimpaired gait. Subjects walking in a crouch gait rely more on proximal muscles, including the gluteus medius and hamstrings, to accelerate the mass center forward during single-limb stance than subjects with unimpaired gait.
Keywords: cerebral palsy, walking, dynamic simulation, crouch gait, induced acceleration
Introduction
Crouch gait, a common movement pattern among individuals with cerebral palsy, is characterized by excessive flexion of the hip, knee, and ankle during the stance phase of gait. This walking pattern is inefficient (Rose et al., 1989; Waters and Mulroy, 1999) and if left untreated can lead to joint pain (Jahnsen et al., 2004), formation of boney deformities (Graham and Selber, 2003), and loss of independent gait (Johnson et al., 1997; Opheim et al., 2009). Clinicians try to identify muscles that can be strengthened, surgically lengthened, or otherwise treated to enable a more erect and efficient walking pattern. Little is known, however, about how individual muscles contribute to joint and mass center motions during crouch gait; thus, it is difficult to design treatment plans that target muscles most likely to improve gait dynamics.
Humans have developed an efficient walking pattern to achieve forward progression while supporting body weight. Several studies (e.g. Anderson and Pandy, 2003; Neptune et al., 2004; Arnold et al., 2005; Kimmel and Schwartz, 2006; Liu et al., 2006) have examined how muscles accelerate the joints and mass center during unimpaired gait. These studies have shown that during early stance the vasti and gluteus maximus support the body and slow forward progression, while in late stance the gastrocnemius and soleus support body weight and propel the body forward (Neptune et al., 2001; Anderson and Pandy, 2003; Liu et al., 2006). Liu et al. (2008) demonstrated that the roles of these muscles are maintained over a range of walking speeds.
Changes in joint kinematics and muscle activation patterns during crouch gait alter how muscles contribute to joint and mass center accelerations. Hicks et al. (2008) analyzed the potential of individual muscles to accelerate the hip and knee, per unit force, in crouched walking postures. This analysis revealed that a crouched posture markedly reduces the potential of several major lower-extremity muscles to generate extension accelerations of the hip and knee and increases the joint flexion accelerations from gravity. While this prior study determined the direction (i.e. flexion or extension) of the accelerations generated by important muscles, the magnitudes of the accelerations generated by muscles depend on muscle forces, which have not been estimated for subjects with crouch gait.
Determining how muscles contribute to joint angular accelerations and mass center accelerations during crouch gait can clarify the role of muscles during this abnormal walking pattern and elucidate biomechanical consequences of treatments such as surgically lengthening muscles. Thus, the goal of the present study was to quantify angular accelerations of the hip, knee, and ankle generated by stance-limb muscles during the single-limb stance phase of crouch gait by creating and analyzing the first subject-specific dynamic simulations of crouch gait. Additionally, we characterized how muscles accelerate the mass center, which provides a holistic view of how muscles contribute to motion of the body during gait. The simulations are freely available at www.simtk.org, enabling other researchers to reproduce the results of this study and to perform additional analyses.
Methods
Subjects
Ten subjects with spastic diplegic cerebral palsy – age: 8.1 ±1.7 yrs, height: 1.25 ±0.09 m, weight: 27.1 ±9.1 kg, leg length: 0.65 ±0.06 m (mean ± SD) – were selected from a database of subjects examined at Gillette Children’s Specialty Healthcare, St. Paul, MN. Each subject included in the study: 1) walked with a mild crouch gait (minimum knee flexion 15–40° during stance), 2) did not walk in equinus and achieved at least 0° of dorsiflexion during his or her physical exam, 3) had no previous surgeries, and 4) had no significant torsional skeletal deformities (less than 30° of tibial torsion and femoral anteversion). All subjects and/or guardians provided informed written consent for the data collection; analyses of the data were performed in accordance with the regulations of all participating institutions.
Motion analysis data were collected and kinematics determined using a 12-camera system (Vicon Motion Systems, Lake Forest, CA) and a standard marker measurement protocol (Davis et al., 1991). Four force plates (AMTI, Watertown, MA) were used to record ground reaction forces and moments, which were sampled at 1080 Hz and low-pass filtered at 20 Hz. Our analysis focused on single support because a lack of consecutive force plate strikes precluded the analysis of double support. Subjects walked at a self-selected walking speed of 0.92 ±0.18 m/s. Surface electromyography (EMG) signals were recorded for nine of the ten subjects from the medial hamstrings, biceps femoris long head, rectus femoris, gastrocnemius, and tibialis anterior (Motion Lab Systems, Baton Rouge, LA). EMG was sampled at 1080 Hz, band-pass filtered between 20 and 400 Hz, rectified, and low-pass filtered at 10 Hz. EMG for each muscle was normalized from zero to one based on the minimum and maximum values of that muscle over all trials for each subject.
Dynamic simulation
A generic musculoskeletal model (lower extremities from Delp et al., 1990 and torso from Anderson and Pandy, 1999) with 19 degrees of freedom and 92 musculotendon actuators was scaled to each subject according to their anthropometric measurements (Figure 1). The degrees of freedom included a ball-and-socket joint located at approximately the third lumbar vertebra between the pelvis and torso, ball-and-socket joints at each hip, a planar joint with coupled translations at each knee, and revolute joints at each ankle. A subject-specific simulation of the single-limb stance phase of gait was then generated using OpenSim, a freely available biomechanical simulation package (Delp et al., 2007, www.simtk.org). Double-support was not included in this study due to a lack of consecutive force plate strikes in the small set of subjects who met our inclusion criteria. Kinematic and ground reaction force data were imported into OpenSim, where the computed muscle control algorithm determined the muscle excitations that generated a forward dynamic simulation that minimized the difference between the experimental and simulated gait kinematics (Thelen et al., 2003; Thelen and Anderson, 2006). Estimated muscle activations were scaled in the same manner as the EMG signals, and compared to the experimentally-measured EMG signals to evaluate the fidelity of the simulation’s muscle activation timing.
Figure 1.
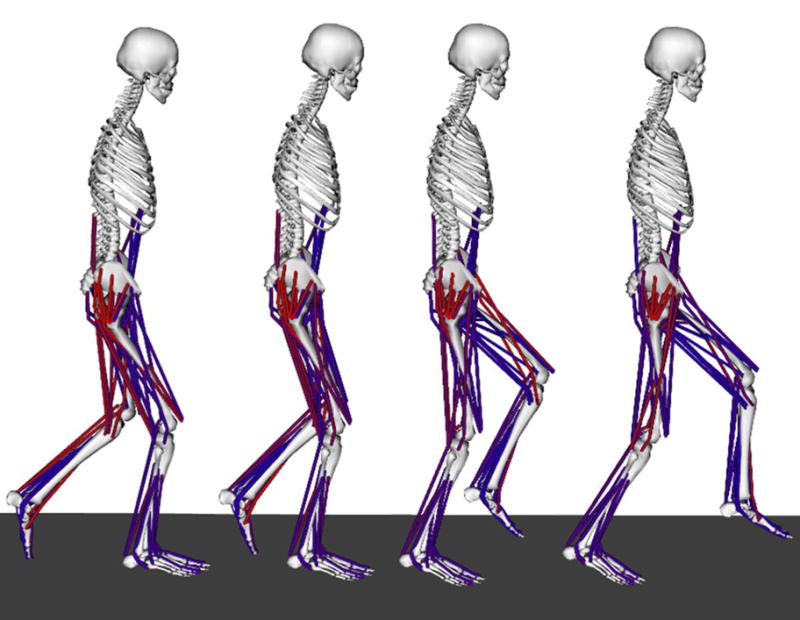
OpenSim model shown at different phases of single-limb stance during crouch gait. The muscles shown in red are highly activated, while those in blue have a low activation level, as determined using the computed muscle control algorithm.
To test the sensitivity of the results to errors in muscle activation timing, we constrained the simulated activations to match the shape and normalized magnitude of the EMG for the 10 muscles for which EMG was recorded for one subject (See Supplementary Figures 1–3). When there was a disagreement between the EMG and simulated activations, the normalized magnitude of the EMG was used to define maximum and minimum bounds on the magnitude of the simulated activations. Although adding these constraints changed the magnitude of the contribution of each muscle the direction (e.g., flexion/extension) did not change. Since these changes in magnitudes did not alter the conclusions drawn from analyzing this simulation, we chose not to constrain activations for other subjects.
A perturbation analysis was used to determine the joint angular accelerations and mass center accelerations generated by each muscle in the model (Liu et al., 2006). In this analysis, one newton of additional force (the perturbation) was applied to each muscle separately and the model’s equations of motion integrated forward in time by 20 ms. These parameters for the perturbation analysis were chosen based upon a sensitivity analysis and experience from previous studies (Goldberg et al., 2004, Neptune et al., 2001). Evaluating the change in position of each joint and the mass center after 20ms, we determined the accelerations generated by each muscle by assuming constant acceleration over this brief period. This quantity represents the potential of each muscle to accelerate each joint and the mass center per newton of force. A muscle’s contribution to joint and mass center accelerations was computed by multiplying the muscle’s potential by its estimated force from the simulation at each time step. Upward accelerations of the mass center were considered to contribute to support of the body and forward accelerations were considered to contribute to progression of the body.
The contributions of each muscle to mass center accelerations in the subjects with crouch gait were compared to two age, size, and speed matched, unimpaired subjects (age: 8.6 ±2.2 yrs; weight: 33.6 ±10.6 kg; speed: 1.08 ±0.1 m/s; leg length: 0.71 ±0.08 m) for whom motion analysis data were also collected at Gillette Children’s Specialty Healthcare. The simulation data for these unimpaired subjects have been previously reported (Liu et al., 2008) and are available at www.simtk.org.
Results
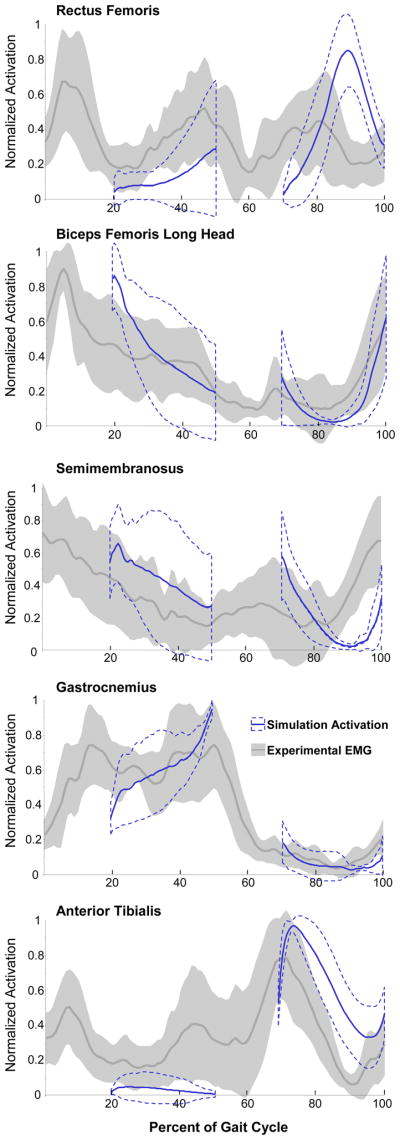
The muscle-driven simulation of each subject (see Movie 1 for an example simulation) tracked all experimental joint angles with an RMS error of less than one degree (Figure 2, Supplementary Figure 4). The resultant joint moments computed by multiplying the estimated muscle forces and moment arms also matched the joint moments computed by inverse dynamics with an RMS error of less than 1 Nm (Supplementary Figure 5). The normalized simulated activations and EMG showed similar on and off patterns during single-limb stance (Figure 3). Differences between estimated excitations and EMG included excessive rectus femoris activity during swing and reduced biceps femoris activity during terminal swing in the simulations.
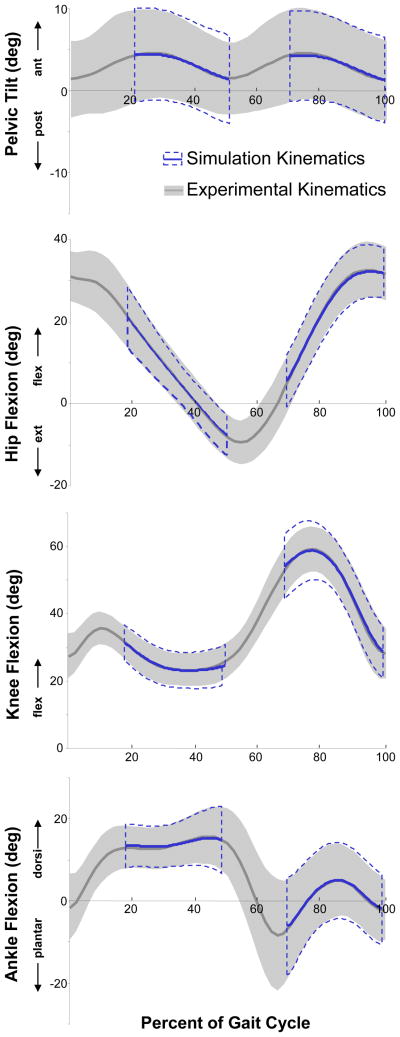
Figure 2.
Comparison of simulated and experimentally-measured joint angles. Average kinematics for pelvic tilt, hip flexion, knee flexion, and ankle dorsiflexion are shown for all 10 subjects. The gray line and shaded regions are the experimentally-measured joint angles (± 1 SD) and the blue line and dashed-lines are the kinematics reproduced by the simulation (± 1 SD). The simulated kinematics are not provided during double support as only single-limb stance was simulated. The simulated kinematics were within 1° of experimental kinematics (RMS error) for all trials.
Figure 3.
Comparison of EMG and simulated activation for the 9 crouch gait subjects for whom EMG data were available. Both the EMG signal and activation were normalized to a range of 0 to 1 between the maximum and minimum values for each subject. The gray line and shaded area represents the EMG data (± 1 SD) over a full gait cycle, while the simulated activations during single-limb stance are shown in blue with the dashed lines showing ± 1 SD. Note that estimated muscle activations are not provided during the double support periods of the gait cycle because only single-limb stance was simulated.
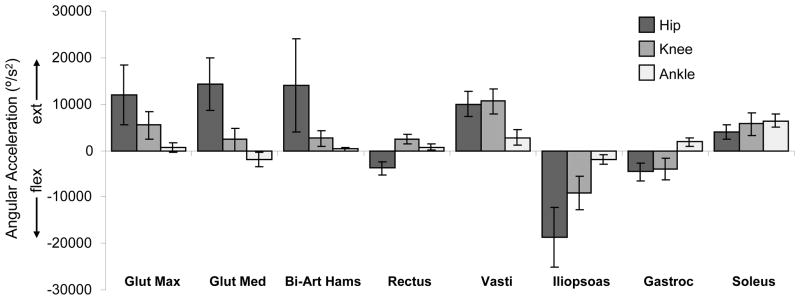
Subjects walking in crouch gait utilized higher muscle forces than the unimpaired subjects. The average total muscle force of all stance-limb muscles during single-limb stance in crouch gait was 8.6 (± 0.6) times body weight versus 5.3 (± 0.7) times body weight in unimpaired gait. The primary contributors to hip extension were the gluteus maximus, posterior gluteus medius, and bi-articular hamstrings, while the primary contributors to knee extension were the vasti, gluteus maximus, and soleus (Figure 4). The iliacus and psoas muscles generated substantial flexion accelerations of all three joints. The rectus femoris generated hip flexion acceleration.
Figure 4.
Average contributions ± 1 SD to hip, knee, and ankle angular accelerations during single-limb stance from the major muscle groups of the stance limb. Positive and negative accelerations correspond to extension and flexion, respectively. Note that the muscles with average values close to zero made small contributions throughout single-limb stance.
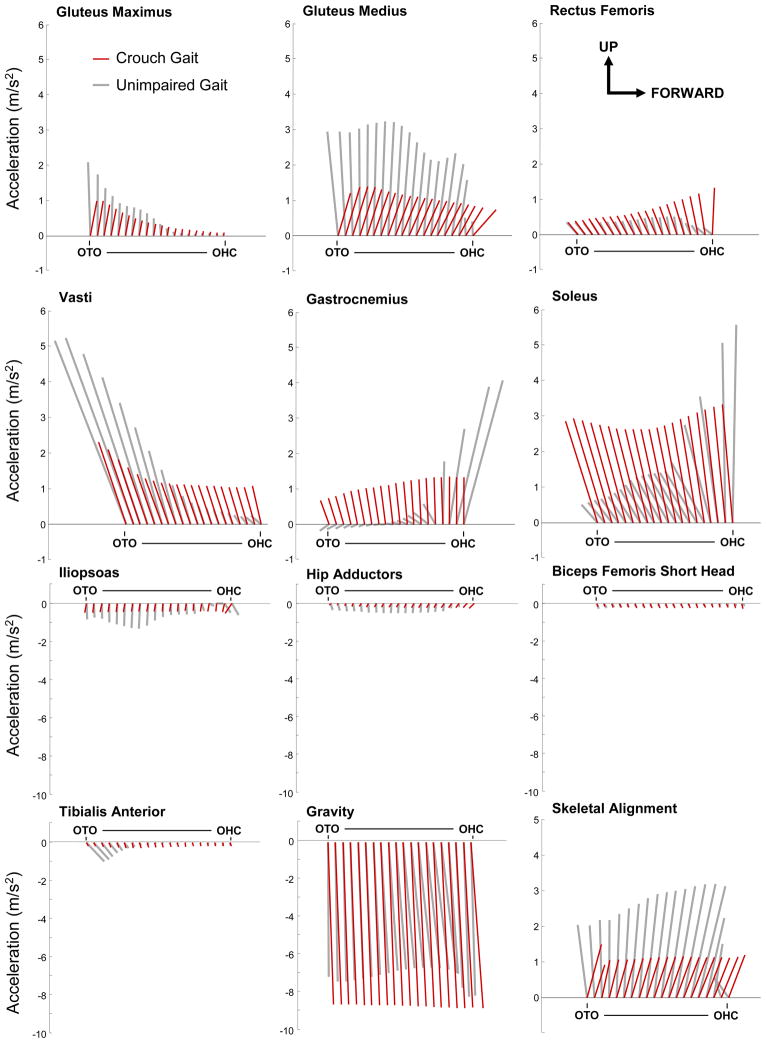
The largest upward accelerations of the mass center were from the soleus, followed by the vasti, then gastrocnemius, gluteus medius, rectus femoris, and gluteus maximus (Figure 5). This subset of muscles provided 90% of the total upward acceleration of the mass center generated by all muscles of the stance limb during crouch gait and unimpaired gait. The ankle plantarflexors contributed more to support during crouch gait, whereas the gluteus medius provided more support during unimpaired gait. The primary muscles that accelerated the mass center downward (Figure 5) included the anterior tibialis, iliopsoas, biceps femoris short head, hip adductors (adductor longus, brevis, and magnus), and medial hamstrings (semimembranosus and semitendinosus). The downward acceleration of the mass center from gravity was larger in crouch gait as less support was provided by skeletal alignment than in unimpaired gait (Figure 5).
Figure 5.
Contributions to mass center accelerations from the muscles providing the largest magnitude accelerations upward and downward and from gravity. The muscles that generated the largest upward acceleration of the mass center during crouch and unimpaired gait included gluteus maximus, gluteus medius, rectus femoris, vastus, gastrocnemius, and soleus. The muscles that accelerated the mass center downward included the iliopsoas, hip adductors, medial hamstrings, biceps femoris short head, and tibialis anterior. Accelerations are shown for single-limb stance from opposite toe-off (OTO) to opposite heel contact (OHC). The vectors represent the average up/down and forward/backward acceleration of the mass center at each 5% of single-limb stance for all subjects. The magnitude of the accelerations in the forward/backward directions has the same scale as the up/down accelerations shown on the vertical-axis. Skeletal alignment was calculated by subtracting the accelerations due to muscles from the accelerations due to the ground reaction force.
Although the muscles that contributed to the vertical acceleration of the mass center were similar in crouch gait and unimpaired gait, the profile of the accelerations differed between the two groups (Figure 5). Accelerations of the mass center generated by muscles during unimpaired gait tended to modulate substantially over the period of single-limb stance, whereas the stance-limb muscles provided more constant contributions to mass center accelerations throughout single-limb stance in crouch gait.
The largest contributors to forward progression during the single-limb stance phase of crouch gait were the bi-articular hamstrings, gluteus medius, and gluteus maximus. The gastrocnemius, rectus femoris, vastus, iliopsoas, and hip adductors slowed forward progression. In contrast, during unimpaired gait, the vasti and soleus slowed forward progression during early single-limb stance while the gastrocnemius accelerated the mass center forward in late single-limb stance. The contributions of all stance-limb muscles to joint and mass center accelerations are provided in Supplementary Figures 6–7.
Discussion
In this study we created the first muscle-actuated simulations of subjects with crouch gait, which provide insight into how individual muscles contribute to joint angular accelerations and mass center accelerations during single-limb stance. Analysis of these simulations indicates that crouch gait is characterized by larger muscle forces than unimpaired gait to support body weight and propel the body forward throughout single-limb stance. These larger forces were necessary to support the body in part due to the fact that the skeleton provided less body weight support in crouch gait, as well as the reduced capacity of muscles to extend the joints (Hicks et al., 2008) in a crouched posture.
Subjects with crouch gait utilized the same muscle groups to support the body as unimpaired gait; however, they employed a different support strategy during single-limb stance. Supporting the body in unimpaired gait requires precise orchestration of muscle activations, with dramatic modulation of muscle activity throughout single-limb stance. In contrast, during crouch gait, the support muscles were active throughout single-limb stance and generated relatively constant accelerations of the mass center. Subjects with crouch gait also used a different strategy to move the mass center forward during single-limb stance, relying more on proximal muscles than the unimpaired subjects.
These different strategies for support and progression during single-limb stance may underlie why some individuals with cerebral palsy adopt a crouch gait. Given the excessive muscle activity common in these subjects, they may adopt a support strategy where muscles are activated consistently throughout single-limb stance. Driving forward progression with more proximal muscles may also allow subjects to rely less on controlling distal leg muscles, which are often more severely effected in patients with cerebral palsy (Carr et al., 1993; Wiley and Damiano, 1998). The use of proximal muscles for forward progression and walking with slightly flexed knees are also characteristics of immature gait in unimpaired children (Farmer, 2003). This suggests that some children with cerebral palsy may adopt crouch gait as a feasible gait pattern given their neurological limitations.
Understanding how individual muscles contribute to joint and mass center accelerations can help to clarify the effects of treatment. For example, the ankle plantarflexors are common targets for surgical lengthening in patients with crouch gait. The gastrocnemius is highly active in stance and has a knee-flexion moment arm, which might play a role in the excessive knee flexion associated with crouch gait. The results from our simulations indicate that although the gastrocnemius and soleus both generate ankle plantarflexion accelerations, they have different effects at the hip and knee. While the soleus contributes to hip and knee extension throughout single-limb stance, the gastrocnemius generates flexion accelerations at both joints. Despite these differences, both muscles accelerate the mass center upward and contribute to support. The contribution of the gastrocnemius to support indicates that the muscle’s role as an extensor of the ankle is more significant to the acceleration of the mass center than its role as a flexor of the hip and knee.
Our subject-specific simulations of crouch gait demonstrate that the soleus is an important crouch-countering muscle. The soleus not only extends all three joints, but provides the largest contribution to body weight support, and thus should not be weakened in this patient population. Furthermore, our results suggest that weakening the gastrocnemius could compromise a patient’s ability to support his or her body weight and may lead to excessive dorsiflexion. While lengthening the gastrocnemius may reduce hip and knee flexion accelerations by reducing the muscle’s force, a patient may still walk in a crouched posture after a gastrocnemius lengthening if the soleus and other muscles cannot compensate for the decreased contribution to support from gastrocnemius. These possible negative effects of weakening the gastrocnemius agree with studies that predicted decreased active moment-generating capacity after lengthening the gastrocnemius (Delp et al., 1995) and that demonstrated increased knee flexion after decreasing gastrocnemius activity (Sutherland et al., 1980).
Testing the accuracy of muscle forces estimated from dynamic simulations is one of the challenges in creating subject-specific simulations. The simulations tracked the experimental kinematics and joint moments closely, but we observed some differences between EMG patterns and simulated activations. Given that maximum voluntary contractions were not available from the study subjects, we could not directly compare the magnitude of the EMG with the estimated activations. Even if the magnitude of a muscle’s force changes, the direction of its acceleration (e.g., flexion/extension) would be the same since, according to the equations of motion, direction is determined by the inertial properties of the model, muscle paths, and posture of the model. We tested the impact of constraining activations to better match the shape and normalized magnitude of the EMG and found that resulting alterations in muscle activations did not impact the conclusions of the study.
Previous investigations have tested the induced acceleration analysis technique used in this study by comparing estimated accelerations to accelerations produced when individual muscles are electrically stimulated (Stewart et al., 2007; Stewart et al., 2008; Hernandez et al., 2008; Hunter et al., 2009). These studies have shown that stimulating a muscle produces accelerations in the same direction as predicted by the induced acceleration analysis. Future studies that use electrical stimulation to examine the accelerations generated by individual muscles during crouch gait could assist in further testing the results presented here.
These simulations were generated for individuals who walked flat-footed in a mild crouch gait. The results of this study may not be extensible to other crouch gait populations, such as patients with equinus, excessive dorsiflexion, or more severe knee flexion. Additionally, this study examined single-limb stance and was not able to explore double-support due to a lack of force plate data. However, single-limb stance is clinically important since an inability to support the mass center during this period may contribute to crouch gait. Future studies examining double-support would provide valuable information about propulsion and the transition from stance to swing.
The muscle-actuated dynamic simulations generated in this study provide insights into the support and progression strategies used in crouch gait. The differences between crouch and unimpaired gait revealed that individuals who walk in a crouch gait utilize similar muscles to support the mass center but different muscles to propel the mass center forward during single-limb stance. The results of this study can be used to identify muscles that are used to support the mass center during crouch gait for interventions such as strength training, and to inform surgical decision-making. Although we can measure muscle activity and kinematics during gait, simulation provides a tool to quantify how the activity of individual muscles contribute to movement and can lead to a deeper understanding of the biomechanics underlying crouch gait.
Supplementary Material
Movie 1. Example simulation of a crouch gait subject.
Acknowledgments
The authors thank the staff of the James R. Gage Center for Gait & Motion Analysis at Gillette Children’s Specialty Healthcare for collecting and sharing motion capture data. This work was funded by NIH R01-HD33929, NIH R01-HD046814, Roadmap for Medical Research U54GM072970, and an NSF Graduate Research Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson FC, Pandy MG. A dynamic optimization solution for vertical jumping in three dimensions. Computer Methods in Biomechanics and Biomedical Engineering. 1999;2:201–31. doi: 10.1080/10255849908907988. [DOI] [PubMed] [Google Scholar]
- Anderson FC, Pandy MG. Individual muscle contributions to support in normal walking. Gait & Posture. 2003;17:159–69. doi: 10.1016/s0966-6362(02)00073-5. [DOI] [PubMed] [Google Scholar]
- Arnold AS, Anderson FC, Pandy MG, Delp SL. Muscular contributions to hip and knee extension during the single limb stance phase of normal gait: a framework for investigating the causes of crouch gait. Journal of Biomechanics. 2005;38:2181–9. doi: 10.1016/j.jbiomech.2004.09.036. [DOI] [PubMed] [Google Scholar]
- Carr LJ, Harrison LM, Evans AL, Stephens JA. Patterns of central motor reorganization in hemiplegic cerebral palsy. Brain. 1993;116:1223–47. doi: 10.1093/brain/116.5.1223. [DOI] [PubMed] [Google Scholar]
- Davis R, Ounpuu S, Tyburski D, Gage J. A gait analysis data collection and reduction technique. Human Movement Science. 1991;10:575–87. [Google Scholar]
- Delp SL, Anderson FC, Arnold AS, Loan P, Habib A, John CT, Guendelman E, Thelen DG. OpenSim: open-source software to create and analyze dynamic simulations of movement. IEEE Transactions on Biomedical Engineering. 2007;54:1940–50. doi: 10.1109/TBME.2007.901024. [DOI] [PubMed] [Google Scholar]
- Delp SL, Loan JP, Hoy MG, Zajac FE, Topp EL, Rosen JM. An interactive graphics-based model of the lower extremity to study orthopaedic surgical procedures. IEEE Transactions on Biomedical Engineering. 1990;37:757–67. doi: 10.1109/10.102791. [DOI] [PubMed] [Google Scholar]
- Delp SL, Statler K, Carroll NC. Preserving plantar flexion strength after surgical treatment for contracture of the triceps surae: a computer simulation study. Journal of Orthopaedic Research. 1995;13:96–104. doi: 10.1002/jor.1100130115. [DOI] [PubMed] [Google Scholar]
- Farmer SE. Key factors in the development of lower limb co-ordination: implications for the acquisition of walking in children with cerebral palsy. Disability & Rehabilitation. 2003;25:807–16. doi: 10.1080/0963828031000106148. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Anderson FC, Pandy MG, Delp SL. Muscles that influcence knee flexion velocity in double support: implications for stiff-knee gait. Journal of Biomechanics. 2004;37:1189–96. doi: 10.1016/j.jbiomech.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Graham H, Selber P. Musculoskeletal aspects of cerebral palsy. Journal of Bone & Joint Surgery, British Volume. 2003;85:157–66. doi: 10.1302/0301-620x.85b2.14066. [DOI] [PubMed] [Google Scholar]
- Hernandez A, Dhaher Y, Thelen DG. In vivo measurement of dynamic rectus femoris function at postures representative of early swing phase. Journal of Biomechanics. 2008;41:137–44. doi: 10.1016/j.jbiomech.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks JL, Schwartz MH, Arnold AS, Delp SL. Crouched postures reduce the capacity of muscles to extend the hip and knee during the single-limb stance phase of gait. Journal of Biomechanics. 2008;41:960–7. doi: 10.1016/j.jbiomech.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter BV, Thelen DG, Dhaher Y. A three-dimentsional biomechanical evaluation of quadriceps and hamstrings function using electrical stimulation. IEEE Transactions on Biomedical Engineering. 2009;17:167–75. doi: 10.1109/TNSRE.2009.2014235. [DOI] [PubMed] [Google Scholar]
- Jahnsen R, Villien L, Aamodt G, Stanghelle JK, Holm I. Musculoskeletal pain in adults with cerebral palsy compared with the general population. Journal of Rehabilitation Medicine. 2004;36:78–84. doi: 10.1080/16501970310018305. [DOI] [PubMed] [Google Scholar]
- Johnson DC, Damiano DL, Abel MF. The evolution of gait in childhood and adolescent cerebral palsy. Journal of Pediatric Orthopaedics. 1997;17:392–6. [PubMed] [Google Scholar]
- Kimmel SA, Schwartz MH. A baseline of dynamic muscle function during gait. Gait & Posture. 2006;23:211–21. doi: 10.1016/j.gaitpost.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Liu MQ, Anderson FC, Pandy MG, Delp SL. Muscles that support the body also modulate forward progression during walking. Journal of Biomechanics. 2006;39:2623–30. doi: 10.1016/j.jbiomech.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Liu MQ, Anderson FC, Schwartz MH, Delp SL. Muscle contributions to support and progression over a range of walking speeds. Journal of Biomechanics. 2008;41:3243–52. doi: 10.1016/j.jbiomech.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neptune RR, Kautz SA, Zajac FE. Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. Journal of Biomechanics. 2001;34:1387–98. doi: 10.1016/s0021-9290(01)00105-1. [DOI] [PubMed] [Google Scholar]
- Neptune RR, Zajac FE, Kautz SA. Muscle mechanical work requirements during normal walking: the energetic cost of raising the body’s center-of-mass is significant. Journal of Biomechanics. 2004;37:817–25. doi: 10.1016/j.jbiomech.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Opheim A, Jahnsen R, Olsson E, Stanghelle JK. Walking function, pain, and fatigue in adults with cerebral palsy: a 7-year follow-up study. Developmental Medicine & Child Neurology. 2009;51:381–8. doi: 10.1111/j.1469-8749.2008.03250.x. [DOI] [PubMed] [Google Scholar]
- Rose J, Gamble JG, Medeiros J, Burgos A, Haskell WL. Energy cost of walking in normal children and in those with cerebral palsy: comparison of heart rate and oxygen uptake. Journal of Pediatric Orthopaedics. 1989;9:276–9. [PubMed] [Google Scholar]
- Stewart C, Postans N, Schwartz MH, Rozumalski A, Roberts A. An exploration of the function of the triceps surae during normal gait using functional electrical stimulation. Gait & Posture. 2007;26:482–8. doi: 10.1016/j.gaitpost.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Stewart C, Postans N, Schwartz MH, Rozumalski A, Roberts AP. An investigation of the action of the hamstring muscles during standing in crouch using functional electrical stimulation (FES) Gait & Posture. 2008;28:372–7. doi: 10.1016/j.gaitpost.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Sutherland DH, Cooper L, Daniel D. The role of the ankle plantar flexors in normal walking. Journal of Bone & Joint Surg, American Volume. 1980;62:354–63. [PubMed] [Google Scholar]
- Thelen DG, Anderson FC. Using computed muscle control to generate forward dynamic simulations of human walking from experimental data. Journal of Biomechanics. 2006;39:1107–15. doi: 10.1016/j.jbiomech.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Thelen DG, Anderson FC, Delp SL. Generating dynamic simulations of movement using computed muscle control. Journal of Biomechanics. 2003;36:321–8. doi: 10.1016/s0021-9290(02)00432-3. [DOI] [PubMed] [Google Scholar]
- Waters RL, Mulroy S. The energy expenditure of normal and pathologic gait. Gait & Posture. 1999;9:207–31. doi: 10.1016/s0966-6362(99)00009-0. [DOI] [PubMed] [Google Scholar]
- Wiley ME, Damiano DL. Lower-extremity strength profiles in spastic cerebral palsy. Developmental Medicine & Child Neurology. 1998;40:100–7. doi: 10.1111/j.1469-8749.1998.tb15369.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie 1. Example simulation of a crouch gait subject.