Abstract
Objective:
To determine the performance of FDG-PET/CT in the detection of relevant colorectal neoplasms (adenomas ≥10 mm, with high-grade dysplasia, cancer) in relation to CT dose and contrast administration and to find a PET cut-off.
Methods:
84 patients, who underwent PET/CT and colonoscopy (n = 79)/sigmoidoscopy (n = 5) for 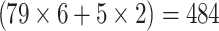 colonic segments, were included in a retrospective study. The accuracy of low-dose PET/CT in detecting mass-positive segments was evaluated by ROC analysis by two blinded independent reviewers relative to contrast-enhanced PET/CT. On a per-lesion basis characteristic PET values were tested as cut-offs.
colonic segments, were included in a retrospective study. The accuracy of low-dose PET/CT in detecting mass-positive segments was evaluated by ROC analysis by two blinded independent reviewers relative to contrast-enhanced PET/CT. On a per-lesion basis characteristic PET values were tested as cut-offs.
Results:
Low-dose PET/CT and contrast-enhanced PET/CT provide similar accuracies (area under the curve for the average ROC ratings 0.925 vs. 0.929, respectively). PET demonstrated all carcinomas (n = 23) and 83% (30/36) of relevant adenomas. In all carcinomas and adenomas with high-grade dysplasia (n = 10) the SUVmax was ≥5. This cut-off resulted in a better per-segment sensitivity and negative predictive value (NPV) than the average PET/CT reviews (sensitivity: 89% vs. 82%; NPV: 99% vs. 98%). All other tested cut-offs were inferior to the SUVmax.
Conclusion:
FDG-PET/CT provides promising accuracy for colorectal mass detection. Low dose and lack of iodine contrast in the CT component do not impact the accuracy. The PET cut-off SUVmax ≥ 5 improves the accuracy.
Keywords: Polyp, Colorectal cancer, PET/CT, Screening
Introduction
Colorectal cancer is a leading cause of morbidity and mortality worldwide [1], despite being curable if detected early and even preventable if dysplastic adenomas as their precursors are eliminated [2–4]. Thus, colorectal screening has been shown to reduce the risk of dying from colorectal cancer. Consequently, colonoscopy was recommended early on for colorectal screening [5]. In 1996 computed tomography colonography (CTC) [6] and in 1997 magnetic resonance colonography (MRC) [7] were also proposed for colorectal screening. They have several advantages: minimally invasive, fast, detect extracolonic disease, and allow computer-aided detection. CT colonography has been recommended in the colorectal screening guidelines since 2008 [8].
With FDG-PET/CT another fascinating tool for colorectal screening is on the horizon. FDG-PET exploits the increased rate of glycolysis in tumour cells to detect diseases. FDG is a glucose analogue that is taken up by cellular glucose transport mechanisms. In the cell, FDG is phosphorylated by hexokinase. In most malignant cells, FDG-6-phosphate then becomes metabolically “trapped” intracellularly because of the relative lack of glucose-6-phosphatase activity in tumours. Thus, FDG accumulation mostly correlates with the grading and the degree of malignancy. Thus, in conjunction with CT, PET/CT brings the advantage of combining metabolic and structural information.
Because only 2.5 polyps in 1,000 develop into cancer per year [9, 10] and because size and shape are the only adequate predictive in vivo criteria for malignancy, PET information about the glucose metabolism could help in identifying relevant colorectal lesions that require polypectomy or resection [5]. Through neglecting PET-negative lesions unnecessary colonoscopies and polypectomies might possibly be prevented. The feasibility of combining PET and CT colonography into PET/CT colonography has been already shown in patients with full-dose CT for tumour staging [11, 12].
The purpose of this study was (a) to determine the performance of FDG-PET/CT in the detection of relevant colorectal neoplasms (adenomas ≥10 mm, with high-grade dysplasia or cancer) in relation to the CT dose and iodine contrast administration and (b) to find a standardised cut-off in PET which might serve as a basis for future computer-aided detection (CAD) applications.
Materials and methods
Patients
In a retrospective study approved by the institutional Ethics Committee, 4,004 consecutive FDG-PET/CT reports from 2,735 patients examined in the period from May 2005 to May 2009 at the University Hospital of Dresden were browsed for those patients who had either a colorectal cancer, a cancer of unknown primary (CUP) syndrome, or focal colorectal FDG uptake further evaluated by colonoscopy or sigmoidoscopy.
PET/CT protocol
PET/CT was performed from the skull base through to the mid-thigh on a 16-slice PET/CT (Biograph 16, Siemens Medical Solutions) and included:
Low-dose (<1 mSv) CT (10 mAs, 120 kV, 16 × 1.5 mm collimation, 0.42 s tube rotation time, 86 mm/s table feed) for attenuation correction, and/or
Normal-dose CT (100 mAs (care dose), 120 kV, 16 × 1.5 mm collimation, 0.75 s tube rotation time, 48 mm/s table feed) with contrast enhancement in the portal venous phase (370 mg/ml iodine concentration, 120 ml contrast volume, 3 ml/s flow, 55 s delay, 30 ml saline flush with 1.5 ml/s flow) and
PET following 74 ± 12 min after the injection of 327 ± 48 MBq FDG with 7–8 table positions each 3 min.
PET images were iteratively reconstructed with 5-mm-thick slices. CT images were reconstructed with 2.5-mm-thick slices.
The bowel was completely unprepped i.e. not cleansed, not distended and not relaxed with spasmolytics (Figs. 1, 2, 3). Only negative oral contrast material was given.
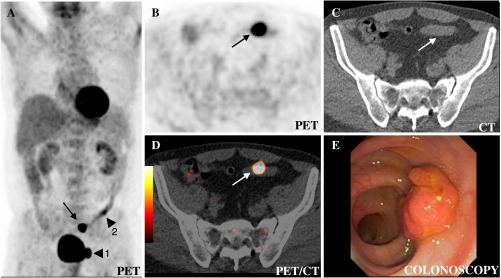
Fig. 1.
Incidental focal colorectal FDG uptake in the sigmoid colon (carcinoma (arrow)) in a 57-year-old man on low-dose PET/CT performed for follow-up after resection of a seminoma. A rotating maximal intensity projection (MIP) (a) allows for screening for focal FDG uptakes that are evaluated further on the multiplanar reconstructions (here axial PET (b), axial low-dose CT (c), axial low-dose PET/CT (d). Besides stool, sphincters and inflammation, the urinary tract (here diverticulum of the bladder (arrowhead 1)) and focal colonic collapse (arrowhead 2) constitute the only physiological pitfalls. Any shortcomings, however, can mostly be differentiated from masses based on CT anatomy and the maximum standardised uptake value (SUVmax). The SUVmax of 24.6 for the lesion in the sigmoid colon (arrow) is a trigger for colonoscopy (Fig. 5)
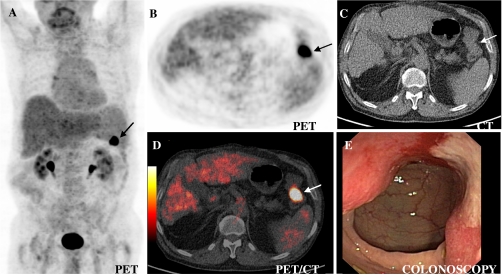
Fig. 2.
Incidental focal colorectal FDG uptake in descending colon (carcinoma (arrow)) in a 72-year-old man on low-dose PET/CT (performed for evaluation of the glucose metabolism in a hepatocellular carcinoma (HCC) as surrogate for dedifferentiation/grading. The maximum standardised uptake value (SUVmax) of 14.4 is a trigger for colonoscopy (Fig. 5)
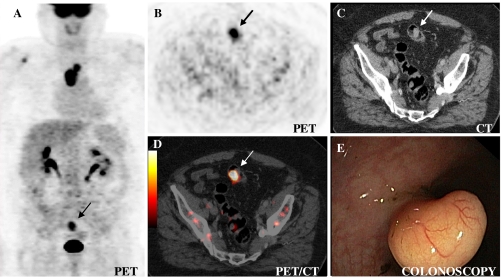
Fig. 3.
Incidental focal colorectal FDG uptake in the sigmoid colon (low-grade adenoma (arrow)) in a 66-year-old man on contrast-enhanced PET/CT performed for staging of oesophageal cancer. The maximum standardised uptake value (SUVmax) of 9.1 is a trigger for colonoscopy (Fig. 5)
In order to increase the number of patients in the analysis also the PET/CT databank of the university hospital Frankfurt was browsed for patients fulfilling the inclusion criteria described above.
Interpretation
Examinations were analysed qualitatively on a per-segment basis and quantitatively on a per-lesion basis.
The qualitative analysis was blindly performed by two independent physicians—a radiologist experienced in CT colonography and less experienced in PET (R1) and a nuclear medicine physician experienced in PET and less experienced in CT (R2). They assessed the PET/CT on a commercially available workstation (Advantage Windows 4.4, General Electric) with two screens each with a quadrant display. The first screen showed only PET images: a rotating maximal intensity projection (MIP) (Fig. 1) in the upper left corner and the orthogonal (coronal, axial and sagittal) multiplanar reconstructions (MPRs) in the other three quadrants. The second screen showed three orthogonal MPRs of the PET/CT and a coronal CT in a lung window. For reviewing PET alone the second computer screen showing the PET/CT was shut down. This ensured that the PET was reviewed separately from the CT in order to compare the subjective PET reviewing with the cut-off-based PET analysis.
The rotating MIP of the PET was used to screen for increased FDG uptake in the abdomen (Fig. 1). Once increased FDG uptake was found, the reviewer placed a cross-reference on the lesion which automatically showed the lesion on the three MPRs of the PET. By scrolling through the MPRs and following the course of the colon the reviewer had to decide if the lesion was in or outside the colon. If the lesion was considered to be in the colon, the reviewer had to assign the lesion to one of the six colonic segments (caecum, ascending, transverse and descending colon, sigmoid and rectum). Subsequently the reviewer had to rate the probability of the colonic segment being mass-positive on a five-point scale (1 = definitely negative, 2 = probably negative, 3 = equivocal, 4 = probably positive, 5 = definitely positive). Segments without increased FDG uptake were rated as definitely negative.
First PET was reviewed alone, then the second screen was turned on and PET was reviewed in combination with low-dose CT as PET/CT. CT was used to decide if the PET lesion was in or outside the colon and if the PET lesion had a sphincter, stool, collapsed bowel or an inflammation as a false-positive correlate. Finally low-dose CT was replaced by contrast-enhanced CT for evaluating contrast-enhanced PET/CT.
Measurements of Hounsfield units (HU) were used to prove stool by air inclusions (minimum HU<0) or a mass by a mean contrast enhancement 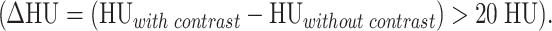
If a wall-adherent mass could be identified on CT (Fig. 3), its height and length were also measured vertically and tangentially with respect to the colonic wall. The larger diameter was used as size. If there was no correlate on CT, the PET lesion was considered a positive finding if it was regarded as a focal lesion (Figs. 1, 2, 3). In this case the size was obtained from colonoscopy or if totally resected, from the histological examination.
The quantitative analysis was performed for each visual colorectal FDG uptake and included the measurement of the maximum standardised uptake value (SUVmax) and mean standardised uptake value (SUVmean) within a volume that contains only voxels with SUV ≥50% of SUVmax.
In addition, the volume of the FDG uptake was measured as so-called metabolic volume or volume of interest (VOI). Currently there is no rationale and no standardisation which border (isocontour) should be used to define the VOI. Therefore, we determined the VOI in relation to six isocontours defined by absolute thresholds (SUV = 4, 7, 10) and relative thresholds depending on the SUVmax (SUV = 25%, 50%, 75% of SUVmax). The value SUV = 4 was chosen as the lowest limit for the absolute threshold because the physiological SUVmax of the liver is in this range. The other cut-off values were equidistantly but otherwise arbitrarily chosen.
The SUV is normalised for activity injected per body weight according to the formula: SUVmax/mean = maximum/mean VOI activity [Bq/ml] / dose injected per patient’s weight [Bq/g] with g = ml for a tissue density of 1 g/ml.
Statistical analysis
Masses were defined as relevant if ≥10 mm in maximum diameter as measured by CT or by colonoscopy or histological examination, or if they revealed high-grade dysplasia, or were cancerous in the histological examination.
The inter-observer agreement in the decision to send the patient to colonoscopy (ratings: 3, 4, 5) or not (ratings: 1, 2) was determined by Cohen’s kappa. P values of kappa below 0.05 indicate a statistically significant difference from only chance agreement.
The performance of PET, low-dose PET/CT and contrast-enhanced PET/CT in detecting mass-positive segments was compared by the area under the receiver operating curve (ROC) for each reviewer as well as for both reviewers by averaging their ROC ratings to a common rating.
The per-segment performance of PET, low-dose PET/CT and contrast-enhanced PET/CT (sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV)) was determined with the dichotomised outcome of histological findings.
Univariate and multivariate logistic regression analyses were used to find cut-off parameters to discern the two outcome groups mentioned above.
The SUVmax were compared with non-parametric analysis of variance and Mann–Whitney U tests. Local p values for the tests done in pairs are given without adjustment. After applying Bonferroni adjustment to avoid an increasing probability of a type I error, p values stay below 0.05—the global alpha for this study.
Finally, PET performance characteristics were determined for a cut-off that included all carcinomas and adenomas with high-grade dysplasia (SUVmax ≥ 5).
Statistical Analysis was performed by using a statistical software package (SPSS Inc., version 16.0, Chicago, USA).
Results
Patient demography
In total, 84 patients (18 female, 66 male) aged 41–91 (mean 65 ± 10 years), who had a total colonoscopy (n = 79) or a sigmoidoscopy (n = 5) as standard of reference for in total 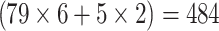 colonic segments, were included in this study. In one case, in which a tumour stenosis in the proximal rectum hindered a full colonoscopy, the postoperative colonoscopy was used as standard of reference for the colonic segments that were preoperatively not assessable. All sigmoidoscopies were performed instead of colonoscopies due to palliative situations.
colonic segments, were included in this study. In one case, in which a tumour stenosis in the proximal rectum hindered a full colonoscopy, the postoperative colonoscopy was used as standard of reference for the colonic segments that were preoperatively not assessable. All sigmoidoscopies were performed instead of colonoscopies due to palliative situations.
Reasons for colonoscopy/sigmoidoscopy referral were: initial staging (n = 14) or follow-up (n = 5) of colorectal cancer, a CUP syndrome (n = 15) or incidental focal colorectal FDG uptakes (n = 50).
Patients with CUP syndrome
The 15 patients with CUP syndrome had malignant lesions at the following sites: liver (n = 6), lymph nodes (n = 4), bone (n = 3), liver, lungs and bones (n = 1) and seminal vesicle (n = 1). Histological examination was available in 13 cases and revealed adenocarcinoma (n = 8), epithelial cancer (n = 3), anaplastic carcinoma (n = 1) and remained unclear in one case (n = 1) with osteolytic bone metastases. In 4 patients the hepatic lesions turned out to be the primary cancer (hepatocellular carcinoma (n = 1) and cholangiocarcinoma (n = 3)). In the remaining patients PET/CT found the primary cancer in 55% (6/11) at the following sites: oesophagus (n = 1), colon (n = 1), nasopharynx (n = 1), tonsil (n = 1), vulva (n = 1) and urinary tract (n = 1). Three primary malignancies (breast cancer (n = 2) and prostate cancer (n = 1)) were not visualised in PET. The origin of hepatic metastases in one patient and of osteolytic bone metastases in another patient remained unclear.
Incidental focal colorectal FDG uptakes
Incidental focal colorectal FDG uptakes were observed in 2.1% (in 50 out of 2,338 patients not scanned for colorectal cancer or CUP). In 50% (25/50) the incidental focal colorectal FDG uptake turned out to be a relevant mass on colonoscopy and even cancer in 16% (8/50).
Per-lesion analysis
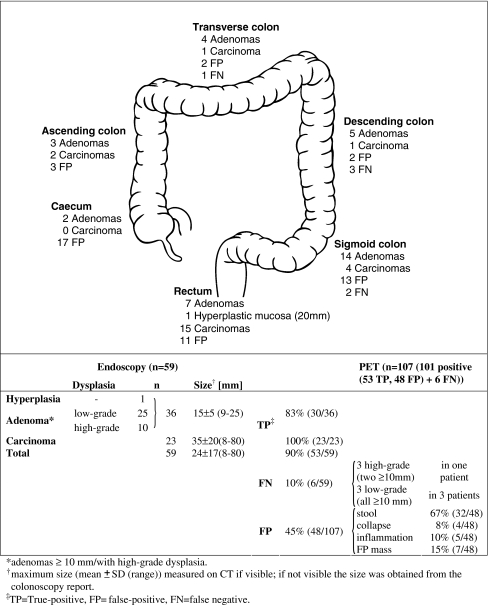
In total, endoscopy revealed 59 relevant masses (36 adenomas and 23 carcinomas) in 43 out of 84 patients. Histological examination of the adenomas revealed in 3% (1/36) no dysplasia, in 69% (25/36) low-grade dysplasia, and in 28% (10/36) high-grade dysplasia (Fig. 4).
Fig. 4.
Summary of findings
PET visibility for relevant masses totalled 90% (53/59): 83% (30/36) for relevant adenomas (mean size (range) 15 ± 5 (9–25) mm) and 100% (23/23) for carcinomas (mean size (range) 35 ± 20 (8–80) mm). PET failed to visualise 10% (6/59) of relevant masses. All masses not visualised on PET were adenomas. Three of the six FDG-negative adenomas were in one patient and showed high-grade dysplasia (two ≥10 mm, one 3 mm). The other three FDG-negative adenomas were in 3 different patients, measured ≥10 mm and revealed low-grade dysplasia. False-positive FDG uptakes totalled 45% (48/107), most (67%) had stool as correlate on CT and most (85%) were located in the caecum, sigmoid or rectum (Fig. 4). Using the CT component, the reviewers correctly identified 85% (41/48) of false-positive FDG uptakes and 15% (7/48) were falsely misinterpreted as masses.
The SUVmax increases with the degree of malignancy, from adenomas with low- and high-grade dysplasia (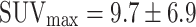 and
and 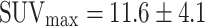 ) to carcinomas
) to carcinomas 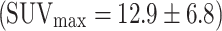 (Fig. 5). The SUVmax of false-positive FDG uptakes (n = 48) were significantly lower
(Fig. 5). The SUVmax of false-positive FDG uptakes (n = 48) were significantly lower 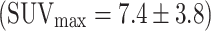 than those of adenomas with high-grade dysplasia
than those of adenomas with high-grade dysplasia 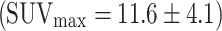 and carcinomas
and carcinomas 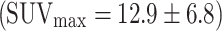 (Fig. 5). SUVmax and SUVmean were found to be the best parameters for distinguishing false-positive (FP) from true-positive (TP) lesions. The SUVmean correlates with the SUVmax (r = 0.97, n = 107 (59 TP and 48 FP)) but shows less difference than the SUVmax between false-positives and masses. Therefore, the isocontour-independent SUVmax was favoured as the cut-off.
(Fig. 5). SUVmax and SUVmean were found to be the best parameters for distinguishing false-positive (FP) from true-positive (TP) lesions. The SUVmean correlates with the SUVmax (r = 0.97, n = 107 (59 TP and 48 FP)) but shows less difference than the SUVmax between false-positives and masses. Therefore, the isocontour-independent SUVmax was favoured as the cut-off.
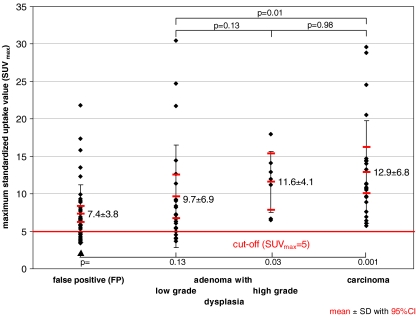
Fig. 5.
Maximum standardised uptake value (SUVmax) in relation to histological examination. The cut-off SUVmax ≥ 5 (red line) includes the detection of all carcinomas and adenomas with high-grade dysplasia, provides higher sensitivity and NPV than the PET and PET/CT reviews (Table 1), and allows for computer-aided detection
In all carcinomas (n = 23) and adenomas with high-grade dysplasia (n = 10) the SUVmax was ≥5 (Fig. 5). In order to include all carcinomas and adenomas with high-grade dysplasia this value was used as cut-off in the comparison between the reviewer-dependent and cut-off-based analysis.
Per-segment analysis (accuracy)
The performance of PET (subjective interpretation vs. cut-off-based analysis), low-dose PET/CT and contrast-enhanced PET/CT is summarised in Table 1: As reflected by the area under the curve low-dose PET/CT proved superior to PET but was not inferior to contrast-enhanced PET/CT. The use of an SUVmax ≥ 5 as a cut-off, which included all carcinomas and adenomas with high-grade dysplasia, resulted in a better per-segment sensitivity and negative predictive value than the average PET and PET/CT reviews (sensitivity 89% vs. 81% and 76–82%; NPV 99% vs. 98% and 97–98%) (Table 1).
Table 1.
Performance of FDG-PET/CT in the detection of relevant colorectal neoplasms (adenomas ≥10 mm, with high-grade dysplasia or cancer)
| Per | Patient | Segment | Lesion (Fig. 4) | ||||
|---|---|---|---|---|---|---|---|
| PET/CT | PET | PET/CT | PET | ||||
| Reviewer | Reviewer | Cut-off (SUVmax≥5) | Low dose | Normala | Reviewer | Cut-off (SUVmax≥5) | |
| n = 84a | n = 484b | n = 398 | n = 404 | n = 59 | n = 101 | ||
| Incidence | 51% (43/84) | 11% (54/484) | 11% (42/398) | 12% (49/404) | (in endoscopy) | (in PET: 53TP, 48FP) | |
| Agreementc | 89% | 91% | 92% | ||||
| AUCd | |||||||
| R1 | 0.845 | 0.882 | 0.875 | ||||
| R2 | 0.877 | 0.887 | 0.888 | ||||
| R1 + R2 | 0.899 | 0.925 | 0.929 | ||||
| Sensitivitye | 91% (39/43) | 81% (44/54) | 89% (48/54) | 76% (32/42) | 82% (40/49) | 90% (53/59) | 94% (50/53)f |
| Specificitye | 80% (33/41) | 93% (399/430) | 93% (401/430) | 97% (345/356) | 97% (344/355) | – | 35% (17/48) |
| NPVe | 89% (33/37) | 98% (399/409) | 99% (401/407) | 97% (345/355) | 98% (344/353) | – | 85% (17/20) |
| PPVe | 83% (39/47) | 59% (44/75) | 62% (48/77) | 74% (32/43) | 78% (40/51) | 52% (53/101) | 62% (50/81) |
| Accuracye | 86% (72/84) | 92% (443/484) | 93% (449/484) | 95% (377/398) | 95% (384/404) | – | 66% (67/101) |
aNormal:=contrast enhanced with full CT dose
bSegments with a standard of reference = 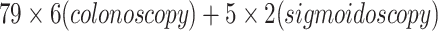
cIn the decision to send the patient for colonoscopy (ratings: 3,4,5) or not (ratings: 1,2) (kappa = 0.630; 0.605; 0.681; p = 0.000)
dArea under the curve (AUC) of the receiver operating characteristic curve (ROC) separate for each reviewer (Ri) and for both reviewers (R1 + R2) with averaged ratings
eObtained from the averaged ratings of two independent reviewers or via the cut-off SUVmax ≥ 5
f3 adenomas with low-grade dysplasia 12 ± 2 mm in size would have been additionally missed with the cut-off SUVmax ≥ 5
Discussion
There is compelling evidence to support screening average-risk individuals aged over 50 to detect and prevent colorectal cancer [2, 3, 8, 13]. By sparing bowel cleansing and distension, by displaying only relevant lesions requiring polypectomy or resection, by taking advantage of computer-aided detection via a standardised cut-off, and by increasing the accuracy in detecting extra-colonic pathological features, PET/CT could surpass CT and MR colonography for colorectal screening.
Clinical impact
Multiple studies have already documented the value of PET/CT for primary staging, restaging, and follow-up of patients with colorectal cancer [14, 15]. Based on its therapeutic impact, its first-line use is increasingly recommended for staging and restaging [15]. For detecting recurrence of colorectal cancer, PET/CT can be more sensitive than tumour markers, so that surveillance of asymptomatic and even tumour-marker-negative patients with PET/CT seems plausible [14, 16–18], in particular when taking into account that PET/CT allows for comprehensive “TNM” follow-up in one examination that is probably more accurate than CT and colonoscopy together. Replacing CT and colonoscopy by PET/CT also seems plausible for evaluation of asymptomatic average-risk patients who present with raised carcinoembryonic antigen (CEA) which indicates a malignancy in 20% with 75% outside the colon [19].
The study suggests that colorectal cancer is always visualized in PET, here with a mean SUVmax of 12.9 ± 6.8 (range 5.7–29.6) (n = 23) (Fig. 5) in accordance with the literature 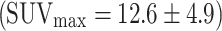 (n = 51) [20–24].
(n = 51) [20–24].
We found that the cut-off-based PET analysis is more accurate than the reviewer-dependent analysis (Table 1). This finding in conjunction with the low incidence of focal colorectal FDG uptakes 2.1% (literature, 2.2% (628 out of 28,253 patients)) but their high predictive value for a mass of 62% (here with the cut-off SUVmax ≥ 5) (literature, 73% (254 out of 349))—of which 46% (literature, 33%) were even cancer [20–22, 25–32]—indicates the recommendation that all patients with focal colorectal FDG uptakes with SUVmax ≥ 5 should automatically undergo a colonoscopy. In other words the overall rate of false-positive FDG uptakes resulting in unnecessary colonoscopy in 0.6% should be accepted for the benefit of detecting masses in 1.6% including even cancer in 0.5% [20–22, 25–32]—in particular if colonoscopy is recommended for screening anyway.
In our study PET/CT incidentally detected additional colorectal cancer in 0.3% (8/2,338) (Figs. 1, 2, 3) and found the primary cancer in the patients with CUP syndrome in 55% (6/11). Ishimori et al. [33] found colorectal cancer as an additional primary malignancy in 0.2% (4/1,912) and other proven carcinomas in 1% including cancer in the head and neck (n = 1), thyroid (n = 6), lung (n = 7), lung and thyroid (n = 1), oesophagus (n = 2), breast (n = 2) and bile duct (n = 1) [33]. We did not evaluate the value of PET/CT in detecting relevant extra-colonic pathologies. But we could demonstrate its value in detecting the primary cancer in patients with CUP syndrome as a surrogate for its potential in detecting relevant extra-colonic pathologies.
PET/CT performed as PET/CT colonography on a cleansed and distended colon [11] as well as on a non-cleansed, only distended colon [12] is also feasible. This has been proposed for patients with an incomplete colonoscopy. Under optimal conditions in a screening setting with a fully cleansed and distended colon, CT colonography is so accurate that the additional value of PET is minimal. Therefore, we evaluated the value of PET/CT in a worst-case scenario i.e. in a non-cleansed and non-distended colon imaged with minimal CT-dose and without contrast material.
Screening issue
Acceptance and compliance
Although the average lifetime risks of diagnosing and dying of colorectal cancer are 5.7% and 2.5%, respectively [34], and although a majority of cases of colorectal cancer can be prevented with colonoscopic removal of the precursor adenomatous polyp [2–4], compliance with colonoscopy is abysmal. Recently, a regional colonoscopy screening programme demonstrated a disappointingly low participation rate of 1.5% despite the benefits and quality of service [35]. On the other hand, the programme underlined the efficacy of screening. Colorectal masses were found in 26% (14,140 out of 54,491) of asymptomatic participants. Cancer was found in 1.3% (692 out of 54,491).
In a CT colonography screening study including 1,452 subjects, the participation rate was 28% [36]. CT colonography is considered less painful and less difficult than colonoscopy and is preferred over colonoscopy [37–39]. Also MR colonography with limited bowel preparation was preferred over colonoscopy due to limited bowel preparation and less pain [40]. However, discomfort associated with cleansing and air filling is still an issue in colonography [36–38], probably impacting compliance with an interval screening programme.
A non-invasive method without cleansing, colon distension and contrast material might possibly enhance compliance with a screening programme. Thus, especially in the case of colorectal cancer with a dwell time of 10 years in the adenoma–carcinoma sequence [9], compliance with a screening programme may compensate for less sensitivity of a single interval examination. Thus, a higher participation rate in a PET/CT programme combined with a high accuracy in detecting relevant extra-colonic findings could compensate for the low PET sensitivity in the detection of small polyps—if small polyps are relevant at all [10].
In CT colonography, relevant extra-colonic findings requiring subsequent medical or surgical interventions were observed in 3.2% (109/3,376) in a screening population [41–43]. Typical examples of important findings are aortic aneurysms, solid renal or hepatic masses, adrenal masses, suspicious lung nodules, hydronephrosis, lymphadenopathy, and ovarian cysts [41, 42, 44]. PET/CT is more specific than contrast-enhanced CT in characterising hepatic masses, adrenal masses, lung nodules and ovarian cysts. Thus, it is more cost-effective than CT colonography for which a mean cost of US $27 ± 8 per patient was calculated for further evaluation of extra-colonic findings [41–43, 45, 46].
Of course, currently the limited availability of PET/CT and its high costs hinder its use for screening. However, if the costs for a non-invasive, 5-min PET/CT examination are lower than those for the sum of the examinations needed to exclude the same amount of cancer entities in the early stage (e.g. MRI, CT, endoscopies for the hollow organs, mammography, inspection for melanoma), the demand for PET/CT as an all-in-one (multiorgan screening) examination might increase.
Target
It is generally accepted that in 80–85% of cancer cases, adenomas constitute the precursors for colorectal cancer [2, 3, 9] and that the progression from adenoma to carcinoma takes 10 years or more [9, 10]. Thus, removal of adenomas at regular intervals in an appropriate time frame constitutes a simple and effective cancer prophylaxis. The fact that the prevalence of polyps is high (30–50%) after age 50 years, increases with age, and that only approximately 3% of the adenomas become malignant [9, 10], render the introduction of a cut-off value in polyp screening reasonable to avoid unnecessary polypectomies.
In CT colonography, the cut-off is related to size and amount relative to 10 mm [26–28]. The sensitivity of PET/CT in visualising polyps ≤10 mm is only 21% (46 out of 219), and the 66% (163 out of 247) sensitivity in detecting masses >10 mm is disappointing compared with that of CT colonography (sensitivity 94%) [24, 27, 30, 47–49]. Despite the high molecular sensitivity of PET for the tracer (10−11–10−12 mole/L), smaller polyps can be negative on PET if their signal is spatially and temporally averaged to normal in the 5 mm3 image voxel and 3-min acquisition time per bed position (partial volume artefact). Hence, it is clear why PET fails to visualize smaller polyps whose signal is blurred in the colonic peristalsis and averaged with that of the signal void of surrounding air in the colon. In this study with improved spatial resolution (4.5 mm in the axial plane), the detection rate for polyps ≥10 mm was 83% (30/36). Current PET/CT provides a higher spatial resolution (2.5 mm) and detector gain (detected counts per second and applied activity (currently 9.1 kcounts/sMBq)) as well as a faster scanning (5 min per whole body), so that detecting smaller polyps may increase as technology progresses and partial volume effects decrease. But the spatial resolution is limited by the linear range of the random walk of the positron, which is a mean of 0.2 mm (maximum 2.4 mm) in the case of 18F. However, lower spatial resolution can be compensated by a higher FDG uptake (hot spot phenomena) so that smaller neoplasms will be detected if their SUVmax is large enough to be differentiated from the background noise (Figs. 1, 2, 3).
Smaller, non-growing and therefore non-glucose-consuming polyps are usually not visible on PET [24]. Thus, with metabolic information, PET/CT is inherently more promising than CT colonography in identifying polyps at risk of malignant transformation or already transformed to cancer. Van Kouwen et al. [50] showed in 24 patients with familial adenomatous polyposis (FAP) that the metabolic PET information identified FAP patients requiring additional examinations to rule out cancer and justified in others a more conservative approach. Other studies also showed a difference in the FDG uptake between polyps and cancer (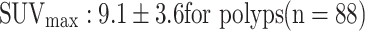 vs.
vs. 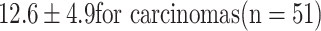 ) [20–24]. This study confirms the correlation between the degree of malignancy and glycolysis rate reflected by SUVmax (Fig. 5). The correlation between the degree of malignancy and glycolysis provides the rationale for an SUVmax-based cut-off.
) [20–24]. This study confirms the correlation between the degree of malignancy and glycolysis rate reflected by SUVmax (Fig. 5). The correlation between the degree of malignancy and glycolysis provides the rationale for an SUVmax-based cut-off.
Computer-aided detection
Computer-aided detection also requires a cut-off. Using a cut-off (SUVmax ≥ 5), that includes all carcinomas and all adenomas with high-grade dysplasia (Fig. 5), resulted in a higher sensitivity and negative predictive value than the reviewer-dependent analysis of the contrast-enhanced PET/CT (sensitivity 89% vs. 82%, NPV 99% vs. 98%) (Table 1). However, the lower specificity of PET in comparison with PET/CT (93% vs. 97%) (Table 1) demonstrates that the PET findings should be still anatomically correlated with the CT to better identify false-positives as stool, gastric or anal sphincters or parts of the urinary system. Administration of contrast did not improve the accuracy (95%) and specificity (97%) of PET/CT (Table 1). Thus, for screening purposes contrast media is not needed and in the case of an equivocal finding in the colorectum the patient should be sent for colonoscopy instead of receiving contrast material and a second CT scan.
We tested different PET values and their combinations for separating true-positives (TP) from false-positives (FP). But the metabolic volumes (VOI) as well as the ratios SUVmax/mean/metabolic volumes, reflecting a kind of density, were inferior to the SUVmax in the differentiation between TP and FP. In principle, the focality of an FDG uptake visualised on 2D maximum intensity projections should mathematically be definable even in 3D. The volume bordered by an SUV of 50% of the SUVmax is the 3D correlate to the full width at half maximum (FWHM) in 1D but also was not useful to improve the specificity. We have also taken into account the slope (gradient) in the SUV distribution by using the differences (Δ) between the VOIs in the ratios SUVmax/mean/ΔVOI. But this cut-off also failed to improve the specificity in differentiating TP from FP.
The failure in finding a better cut-off could possibly be explained by the fact that 59% (50/84) of the patients were already selected through the focal criterion, which was visually defined. In our study the 50 patients with incidental focal colorectal FDG uptake were visually filtered out from 2,338 patients. It is conceivable that this pre-selection can be performed by a computer, which filters out only the patients with an abdominal FDG uptake over a certain cut-off. Then, the urinary system as the only physiological cause of increased FDG uptake in the abdomen needs to be excluded by a human interface. But the urinary system can possibly also be identified by a computer based on SUVmax criteria (e.g. high value and homogeneous distribution) in conjunction with anatomical criteria (e.g. localisation and shape). Consequently, computer-aided detection of focal FDG uptakes in PET/CT is easier to realise than computer-aided detection on CT/MR colonography or even colonoscopy.
Dose issue: detection vs. initiation of cancer
Whether in the clinical context of staging, preoperative evaluation, follow-up and evaluation of screening findings or in a context of screening risk groups (e.g. smokers) or even asymptomatic persons not at risk, PET/CT is probably most sensitive in the detection of malignancies [25, 29, 51, 52]. This is supported by the fact that PET/CT is used if not primarily at least as ultima ratio to find the primary cancer in patients with CUP syndrome [53].
Combined PET and tumour marker screening revealed cancer in 1.5% (986 out of 67,510) of asymptomatic people. Of these, PET detected 668 cancer (sensitivity 68%). They were mostly cancers in the thyroid (25%), colon (23%), lung (21%) and breast (10%) [54–60]. When additionally using the CT component, which is automatically included nowadays for attenuation correction, small lung cancer as well as non-oncological pathological features can also be detected.
Considering the ratio of extra-colonic pathological features to colonic malignancies, PET/CT is expected to detect more extra-colonic pathological features than miss colonic malignancies. Thus, the advantage of PET/CT in detecting pathological features in and outside the colon outweighs both its weakness in detecting smaller, less malignant polyps as well as the 0.024% radiation risk of initiating a malignancy in 20–30 years. The radiation risk is calculated from 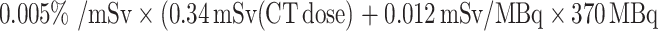 (PET dose)) [61]. Cancer is responsible for death in more than 25% of the population in industrialised nations [62]. The probability of detecting cancer increases with age while the probability of dying from radiation-induced cancer decreases with age due to the latency period of 20–30 years. Therefore, the individual benefit-to-cost ratio of PET/CT screening increases with age. In the worst case, if both the radiation-induced cancer mortality (0.024%) and the natural cancer mortality (25%) are not age-related, to be justified PET/CT needs to prevent more than 0.6% of the natural 25% of cancer mortality.
(PET dose)) [61]. Cancer is responsible for death in more than 25% of the population in industrialised nations [62]. The probability of detecting cancer increases with age while the probability of dying from radiation-induced cancer decreases with age due to the latency period of 20–30 years. Therefore, the individual benefit-to-cost ratio of PET/CT screening increases with age. In the worst case, if both the radiation-induced cancer mortality (0.024%) and the natural cancer mortality (25%) are not age-related, to be justified PET/CT needs to prevent more than 0.6% of the natural 25% of cancer mortality.
Limitation and future work
The study is retrospective. Further prospectively designed studies should clarify:
If omission of oral contrast reduces the FDG uptake in the normal colonic wall and its excretion into stool (direct or via dissociation of FDG-loaded mucosa cells) [63],
If the cut-off is dependent on the scanner type and requires a cross-calibration between different scanners [64],
If an intrinsic normalisation to the mean SUV of the liver improves the accuracy of the cut-off [65], and
If low dose, non-enhanced PET/CT is more cost-effective than CT in identifying (detecting and classifying) relevant extra-colonic pathological features [66].
Another limitation of this retrospective study is the selection bias. Only patients with a high likelihood of being further evaluated with colonoscopy were filtered out of the PET database of 4,004 reports. Thus, the database was only browsed for patients referred for initial staging or follow-up of colorectal cancer or for further evaluation of a CUP syndrome or incidental focal colorectal FDG uptakes. The other, mostly oncological PET patients were unlikely to undergo additional screening colonoscopy within the next 3 months after the PET/CT. Therefore, we limited the search only to the subgroup described above and did not check if other PET patients coincidentally underwent a colonoscopy. However, by including patients with CUP syndrome as a “normal collective” (without a colorectal mass), dividing the colon into six segments, considering each segment as independent and performing a per-segment analysis, we sought to lower the incidence (43/84 (51%) per patient vs. 54/484 (11%) per segment) and thereby to minimise any bias that might be associated with the patient selection. A prospective study including a normal collective undergoing PET/CT followed by colonoscopy would overcome the selection bias. However, the meaning of the SUVmax as cut-off should not be biased by the patient selection.
Conclusion
FDG-PET/CT provides promising accuracy for colorectal mass detection. Low dose and lack of iodine contrast do not impact the accuracy. A standardised PET cut-off (e.g. SUVmax ≥ 5) improves the accuracy.
Prospective studies with more polyps and improved PET technology seem to be warranted—not only to obtain a test data set for finding a better cut-off, but also to figure out if PET/CT is sufficient for ruling out relevant colorectal neoplasms.
Acknowledgements
The authors like to thank the German Federal Ministry of Education and Research (BMDF) for providing the PET/CT (Contract 03 ZIK 04).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- AUC
area under the curve
- CAD
computer-aided detection
- CUP
cancer of unknown primary
- CEA
carcinoembryonic antigen
- CTC
computed tomography colonography
- MIP
maximum intensity projection
- MPR
multiplanar reconstruction
- MRC
magnetic resonance colonography
- FDG
2-[18F]fluoro-2-deoxy-d-glucose (FDG)
- PET/CT
positron emission tomography/computed tomography
- ROC
receiver operating curve
- SUV
standardised uptake value
- VOI
volume of interest
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 3.Toribara NW, Sleisenger MH. Screening for colorectal cancer. N Engl J Med. 1995;332:861–867. doi: 10.1056/NEJM199503303321306. [DOI] [PubMed] [Google Scholar]
- 4.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 5.Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology. 1997;112:594–642. doi: 10.1053/gast.1997.v112.agast970594. [DOI] [PubMed] [Google Scholar]
- 6.Hara AK, Johnson CD, Reed JE, et al. Detection of colorectal polyps by computed tomographic colography: feasibility of a novel technique. Gastroenterology. 1996;110:284–290. doi: 10.1053/gast.1996.v110.pm8536869. [DOI] [PubMed] [Google Scholar]
- 7.Luboldt W, Bauerfeind P, Steiner P, Fried M, Krestin GP, Debatin JF. Preliminary assessment of three-dimensional magnetic resonance imaging for various colonic disorders. Lancet. 1997;349:1288–1291. doi: 10.1016/S0140-6736(96)11332-5. [DOI] [PubMed] [Google Scholar]
- 8.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Eide TJ. Natural history of adenomas. World J Surg. 1991;15:3–6. doi: 10.1007/BF01658952. [DOI] [PubMed] [Google Scholar]
- 10.Nusko G, Mansmann U, Partzsch U, et al. Invasive carcinoma in colorectal adenomas: multivariate analysis of patient and adenoma characteristics. Endoscopy. 1997;29:626–631. doi: 10.1055/s-2007-1004268. [DOI] [PubMed] [Google Scholar]
- 11.Veit-Haibach P, Kuehle CA, Beyer T, et al. Diagnostic accuracy of colorectal cancer staging with whole-body PET/CT colonography. JAMA. 2006;296:2590–2600. doi: 10.1001/jama.296.21.2590. [DOI] [PubMed] [Google Scholar]
- 12.Nagata K, Ota Y, Okawa T, Endo S, Kudo SE. PET/CT colonography for the preoperative evaluation of the colon proximal to the obstructive colorectal cancer. Dis Colon Rectum. 2008;51:882–890. doi: 10.1007/s10350-008-9236-1. [DOI] [PubMed] [Google Scholar]
- 13.Brenner H, Hoffmeister M, Brenner G, Altenhofen L, Haug U. Expected reduction of colorectal cancer incidence within 8 years after introduction of the German screening colonoscopy programme: estimates based on 1,875,708 screening colonoscopies. Eur J Cancer. 2009;14:14. doi: 10.1016/j.ejca.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 14.Shen YY, Liang JA, Chen YK, Tsai CY, Kao CH. Clinical impact of 18F-FDG-PET in the suspicion of recurrent colorectal cancer based on asymptomatically elevated serum level of carcinoembryonic antigen (CEA) in Taiwan. Hepatogastroenterology. 2006;53:348–350. [PubMed] [Google Scholar]
- 15.Vriens D, de Geus-Oei LF, van der Graaf WT, Oyen WJ. Tailoring therapy in colorectal cancer by PET-CT. Q J Nucl Med Mol Imaging. 2009;53:224–244. [PubMed] [Google Scholar]
- 16.Chen LB, Tong JL, Song HZ, Zhu H, Wang YC. (18)F-DG PET/CT in detection of recurrence and metastasis of colorectal cancer. World J Gastroenterol. 2007;13:5025–5029. doi: 10.3748/wjg.v13.i37.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorensen N, Jensen A, Wille-Jorgensen P, et al. Strict follow-up programme including CT and F-FDG-PET after curative surgery for colorectal cancer. Colorectal Dis. 2009;11:11. doi: 10.1111/j.1463-1318.2008.01565.x. [DOI] [PubMed] [Google Scholar]
- 18.Tan E, Gouvas N, Nicholls RJ, Ziprin P, Xynos E, Tekkis PP. Diagnostic precision of carcinoembryonic antigen in the detection of recurrence of colorectal cancer. Surg Oncol. 2009;18:15–24. doi: 10.1016/j.suronc.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Lim YK, Kam MH, Eu KW. Carcinoembryonic antigen screening: how far should we go? Singapore Med J. 2009;50:862–865. [PubMed] [Google Scholar]
- 20.Chen YK, Kao CH, Liao AC, Shen YY, Su CT. Colorectal cancer screening in asymptomatic adults: the role of FDG PET scan. Anticancer Res. 2003;23:4357–4361. [PubMed] [Google Scholar]
- 21.Israel O, Yefremov N, Bar-Shalom R, et al. PET/CT detection of unexpected gastrointestinal foci of 18F-FDG uptake: incidence, localization patterns, and clinical significance. J Nucl Med. 2005;46:758–762. [PubMed] [Google Scholar]
- 22.Gutman F, Alberini JL, Wartski M, et al. Incidental colonic focal lesions detected by FDG PET/CT. AJR Am J Roentgenol. 2005;185:495–500. doi: 10.2214/AJR.04.1654. [DOI] [PubMed] [Google Scholar]
- 23.Nakajo M, Jinnouchi S, Tashiro Y, Shirahama H, Sato E, Koriyama C. Effect of clinicopathologic factors on visibility of colorectal polyps with FDG PET. AJR Am J Roentgenol. 2009;192:754–760. doi: 10.2214/AJR.08.1304. [DOI] [PubMed] [Google Scholar]
- 24.Ravizza D, Bartolomei M, Santoro L, et al. Positron emission tomography for the detection of colorectal adenomas. Dig Liver Dis. 2009;28:28. doi: 10.1016/j.dld.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 25.Zhuang H, Hickeson M, Chacko TK, et al. Incidental detection of colon cancer by FDG positron emission tomography in patients examined for pulmonary nodules. Clin Nucl Med. 2002;27:628–632. doi: 10.1097/00003072-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Agress H, Jr, Cooper BZ. Detection of clinically unexpected malignant and premalignant tumors with whole-body FDG PET: histopathologic comparison. Radiology. 2004;230:417–422. doi: 10.1148/radiol.2302021685. [DOI] [PubMed] [Google Scholar]
- 27.Kamel EM, Thumshirn M, Truninger K, et al. Significance of incidental 18F-FDG accumulations in the gastrointestinal tract in PET/CT: correlation with endoscopic and histopathologic results. J Nucl Med. 2004;45:1804–1810. [PubMed] [Google Scholar]
- 28.Pandit-Taskar N, Schoder H, Gonen M, Larson SM, Yeung HW. Clinical significance of unexplained abnormal focal FDG uptake in the abdomen during whole-body PET. AJR Am J Roentgenol. 2004;183:1143–1147. doi: 10.2214/ajr.183.4.1831143. [DOI] [PubMed] [Google Scholar]
- 29.Even-Sapir E, Lerman H, Gutman M, et al. The presentation of malignant tumours and pre-malignant lesions incidentally found on PET-CT. Eur J Nucl Med Mol Imaging. 2006;33:541–552. doi: 10.1007/s00259-005-0056-4. [DOI] [PubMed] [Google Scholar]
- 30.Hemandas AK, Robson NK, Hickish T, Talbot RW. Colorectal tubulovillous adenomas identified on fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography scans. Colorectal Dis. 2008;10:386–389. doi: 10.1111/j.1463-1318.2007.01261.x. [DOI] [PubMed] [Google Scholar]
- 31.Lee ST, Tan T, Poon AM, et al. Role of low-dose, noncontrast computed tomography from integrated positron emission tomography/computed tomography in evaluating incidental 2-deoxy-2-[F-18]fluoro-d-glucose-avid colon lesions. Mol Imaging Biol. 2008;10:48–53. doi: 10.1007/s11307-007-0117-0. [DOI] [PubMed] [Google Scholar]
- 32.Lee JC, Hartnett GF, Hughes BG, Ravi Kumar AS. The segmental distribution and clinical significance of colorectal fluorodeoxyglucose uptake incidentally detected on PET-CT. Nucl Med Commun. 2009;30:333–337. doi: 10.1097/MNM.0b013e32832999fa. [DOI] [PubMed] [Google Scholar]
- 33.Ishimori T, Patel PV, Wahl RL. Detection of unexpected additional primary malignancies with PET/CT. J Nucl Med. 2005;46:752–757. [PubMed] [Google Scholar]
- 34.Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 35.Mansman U, Crispin A, Henschel V, et al. Epidemiology and quality control of 245,000 outpatient colonoscopies. Dtsch Arztebl. 2008;105:434–440. doi: 10.3238/arztebl.2008.0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edwards JT, Mendelson RM, Fritschi L, et al. Colorectal neoplasia screening with CT colonography in average-risk asymptomatic subjects: community-based study. Radiology. 2004;230:459–464. doi: 10.1148/radiol.2302021422. [DOI] [PubMed] [Google Scholar]
- 37.Svensson MH, Svensson E, Lasson A, Hellstrom M. Patient acceptance of CT colonography and conventional colonoscopy: prospective comparative study in patients with or suspected of having colorectal disease. Radiology. 2002;222:337–345. doi: 10.1148/radiol.2222010669. [DOI] [PubMed] [Google Scholar]
- 38.van Gelder RE, Birnie E, Florie J, et al. CT colonography and colonoscopy: assessment of patient preference in a 5-week follow-up study. Radiology. 2004;233:328–337. doi: 10.1148/radiol.2331031208. [DOI] [PubMed] [Google Scholar]
- 39.Taylor SA, Halligan S, Saunders BP, Bassett P, Vance M, Bartram CI. Acceptance by patients of multidetector CT colonography compared with barium enema examinations, flexible sigmoidoscopy, and colonoscopy. AJR Am J Roentgenol. 2003;181:913–921. doi: 10.2214/ajr.181.4.1810913. [DOI] [PubMed] [Google Scholar]
- 40.Florie J, Birnie E, van Gelder RE, et al. MR colonography with limited bowel preparation: patient acceptance compared with that of full-preparation colonoscopy. Radiology. 2007;245:150–159. doi: 10.1148/radiol.2451061244. [DOI] [PubMed] [Google Scholar]
- 41.Gluecker TM, Johnson CD, Wilson LA, et al. Extracolonic findings at CT colonography: evaluation of prevalence and cost in a screening population. Gastroenterology. 2003;124:911–916. doi: 10.1053/gast.2003.50158. [DOI] [PubMed] [Google Scholar]
- 42.Yee J, Kumar NN, Godara S, et al. Extracolonic abnormalities discovered incidentally at CT colonography in a male population. Radiology. 2005;236:519–526. doi: 10.1148/radiol.2362040166. [DOI] [PubMed] [Google Scholar]
- 43.Pickhardt PJ, Hanson ME, Vanness DJ, et al. Unsuspected extracolonic findings at screening CT colonography: clinical and economic impact. Radiology. 2008;249:151–159. doi: 10.1148/radiol.2491072148. [DOI] [PubMed] [Google Scholar]
- 44.Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349:2191–2200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 45.Flicker MS, Tsoukas AT, Hazra A, Dachman AH. Economic impact of extracolonic findings at computed tomographic colonography. J Comput Assist Tomogr. 2008;32:497–503. doi: 10.1097/RCT.0b013e3181692091. [DOI] [PubMed] [Google Scholar]
- 46.Hara AK, Johnson CD, MacCarty RL, Welch TJ. Incidental extracolonic findings at CT colonography. Radiology. 2000;215:353–357. doi: 10.1148/radiology.215.2.r00ap33353. [DOI] [PubMed] [Google Scholar]
- 47.Yasuda S, Fujii H, Nakahara T, et al. 18F-FDG PET detection of colonic adenomas. J Nucl Med. 2001;42:989–892. [PubMed] [Google Scholar]
- 48.van Kouwen MC, Nagengast FM, Jansen JB, Oyen WJ, Drenth JP. 2-(18F)-fluoro-2-deoxy-d-glucose positron emission tomography detects clinical relevant adenomas of the colon: a prospective study. J Clin Oncol. 2005;23:3713–3717. doi: 10.1200/JCO.2005.02.401. [DOI] [PubMed] [Google Scholar]
- 49.Friedland S, Soetikno R, Carlisle M, Taur A, Kaltenbach T, Segall G. 18-Fluorodeoxyglucose positron emission tomography has limited sensitivity for colonic adenoma and early stage colon cancer. Gastrointest Endosc. 2005;61:395–400. doi: 10.1016/S0016-5107(04)02775-0. [DOI] [PubMed] [Google Scholar]
- 50.van Kouwen MC, Drenth JP, van Krieken JH, et al. Ability of FDG-PET to detect all cancers in patients with familial adenomatous polyposis, and impact on clinical management. Eur J Nucl Med Mol Imaging. 2006;33:270–274. doi: 10.1007/s00259-005-1955-0. [DOI] [PubMed] [Google Scholar]
- 51.Truijers M, Pol JA, Kurvers H, Bredie S, Oyen WJ, Blankensteijn JD. Incidental finding of malignancy in patients preoperatively evaluated for aneurysm wall pathology using PET/CT. J Vasc Surg. 2009;49:1313–1315. doi: 10.1016/j.jvs.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 52.Choi JY, Lee KS, Kwon OJ, et al. Improved detection of second primary cancer using integrated [18F] fluorodeoxyglucose positron emission tomography and computed tomography for initial tumor staging. J Clin Oncol. 2005;23:7654–7659. doi: 10.1200/JCO.2005.01.4340. [DOI] [PubMed] [Google Scholar]
- 53.Yapar Z, Kibar M, Yapar AF et al (2010) The value of 18F-fluorodeoxyglucose positron emission tomography/computed tomography in carcinoma of an unknown primary: diagnosis and follow-up. Nucl Med Commun 31:59–66 [DOI] [PubMed]
- 54.Yasuda S, Ide M, Fujii H, et al. Application of positron emission tomography imaging to cancer screening. Br J Cancer. 2000;83:1607–1611. doi: 10.1054/bjoc.2000.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shen YY, Su CT, Chen GJ, Chen YK, Liao AC, Tsai FS. The value of 18F-fluorodeoxyglucose positron emission tomography with the additional help of tumor markers in cancer screening. Neoplasma. 2003;50:217–221. [PubMed] [Google Scholar]
- 56.Chen YK, Ding HJ, Su CT, et al. Application of PET and PET/CT imaging for cancer screening. Anticancer Res. 2004;24:4103–4108. [PubMed] [Google Scholar]
- 57.Ide M. Cancer screening with FDG-PET. Q J Nucl Med Mol Imaging. 2006;50:23–27. [PubMed] [Google Scholar]
- 58.Minamimoto R, Senda M, Uno K, et al. Performance profile of FDG-PET and PET/CT for cancer screening on the basis of a Japanese nationwide survey. Ann Nucl Med. 2007;21:481–498. doi: 10.1007/s12149-007-0061-8. [DOI] [PubMed] [Google Scholar]
- 59.Kojima S, Zhou B, Teramukai S, et al. Cancer screening of healthy volunteers using whole-body 18F-FDG-PET scans: the Nishidai clinic study. Eur J Cancer. 2007;43:1842–1848. doi: 10.1016/j.ejca.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 60.Nishizawa S, Kojima S, Teramukai S, et al. Prospective evaluation of whole-body cancer screening with multiple modalities including [18F]fluorodeoxyglucose positron emission tomography in a healthy population: a preliminary report. J Clin Oncol. 2009;27:1767–1773. doi: 10.1200/JCO.2008.18.2238. [DOI] [PubMed] [Google Scholar]
- 61.Hays MT, Watson EE, Thomas SR, Stabin M. MIRD dose estimate report no. 19: radiation absorbed dose estimates from (18)F-FDG. J Nucl Med. 2002;43:210–214. [PubMed] [Google Scholar]
- 62.Becker N, Wahrendorf J (1998) Krebsatlas der Bundesrepublik Deutschland 1981–1990. Springer, Berlin. http://www.dkfz-heidelberg.de/de/krebsatlas/gesamt/mort_2.html. Accessed 24 Sept 2009
- 63.Otsuka H, Graham MM, Kubo A, Nishitani H. The effect of oral contrast on large bowel activity in FDG-PET/CT. Ann Nucl Med. 2005;19:101–108. doi: 10.1007/BF03027388. [DOI] [PubMed] [Google Scholar]
- 64.Boellaard R, Oyen WJ, Hoekstra CJ, et al. The Netherlands protocol for standardisation and quantification of FDG whole body PET studies in multi-centre trials. Eur J Nucl Med Mol Imaging. 2008;35:2320–2333. doi: 10.1007/s00259-008-0874-2. [DOI] [PubMed] [Google Scholar]
- 65.Paquet N, Albert A, Foidart J, Hustinx R. Within-patient variability of (18)F-FDG: standardized uptake values in normal tissues. J Nucl Med. 2004;45:784–788. [PubMed] [Google Scholar]
- 66.Hassan C, Pickhardt PJ, Laghi A, et al. Impact of whole-body CT screening on the cost-effectiveness of CT colonography. Radiology. 2009;251:156–165. doi: 10.1148/radiol.2511080590. [DOI] [PubMed] [Google Scholar]