Abstract
Psoriasis is a common disease with the population prevalence ranging from 2% to 3%. Its prevalence in the population is affected by genetic, environmental, viral, infectious, immunological, biochemical, endocrinological, and psychological factors, as well as alcohol and drug abuse. In the recent years, psoriasis has been recognised as a systemic disease associated with numerous multiorgan abnormalities and complications. Dyslipidemia is one of comorbidities in psoriatic patients. Lipid metabolism studies in psoriasis have been started at the beginning of the 20th century and are concentrated on skin surface lipids, stratum corneum lipids and epidermal phospholipids, serum lipids, dermal low-density lipoproteins in the psoriatic skin, lipid metabolism, oxidative stress and correlations between inflammatory parameters, lipid parameters and clinical symptoms of the disease. On the basis of the literature data, psoriasis can be described as an immunometabolic disease.
1. Introduction
Psoriasis is a common disease affecting, as presumed, approximately 120–180 million people worldwide [1]. Around 150,000 new cases of psoriasis are reported annually. There are fewer reports on the incidence of psoriasis, but in recent studies an increasing trend over the last 3 decades was shown [1, 2]. The population prevalence of psoriasis has been reported to range from 2% to 3%. However, in some countries there is a higher prevalence rate for psoriasis, for example in Kazakhstan, Trinidad and Tobago, Paraguay, Kenya, Tanzania, Egypt, and Kuwait [3]. Four hundred people die annually from psoriasis-related causes in the Unites States [1]. Psoriasis prevalence in the population is affected by genetic, environmental, viral, infectious, immunological, biochemical, endocrinological, and psychological (trauma, stress) factors as well as alcohol and drug abuse [4, 5].
In the recent years, psoriasis has been recognised as a systemic disease associated with numerous multiorgan abnormalities and complications. In psoriatic patients an increased risk of cardiovascular abnormalities, hypertension, dyslipidemia, atherosclerosis, diabetes mellitus type 2, obesity, chronic obturative pulmonary disease, cerebral stroke, osteoporosis, cancer, and depression was noticed [6–8].
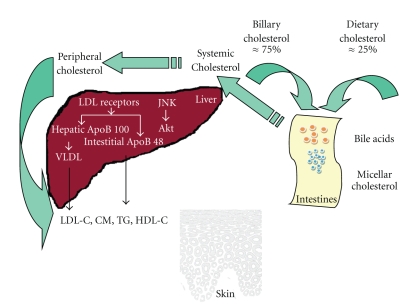
Lipid metabolism research studies in psoriasis have been started at the beginning of the 20th century from the quantitative analysis of serum cholesterol in psoriatic patients [9]. The abnormal fat metabolism was considered to be an important factor in the etiopathogenesis of psoriasis. Grütz and Burger examined the development of psoriatic skin manifestations as a symptom comparable to xanthomatosis [9, 10]. Melczer found changes in the composition of phospholipids in psoriatic foci and suggested that inflammation, congestion, and parakeratosis resulted from lipid deposition in the reticular-endothelial system [11]. It was also suggested that continuous separation of psoriatic scales caused the permanent loss of lipids which might affect serum lipid abnormalities [11, 12]. Lipid metabolism is a complex process which takes place in different human organs and peripheral blood (Figure 1) [13]. Its disturbances in psoriasis need further studies to be fully elucidated. There are some new methods for diagnosis of cholesterol in the healthy skin available; however its exact usefulness should be carefully recognised [14, 15].
Figure 1.
Cholesterol trafficking in human organism. CM: chylomicrones, HDL-C: high-density lipoproteins cholesterol, LDL-C: low-density lipoproteins cholesterol, VLDL-C: very low-density lipoproteins cholesterol, TG: triglycerides, JNK: Janus-family tyrosine kinase, and Akt: kinase Akt. The figure is adapted after permission from [13]. The complete electronic version of this article can be found online at: http://www.lipidworld.com/content/8/1/41.
Nowadays the studies are concentrated on the skin surface lipids, epidermal lipids (including stratum corneum lipids, and epidermal phospholipids), serum lipids, dermal low-density lipoproteins in the psoriatic skin, lipid metabolism, oxidative stress and correlations between inflammatory parameters, lipid parameters, and clinical symptoms of the disease (Figure 2) [10–12, 16–19]. The aim of this study is to present an update of the lipid studies in psoriasis on the basis of the literature review.
Figure 2.
Influence of the psoriasis associated dyslipidemia on human organs. This figure is based (after permission) on the figure from [19].
2. Skin Surface and Epidermal Lipids
The stratum corneum consists of corneocytes and intracellular lipids, mainly ceramides, sterols, and free fatty acids which form the barrier for diffusion of substances into the skin [20–23]. The lipids are organised into multilamellar intercellular membranes derived from glycerophospholipids, glucocerebrosides, sphingomyelin of the stratum granulosum-stratum corneum interface [23, 24]. Then the precursors are converted to ceramides and free fatty acids by the hydrolytic enzymes [25, 26]. In psoriasis, alterations in ceramide content have been demonstrated [27] and abnormal lipid structures reported [28]. Total lipids, phospholipids, triacylglycerols, and cholesterol were found to increase both in blood and in epidermis of psoriatic patients [29, 30]. The proportion of an esterified fraction decreased mainly in the normally appearing epidermis areas, especially in severe psoriasis [31]. In the gas liquid chromatography, significantly lower spectrum of short-chain fatty acids (SCFAs) levels were detected in both psoriatic and uninvolved areas [32]. The correlation was found between increased levels of free and total cholesterol as well as phospholipids in the epidermis and the severity of psoriasis [31, 32].
The main features of the corneous layer observed under the scanning electron microscope include widened intracellular spaces, lack of resistant intercellular junctions, impaired intracellular adhesion, which may result in markedly abnormal cholesterol homeostasis [33, 34]. In the lipid thin-layer chromatography, an increased amount of total phospholipids was found in the involved psoriatic epidermis whereas the decrease of phosphatidylserine and the increase of phosphatidylinositol were observed in psoriatic lesions and in the lesion-free epidermis [35].
Lacroix demonstrated significant amount of cholesterol in scaly plagues and in serum. He suggested that psoriasis might be the form of cholesterol elimination through the skin [9]. The regulation of cellular cholesterol metabolism is already fully developed in the foetal life. The maintenance of its steady cellular levels is an important element of cellular and systemic homeostasis. It is already known that this homeostasis is disturbed in psoriasis [10]. Every day about 85 mg of cholesterol is secreted through the healthy skin. In psoriasis, the patients lost daily 12–23.5-fold more lipids with the scales than healthy subjects [18, 36, 37].
3. Serum Lipids
Serum lipids levels were examined in many different groups of psoriatic patients in comparison to relevant healthy controls [9–11, 16, 18, 38–48]. The blood lipid results are considerably dependent on group matching (age, gender, and ethnic and cultural factors). In most of the studies, a statistically significant elevated level of total cholesterol (TC), low-density lipoprotein (LDL) cholesterol and/or triglycerides (TG) in psoriatic patients was demonstrated comparing to a healthy control group [11, 16, 18, 39, 40, 43–47, 49–52]. Moreover, there was a decrease of high density lipoprotein (HDL) cholesterol in the serum of psoriatic patients [43, 48, 50–53]. Only in a few studies no differences in lipid serum levels between psoriatic patients and healthy controls were observed [38, 42, 54].
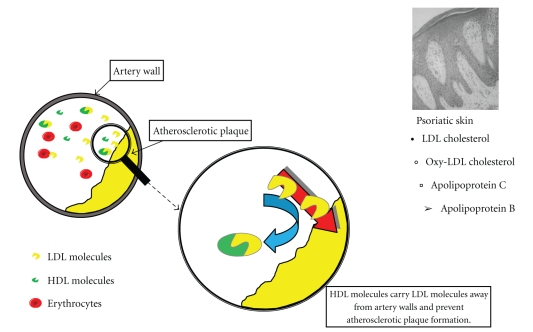
Nowadays there is an increased interest in HDL cholesterol, because clinical and epidemiological studies showed an inverse relationship between the level of HDL and the development of atherosclerosis [55]. HDL is a very important factor in reverse cholesterol transport (RCT). It takes part in the transport of cholesterol produced or accumulated in the peripheral tissues to the liver or other steroidogenic tissues and exerts the antioxidant, anti-inflammatory, antithrombotic and fibrinolytic action [55]. It should be underlined that neither HDL nor LDL is “bad cholesterol,” because both are essential for the proper transport of cholesterol (Figure 3).
Figure 3.
Lipoprotein replacement in circulation from artery walls and peripheral blood into psoriatic skin lesions. Based and modified with permission from figures from [56]. Available from http://www.biolsci.org/v05p0474.htm.
Results of the studies present a decrease of cholesterol and phospholipids levels connected with HDL fraction independently of psoriasis severity and duration [36]. In psoriasis, a decrease of HDL synthesis and HDL structural changes can be observed, due to various biochemical disturbances, such as abnormalities of receptor function, changes of hepatic structure and function, activity changes of hepatocyte membranes, impaired RCT, esterification, and lipases [36]. It can be hypothesised that HDL structural changes are caused by a decrease of cholesterol and phospholipids level as well as an increase of apolipoprotein A (apoA) concentration in the HDL coat. So far, all the studies were based on the quantitative evaluation of lipids in the psoriatic patient serum and epidermis. Further studies are needed to specify the role of disturbances of HDL structure and composition as well as connections between lipid abnormalities and the immune response in psoriasis.
The studies concerning the concentration of serum phospholipids in the psoriatic patients present different results. A decrease of concentration of total phospholipids, as well as phosphatidylethanolamine, lecithin, the lecithin : cholesterol ratio and linolenic acid, docosatetraenoic acid, docosapentaenoic acid, and docosahexaenoic acid in the serum was observed [57–61]. There was also an increased level of some fractions of serum phospholipids (e.g., lysolecithin) and palmitic acid, palmitoleic acid, and dihomo-γ-linolenic acid (DHLA) [57, 58, 62–64]. Some reports, however, do not present any differences in the level of serum phospholipids between psoriatic patients and healthy control group [65]. Our results did not show any statistically significant differences in the level of total phospholipids, but the decreasing tendency of its level was seen in both normolipidemic and hyperlipidemic patients [10].
4. Apolipoproteins
Apolipoproteins are the protein part of lipoproteins, and their composition is specific for each lipoprotein. They have a different molecular structure, amino acid composition, and antiatherosclerotic properties. In psoriatic patients, different results concerning apolipoproteins apoA1, apoB, and apoE were presented [16, 41, 66, 67]. Apolipoprotein A1 has been immunocytochemically detected at the psoriatic skin dermoepidermal junction, vascular walls, and the perivascular region of papillary dermis. Apolipoprotein B100 and apolipoprotein E were observed intracellularly both in normal epidermis and psoriatic epidermis, and they were also detected in parakeratotic regions in the horny layer [68].
ApoA1 plays the main part in the reverse cholesterol transport from the peripheral cells to the liver. Its decreased level has an influence on the higher risk of atherosclerosis development [69]. ApoA2 stabilizes the HDL structure and is considered as the lecithin : cholesterol acetyltransferase (LCAT) inhibitor. Its role concerning atherosclerosis is controversial, because it was shown that apoA1 impaired the inflow of cholesterol from adipocytes to the extracellular space [70]. Elevated levels of apolipoproteins A1 and A2 accompany the intake of alcohol. The level of apoA1 increases also in familiar hyperproteinemia, in pregnancy, during estrogen therapy, and during physical exercise.
Elevated levels of apolipoprotein B are associated with the increased risk of atherosclerosis, due to its role in the cholesterol accumulation in the endothelium, which initiates the atheromatous process. Apo B elevated levels are observed in the hyperlipidemia type IIa, IIb, IV, and V, in nephritic syndrome, pregnancy, familiar hyperapo-ß-lipoproteinemia, biliary obstruction, smokers, and dialyzed patients on treatment with diuretics ß-blockers, cyclosporine, or glucocorticoids [71].
Apolipoprotein C3 (apoC3) is suggested to inhibit lipoprotein lipase [72, 73] and hepatic triglyceride lipase [74], enzymes responsible for the clearance of triglyceride rich particles from the plasma. Furthermore, apoC3 was shown to inhibit the hepatic uptake of triglyceride rich particles [75]. Apo C3 also appears to interfere with HDL receptor-mediated uptake of lipoproteins. It is known that an increase in apoC3 levels induces the development of hypertriglyceridemia.
In most studies, elevated levels of apoA1, apoB [16, 43], apoC3, and apoE [41, 76–78] were detected in the serum of psoriatic patients compared to the healthy control group. However, there are also contrary results showing decreased levels of apolipoproteins [79]. Many authors did not show any differences in apoA1, apoA2, and apoB levels between psoriatic patients and the control group [10, 76, 80]. It was also reported that apoA1 sequestration in the inflamed tissues might lead to reduced HDL-C serum levels and thus increase the risk of cardiovascular disease in psoriatic patients [81].
Apolipoprotein E (ApoE) is a glycoprotein involved in the regulation of triglycerides and low-density lipoprotein (LDL) levels [67]. ApoE can modulate mitogen-activated T-lymphocyte proliferation in vitro and provides protection against some infections [82, 83]. The role of the apoE gene in psoriasis was suggested, because in psoriatic skin there is the downregulation of ApoE expression and the normalization of ApoE levels precedes clinical improvement [67]. Furthermore, in a Japanese population the epsilon 2 allele was found to be significantly more frequent in psoriatic patients than in controls, suggesting that there may be a relationship between these particular alleles and development of psoriasis [84]. It is believed that the epsilon 4 allele could be a risk factor for developing a severe form of psoriasis [85].
5. Oxidative Stress
Reactive oxygen species (ROSs) such as hydroxyl radical (HO∙), peroxyl radicals (ROO∙), superoxide anion (O2 ∙−),hydrogen peroxide (H2O2), nitrogen oxide (NO∙), and hypochlorous acid (HOCl) are constantly produced as a result of metabolic reactions in living systems [86]. Oxidative stress may be defined as an imbalance between cellular production of ROS and antioxidant defence mechanisms. It leads to oxidative damage of lipids and proteins contributing to barrier integrity, which is essential for healthy skin conditions [18, 87, 88]. The skin antioxidant system consists of a network of both enzymatic (glutathione peroxidase (GSH-Px), catalase (CAT), superoxide dismutase (SOD), and paraoxonase (PON1)) and nonenzymatic antioxidants. Nonenzymatic antioxidants (glutathione, β-carotene, ascorbic acid, and tocopherols) present in cells are regarded as protectors against the lipid peroxidation [88].
Increased production of oxygen metabolites, overwhelming the antioxidant capacity of the body, is an important feature in psoriasis [87]. Early and active psoriatic lesions are characterized by the intraepidermal penetration of activated polymorphonuclear leucocytes which leads to ROS production provided by NADPH oxidase and proteolytic enzymes [88]. The production of ROS can be indirectly assessed by the levels of lipid peroxidation products such as lipid hydroperoxide (LHP), malondialdehyde (MDA), oxidized low-density lipoprotein (ox-LDL), and thiobarbituric acid (TBA) [87]. Patients with psoriasis exhibit increased concentrations of MDA [51, 87, 89, 90] and ox-LDL [18] in the tissues and higher levels of TBA [43, 52, 87] and anti-ox-LDL autoantibody (AuAb-oxLDL) [50, 51, 87] in the blood. The lipid peroxidation markers were found significantly higher in the patients with severe or active psoriasis (PASI > 3) than in the patients with mild or inactive psoriasis (PASI < 3) [43]. The accumulation of ox-LDL was detected in the upper epidermis of the involved skin from psoriatic patients by direct immune-fluorescent method [18]. Ox-LDLs are able to initiate inflammation and to influence the adhesion of endothelial cells and on oxidant status of the blood vessels cells, which is important in the development of early atherogenesis [53]. They are also antigenic and can elicit an immune response with a generation of circulating antibodies AuAb-oxLDL and β2-GP1-dependent anticardiolipin antibodies (aCL), as a consequence of structural similarity between ox-LDL surface structure and β2-GP1-anionic phospholipid complex, the antigenic target for aCL [91]. The level of AuAb-oxLDL has been suggested to reflect the in vivo oxidation of LDL. The importance of AuAb-oxLDL in diseases such as myocardial infarct, atherosclerosis, diabetes mellitus, renal failure, systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), Behςet's disease, and psoriasis was suggested [51]. aCL level is increased in psoriatic patients. It could be a useful marker in predicting atherosclerosis risk, because it may promote atherosclerotic lesions [91]. In plasma and red blood cells (RBCs) of psoriatic patients, increased levels of MDA were observed which indicates an advanced peroxidative process in erythrocyte membranes. The increased peroxidation of lipid bilayer caused by a decrease of antioxidant enzyme activities may be the essential mechanism of the membrane fluidity decrease observed in association with the exacerbation of the disease [88, 89, 92]. The impaired antioxidant status is shown by decreased serum levels of erythrocyte SOD [51, 90] and GSH-Px activities [51, 90, 92, 93] of increased PON1 activity [54] and of increased [90] or decreased [51] serum CAT activity in patients with psoriasis. Nonenzymatic antioxidants were also decreased [51, 92, 93]. Changes in the elastase neutrophil ratio illustrating an increase in neutrophil function can be a marker of psoriasis [43]. In general, total antioxidant status (TAS) in psoriasis is reduced [43, 51], or there are no significant differences between patients and healthy controls [52, 54, 89].
A high serum total homocysteine (tHcy) level was observed in patients with psoriasis. The main mechanisms of hyperhomocysteinemia engaged in the development of atherothrombosis are endothelial injury, platelet activation, oxidative modification of low-density lipoproteins, and endothelial-leukocyte interactions [94, 95]. There was a positive relationship between an increased level of AuAb-oxLDL and plasma tHcy levels which may play an important role in development of atherothrombotic complications in psoriatic patients [96].
Oxidative stress may have a pivotal role in both therapeutic mechanisms and side effects induced by anthralin. Systemic antioxidant administration may provide an opportunity for therapeutic intervention against anthralin-associated toxicities [88]. Lipid peroxidation is the earliest response mediating activation of downstream signalling events in peripheral blood mononuclear cells (PBMCs) and keratinocytes by anthralin. It leads to the activation of c-jun-N-terminal kinase (JNK), event relevant for the regulation of cellular proliferation and apoptosis [97].
It is well known that phototherapy is recommended in the psoriasis treatment. However, both ultraviolet A and B radiation (UVA and UVB) apart from therapeutic and immunomodulating action induce production of ROS and increase lipid peroxidation [53]. There was a difference between the effect of phototherapy on lipid parameters in patients with mild or moderate psoriasis (PASI1 from 5.4 to 22.1; mean 15.2 ± 4.9) and severe psoriasis (PASI 2, PASI 22.5 to 49.2; mean 30.3 ± 5.8). Exacerbated skin manifestations of psoriasis are accompanied by an increase of dyslipidaemia and oxidation processes. Therefore patients with severe psoriasis are exposed to higher risk of atherosclerosis. PASI2 patients have higher level of AuAb-oxLDL than PASI1 patients. Phototherapy increased TC, LDL, and AuAb-oxLDL level in PASI1 patients. Level of ox-LDL was decreased after phototherapy in patients with severe psoriasis and it was accompanied by increase of ferric reducing ability of plasma (FRAP) and negative correlation with AuAb-oxLDL level. It can be explained by therapeutic action of phototherapy and reduction of inflammatory processes [53].
6. Peroxisome Proliferator-Activated Receptors (PPARs) and Liver X Receptors (LXRs)
The epidermis is a very active site of lipid metabolism, and all peroxisome proliferator-activated receptor (PPAR) and liver X receptor (LXR) isoforms are expressed in the epidermis. An increased expression of PPARβ/δ and a decreased expression of PPARα and PPARγ were observed in the lesional skin of patients with psoriasis and atopic dermatitis [98–100]. Since the prevalence of metabolic syndrome is increased in psoriasis [101], a combination of insulin resistance, obesity, or chronic inflammation may trigger the expression of PPARβ/δ, which in turn contributes to a nonterminated regenerative skin phenotype. This disease mechanism would be expected to be aggravated by acute inflammation, or stress via the induction of PPARβ/δ by TNFα and stress-activated kinase [102].
PPARs α, β/δ, γ, and LXRs α and β belong to the nuclear steroid hormone receptor superfamily, which are regulated by fatty acid derivatives capable of controlling lipid and lipoprotein metabolism, cell proliferation, differentiation, and apoptosis of various cell types, including keratinocytes and sebaceous gland cells. These receptors play also a role in cutaneous carcinogenesis [100].
An activation of PPARs and LXRs leads to stimulation of epidermal lipid synthesis, formation and secretion of lamellar bodies, and activation of enzymes required for the extracellular processing of lipids in the stratum corneum, resulting in the formation of lamellar membranes that mediate permeability barrier function. PPARγ activation appeared to have the least effect on epidermal lipid synthesis among the PPAR and LXR activators tested. PPARβ/δ is the key PPAR isoform involved in lamellar body formation and secretion as well as in lipid storage [103, 104].
PPAR-α can also modulate the inflammatory response by inhibiting cytokine secretion, maturation, and migration and the T-cell-stimulatory activity of the epidermal antigen-presenting cell, the Langerhans cell. This was associated with decreased levels of phosphorylated nuclear factor-κB (NF-κB) [105]. Moreover, PPAR-α activation induces antioxidant enzymes, such as catalase or SOD, which would reduce the oxidative stress and the activation of mediators of inflammatory response [88]. The anti-inflammatory role of PPARβ/δ and PPARγ in the skin is uncertain, but it is suggested that they downregulate inflammation. LXR activators have a potent anti-inflammatory activity in both the irritant and allergic contact models of cutaneous inflammation [106, 107]. These findings suggest the possibility of PPAR-α activators as novel nonsteroidal anti-inflammatory drugs in the topical treatment of common inflammatory diseases such as atopic dermatitis, psoriasis, acne, and photodermatitis. A great improvement of skin lesions and also of psoriatic arthritis had been initially documented in patients with psoriasis treated with the oral PPARγ activators troglitazone [108, 109] or pioglitazone [110–112]. In contrast, topical treatment of psoriatic skin with the PPAR activators tetradecylthioacetic acid and rosiglitazone did not show a significant effect [113, 114].
LXR and PPAR influence also the synthesis of cholesterol sulfate, which is a potent regulator of epidermal differentiation and corneocyte desquamation. The stimulation of both the cellular and extracellular components of the stratum corneum by PPARα and LXR activators results in the generation of a mature, functionally competent stratum corneum earlier in fetal development. Moreover, in a mouse model of epidermal hyperproliferation induced by repeated barrier disruption to the flank skin of hairless mice [115], topical PPARα activation inhibited proliferation and increased keratinocyte apoptosis. The activation of PPARα in the epidermis decreases keratinocyte proliferation. The absence of PPARβ/δ leads to increased keratinocyte proliferation and under some experimental conditions PPARβ/δ activators inhibit keratinocyte proliferation. It has been demonstrated that activation of PPARβ/δ induces endothelial cell proliferation and angiogenesis [116]. It was suggested that in the hyperproliferative epidermis of psoriatic skin, PPARβ/δ overexpression mediates keratinocyte proliferation via NF-κB [98]. The proliferative state of the keratinocytes may determine the effect of PPARγ activation on keratinocyte proliferation. A proapoptotic effect of PPARγ in T cells has been observed [117], and activation of PPARγ has an inhibitory effect on psoriasis, whereas this is not the case with PPARβ/δ activation [118, 119]. In LXRs deficient mice, thinning of the epidermis was observed [120].
7. Cardiovascular Disease (CVD)
In patients with psoriasis one observes an increased risk of cardiovascular disease which can be explained by several possible biological factors [6, 121–125]. Psoriasis is associated with traditional risk factors of CVD such as increased BMI, hyperlipidemia, hypertension, type 2 diabetes mellitus, and cigarette smoking [124–126]. Obesity has been shown to be an independent risk factor for the development of psoriasis and is also associated with more severe psoriasis and cardiovascular complications [125]. The persistent skin inflammation may contribute to a dyslipidemia and premature atherosclerosis [126, 127]. The duration of disease and its severity are related to the incidence of cardiovascular diseases, such as myocardial infarction, coronary artery disease and stroke [16, 38–40, 43–47, 54, 101, 121, 122, 127–135]. In psoriatic patients, lipid abnormalities are correlated with increased mortality due to myocardial infarction and stroke [129, 134]. Elevated level of C-reactive protein (CRP) is a risk factor for CVD and it can predict long-term risk for cardiovascular events [136]. The treatment for psoriasis such as retinoids and cyclosporine may be also responsible for initiation of hyperlipidemia which can promote future CVD [137–141]. Methotrexate use is associated with hyperhomocysteinemia, also a risk factor for cardiovascular disease [142].
There was a strong association observed between arterial stiffness, which is endothelial dysfunction marker, and the risk of cardiovascular events. Pulse wave velocity (PWV) is the gold standard measurement of arterial stiffness and in the patients with psoriasis and psoriatic arthritis an increased femoral-carotid PWV was observed [127, 143]. There were also functional and structural changes in the myocardium, changes in electrocardiographic activity, such as increased P wave dispersion, and structural changes in coronary vessels in psoriatic patients (Figure 4) [7, 19, 144].
Figure 4.
Psoriasis and cardiovascular abnormalities. This figure is a modified one (after permission) from [19].
8. NTproBNP
In recent years, the probable usefulness of NTproBNP as a biomarker of heart failure (HF) has been established. There was a positive correlation observed between NT-pro BNP in blood serum of psoriatic patients and heart diseases as well as acceptation of the disease [145].
9. Lipid and Immunologic Abnormalities
In psoriasis, the association between lipid and immunologic abnormalities was observed, that is why the disease could be described as an immunometabolic syndrome [128, 146]. Psoriasis is a chronic inflammation characterized by increased Th-1 and Th-17 T cell activity [128]. The significant role of cytokines, such as TNF-α, IL-6, IL-8, IFN-gamma, IL-1, and IL-17 in the generation of proatheromatous abnormalities (dyslipidemia, insulin resistance, endothelial dysfunction, clotting system activation, and pro-oxidative stress) was reported [127, 128, 146, 147]. TNF-α is a potent activator of c-Jun amino-terminal kinase,which stimulates the main regulator of proinflammatory activity protein-1 and is connected with obesity [128]. TNF-α can also lead to insulin resistance by inhibiting phosphorylation of insulin receptor tyrosine and of insulin receptor substrate 1. Treatment with TNF-α inhibitors affects the increase of HDL level [128]; in particular, TNF may affect endothelium dysfunction by decreasing the levels of nitric oxide synthase and cyclooxygenase 1 [127].
10. Effects of Antipsoriatics and Hypolipemic Drugs on Psoriasis
Antipsoriatic drugs can be also responsible for the lipid profile disturbances in psoriatic patients, because of their action on the circulating lipids [148–156]. Retinoids have the most potent activity on increasing the levels of triglycerides, total cholesterol, LDL cholesterol, and VLDL cholesterol and simultaneously decreasing the HDL fraction [137–140]. There are some reports that the diet enriched with fish oil can reduce side effects of these drugs [157, 158]. Cyclosporin has milder effects on the lipid profile, but it can also lead to some abnormalities for example TG elevation [159]. TNF-α inhibitors can cause an increase of serum triglyceride levels, but they have beneficial effects on the increase of HDL level and are able to decrease blood insulin levels [141, 160–162].
Hyperlipidemia is treated with statins which effectively reduce CRP and TNF-α levels as well as decrease levels of low-density lipoproteins and alleviate the arterial stiffness. Statins also downregulate adhesion molecules such as LFA-1 and ICAM-1 on leukocytes and endothelial cells which are essential in leukocyte activation, leukocyte migration to inflammatory sites, and immunologic cytotoxicity [163]. Statins have the inhibiting action on the expression of MHC II molecules, chemokine receptors on Th-1 cell and the production of NO [163]. These drugs are generally beneficial for psoriatic patients and reduce the risk of cardiovascular diseases. However, there was also a case of exacerbation of psoriasis after the treatment with three different statins and bezafibrate [164]. Fibrates, used to decrease cholesterol levels, may also affect rapid and acute development of clinical symptoms of psoriasis.
11. Summary
The lipid disturbances are recognised as a very important part in the pathogenesis of psoriasis. The results of the majority of the studies are coherent and indicate that the increased total cholesterol, LDL cholesterol and/or triglycerides, and decreased HDL cholesterol in psoriatic patients' serum the composition of apolipoproteins, and increased production of oxygen metabolites are features of the metabolic syndrome. These factors have also a great impact on some comorbidities observed in psoriatic patients especially on cardiovascular diseases. These lipid disturbances are also connected with immunological abnormalities, that is why psoriasis could be classified as an immunometabolic disease. In spite of the intensive investigations, the explanation of the steps of disease mechanisms in psoriasis have not been recognised so far. On the basis the literature data, further studies should be designed to connect the lipid and immunological disturbances.
The review of the last years suggests an introduction of some new therapeutic methods for psoriatic patients as for example statins. Their immunomodulatory activities like influence on T cells and antigen presenting cells function, influence on leukocyte adhesion and endothelial cell function are discussed. In many papers the importance of reduction of animal fat, introduction of fish and plant oil, preparations with the omega-6 and omega-3 fatty acids as well as BMI reduction, prevention of obesity and quitting addictions were suggested.
References
- 1.Icen M, Crowson CS, McEvoy MT, Dann FJ, Gabriel SE, Kremers HM. Trends in incidence of adult-onset psoriasis over three decades: a population-based study. Journal of the American Academy of Dermatology. 2009;60(3):394–401. doi: 10.1016/j.jaad.2008.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson FC, Icen M, Crowson CS, McEvoy MT, Gabriel SE, Kremers HM. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population-based study. Arthritis Care and Research. 2009;61(2):233–239. doi: 10.1002/art.24172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chandran V, Raychaudhuri SP. Geoepidemiology and environmental factors of psoriasis and psoriatic arthritis. Journal of Autoimmunity. 2010;34(3):J314–J321. doi: 10.1016/j.jaut.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Romanowska M, Al Yacoub N, Seidel H, et al. PPARδ enhances keratinocyte proliferation in psoriasis and induces heparin-binding EGF-like growth factor. Journal of Investigative Dermatology. 2008;128(1):110–124. doi: 10.1038/sj.jid.5700943. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y-J, Shen J-L, Wu C-Y, Chang Y-T, Chen C-M, Lee F-Y. Elevated plasma osteopontin level is associated with occurrence of psoriasis and is an unfavorable cardiovascular risk factor in patients with psoriasis. Journal of the American Academy of Dermatology. 2009;60(2):225–230. doi: 10.1016/j.jaad.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 6.Nijsten T, Wakkee M. Complexity of the association between psoriasis and comorbidities. Journal of Investigative Dermatology. 2009;129(7):1601–1603. doi: 10.1038/jid.2009.55. [DOI] [PubMed] [Google Scholar]
- 7.Pietrzak A, Janowski K, Łopatynski J, et al. Psoriasis and heart. Something new under the sun. Giornale Italiano di Dermatologia e Venereologia. 2006;141(5):457–463. [Google Scholar]
- 8.Pietrzak A, Lecewicz-Toruń B, Kądziela-Wypyska G. Changes in the digestive system in patients suffering from psoriasis. Annales Universitatis Mariae Curie-Sklodowska. Sectio D. 1998;53:187–194. [PubMed] [Google Scholar]
- 9.Chibowska M. Role of serum lipids in pseriasis. Przeglad Dermatologiczny. 1970;57(2):255–260. [PubMed] [Google Scholar]
- 10.Pietrzak A, Toruniowa B, Pietrzak B, Chwaluk J. Lipid profile in psoriatic patients according to sex and age. Przeglad Dermatologiczny. 1994;81(5):441–449. [Google Scholar]
- 11.Pietrzak A, Jastrzebska I, Krasowska D, et al. Serum pancreatic lipase [EC 3.1.1.3] activity, serum lipid profile and peripheral blood dendritic cell populations in normolipidemic males with psoriasis. Journal of Molecular Catalysis B. 2006;40(3-4):144–154. [Google Scholar]
- 12.Wilkinson DI. Lipid metabolism in psoriasis. In: Farber EM, Cox AJ, Jacobs PH, editors. Proceedings of the International Symposium on Psoriasis. Stanford, Calif, USA: Stanford University Press; 1971. pp. 277–287. [Google Scholar]
- 13.Yamaoka-Tojo M, Tojo T, Kosugi R, et al. Effects of ezetimibe add-on therapy for high-risk patients with dyslipidemia. Lipids in Health and Disease. 2009;8:p. 41. doi: 10.1186/1476-511X-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zawydiwski R, Sprecher DL, Evelegh MJ, Horsewood P, Carte C, Patterson M. A novel test for the measurement of skin cholesterol. Clinical Chemistry. 2001;47(7):1302–1304. [PubMed] [Google Scholar]
- 15.Stein JH, Tzou WS, DeCara JM, et al. Usefulness of increased skin cholesterol to identify individuals at increased cardiovascular risk (from the predictor of advanced subclinical atherosclerosis study) American Journal of Cardiology. 2008;101(7):986–991. doi: 10.1016/j.amjcard.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 16.Mallbris L, Granath F, Hamsten A, Ståhle M. Psoriasis is associated with lipid abnormalities at the onset of skin disease. Journal of the American Academy of Dermatology. 2006;54(4):614–621. doi: 10.1016/j.jaad.2005.11.1079. [DOI] [PubMed] [Google Scholar]
- 17.Ishikawa H, Sato H. Demonstration of beta-lipoproteins in the psoriatic skin by immunofluorescent technique. Archiv für Dermatologische Forschung. 1974;30(8):191–206. doi: 10.1007/BF00604821. [DOI] [PubMed] [Google Scholar]
- 18.Tekin NS, Tekin IO, Barut F, Sipahi EY. Accumulation of oxidized low-density lipoprotein in psoriatic skin and changes of plasma lipid levels in psoriatic patients. Mediators of Inflammation. 2007;2007:5 pages. doi: 10.1155/2007/78454. Article ID 78454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pietrzak A, Chodorowska G, Szepietowski J, Zalewska-Janowska A, Krasowska D, Hercogová J. Psoriasis and serum lipid abnormalities. Dermatologic Therapy. 2010;23(2):160–173. doi: 10.1111/j.1529-8019.2010.01311.x. [DOI] [PubMed] [Google Scholar]
- 20.Jungersted JM, Hellgren LI, Jemec GBE, Agner T. Lipids and skin barrier function—a clinical perspective. Contact Dermatitis. 2008;58(5):255–262. doi: 10.1111/j.1600-0536.2008.01320.x. [DOI] [PubMed] [Google Scholar]
- 21.Bleck O, Abeck D, Ring J, et al. Two ceramide subfractions detectable in Cer(AS) position by HPTLC in skin surface lipids of non-lesional skin of atopic eczema. Journal of Investigative Dermatology. 1999;113(6):894–900. doi: 10.1046/j.1523-1747.1999.00809.x. [DOI] [PubMed] [Google Scholar]
- 22.Landmann L. Epidermal permeability barrier: transformation of lamellar granule-disks into intercellular sheets by a membrane-fusion process, a freeze-fracture study. Journal of Investigative Dermatology. 1986;87(2):202–209. doi: 10.1111/1523-1747.ep12695343. [DOI] [PubMed] [Google Scholar]
- 23.Elias PM, Menon GK. Structural and lipid biochemical correlates of the epidermal permeability barrier. Advances in Lipid Research. 1991;24:1–26. doi: 10.1016/b978-0-12-024924-4.50005-5. [DOI] [PubMed] [Google Scholar]
- 24.Grayson S, Elias PM. Isolation and lipid biochemical characterization of stratum corneum membrane complexes: implications for the cutaneous permeability barrier. Journal of Investigative Dermatology. 1982;78(2):128–135. doi: 10.1111/1523-1747.ep12505953. [DOI] [PubMed] [Google Scholar]
- 25.Man MQM, Feingold KR, Thornfeldt CR, Elias PM. Optimization of physiological lipid mixtures for barrier repair. Journal of Investigative Dermatology. 1996;106(5):1096–1101. doi: 10.1111/1523-1747.ep12340135. [DOI] [PubMed] [Google Scholar]
- 26.Holleran WM, Feingold KR, Man MQM, Gao WN, Lee JM, Elias PM. Regulation of epidermal sphingolipid synthesis by permeability barrier function. Journal of Lipid Research. 1991;32(7):1151–1158. [PubMed] [Google Scholar]
- 27.Motta S, Monti M, Sesana S, Mellesi L, Ghidoni R, Caputo R. Abnormality of water barrier function in psoriasis: role of ceramide fractions. Archives of Dermatology. 1994;130(4):452–456. [PubMed] [Google Scholar]
- 28.Ghadially R, Reed JT, Elias PM. Stratum corneum structure and function correlates with phenotype in psoriasis. Journal of Investigative Dermatology. 1996;107(4):558–564. doi: 10.1111/1523-1747.ep12582813. [DOI] [PubMed] [Google Scholar]
- 29.Khyshiktuev BS, Falko EV. Alterations in the parameters of lipid metabolism in different biological objects in psoriatic patients during exacerbation and remission. Vestnik Dermatologii i Venerologii. 2005;6:40–43. [Google Scholar]
- 30.Ansidei V, Binazzi M, Cantelmi A, Gaiti A, Porcellati G. Phospholipid involvement in psoriatic epidermis. Italian Journal of Biochemistry. 1981;30(1):40–45. [PubMed] [Google Scholar]
- 31.Fortinskaia ES, Torkhovskaia TI, Sharapova GI, Loginova TK, Kliuchnikova ZI, Khalilov EM. Features of distribution of free and esterified cholesterol in the epidermis, biological membranes and plasma lipoproteins in psoriasis. Klinicheskaia Laboratornaia Diagnostika. 1996;(4):38–43. [PubMed] [Google Scholar]
- 32.Khyshiktuyev BS, Karavayeva TM, Falko YeV. Variability of quantitative changes in short-chain fatty acids in the serum and epidermis in psoriasis. Klinichescheskaya Laboratornaya Diagnostika. 2008;(8):22–24. [PubMed] [Google Scholar]
- 33.Maxfield FR, Tabas I. Role of cholesterol and lipid organization in disease. Nature. 2005;438(7068):612–621. doi: 10.1038/nature04399. [DOI] [PubMed] [Google Scholar]
- 34.Orfanos CE. Scanning electron microscopy in psoriasis. In: Farber EM, Cox AJ, Jacobs PH, editors. Proceedings of the International Symposium on Psoriasis. Stanford, Calif, USA: Stanford University Press; 1971. pp. 169–185. [Google Scholar]
- 35.Tsambaos D, Kalofoutis A, Stratigos J. Thin-layer chromatography of phospholipid components of normal and psoriatic epidermis. British Journal of Dermatology. 1977;97(2):135–138. doi: 10.1111/j.1365-2133.1977.tb15058.x. [DOI] [PubMed] [Google Scholar]
- 36.Wakkee M. Psoriasis: comorbidity and treatment. Rotterdam, The Netherlands: Erasmus University Rotterdam; 2010. Doctoral thesis. [Google Scholar]
- 37.Ponec M, Havekes L, Kempenaar J, Vermeer BJ. Cultured human skin fibroblasts and keratinocytes: differences in the regulation of cholesterol synthesis. Journal of Investigative Dermatology. 1983;81(2):125–130. doi: 10.1111/1523-1747.ep12542979. [DOI] [PubMed] [Google Scholar]
- 38.Farshchian M, Zamanian A, Farshchian M, Monsef A-R, Mahjub H. Serum lipid level in Iranian patients with psoriasis. Journal of the European Academy of Dermatology and Venereology. 2007;21(6):802–805. doi: 10.1111/j.1468-3083.2006.02099.x. [DOI] [PubMed] [Google Scholar]
- 39.Gisondi P, Tessari G, Conti A, et al. Prevalence of metabolic syndrome in patients with psoriasis: a hospital-based case-control study. British Journal of Dermatology. 2007;157(1):68–73. doi: 10.1111/j.1365-2133.2007.07986.x. [DOI] [PubMed] [Google Scholar]
- 40.Cohen AD, Sherf M, Vidavsky L, Vardy DA, Shapiro J, Meyerovitch J. Association between psoriasis and the metabolic syndrome: a cross-sectional study. Dermatology. 2008;216(2):152–155. doi: 10.1159/000111512. [DOI] [PubMed] [Google Scholar]
- 41.Tam L-S, Tomlinson B, Chu TT-W, et al. Cardiovascular risk profile of patients with psoriatic arthritis compared to controls—the role of inflammation. Rheumatology. 2008;47(5):718–723. doi: 10.1093/rheumatology/ken090. [DOI] [PubMed] [Google Scholar]
- 42.Ferretti G, Simonetti O, Offidani AM, et al. Changes of plasma lipids and erythrocyte membrane fluidity in psoriatic children. Pediatric Research. 1993;33(5 I):506–509. doi: 10.1203/00006450-199305000-00017. [DOI] [PubMed] [Google Scholar]
- 43.Rocha-Pereira P, Santos-Silva A, Rebelo I, Figueiredo A, Quintanilha A, Teixeira F. Dislipidemia and oxidative stress in mild and in severe psoriasis as a risk for cardiovascular disease. Clinica Chimica Acta. 2001;303(1-2):33–39. doi: 10.1016/s0009-8981(00)00358-2. [DOI] [PubMed] [Google Scholar]
- 44.Akhyani M, Ehsani AH, Robati RM, Robati AM. The lipid profile in psoriasis: a controlled study. Journal of the European Academy of Dermatology and Venereology. 2007;21(10):1330–1332. doi: 10.1111/j.1468-3083.2007.02260.x. [DOI] [PubMed] [Google Scholar]
- 45.Javidi Z, Meibodi NT, Nahidi Y. Serum lipids abnormalities and psoriasis. Indian Journal of Dermatology. 2007;52(2):89–92. [Google Scholar]
- 46.Amin T, Saied E, Abdou SH. Atherosclerotic risk in psoriasis. Journal of Pan-Arab League of Dermatologists. 2005;16(2):39–45. [Google Scholar]
- 47.Bajaj DR, Mahesar SM, Devrajani BR, Iqbal MP. Lipid profile in patients with psoriasis presenting at Liaquat University Hospital Hyderabad. Journal of the Pakistan Medical Association. 2009;59(8):512–515. [PubMed] [Google Scholar]
- 48.Reynoso-von Drateln C, Martínez-Abundis E, Balcázar-Muñoz BR, Bustos-Saldaña R, González-Ortiz M. Lipid profile, insulin secretion, and insulin sensitivity in psoriasis. Journal of the American Academy of Dermatology. 2003;48(6):882–885. doi: 10.1067/mjd.2003.446. [DOI] [PubMed] [Google Scholar]
- 49.Piskin S, Gurkok F, Ekuklu G, Senol M. Serum lipid levels in psoriasis. Yonsei Medical Journal. 2003;44(1):24–26. doi: 10.3349/ymj.2003.44.1.24. [DOI] [PubMed] [Google Scholar]
- 50.Örem A, Çimşit G, Değer O, Örem C, Vanizor B. The significance of autoantibodies against oxidatively modified low-density lipoprotein (LDL) in patients with psoriasis. Clinica Chimica Acta. 1999;284(1):81–88. doi: 10.1016/s0009-8981(99)00062-5. [DOI] [PubMed] [Google Scholar]
- 51.Vanizor Kural B, Örem A, Çimşit GU, Yandi YE, Calapoǧlu M. Evaluation of the atherogenic tendency of lipids and lipoprotein content and their relationships with oxidant-antioxidant system in patients with psoriasis. Clinica Chimica Acta. 2003;328(1-2):71–82. doi: 10.1016/s0009-8981(02)00373-x. [DOI] [PubMed] [Google Scholar]
- 52.Coimbra S, Oliveira H, Reis F, et al. Circulating levels of adiponectin, oxidized LDL and C-reactive protein in Portuguese patients with psoriasis vulgaris, according to body mass index, severity and duration of the disease. Journal of Dermatological Science. 2009;55(3):202–204. doi: 10.1016/j.jdermsci.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 53.Hadas E, Bożek A, Jarząb J. Impact of phototherapy on selected lipid profile indices in psoriatic patients allowing for intensification of the disease. Postępy Dermatologii i Alergologii. 2007;24(5):215–223. [Google Scholar]
- 54.Toker A, Kadi M, Yildirim AK, Aksoy H, Akçay F. Serum lipid profile paraoxonase and arylesterase activities in psoriasis. Cell Biochemistry and Function. 2009;27(3):176–180. doi: 10.1002/cbf.1553. [DOI] [PubMed] [Google Scholar]
- 55.Kuliszkiewicz-Janus M, Mohamed AS, Abod N. The biology of HDL lipoprotein and its antisclerotic activity. Postepy Higieny i Medycyny Doświadczalnej. 2006;60:307–315. [PubMed] [Google Scholar]
- 56.Daniels TF, Killinger KM, Michal JJ, Wright RW, Jr., Jiang Z. Lipoproteins, cholesterol homeostasis and cardiac health. International Journal of Biological Sciences. 2009;5(5):474–488. doi: 10.7150/ijbs.5.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Niestierienko GB, Kogon GH, Ulanowska FD. Lipidnyj obmien u bolnych sizmienieniami kostno-sustawnowo apparata pri psoriaticzieskoj boliezni. Vestnik Dermatologii i Venerologii. 1971;6:p. 31. [Google Scholar]
- 58.Wilienczik BT. Frakcji fosfolipidow syworotki krowi bolnych psoriazom i egziemoj. Vestnik Dermatologii i Venerologii. 1971;6:p. 71. [Google Scholar]
- 59.Panfilova TS, Tananova GV. Lipid metabolic indices of children with psoriasis. Vestnik Dermatologii i Venerologii. 1983;(6):60–61. [PubMed] [Google Scholar]
- 60.Vahlquist C, Berne B, Boberg M. The fatty-acid spectrum in plasma and adipose tissue in patients with psoriasis. Archives of Dermatological Research. 1985;278(2):114–119. doi: 10.1007/BF00409217. [DOI] [PubMed] [Google Scholar]
- 61.Iwanowa IP, Mariejewa TE. Naruszienija pierieisnowo okislienija lipidow aktiwnosti lizosomalnych gidrolaz i ich korriekcja u bolnych psoriazom. Vestnik Dermatologii i Venerologii. 1987;4:p. 26. [Google Scholar]
- 62.Chibowska M. Biochemical studies of serum lipids and histochemical studies on lipids of the soft palate mucosa in palatal lipidophilia and psoriasis with associated prelipidophilia. Lublin, Poland: Klinika Dermatologii Akademii Medycznej w Lublinie; 1967. Doctoral thesis. [PubMed] [Google Scholar]
- 63.Dowzanski SI, Szierstniewa WN, Graszkina IG. Sierdieczno sosudiataja sistiema i lipidnyjobmien u bolnych psoriazom. Vestnik Dermatologii i Venerologii. 1982;17 [Google Scholar]
- 64.Mingrone G, Greco AV, Venier A, Peruzzi E, Serri F. Lipid synthesis from the liver in patients with psoriasis. Archives of Dermatological Research. 1980;268(3):271–276. doi: 10.1007/BF00404288. [DOI] [PubMed] [Google Scholar]
- 65.Brenner S, Krakowski A, Levtov O, Heldenberg D, Werbin B, Tamir I. Serum lipids in patients with psoriasis. Dermatologica. 1975;150:p. 196. doi: 10.1159/000251409. [DOI] [PubMed] [Google Scholar]
- 66.Campalani E, Allen MH, Fairhurst D, et al. Apolipoprotein E gene polymorphisms are associated with psoriasis but do not determine disease response to acitretin. British Journal of Dermatology. 2006;154(2):345–352. doi: 10.1111/j.1365-2133.2005.06950.x. [DOI] [PubMed] [Google Scholar]
- 67.Karpouzis A, Caridha R, Tripsianis G, Michailidis C, Martinis G, Veletza SV. Apolipoprotein e gene polymorphism in psoriasis. Archives of Dermatological Research. 2009;301(6):405–410. doi: 10.1007/s00403-009-0968-0. [DOI] [PubMed] [Google Scholar]
- 68.Miyauchi H. Immunohistochemical study for the localization of apolipoprotein AI, B100, and E in normal and psoriatic skin. Igaku Kenkyu. Acta Medica. 1991;61(2):79–86. [PubMed] [Google Scholar]
- 69.Frank PG, Marcel YL. Apolipoprotein A-I: structure-function relationships. Journal of Lipid Research. 2000;41(6):853–872. [PubMed] [Google Scholar]
- 70.Barbaras R, Puchois P, Fruchart J-C, Ailhaud G. Cholesterol efflux from cultured adipose cells is mediated by LpAI particles but not by LpAI:AII particles. Biochemical and Biophysical Research Communications. 1987;142(1):63–69. doi: 10.1016/0006-291x(87)90451-7. [DOI] [PubMed] [Google Scholar]
- 71.Blanche PJ, Gong EL, Forte TM, Nichols AV. Characterization of human high-density lipoproteins by gradient gel electrophoresis. Biochimica et Biophysica Acta. 1981;665(3):408–419. doi: 10.1016/0005-2760(81)90253-8. [DOI] [PubMed] [Google Scholar]
- 72.Brown WV, Baginsky ML. Inhibition of lipoprotein lipase by an apoprotein of human very low density lipoprotein. Biochemical and Biophysical Research Communications. 1972;46(2):375–382. doi: 10.1016/s0006-291x(72)80149-9. [DOI] [PubMed] [Google Scholar]
- 73.Krauss RM, Herbert PN, Levy RI, Fredrickson DS. Further observations on the activation and inhibition of lipoprotein lipase by apolipoproteins. Circulation Research. 1973;33(4):403–411. doi: 10.1161/01.res.33.4.403. [DOI] [PubMed] [Google Scholar]
- 74.Kinnunen PKJ, Ehnholm C. Effect of serum and C apoproteins from very low density lipoproteins on human postheparin plasma hepatic lipase. FEBS Letters. 1976;65(3):354–357. doi: 10.1016/0014-5793(76)80145-7. [DOI] [PubMed] [Google Scholar]
- 75.Windler E, Havel RJ. Inhibitory effects of C apolipoproteins from rats and humans on the uptake of triglyceride-rich lipoproteins and their remnants by the perfused rat liver. Journal of Lipid Research. 1985;26(5):556–565. [PubMed] [Google Scholar]
- 76.Pietrzak A, Kądzielewski J, Janowski K, et al. Lipoprotein (a) in patients with psoriasis: associations with lipid profiles and disease severity. International Journal of Dermatology. 2009;48(4):379–387. doi: 10.1111/j.1365-4632.2009.03994.x. [DOI] [PubMed] [Google Scholar]
- 77.Xiao T, Yang C, Xiao Y, Song F. Serum apolipoprotein levels of psoriatic patients with normal serum lipid levels. Chinese Medical Sciences Journal. 1997;12(4):224–228. [PubMed] [Google Scholar]
- 78.Seishima M, Seishima M, Mori S, Noma A. Serum lipid and apolipoprotein levels in patients with psoriasis. British Journal of Dermatology. 1994;130(6):738–742. doi: 10.1111/j.1365-2133.1994.tb03411.x. [DOI] [PubMed] [Google Scholar]
- 79.Barba A, Schena D, Ferrari S, Grigolini L. Observations on lipid metabolism in patients affected with psoriasis. Preliminary findings. Giornale Italiano di Dermatologia e Venereologia. 1987;122(3):85–89. [PubMed] [Google Scholar]
- 80.Leren TP, Maartmann-Moe K, Thune P, Berg K. Low density lipoprotein receptors in cultured skin fibroblasts from psoriasis patients. Clinical Genetics. 1984;25(3):230–241. doi: 10.1111/j.1399-0004.1984.tb01983.x. [DOI] [PubMed] [Google Scholar]
- 81.Oliveiro F, Sfriso P, Baldo G, et al. Apolipoprotein A-I and cholesterol in synovial fluid of patients with rheumatoid arthritis, psoriatic arthritis and osteoarthritis. Clinical and Experimental Rheumatology. 2009;27(1):79–83. [PubMed] [Google Scholar]
- 82.Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240(4852):622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 83.Mahley RW, Rall SC., Jr. Apolipoprotein E: far more than a lipid transport protein. Annual Review of Genomics and Human Genetics. 2000;1(2000):507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- 84.Furumoto H, Nakamura K, Imamura T, et al. Association of apolipoprotein allele ε2 with psoriasis vulgaris in Japanese population. Archives of Dermatological Research. 1997;289(9):497–500. doi: 10.1007/s004030050229. [DOI] [PubMed] [Google Scholar]
- 85.Coto-Segura P, Coto E, Alvarez V, Santos-Juanes J. Apolipoprotein ε4 allele is associated with psoriasis severity: reply. Archives of Dermatological Research. 2010;302(3):237–238. doi: 10.1007/s00403-010-1031-x. [DOI] [PubMed] [Google Scholar]
- 86.Geronikaki AA, Gavalas AM. Antioxidants and inflammatory disease: synthetic and natural antioxidants with anti-inflammatory activity. Combinatorial Chemistry and High Throughput Screening. 2006;9(6):425–442. doi: 10.2174/138620706777698481. [DOI] [PubMed] [Google Scholar]
- 87.Rashmi R, Rao KSJ, Basavaraj KH. A comprehensive review of biomarkers in psoriasis. Clinical and Experimental Dermatology. 2009;34(6):658–663. doi: 10.1111/j.1365-2230.2009.03410.x. [DOI] [PubMed] [Google Scholar]
- 88.Briganti S, Picardo M. Antioxidant activity, lipid peroxidation and skin diseases. What’s new. Journal of the European Academy of Dermatology and Venereology. 2003;17(6):663–669. doi: 10.1046/j.1468-3083.2003.00751.x. [DOI] [PubMed] [Google Scholar]
- 89.Nassiri S, Malekzad F, Sarlak M, et al. Interplay among antioxidants and oxidants in psoriasis. Iranian Journal of Dermatology. 2009;12:56–59. [Google Scholar]
- 90.Yildirim M, Inaloz HS, Baysal V, Delibas N. The role of oxidants and antioxidants in psoriasis. Journal of the European Academy of Dermatology and Venereology. 2003;17(1):34–36. doi: 10.1046/j.1468-3083.2003.00641.x. [DOI] [PubMed] [Google Scholar]
- 91.Campanati A, Simonetti O, Silvestri B, et al. Anticardiolipin antibodies expression in psoriasis. Giornale Italiano di Dermatologia e Venereologia. 2004;139(3):165–170. [Google Scholar]
- 92.Kökçam I, Nazıroǧlu M. Antioxidants and lipid peroxidation status in the blood of patients with psoriasis. Clinica Chimica Acta. 1999;289(1-2):23–31. doi: 10.1016/s0009-8981(99)00150-3. [DOI] [PubMed] [Google Scholar]
- 93.Naziroglu M, Kokcam I. Antioxidants and lipid peroxidation status in the blood of patients with alopecia. Cell Biochemistry and Function. 2000;18(3):169–173. doi: 10.1002/1099-0844(200009)18:3<169::AID-CBF870>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 94.Lynch SM, Campione AL, Moore MK. Plasma thiols inhibit hemin-dependent oxidation of human low-density lipoprotein. Biochimica et Biophysica Acta. 2000;1485(1):11–22. doi: 10.1016/s1388-1981(00)00030-5. [DOI] [PubMed] [Google Scholar]
- 95.Thambyrajah J, Townend JN. Homocysteine and atherothrombosis—mechanisms for injury. European Heart Journal. 2000;21(12):967–974. doi: 10.1053/euhj.1999.1914. [DOI] [PubMed] [Google Scholar]
- 96.Vanizor Kural B, Örem A, Çimşit G, Uydu HA, Yandi YE, Alver A. Plasma homocysteine and its relationships with atherothrombotic markers in psoriatic patients. Clinica Chimica Acta. 2003;332(1-2):23–30. doi: 10.1016/s0009-8981(03)00082-2. [DOI] [PubMed] [Google Scholar]
- 97.Peus D, Beyerle A, Rittner HL, et al. Anti-psoriatic drug anthralin activates JNK via lipid peroxidation: mononuclear cells are more sensitive than keratinocytes. Journal of Investigative Dermatology. 2000;114(4):688–692. doi: 10.1046/j.1523-1747.2000.00934.x. [DOI] [PubMed] [Google Scholar]
- 98.Westergaard M, Henningsen J, Johansen C, et al. Expression and localization of peroxisome proliferator-activated receptors and nuclear factor κB in normal and lesional psoriatic skin. Journal of Investigative Dermatology. 2003;121(5):1104–1117. doi: 10.1046/j.1523-1747.2003.12536.x. [DOI] [PubMed] [Google Scholar]
- 99.Plager DA, Leontovich AA, Henke SA, et al. Early cutaneous gene transcription changes in adult atopic dermatitis and potential clinical implications. Experimental Dermatology. 2007;16(1):28–36. doi: 10.1111/j.1600-0625.2006.00504.x. [DOI] [PubMed] [Google Scholar]
- 100.Schmuth M, Jiang YJ, Dubrac S, Elias PM, Feingold KR. Peroxisome proliferator-activated receptors and liver X receptors in epidermal biology. Journal of Lipid Research. 2008;49(3):499–509. doi: 10.1194/jlr.R800001-JLR200. [DOI] [PubMed] [Google Scholar]
- 101.Sommer DM, Jenisch S, Suchan M, Christophers E, Weichenthal M. Increased prevalence of the metabolic syndrome in patients with moderate to severe psoriasis. Archives of Dermatological Research. 2006;298(7):321–328. doi: 10.1007/s00403-006-0703-z. [DOI] [PubMed] [Google Scholar]
- 102.Tan M-H, Gordon M, Lebwohl O, George J, Lebwohl MG. Improvement of pyoderma gangrenosum and psoriasis associated with Crohn disease with anti-tumor necrosis factor α monoclonal antibody. Archives of Dermatology. 2001;137(7):930–933. [PubMed] [Google Scholar]
- 103.Schmuth M, Haqq CM, Cairns WJ, et al. Peroxisome proliferator-activated receptor (PPAR)-β/δ stimulates differentiation and lipid accumulation in keratinocytes. Journal of Investigative Dermatology. 2004;122(4):971–983. doi: 10.1111/j.0022-202X.2004.22412.x. [DOI] [PubMed] [Google Scholar]
- 104.Man MQM, Choi E-H, Schmuth M, et al. Basis for improved permeability barrier homeostasis induced by PPAR and LXR activators: liposensors stimulate lipid synthesis, lamellar body secretion, and post-secretory lipid processing. Journal of Investigative Dermatology. 2006;126(2):386–392. doi: 10.1038/sj.jid.5700046. [DOI] [PubMed] [Google Scholar]
- 105.Dubrac S, Stoitzner P, Pirkebner D, et al. Peroxisome proliferator-activated receptor-α activation inhibits Langerhans cell function. Journal of Immunology. 2007;178(7):4362–4372. doi: 10.4049/jimmunol.178.7.4362. [DOI] [PubMed] [Google Scholar]
- 106.Fowler AJ, Sheu MY, Schmuth M, et al. Liver X receptor activators display anti-inflammatory activity in irritant and allergic contact dermatitis models: liver-X-receptor-specific inhibition of inflammation and primary cytokine production. Journal of Investigative Dermatology. 2003;120(2):246–255. doi: 10.1046/j.1523-1747.2003.12033.x. [DOI] [PubMed] [Google Scholar]
- 107.Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nature Medicine. 2003;9(2):213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 108.Ellis CN, Varani J, Fisher GJ, et al. Troglitazone improves psoriasis and normalizes models of proliferative skin disease: ligands for peroxisome proliferator-activated receptor-γ inhibit keratinocyte proliferation. Archives of Dermatology. 2000;136(5):609–616. doi: 10.1001/archderm.136.5.609. [DOI] [PubMed] [Google Scholar]
- 109.Pershadsingh HA, Benson SC, Ellis CN. Improvement in psoriasis with rosiglitazone in a diabetic and a nondiabetic patient. Skinmed. 2005;4(6):386–390. doi: 10.1111/j.1540-9740.2005.04434.x. [DOI] [PubMed] [Google Scholar]
- 110.Bongartz T, Coras B, Vogt T, Schölmerich J, Müller-Ladner U. Treatment of active psoriatic arthritis with the PPARγ ligand pioglitazone: an open-label pilot study. Rheumatology. 2005;44(1):126–129. doi: 10.1093/rheumatology/keh423. [DOI] [PubMed] [Google Scholar]
- 111.Robertshaw H, Friedmann PS. Pioglitazone: a promising therapy for psoriasis. British Journal of Dermatology. 2005;152(1):189–191. doi: 10.1111/j.1365-2133.2005.06369.x. [DOI] [PubMed] [Google Scholar]
- 112.Shafiq N, Malhotra S, Pandhi P, Gupta M, Kumar B, Sandhu K. Pilot trial: pioglitazone versus placebo in patients with plaque psoriasis (the P6) International Journal of Dermatology. 2005;44(4):328–333. doi: 10.1111/j.1365-4632.2005.02504.x. [DOI] [PubMed] [Google Scholar]
- 113.Kuenzli S, Saurat J-H. Effect of topical PPARβ/δ and PPARγ agonists on plaque psoriasis: a pilot study. Dermatology. 2003;206(3):252–256. doi: 10.1159/000068897. [DOI] [PubMed] [Google Scholar]
- 114.Kuenzli S, Saurat J-H. Peroxisome proliferator-activated receptors as new molecular targets in psoriasis. Current Drug Targets: Inflammation & Allergy. 2004;3(2):205–211. doi: 10.2174/1568010043343976. [DOI] [PubMed] [Google Scholar]
- 115.Denda M, Wood LC, Emami S, et al. The epidermal hyperplasia associated with repeated barrier disruption by acetone treatment or tape stripping cannot be attributed to increased water loss. Archives of Dermatological Research. 1996;288(5-6):230–238. doi: 10.1007/BF02530090. [DOI] [PubMed] [Google Scholar]
- 116.Piqueras L, Reynolds AR, Hodivala-Dilke KM, et al. Activation of PPARβ/δ induces endothelial cell proliferation and angiogenesis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27(1):63–69. doi: 10.1161/01.ATV.0000250972.83623.61. [DOI] [PubMed] [Google Scholar]
- 117.Harris SG, Phipps RP. Induction of apoptosis in mouse T cells upon peroxisome proliferator-activated receptor gamma (PPAR-γ) binding. Advances in Experimental Medicine and Biology. 2002;507:421–425. doi: 10.1007/978-1-4615-0193-0_65. [DOI] [PubMed] [Google Scholar]
- 118.Kuenzli S, Saurat J-H. Peroxisome proliferator-activated receptors in cutaneous biology. British Journal of Dermatology. 2003;149(2):229–236. doi: 10.1046/j.1365-2133.2003.05532.x. [DOI] [PubMed] [Google Scholar]
- 119.Malhotra S, Bansal D, Shafiq N, Pandhi P, Kumar B. Potential therapeutic role of peroxisome proliferator activated receptor-γ agonists in psoriasis. Expert Opinion on Pharmacotherapy. 2005;6(9):1455–1461. doi: 10.1517/14656566.6.9.1455. [DOI] [PubMed] [Google Scholar]
- 120.Kömüves LG, Schmuth M, Fowler AJ, et al. Oxysterol stimulation of epidermal differentiation is mediated by liver X receptor-β in murine epidermis. Journal of Investigative Dermatology. 2002;118(1):25–34. doi: 10.1046/j.0022-202x.2001.01628.x. [DOI] [PubMed] [Google Scholar]
- 121.Yalcin H, Balci DD, Ucar E, et al. Myocardial perfusion is preserved in patients with psoriasis without clinically evident cardiovascular disease. Journal of the European Academy of Dermatology and Venereology. 2009;23(7):798–802. doi: 10.1111/j.1468-3083.2009.03178.x. [DOI] [PubMed] [Google Scholar]
- 122.Brauchli YB, Jick SS, Miret M, Meier CR. Psoriasis and risk of incident myocardial infarction, stroke or transient ischaemic attack: an inception cohort study with a nested case-control analysis. British Journal of Dermatology. 2009;160(5):1048–1056. doi: 10.1111/j.1365-2133.2008.09020.x. [DOI] [PubMed] [Google Scholar]
- 123.Mastrolonardo M, Picardi A, Alicino D, Bellomo A, Pasquini P. Cardiovascular reactivity to experimental stress in psoriasis: a controlled investigation. Acta Dermato-Venereologica. 2006;86(4):340–344. doi: 10.2340/00015555-0099. [DOI] [PubMed] [Google Scholar]
- 124.Kaplan MJ. Cardiometabolic risk in psoriasis: differential effects of biologic agents. Vascular Health and Risk Management. 2008;4(6):1229–1235. doi: 10.2147/vhrm.s3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Azfar RS, Gelfand JM. Psoriasis and metabolic disease: epidemiology and pathophysiology. Current Opinion in Rheumatology. 2008;20(4):416–422. doi: 10.1097/BOR.0b013e3283031c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kremers HM, McEvoy MT, Dann FJ, Gabriel SE. Heart disease in psoriasis. Journal of the American Academy of Dermatology. 2007;57(2):347–354. doi: 10.1016/j.jaad.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 127.Gisondi P, Fantin F, Del Giglio M, et al. Chronic plaque psoriasis is associated with increased arterial stiffness. Dermatology. 2009;218(2):110–113. doi: 10.1159/000182256. [DOI] [PubMed] [Google Scholar]
- 128.Dreiher J, Weitzman D, Shapiro J, Davidovici B, Cohen AD. Psoriasis and chronic obstructive pulmonary disease: a case-control study. British Journal of Dermatology. 2008;159(4):956–960. doi: 10.1111/j.1365-2133.2008.08749.x. [DOI] [PubMed] [Google Scholar]
- 129.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. Journal of the American Medical Association. 2006;296(14):1735–1741. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 130.Herron MD, Hinckley M, Hoffman MS, et al. Impact of obesity and smoking on psoriasis presentation and management. Archives of Dermatology. 2005;141(12):1527–1534. doi: 10.1001/archderm.141.12.1527. [DOI] [PubMed] [Google Scholar]
- 131.Huerta C, Rivero E, García Rodríguez LA. Incidence and risk factors for psoriasis in the general population. Archives of Dermatology. 2007;143(12):1559–1565. doi: 10.1001/archderm.143.12.1559. [DOI] [PubMed] [Google Scholar]
- 132.Kaye JA, Li L, Jick SS. Incidence of risk factors for myocardial infarction and other vascular diseases in patients with psoriasis. British Journal of Dermatology. 2008;159(4):895–902. doi: 10.1111/j.1365-2133.2008.08707.x. [DOI] [PubMed] [Google Scholar]
- 133.Ludwig RJ, Herzog C, Rostock A, et al. Psoriasis: a possible risk factor for development of coronary artery calcification. British Journal of Dermatology. 2007;156(2):271–276. doi: 10.1111/j.1365-2133.2006.07562.x. [DOI] [PubMed] [Google Scholar]
- 134.Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM. Prevalence of cardiovascular risk factors in patients with psoriasis. Journal of the American Academy of Dermatology. 2006;55(5):829–835. doi: 10.1016/j.jaad.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 135.Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. Journal of Investigative Dermatology. 2009;129(10):2411–2418. doi: 10.1038/jid.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Koenig W. Predicting risk and treatment benefit in atherosclerosis: the role of C-reactive protein. International Journal of Cardiology. 2005;98(2):199–206. doi: 10.1016/j.ijcard.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 137.Corbetta S, Angioni R, Cattaneo A, Becke-Peccoz P, Spada A. Effects of retinoid therapy on insulin sensitivity, lipid profile and circulating adipocytokines. European Journal of Endocrinology. 2006;154(1):83–86. doi: 10.1530/eje.1.02057. [DOI] [PubMed] [Google Scholar]
- 138.Gupta AK, Goldfarb MT, Ellis CN, Voorhees JV. Side-effect profile of acitretin therapy in psoriasis. Journal of the American Academy of Dermatology. 1989;20(6):1088–1093. doi: 10.1016/s0190-9622(89)70138-9. [DOI] [PubMed] [Google Scholar]
- 139.Vahlquist A, Törmä H. Retinoids and keratinization. Current concepts. International Journal of Dermatology. 1988;27(2):81–95. doi: 10.1111/j.1365-4362.1988.tb01278.x. [DOI] [PubMed] [Google Scholar]
- 140.Ellis CN, Kang S, Vinik AI, et al. Glucose and insulin responses are improved in patients with psoriasis during therapy with etretinate. Archives of Dermatology. 1987;123(4):471–475. [PubMed] [Google Scholar]
- 141.Ohtsuka T. The correlation between response to oral cyclosporin therapy and systemic inflammation, metabolic abnormality in patients with psoriasis. Archives of Dermatological Research. 2008;300(10):545–550. doi: 10.1007/s00403-008-0887-5. [DOI] [PubMed] [Google Scholar]
- 142.Strober BE, Menon K. Folate supplementation during methotrexate therapy for patients with psoriasis. Journal of the American Academy of Dermatology. 2005;53(4):652–659. doi: 10.1016/j.jaad.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 143.Soy M, Yildiz M, Uyanik MS, Karaca N, Güfer G, Piskin S. Susceptibility to atherosclerosis in patients with psoriasis and psoriatic arthritis as determined by carotid-femoral (aortic) pulse-wave velocity measurement. Revista Española de Cardiología. 2009;62(1):96–99. [PubMed] [Google Scholar]
- 144.Gunes Y, Tuncer M, Calka O, et al. Increased frequency of pulmonary hypertension in psoriasis patients. Archives of Dermatological Research. 2008;300(8):435–440. doi: 10.1007/s00403-008-0859-9. [DOI] [PubMed] [Google Scholar]
- 145.Pietrzak A, Borowik M, Chodorowska G, et al. Lipid profile and serum NT—proBNP level, and disease adaptation in psoriatic patients. In: Janowski K, Gierus J, editors. Sick Person—Biopsychosocial Aspects. Lublin, Poland: Wydawnictwo Drukarnia Best Print s.c.; 2009. pp. 348–360. [Google Scholar]
- 146.Wysocki J, Skoczyński S, Strózik A, Hochuł B, Zyguła M. Metabolic or immunometabolic syndrome? Wiadomosci Lekarskie. 2005;58(1-2):124–127. [PubMed] [Google Scholar]
- 147.Zalewska A, Głowacka E, Wyczółkowska J, Tchórzewski H, Narbutt J, Sysa-Jědrzejowska A. Interleukin 6 and 8 levels in plasma and fibroblast cultures in psoriasis. Mediators of Inflammation. 2006;2006:6 pages. doi: 10.1155/MI/2006/81767. Article ID 81767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Edwards BD, Bhatnagar D, Mackness MI, et al. Effect of low-dose cyclosporin on plasma lipoproteins and markers of cholestasis in patients with psoriasis. Quarterly Journal of Medicine. 1995;88(2):109–113. [PubMed] [Google Scholar]
- 149.Lassus A, Dahlgren A-L, Halpern MJ, Santalahti J, Happonen H-P. Effects of dietary supplementation with polyunsaturated ethyl ester lipids (Angiosan) in patients with psoriasis and psoriatic arthritis. Journal of International Medical Research. 1990;18(1):68–73. doi: 10.1177/030006059001800109. [DOI] [PubMed] [Google Scholar]
- 150.Meffert H, Bräutigam M, Färber L, Weidinger G. Low-dose (1.25 mg/kg) cyclosporin A: treatment of psoriasis and investigation of the influence on lipid profile. Acta Dermato-Venereologica. 1997;77(2):137–141. doi: 10.2340/0001555577137141. [DOI] [PubMed] [Google Scholar]
- 151.Miquel J, Bernd A, Sempere J, Díaz-Alperi J, Ramírez A. The curcuma antioxidants: pharmacological effects and prospects for future clinical use. A review. Archives of Gerontology and Geriatrics. 2002;34(1):37–46. doi: 10.1016/s0167-4943(01)00194-7. [DOI] [PubMed] [Google Scholar]
- 152.Osmancevic A, Nilsen L, Landin-Wilhelmsen K, et al. Effect of climate therapy at Gran Canaria on vitamin D production, blood glucose and lipids in patients with psoriasis. Journal of the European Academy of Dermatology and Venereology. 2009;23(10):1133–1140. doi: 10.1111/j.1468-3083.2009.03245.x. [DOI] [PubMed] [Google Scholar]
- 153.Reiss F, Jaimovich L. The influence of sitosterol upon psoriasis vulgaris: observations on electrophoretic pattern. Dermatologica. 1958;17(5):393–401. doi: 10.1159/000255614. [DOI] [PubMed] [Google Scholar]
- 154.Sattar N, Crompton P, Cherry L, Kane D, Lowe G, McInnes IB. Effects of tumor necrosis factor blockade on cardiovascular risk factors in psoriatic arthritis: a double-blind, placebo-controlled study. Arthritis and Rheumatism. 2007;56(3):831–839. doi: 10.1002/art.22447. [DOI] [PubMed] [Google Scholar]
- 155.Vallesi G, Raggi F, Rufini S, Gizzi S, Ercolani E, Rossi R. Effects of cyclotronic ion resonance on human metabolic processes: a clinical trial and one case report. Electromagnetic Biology and Medicine. 2007;26(4):283–288. doi: 10.1080/15368370701768823. [DOI] [PubMed] [Google Scholar]
- 156.Shrinsky IV, Shrinskt VS. Efficacy of simvastin in plaque psoriasis: a pilot study. The Journal of American Academy of Dermatology. 2007;57(3):529–531. doi: 10.1016/j.jaad.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 157.Ashley JM, Lowe NJ, Borok ME, Alfin-Slater RB. Fish oil supplementation results in decreased hypertriglyceridemia in patients with psoriasis undergoing etretinate or acitretin therapy. Journal of the American Academy of Dermatology. 1988;19(1 I):76–82. doi: 10.1016/s0190-9622(88)70154-1. [DOI] [PubMed] [Google Scholar]
- 158.Marsden JR. Effect of dietary fish oil on hyperlipidaemia due to isotretinoin and etretinate. Human Toxicology. 1987;6(3):219–222. doi: 10.1177/096032718700600308. [DOI] [PubMed] [Google Scholar]
- 159.Grossman RM, Delaney RJ, Brinton EA, Carter DM, Gottlieb AB. Hypertriglyceridemia in patients with psoriasis treated with cyclosporine. Journal of the American Academy of Dermatology. 1991;25(4):648–651. doi: 10.1016/0190-9622(91)70247-y. [DOI] [PubMed] [Google Scholar]
- 160.Cauza E, Cauza K, Hanusch-Enserer U, Etemad M, Dunky A, Kostner K. Intravenous anti TNF-α antibody therapy leads to elevated triglyceride and reduced HDL-cholesterol levels in patients with rheumatoid and psoriatic arthritis. Wiener Klinische Wochenschrift. 2002;114(23-24):1004–1007. [PubMed] [Google Scholar]
- 161.Antoniou C, Dessinioti C, Katsambas A, Stratigos AJ. Elevated triglyceride and cholesterol levels after intravenous antitumour necrosis factor-α therapy in a patient with psoriatic arthritis and psoriasis vulgaris. British Journal of Dermatology. 2007;156(5):1090–1091. doi: 10.1111/j.1365-2133.2007.07835.x. [DOI] [PubMed] [Google Scholar]
- 162.Spanakis E, Sidiropoulos P, Papadakis J, et al. Modest but sustained increase of serum high density lipoprotein cholesterol levels in patients with inflammatory arthritides treated with infliximab. Journal of Rheumatology. 2006;33(12):2440–2446. [PubMed] [Google Scholar]
- 163.Rajpara AN, Goldner R, Gaspari A. Psoriasis: can statins play a dual role? Dermatology Online Journal. 2010;16(2, article 2) [PubMed] [Google Scholar]
- 164.Jacobi TC, Highet A. A clinical dilemma while treating hypercholesterolaemia in psoriasis. British Journal of Dermatology. 2003;149(6):1305–1306. doi: 10.1111/j.1365-2133.2003.05675.x. [DOI] [PubMed] [Google Scholar]