Abstract
Background:
There is discordance among studies assessing the impact of race on outcome of patients with Triple Negative Breast Cancer (TNBC). We assessed survival outcomes for African American (AA) versus Caucasian (CA) women with TNBC treated at an urban cancer center in Memphis, TN with a predominant AA patient population.
Methods:
Patients with Stage I-III TNBC were identified from our breast database. Event free survival (EFS) and Breast cancer specific survival (BCSS) were the primary outcome measures. Cox proportional hazards models were fitted for EFS and BCSS.
Results:
Of the 124 patients, 71% were AA. No significant association between race and stage (P = 0.21) or menopausal status (P = 0.15) was observed. Median age at diagnosis was significantly lower for AA versus CA women (49.5 vs. 55 years, P = 0.024). 92% of the patients received standard neo/adjuvant chemotherapy, with no significant difference in duration and type of chemotherapy between the races. With a median follow up of 23 months, 28% of AA vs. 19% of CA women had an event (P = 0.37). 3 year EFS and BCSS trended favorably towards CA race (77% vs. 64%, log rank P = 0.20 and 92% vs. 76%, P = 0.13 respectively) with a similar trend noted on multiple variable modeling (EFS: HR 0.62, P = 0.29; BCSS: HR 0.36, P = 0.18). AA women ≥50 years at diagnosis had a significantly worse BCSS than the CA women in that age group (P = 0.012).
Conclusion:
Older AA women with TNBC have a significantly worse breast cancer specific survival than their CA counterparts. Overall, there is a trend towards lower survival for AA women compared to Caucasians despite uniformity of tumor phenotype and treatment. The high early event rate, irrespective of race, underscores the need for effective therapies for women with TNBC.
Keywords: triple negative, racial differences, biology, uniform treatment, survival
Introduction
Triple negative breast cancer (TNBC) is characterized by a lack of histologic expression of estrogen and progesterone receptors and HER2 protein on the surface of tumor cells (ER, PR and HER2 negative). TNBC is more likely to have aggressive clinicopathologic features like a higher nuclear and histologic grade, higher mitotic index, advanced stage, and a younger age at presentation.1,2 By gene expression analysis, a majority of the TNBCs cluster with the “basal-like” tumors, which have a worse prognosis compared to the hormone receptor positive subgroups (luminal A and luminal B).3,4 There is a debate in literature, however, as to the true biologic origin of these tumors and they likely constitute a more heterogeneous group than presently recognized. 5 Epidemiological studies show the highest prevalence of TNBC among African American (AA) women, especially younger premenopausal African Americans.1,6 This may, in part, explain a higher mortality rate despite a lower incidence of breast cancer overall among African Americans, though other factors like socioeconomic, cultural and treatment differences have been linked to this disparity as well.7–10 This racial disparity in survival, however, persists after adjustment for treatment distribution and access to health care.11,12
There are limited reports in literature that specifically address the question of whether African American race independently confers a worse outcome in patients with a triple negative phenotype. Two population based studies have shown worse survival associated with AA race for patients with triple negative tumors.13,14 Contrary to that, in a cohort of patients with TNBC treated uniformly with pre-operative chemotherapy at a tertiary cancer center, an independent effect of race on survival was not observed after adjustment for other variables.15
We aimed to examine the association between race and survival outcomes of women with TNBC who were treated at the University of Tennessee Cancer Institute. Our institution caters to a large inner city population with a predominant African American representation. We analyzed any association between race and specific tumor and treatment characteristics for this cohort of patients. This report summarizes our findings.
Patients and Methods
All female patients with a diagnosis of invasive breast cancer treated at the University of Tennessee Cancer Institute, Memphis, between September 2003 and December 2008 were screened by a search of our electronic medical record system. Patients who had ER, PR and HER2 negative tumors were identified. Pathology reports were reviewed to confirm triple negative status of the tumors. ER and PR were considered as negative if the percentage of cells staining positive by immunohistochemistry (IHC) were below the minimum cut-off required for a positive result by the manufacturer’s standard. A documented absence of HER2 gene amplification by fluorescence in situ hybridization was required in case of 2+ staining by IHC. Patients were excluded if their race was reported as other than AA or Caucasian, if they had incomplete information for stage at presentation and receptor status, had stage IV disease at presentation, did not undergo definitive breast surgery, or were lost to follow up prior to initiation of any therapy for breast cancer. Individual patient medical records were reviewed to collect demographic, clinical and treatment information.
Information on the following variables was recorded: patient characteristics including race, age at diagnosis and menopausal status; tumor characteristics including tumor size, modified Scarff-Bloom-Richardson grade, stage at diagnosis according to the AJCC staging criteria, sixth edition (2002); treatment details including receipt or non receipt of chemotherapy in the adjuvant or neo-adjuvant setting, duration and type of chemotherapy received, type and date of definitive surgery, details of axillary staging, and receipt of adjuvant radiation therapy. The study was approved by the Institutional Review Board at the University of Tennessee Health Science Center.
Statistical Analysis
Survival Outcome measures for this analysis were Event Free Survival (EFS) and Breast Cancer Specific Survival (BCSS) for the whole cohort and separately for AA and Caucasian patients. EFS was measured from the date of definitive breast cancer surgery to the date of first recurrence (locoregional, contralateral or distant) or death from breast cancer, whichever occurred first. BCSS was measured from the date of definitive breast cancer surgery to the date of death from breast cancer. Patients who were alive and free of recurrence were censored at the date of the last follow up for this analysis. Patients who had no evidence of recurrence of breast cancer prior to death were also censored at the date of last follow up. Survival estimates for the entire cohort and for the two races were obtained by the Kaplan-Meier method and the log rank test was employed for comparing the survival curves.
We investigated associations between race and patient, tumor and treatment variables using Fisher’s exact test. Differences in the medians between two groups of continuous variables were tested by the Wilcoxon-Mann-Whitney test. Cox proportional hazards models were fitted separately for EFS and BCSS to determine the relationship of patient, tumor and treatment variables with survival outcomes. Variables that attained a significance level of 0.15 in the univariate models were considered for inclusion in the multiple variable models. The best multiple variable models for each outcome were chosen by step-wise selection. Race was included in all models, regardless of significance, because our primary objective was to determine the influence of race on survival outcomes. A P-value <0.05 was considered significant in the final multiple variable models for both survival outcomes. Due to the retrospective nature of this study, sample size was limited and no power calculations were made. All analyses were performed using SAS Version 9.2.
Results
Patient characteristics
Between September 2003 and December 2008, 147 patients with a diagnosis of triple negative breast cancer were referred to the University of Tennessee Cancer Institute. Twenty three patients were excluded from this analysis because they met one or more of the exclusion criteria described under the methods section: 13 with stage IV disease, 2 with race other than AA or Caucasian, 2 with incomplete staging information, 3 who progressed to stage IV disease prior to definitive surgery, 1 patient with a squamous cell histology, and 2 lost to follow up immediately after definitive surgery. Table 1 summarizes the characteristics of the 124 patients included in this report.
Table 1.
Patient characteristics.
| Characteristic* | AA N = 88 (71%) | Caucasian N = 36 (29%) | Total N = 124 (100%) | P-value |
|---|---|---|---|---|
| Age groups | ||||
| <50 | 44 (50) | 11 (31) | 55 (44) | 0.07 |
| ≥50 | 44 (50) | 25 (69) | 69 (56) | |
| Menopausal status | ||||
| Post | 53 (60) | 27 (75) | 80 (65) | 0.15 |
| Pre | 35 (40) | 9 (25) | 44 (35) | |
| Nodal status | ||||
| Negative | 41 (47) | 23 (64) | 64 (52) | 0.11 |
| Positive | 46 (53) | 13 (36) | 59 (48) | |
| Stage | ||||
| I | 18 (21) | 12 (33) | 30 (24) | 0.21 |
| II | 53 (61) | 16 (45) | 69 (56) | |
| III | 16 (18) | 8 (22) | 24 (20) | |
| Grade | ||||
| I/II | 9 (11) | 4 (12) | 13 (11) | 0.99 |
| III | 73 (89) | 30 (88) | 103 (89) | |
| Surgery | ||||
| Mastectomy | 41 (47) | 22 (61) | 63 (51) | 0.17 |
| Lumpectomy | 47 (53) | 14 (39) | 61 (49) | |
| Neo/Adjuvant chemotherapy | 80 (91) | 34 (94) | 114 (92) | 0.7 |
| Yes | 8 (9) | 2 (6) | 10 (8) | |
| No | ||||
| Adjuvant radiotherapy | 57 (65) | 24 (67) | 79 (66) | 0.99 |
| Yes | 31 (35) | 12 (33) | 41 (34) | |
| No | ||||
Due to missing data, the numbers for some of the baseline characteristics do not add up to the column totals.
Of the 124 patients, 71% were AA. The median age at diagnosis for the whole cohort was 51 years (range 26–82 years). There was a trend towards a greater proportion of AA women diagnosed at age younger than 50 as compared to Caucasians (50% vs. 31%, Fisher’s exact P value = 0.07). Median age at diagnosis for AA women was significantly lower than the Caucasian women {49.5 years (range 28–77) vs. 55 years (range 26–82), P value = 0.024}. There was no significant association between menopausal status and race (P value = 0.15). Stage at presentation did not differ by race (P value = 0.21).
Neoadjuvant/Adjuvant Treatment
Of the 124 patients, 114 (92%) and 79 (66%) received adjuvant chemotherapy and radiotherapy respectively. There was no difference in the proportion of patients who received neoadjuvant/adjuvant therapy by race (Table 1). The median number of chemotherapy cycles was 7 (range 0–10), and did not differ by race (Wilcoxon Mann Whitney test P value = 0.68).
The proportion of patients who received both anthracyclines and taxanes as part of their chemotherapy regimen was not significantly different between the races (65% of AA patients versus 56% of CA patients, Chi- square P value = 0.36).
Survival results
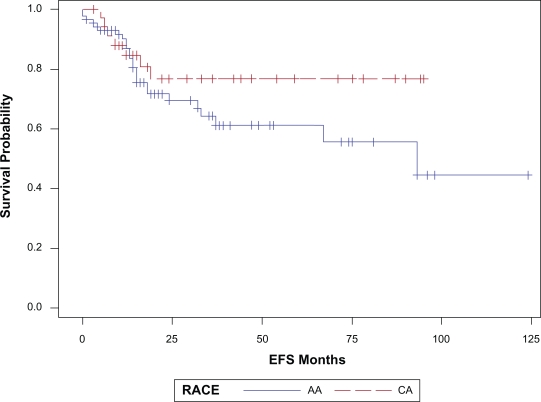
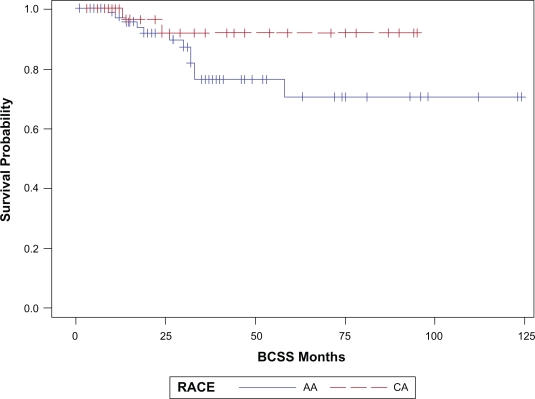
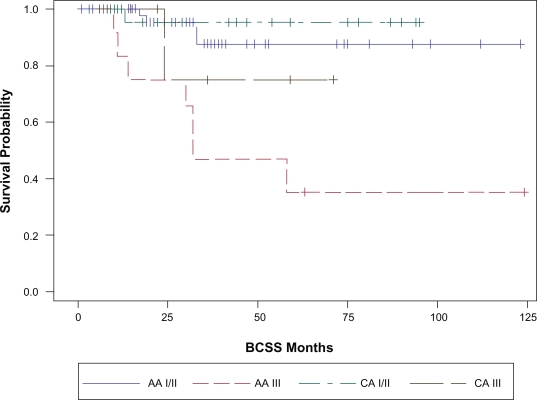
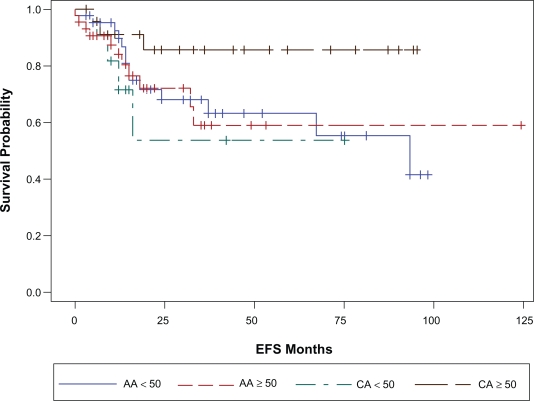
With a median follow up of 23 months (range 1–124 months), 32 patients (26%) have had a breast cancer event, i.e. recurrence or death related to breast cancer (Table 2). The site of first relapse i.e. locoregional (LR) versus systemic ±locoregional (LRS) did not differ by race. The overall 3 year EFS and BCSS estimates for the cohort were 68% (SE, 5%) and 81% (SE, 5%) respectively. The three-year EFS and BCSS estimates for AA race were 64% and 76% respectively. The corresponding 3- year EFS and BCSS estimates for Caucasians were 77% and 92% respectively (Table 3). Figures 1a and 1b illustrate the Kaplan-Meier survival curves with the corresponding log-rank P-values for EFS and BCSS. Though the difference in survival between the two races did not reach statistical significance, both the unadjusted and stage adjusted survival (Figs. 2a and 2b) trend towards a better outcome for the Caucasian patients. When analyzed by age at diagnosis (<50 vs. ≥50), among the patients in the older age group, BCSS was significantly lower for AA patients compared to the CA patients (Fig. 3b; log-rank P-value = 0.012). There was no statistically significant survival difference noted between the races among the younger patients (Fig. 3a).
Table 2.
Patient outcomes.
| Outcome measure | AA N = 88 (71%) | Caucasian N = 36 (29%) | Total N = 124 (100%) | P-value* |
|---|---|---|---|---|
| Breast cancer event | ||||
| Yes | 25 (28) | 7 (19) | 32 (26) | 0.37* |
| No | 63 (72) | 29 (81) | 92 (74) | |
| Breast cancer death | ||||
| Yes | 12 (14) | 2 (6) | 14 (11) | 0.35* |
| No | 76 (86) | 34 (94) | 110 (89) | |
| Site of 1st relapse | ||||
| LR | 10 (40) | 2 (29) | 12 (38) | 0.68* |
| LRS | 15 (60) | 5 (71) | 20 (63) | |
Fisher’s exact P values.
Table 3.
Survival estimates.
| AA | Caucasian | Overall | P-value** | |
|---|---|---|---|---|
| 3 year EFS, % (SE) | 64 (7) | 77 (8) | 68 (5) | 0.20 |
| Node negative | 73 (10) | 79 (10) | 0.97 | |
| Node positive | 53 (9) | 75 (13) | 0.19 | |
| 3 year BCSS, % (SE) | 76 (6) | 92 (6) | 81 (5) | 0.13 |
| Node negative | 89 (7) | 91 (9) | 0.95 | |
| Node positive | 70 (9) | 92 (8) | 0.15 |
Log rank P values comparing the entire survival curves between groups.
Figure 1a.
EFS by race, P = 0.20.
Figure 1b.
BCSS by race, P = 0.13.
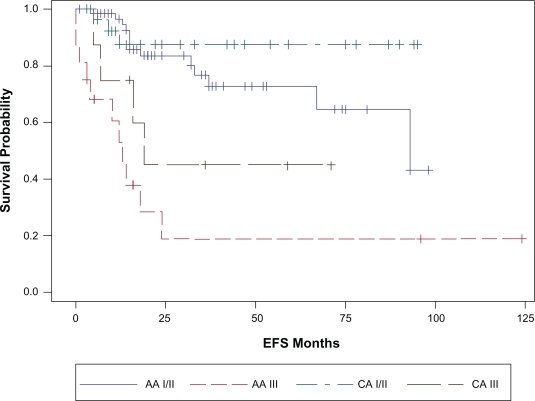
Figure 2a.
Stage adjusted EFS by race. Stage I/II CA vs. AA P = 0.25; Stage II CA vs. AA P = 0.18.
Figure 2b.
Stage adjusted BCSS by race. Stage I/II CA vs. AA P = 0.56; Stage II CA vs. AA P = 0.22.
Figure 3b.
Age adjusted BCSS by race. Age < 50 years CA vs. AA P = 0.37; ≥50 years CA vs. AA P = 0.01
Figure 3a.
Age adjusted EFS by race. Age < 50 years CA vs. AA P = 0.53; ≥50 years CA vs. AA P = 0.09.
Cox proportional hazard modeling
The following variables were included in the univariate models for EFS and BCSS: race (Caucasian vs. AA), lymph node status (positive vs. negative), stage at presentation (III vs. I or II), tumor grade (3 vs. 1 & 2), menopausal status (pre vs. post menopausal), age at diagnosis (≥50 vs. <50), adjuvant radiation (not received vs. received), adjuvant chemotherapy (received vs. not received), and the type of surgery (mastectomy vs. segmental resection). Only the variables that were retained in the final multiple variable models for EFS and BCSS are shown in Table 4. After controlling for other variables, though not statistically significant the survival outcomes trended in favor of the Caucasian race for both EFS (HR 0.62, P = 0.29) and BCSS (HR 0.36, P = 0.18). Stage at presentation retained significance after adjustment for other variables in the final models for both EFS and BCSS.
Table 4.
Multiple variable cox proportional hazard models.*
|
Event free survival | ||
|---|---|---|
| Variable | HR | P-value |
| Stage | 8.34 | <0.0001 |
| XRT | 3.09 | 0.0031 |
| Menopausal status | 2.33 | 0.0287 |
| Race | 0.62 | 0.29 |
| Breast cancer specific survival | ||
| Stage | 6.82 | 0.0008 |
| Race | 0.36 | 0.18 |
The final models reflect only the variables retained by the process of step-wise selection, from among the variables which met the significance level for inclusion in univariate modeling.
Discussion
TNBC represents a biologically distinct subset of breast cancer, with high chemotherapy responsiveness, but paradoxically poor long term survival with the greatest hazard of recurrence during the first 2–3 years after diagnosis.16 That over a quarter of our patients had a breast cancer event with a median follow up of 23 months, underscores the distinct natural history of TNBC as compared to the luminal subtypes where a continued hazard of recurrence is observed even beyond the first 5 years after diagnosis.17
There is an association between African ancestry and the likelihood of developing a tumor with a triple negative phenotype. Non African American black women have also been found to have a higher prevalence of TNBC, a younger age at diagnosis and worse survival than their white counterparts.18,19 An analysis of the distribution of the risk factors associated with a higher likelihood of development of TNBC shows an increased prevalence of these risk factors among the AA women in the general population compared to white women.20 As opposed to luminal breast cancer, increased parity, and a younger age at first full term pregnancy are associated with an increased risk of developing TNBC. AA women were also more likely to never have breast fed, and reported a shorter duration of breast feeding per child than white women, which are also factors associated with the risk of developing TNBC.
There are few reported studies that specifically address the issue of race and survival for women with TNBC. Bauer et al13 and Lund et al14 have reported a worse survival for AA women overall with breast cancer and within the triple negative subtype, after controlling for socioeconomic factors, treatment delay and tumor characteristics. A recent report, by Dawood et al of four hundred and seventy one patients with TNBC treated with standard neo-adjuvant chemotherapy at the MD Anderson Cancer Center found no detriment in survival or likelihood of attaining a pathological complete remission (pCR) associated with AA race.15 The authors suggested that treatment disparities might have resulted in poorer survival for AA women with TNBC in the other reported datasets, while their cohort of uniformly treated patients had similar outcomes irrespective of race. The odds of survival, in fact, tended to be more favorable for AA race compared to white and other races. The 3 year relapse free and overall survival rates however were low (63% and 71% respectively) for the entire cohort, independent of race. However, in another report, among a smaller cohort of 38 TNBC patients treated with standard anthracycline and taxane based neoadjuvant therapy, AA race was associated with a lower pCR rate and a worse survival probability compared to white and other races.21 All the above studies have differences in methodology, sample size and the variables analyzed. Thus, a direct comparison of these results with our data is not likely to be meaningful. However, we would like to point out similarities and differences between the cohort from our institution and the other reported datasets.
Our cohort of TNBC patients is unique in that the majority of our cohort is comprised of AA women (71% as compared to 21% in the report by Dawood et al 24% in the series by Lund et al 40% in the dataset from Carey et al and 10% in the California cancer registry study by Bauer et al). This is representative of our overall breast cancer population at UTCI. Of the 200 new breast cancer patients seen over the last 18 months, 60% were AA. Unlike the population based cohorts reported by Lund et al14 and Carey et al1 but similar to the single institution study by Dawood et al our cohort is comprised entirely of patients with triple negative tumors for whom detailed staging and treatment history is available. In our study, stage at presentation did not differ between the two races though numerically more AA patients presented with node positive disease at diagnosis compared to the Caucasian patients(53% vs. 36% respectively, P = 0.11). There was no difference in the likelihood of receiving adjuvant chemotherapy and radiotherapy or the duration and type of chemotherapy received by race. Unlike the MD Anderson study, controlling for these among other factors still resulted in a trend towards better survival for the Caucasian patients compared to the AA patients (HR 0.62 for EFS, and 0.36 for BCSS). A longer follow up is needed to see if these differences become statistically significant over time. Our results suggest that treatment disparity alone doesn’t account for the observed differences in survival between the two races. The cohort from MD Anderson may also represent a select group of patients referred from different geographic areas with a different socioeconomic background and baseline risk factors than our patients. Our cohort represents a geographically uniform group treated at an urban cancer center in the Memphis metropolitan area with a sizeable African American patient population. Our findings are significant in this regard that our patients are more likely to represent the AA women with TNBC in the community and the survival disadvantage that these women face likely represents a complex interplay between genetics and their unique socioeconomic, cultural and environmental risks.
An interesting observation in our cohort is the age and menopausal status of our patients, with 65% being postmenopausal and over half of the patients being ≥age 50 at diagnosis, contrary to the previously observed associations between TNBC and younger age and premenopausal status.13,22
In addition, we found that AA race was significantly associated with a worse BCSS for those patients ≥50 years at diagnosis. The significance of this observation is not entirely clear at this time, but merits further investigation to understand the unique hormonal milieu that can influence the risk of development and outcomes from particular subtypes of breast cancer in different age and race groups.
A range of molecularly heterogeneous tumors sharing a common phenotype, the so called TNBC, may account for differences seen in clinical outcomes between subsets of patients. Recent molecular characterization of this phenotype has led to interesting insights into the biology of these complex tumors and some potential targets for treatment.23 Beyond the tumor itself, racial differences in outcomes can stem from varying tumor-host interactions,24 and pharmacogenomic differences in efficacy and toxicity of systemic therapies.25–27
The pattern of relapse noted in our study is also in keeping with other reports of a propensity for systemic relapses for patients with TNBC compared to the luminal subtypes.28,29 Overall, 62% of all first relapses in our cohort were systemic relapses, with no difference noted between the two races. Thus, better systemic therapies are needed for TNBC that can positively impact distant disease free survival and overall survival for these patients.
Advances in the understanding of the biology of these tumors and the tumor microenvironment are already being translated into clinical practice. Encouraging results have been seen with the first in-class PARP (poly ADP-ribose polymerase-1) inhibitor drugs for patients with TNBC30 and those with genetic mutations in the DNA repair genes BRCA-1 and 2.31 Several other compounds like the CHK1 and AURKB inhibitors, and inhibitors of the VEGF, EGFR and IGF pathways are currently under investigation for the treatment of TNBC and offer hope for the future.
Conclusion
In conclusion, our study adds to the growing body of literature examining differences in outcomes within the broad category of tumors classified as triple negative. We acknowledge that there are limitations to our study, given its retrospective nature and a relatively small sample size. However, our findings support the need for molecular sub-classification, and search for effective targeted therapies for TNBC.
Acknowledgments
The authors would like to thank Matthew Smeltzer for statistical analysis and interpretation.
Footnotes
Disclosures
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Carey LA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. Jama. 2006;295(21):2492–502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 2.Tian XS, et al. Clinicopathologic and prognostic characteristics of triple-negative breast cancer. Onkologie. 2008;31(11):610–4. doi: 10.1159/000162288. [DOI] [PubMed] [Google Scholar]
- 3.Perou CM, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 4.Sorlie T, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98(19):10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gusterson B. Do ‘basal-like’ breast cancers really exist? Nat Rev Cancer. 2009;9(2):128–34. doi: 10.1038/nrc2571. [DOI] [PubMed] [Google Scholar]
- 6.Morris GJ, et al. Differences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients: a single-institution compilation compared with the National Cancer Institute’s Surveillance, Epidemiology, and End Results database. Cancer. 2007;110(4):876–84. doi: 10.1002/cncr.22836. [DOI] [PubMed] [Google Scholar]
- 7.DeLancey JO, et al. Recent trends in Black-White disparities in cancer mortality. Cancer Epidemiol Biomarkers Prev. 2008;17(11):2908–12. doi: 10.1158/1055-9965.EPI-08-0131. [DOI] [PubMed] [Google Scholar]
- 8.Jemal A, et al. Cancer statistics. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 9.Ward E, et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin. 2008;58(1):9–31. doi: 10.3322/CA.2007.0011. [DOI] [PubMed] [Google Scholar]
- 10.Smith ER, et al. Breast cancer survival among economically disadvantaged women: the influences of delayed diagnosis and treatment on mortality. Cancer Epidemiol Biomarkers Prev. 2008;17(10):2882–90. doi: 10.1158/1055-9965.EPI-08-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polite BN, et al. Racial differences in clinical outcomes from metastatic breast cancer: a pooled analysis of CALGB 9342 and 9840—Cancer and Leukemia Group B. J Clin Oncol. 2008;26(16):2659–65. doi: 10.1200/JCO.2007.13.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jatoi I, Becher H, Leake CR. Widening disparity in survival between white and African-American patients with breast carcinoma treated in the US Department of Defense Healthcare system. Cancer. 2003;98(5):894–9. doi: 10.1002/cncr.11604. [DOI] [PubMed] [Google Scholar]
- 13.Bauer KR, et al. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109(9):1721–8. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 14.Lund MJ, et al. Race and triple negative threats to breast cancer survival: a population-based study in Atlanta, GA. Breast Cancer Res Treat. 2009;113(2):357–70. doi: 10.1007/s10549-008-9926-3. [DOI] [PubMed] [Google Scholar]
- 15.Dawood S, et al. Triple receptor-negative breast cancer: the effect of race on response to primary systemic treatment and survival outcomes. J Clin Oncol. 2009;27(2):220–6. doi: 10.1200/JCO.2008.17.9952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liedtke C, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26(8):1275–81. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 17.Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 18.Bowen RL, et al. Early onset of breast cancer in a group of British black women. Br J Cancer. 2008;98(2):277–81. doi: 10.1038/sj.bjc.6604174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huo D, et al. Population differences in breast cancer: survey in indigenous african women reveals over-representation of triple-negative breast cancer. J Clin Oncol. 2009;27(27):4515–21. doi: 10.1200/JCO.2008.19.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Millikan RC, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109(1):123–39. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balmanoukian A, et al. African American Women Who Receive Primary Anthracycline- and Taxane-Based Chemotherapy for Triple-Negative Breast Cancer Suffer Worse Outcomes Compared With White Women. 2009. pp. e35–7. [DOI] [PMC free article] [PubMed]
- 22.Ihemelandu CU, et al. Molecular breast cancer subtypes in premenopausal and postmenopausal African-American women: age-specific prevalence and survival. J Surg Res. 2007;143(1):109–18. doi: 10.1016/j.jss.2007.03.085. [DOI] [PubMed] [Google Scholar]
- 23.Sparano JA, et al. Genotypic characterization of phenotypically defined triple-negative breast cancer. 2009;500 [Google Scholar]
- 24.Martin DN, et al. Differences in the tumor microenvironment between African-American and European-American breast cancer patients. PLoS One. 2009;4(2):e4531. doi: 10.1371/journal.pone.0004531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaremko M, et al. Polymorphism of the DNA repair enzyme XRCC1 is associated with treatment prediction in anthracycline and cyclophosphamide/ methotrexate/5-fluorouracil-based chemotherapy of patients with primary invasive breast cancer. Pharmacogenet Genomics. 2007;17(7):529–38. doi: 10.1097/FPC.0b013e32801233fc. [DOI] [PubMed] [Google Scholar]
- 26.Lal S, et al. Novel SLC22 A16 polymorphisms and influence on doxorubicin pharmacokinetics in Asian breast cancer patients. Pharmacogenomics. 2007;8(6):567–75. doi: 10.2217/14622416.8.6.567. [DOI] [PubMed] [Google Scholar]
- 27.Phan VH, et al. Ethnic differences in drug metabolism and toxicity from chemotherapy. Expert Opin Drug Metab Toxicol. 2009;5(3):243–57. doi: 10.1517/17425250902800153. [DOI] [PubMed] [Google Scholar]
- 28.Millar EKA, et al. Prediction of Local Recurrence, Distant Metastases, and Death After Breast-Conserving Therapy in Early-Stage Invasive Breast Cancer Using a Five-Biomarker Panel. J Clin Oncol. 2009:4701–8. doi: 10.1200/JCO.2008.21.7075. [DOI] [PubMed] [Google Scholar]
- 29.Moran MS, et al. Long-term outcomes and clinicopathologic differences of African-American versus white patients treated with breast conservation therapy for early-stage breast cancer. Cancer. 2008;113(9):2565–74. doi: 10.1002/cncr.23881. [DOI] [PubMed] [Google Scholar]
- 30.O’Shaughnessy J, et al. Efficacy of BSI-201, a poly (ADP-ribose) polymerase-1 (PARP1) inhibitor, in combination with gemcitabine/carboplatin (G/C) in patients with metastatic triple-negative breast cancer (TNBC): Results of a randomized phase II trial. J Clin Oncol. 2009;27:3. [Google Scholar]
- 31.Tutt A, et al. Phase II trial of the oral PARP inhibitor olaparib in BRCA-deficient advanced breast cancer. J clin Oncol. 2009;27:CRA501. [Google Scholar]