Abstract
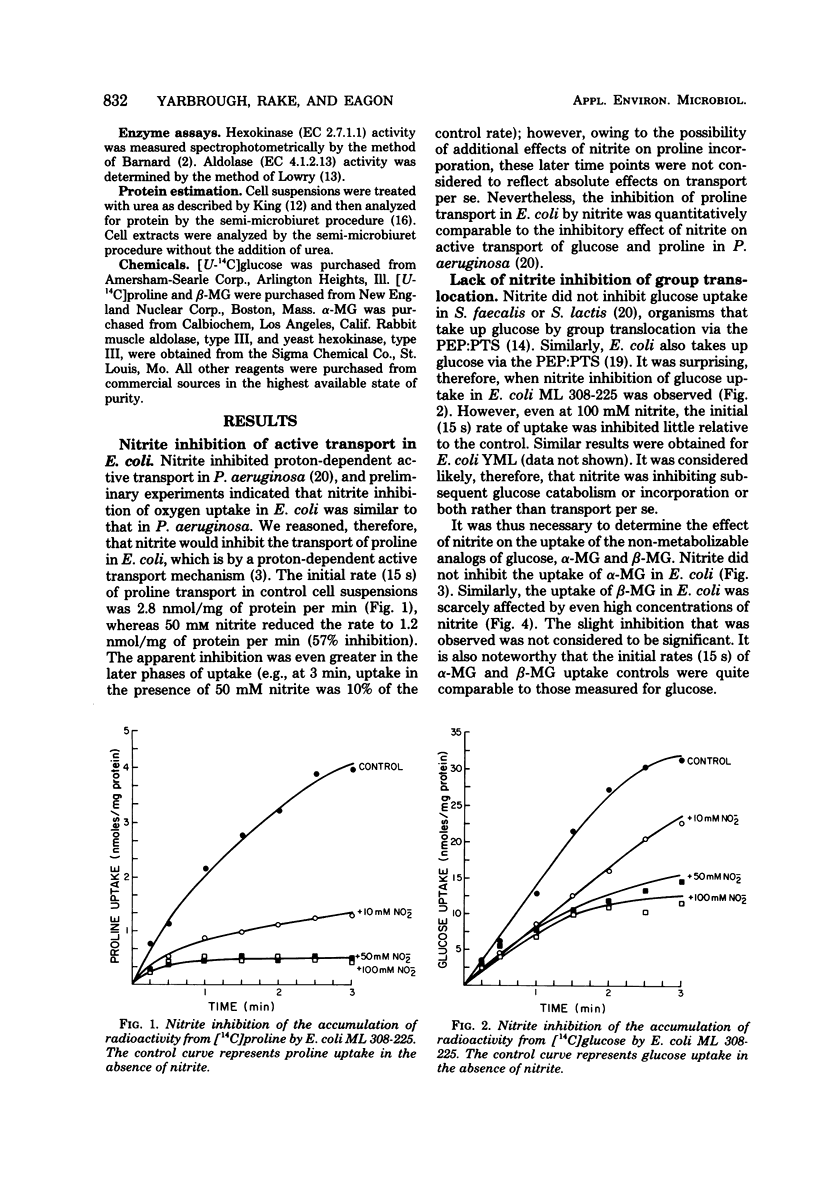
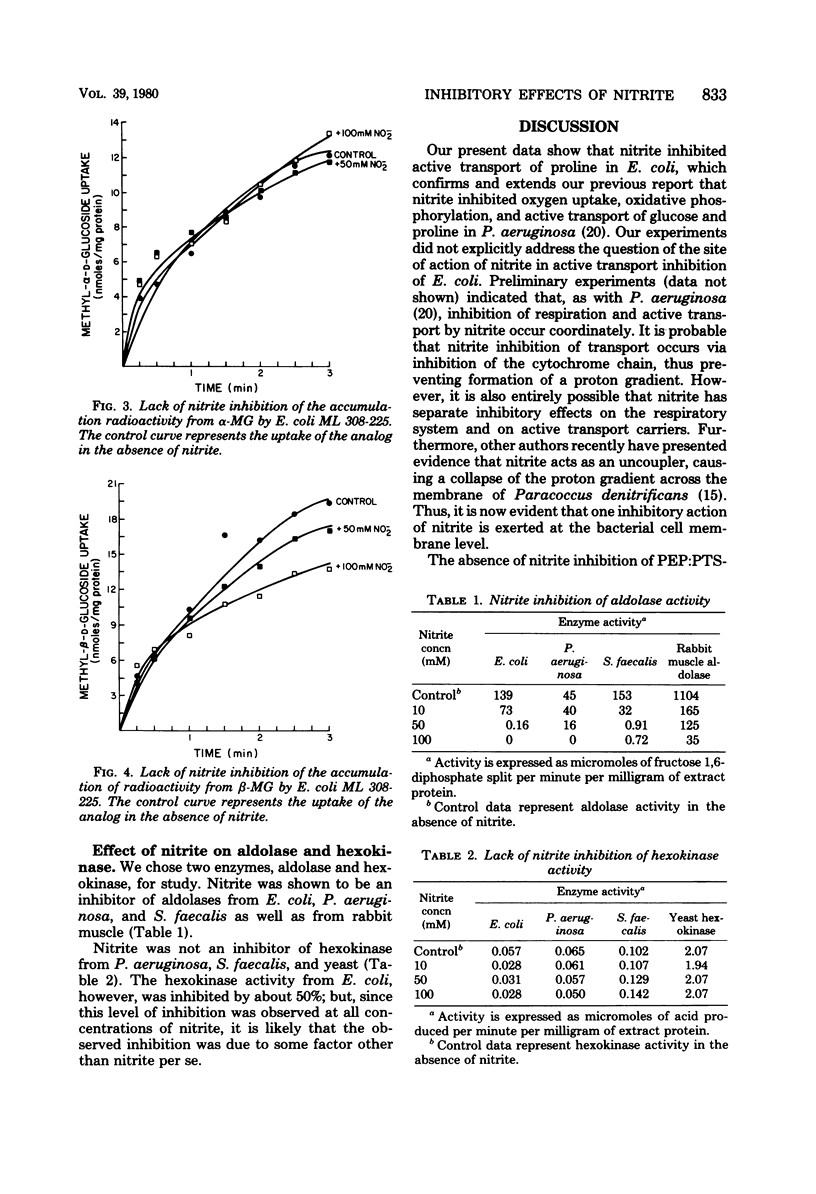
Nitrite inhibited active transport of proline in Escherichia coli but not group translocation of sugar via the phosphoenolpyruvate:phosphotransferase system. These results were consistent with previous results that nitrite inhibits active transport, oxygen uptake, and oxidative phosphorylation in aerobic bacteria. Nitrite also inhibited aldolase (EC 4.1.2.13) from E. coli, Pseudomonas aeruginosa, Streptococcus faecalis, and rabbit muscle. Thus, these various data showed that nitrite has more than one site of attack in the bacterial cell. These data also indicated that nitrite is inhibitory to a wide range of physiological types of bacteria.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAALSRUD K., BAALSRUD K. S. Studies on Thiobacillus denitrificans. Arch Mikrobiol. 1954;20(1):34–62. doi: 10.1007/BF00412265. [DOI] [PubMed] [Google Scholar]
- Barnard E. A. Hexokinases from yeast. Methods Enzymol. 1975;42:6–20. doi: 10.1016/0076-6879(75)42085-7. [DOI] [PubMed] [Google Scholar]
- Berger E. A. Different mechanisms of energy coupling for the active transport of proline and glutamine in Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1514–1518. doi: 10.1073/pnas.70.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkerd E. F., Kolari O. E. The history and use of nitrate and nitrite in the curing of meat. Food Cosmet Toxicol. 1975 Dec;13(6):655–661. doi: 10.1016/0015-6264(75)90157-1. [DOI] [PubMed] [Google Scholar]
- CASTELLANI A. G., NIVEN C. F., Jr Factors affecting the bacteriostatic action of sodium nitrite. Appl Microbiol. 1955 May;3(3):154–159. doi: 10.1128/am.3.3.154-159.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HADJIPETROU L. P., STOUTHAMER A. H. ENERGY PRODUCTION DURING NITRATE RESPIRATION BY AEROBACTER AEROGENES. J Gen Microbiol. 1965 Jan;38:29–34. doi: 10.1099/00221287-38-1-29. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Inhibition of membrane transport in Streptococcus faecalis by uncouplers of oxidative phosphorylation and its relationship to proton conduction. J Bacteriol. 1968 Dec;96(6):2025–2034. doi: 10.1128/jb.96.6.2025-2034.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto M., Umeyama M., Chiba S. Alteration of fermentation products from butyrate to acetate by nitrate reduction in Clostridium perfringens. Z Allg Mikrobiol. 1974;14(2):115–121. doi: 10.1002/jobm.3630140206. [DOI] [PubMed] [Google Scholar]
- MCGILVERY R. W., MOKRASCH L. C. Purification and properties of fructose-1, 6-diphosphatase. J Biol Chem. 1956 Aug;221(2):909–917. [PubMed] [Google Scholar]
- McKay L. L., Walter L. A., Sandine W. E., Elliker P. R. Involvement of phosphoenolpyruvate in lactose utilization by group N streptococci. J Bacteriol. 1969 Aug;99(2):603–610. doi: 10.1128/jb.99.2.603-610.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer E. M., van der Zwaan J. W., Wever R., Stouthamer A. H. Anaerobic respiration and energy conservation in Paracoccus denitrificans. Functioning of iron-sulfur centers and the uncoupling effect of nitrite. Eur J Biochem. 1979 May 2;96(1):69–76. doi: 10.1111/j.1432-1033.1979.tb13014.x. [DOI] [PubMed] [Google Scholar]
- O'Leary V., Solberg M. Effect of sodium nitrite inhibition on intracellular thiol groups and on the activity of certain glycolytic enzymes in Clostridium perfringens. Appl Environ Microbiol. 1976 Feb;31(2):208–212. doi: 10.1128/aem.31.2.208-212.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gent-Ruijters M. L., DeVries W., Southamer A. H. Influence of nitrate on fermentation pattern, molar growth yields and synthesis of cytochrome b in Propionibacterium pentosaceum. J Gen Microbiol. 1975 May;88(1):36–48. doi: 10.1099/00221287-88-1-36. [DOI] [PubMed] [Google Scholar]



