Abstract
Background
Excessive proliferation and impaired apoptosis of pulmonary artery smooth muscle cells (PASMC) contributes to vascular obstruction in patients and fawn-hooded rats (FHR) with pulmonary arterial hypertension (PAH). Expression and activity of mitochondrial superoxide dismutase-2 (SOD2), the major generator of H2O2, is known to be reduced in PAH; however, the mechanism and therapeutic relevance of this is unknown.
Methods and Results
SOD2 expression in PASMC is decreased in PAH patients and FHR with PAH. FHR PASMC have higher proliferation and lower apoptosis rates than Sprague-Dawley PASMC. Moreover, FHR PASMC have hyperpolarized mitochondria, low H2O2 production and a reduced cytoplasmic and mitochondrial redox state. Administration of SOD2 siRNA to normal PASMC recapitulates the FHR-PAH phenotype, hyperpolarizing mitochondria, decreasing H2O2 and inhibiting caspase activity. Conversely, SOD2 over-expression in FHR PASMC, or therapy with the SOD-mimetic MnTBAP, reverses the hyperproliferative PAH phenotype. Importantly, SOD-mimetic therapy regresses PAH in vivo. Investigation of the SOD2 gene revealed no mutation, suggesting a possible epigenetic dysregulation. Genomic bisulfite sequencing demonstrates selective hypermethylation of a CpG island in an enhancer region of intron 2 and another in the promoter. Differential methylation occurs selectively in PA versus aortic SMC and is reversed by the DNA methyltransferase inhibitor, 5-aza-2′-deoxycytidine, restoring both SOD2 expression and the proliferation/apoptosis ratio. The expression of the enzymes that mediate gene methylation, DNA methyltransferases 1 and 3B, is upregulated in FHR lungs.
Conclusions
Tissue-specific, epigenetic SOD2 deficiency initiates and sustains a heritable form of PAH by impairing redox signaling and creating a proliferative, apoptosis-resistant PASMC. SOD augmentation regresses experimental PAH. The discovery of an epigenetic component to PAH may offer new therapeutic targets.
Keywords: Pulmonary arterial hypertension, Voltage-gated potassium channels (Kv1.5), Hypoxia-inducible factor-1α (HIF-1α), Epigenetic gene methylation, DNA methyltransferase
Introduction
Pulmonary arterial hypertension (PAH) is a syndrome in which obstructed, constricted small pulmonary arteries (PA) and increased pulmonary vascular resistance ultimately lead to right ventricular hypertrophy and failure. Despite important advances in understanding the mechanism of PAH, such as the discovery of mutations in bone morphogenetic protein receptors in familial PAH, and the advent of effective oral therapies, such as phosphodiesterase-5 inhibitors and endothelin antagonists, mortality remains high (15% at 1-year).
PAH may be viewed, in part, as a disease of excess proliferation and impaired apoptosis of the pulmonary artery smooth muscle cell (PASMC), reviewed in1. While this study focuses on PASMC, many cell types are abnormal in PAH. There is infiltration of the lung with inflammatory cells, disorganized endothelial proliferation in plexiform lesions, activation of myofibroblasts and disruption of the extracellular matrix. The animal model used in this study (the fawn-hooded rat, FHR) develops spontaneous PAH, characterized by excessive proliferation and impaired apoptosis of the PASMC and therefore this model is useful to study the role of PASMC in PAH. Moreover, FHR share with human PAH PASMC a disrupted mitochondrial network and low expression and activity of superoxide dismutase-2 (SOD2)2.
The proliferation/apoptosis imbalance in PAH suggests similarities to cancer3 although it should be noted that endothelial cells and PASMC do not invade the surrounding tissue or metastasize. Nevertheless, FHR-PAH and certain cancers share a metabolic profile that creates a “pseudohypoxic” cellular environment which favors proliferation. Relevant abnormalities include: 1) decreased superoxide dismutase-2 (SOD2)2, 4 2) normoxic activation of the transcription factor, hypoxia-inducible factor-1α (HIF-1α)2, 5, 6and 3) HIF-1α induced activation of pyruvate dehydrogenase kinase2, 7 which inhibits the Krebs’ cycle, shifting metabolism toward glycolysis2, 7. As a consequence, electron flux is reduced and generation of reactive oxygen species (ROS) in the mitochondria decreases.
There is evidence that implicates SOD2 in the development of idiopathic PAH. SOD2 downregulation precedes PAH in FHR2. In rats, the SOD2 gene is located on chromosome 1 and FH-BN1 consomic rats (identical to FHR save for introgression of a normal chromosome 1 do not develop PAH)2. Likewise, in human PAH, SOD2 deficiency is noted in PAs and plexiform lesions2, 4. Biological plausibility for an etiologic role for SOD2 is supported by the recent recognition that SOD2 is a putative tumor-suppressor gene and that epigenetic silencing of SOD2 increases proliferation of cancer cells8, 9.
SOD2 is the gatekeeper that regulates physiologic production of H2O2 (produced from mitochondrial superoxide during respiration). H2O2 is less toxic than superoxide and its greater diffusion radius allows it to serve as a signaling molecule. H2O2 modulates the activity of transcription factors such as HIF-1α (which it inhibits)10, 11, and sulfhydryl rich proteins, including the voltage-gated potassium channel, Kv1.512 (which it activates). Because the FHR’s PAH is heritable and yet sequencing showed the SOD2 gene has no mutations, we hypothesized that epigenetic suppression of SOD2 may initiate and/or sustain PAH. We report here that selective hypermethylation of CpG islands in the SOD2 gene promotes a proliferative, anti-apoptotic phenotype in PASMC. Remarkably, induction of SOD2 deficiency is sufficient to create a PAH phenotype in normal PASMC whilst correction of SOD2 deficiency has therapeutic benefit in FHR PASMC and regresses PAH in vivo. This epigenetic mechanism may have significant mechanistic and therapeutic implications in human PAH.
Methods
Animal studies
The University of Chicago Animal Care Committee approved all protocols. FHR were purchased from Charles Rivers Laboratories and then bred in our facility. FHR were studied at approximately age 40-weeks. Age-matched FH-BN1 rats and weight-matched Sprague Dawley rats (SDR) served as controls.
Statistics
Sample size for all experiments was ≥10/group, unless stated otherwise. Values are stated as mean±SEM. Inter-group differences between 2 groups were assessed by an unpaired Students’ t-test while an ANOVA with post hoc analysis using Fisher’s PLSD test was used for comparison amongst multiple groups. Normality was confirmed with a Kolmogorov-Smirnov or D’Agostino & Pearson omnibus test. For data that was not normally distributed (pulmonary artery muscularization after control or MnTBAP treatment), we used a Mann-Whitney test. A Fisher’s exact test was used to compare the number of muscularized vessels between MnTBAP and untreated FHR. A p<0.05 was considered statistically significant.
Detailed methods for cell culture, quantitative PCR, catheterization, echocardiography, functional capacity (treadmill), PASMC proliferation and apoptosis assays have been published previously7 and can be found in the supplemental methods. The methodology for quantitative lung histology is also found in the supplemental methods.
ROS in isolated resistance PAs and PASMC
ROS were measured in isolated resistance PAs using L-012 chemiluminescence or Electron Paramagnetic Resonance (EPR).
EPR
Isolated resistance PAs were incubated with a derivative of cyclic hydroxylamine (CMH) (200μM in Krebs-HEPES buffer with Desferrioxamine and DETC at 37°C). Changes in ROS production during a 5-minute acquisition period were quantified by measuring the peak height of EPR triplet signal, a measure of 3-methoxycarbonyl-proxyl nitroxide (CM•) kinetics.
Enhanced chemiluminescence
The PA was incubated in L-012 (100μM for 30-minutes, 37°C, Wako Chemicals), a superoxide-sensitive chemiluminescence enhancer that doesn’t undergo redox cycling2.
Amplex Red
Measurement of H2O2 production was performed in PASMC or PA rings using the fluorometric Amplex Red assay2.
Immunoblotting and immunofluorescence
Immunoblotting was performed on 25μg of protein pooled from n=3 4th division pulmonary arteries or isolated PASMC (on-line supplement)2.
Small interfering RNA (siRNA)
PASMC cells were grown to ~50% confluence and then exposed to anti-SOD2 siRNA. Several “on-target” siRNAs (Applied Biosystems) were tested and the optimal dose to achieve maximal knockdown with minimal toxicity was established. For each experiment there was a scrambled siRNA control. Details are given in the on-line supplement.
Replication deficient adenoviruses
FHR PASMCs were infected with serotype 5 recombinant adenovirus carrying the human SOD2 gene under a CMV promoter as described2 (see on-line supplement).
Redox-sensitive GFP constructs
Redox-sensitive GFP constructs (roGFP2) targeted either to the cytoplasm or mitochondria (a kind gift of Dr. Remington, University of Oregon) were transfected into PASMCs using Fugene HD transfection reagent (Roche). By using different excitation wavelengths (400 and 490nm) and measuring emission at 535nm, the redox status of cells was assessed (the higher the ratio 400/490nm, the more oxidized the PASMCs)13.
O2-consumption
O2-consumption was measured using a Mitocell MT200 chamber and 782 oxygen meter (Strathkelvin Instruments, North Lanarkshire, Scotland). Cells (106 in 500μl) were studied in culture medium at 37°C during a 10-minute protocol.
Metalloporphyrin Mn(III)tetrakis (4-benzoic acid) porphyrin (MnTBAP) therapy in vivo
MnTBAP is a synthetic, nontoxic, cell-permeable SOD mimetic. A daily 10 mg/kg dose of MnTBAP was selected based on preliminary data and the literature. Similar doses (10mg/kg IP every 3 days) are well tolerated and prevent proliferation of premalignant cells in a rat model of Barrett’s esophagus14. Once FHR had established PAH, as confirmed by a pulmonary artery acceleration time (PAAT) <25ms, they were randomized to receive MnTBAP versus vehicle for 4 weeks. Assessment of functional capacity (a graded treadmill test) and RV mass and function (echo) was performed, prior to and following therapy. The severity of PAH at study termination was determined by echo-Doppler and high-fidelity catheterization (age 44 weeks).
Bisulfite sequencing
Genomic DNA was isolated from resistance PAs of FH-BN1 consomic rats and FHR (treated with vehicle or 5-aza-2′-deoxycytidine; 1 mg/kg body weight, i.p. injection every 3 days for 2-weeks). Genomic DNA was digested with the restriction enzyme HindIII and then modified with sodium bisulfite using Zymo Research EZ DNA Methylation™ Kit. We designed primers specific to bisulfite modified DNA in 6 segments of the SOD2 promoter region approximately 2 kb upstream of the transcription initiation site and one segment within intron 2 (Supplemental Table 1). PCR products were isolated and purified by agarose gel electrophoresis using a QIAquick® Gel Isolation Kit (Qiagen) and cloned into the TOPO TA expression vector (Invitrogen). After transformation of one-shot, chemically competent E. coli cells, 10-15 clones were selected for sequencing. Sequences were then analyzed for methylation at all CpG locations.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
RESULTS
SOD2 expression is reduced in PASMC of FHR and patients with PAH
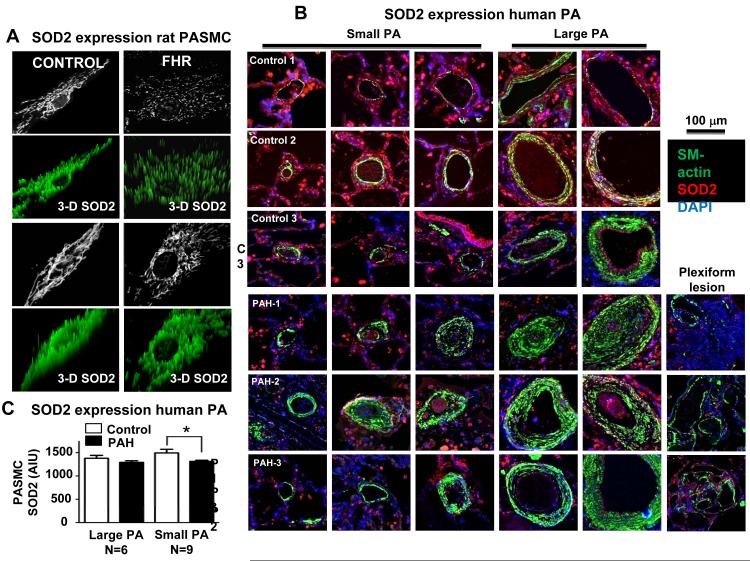
Immunostaining revealed a marked reduction in SOD2 expression and fragmentation of the mitochondrial reticulum in FHR PASMC (Figure 1A). In lung tissue harvested at autopsy, SOD2 expression was reduced within the media and adventitia of small PAs and in plexiform lesions (Figure 1B-C) of PAH versus control patients (without lung disease).
Figure 1. Decreased SOD2 expression in FHR and patients with WHO Category-1 PAH.
A) Confocal microscopy shows decreased PASMC SOD2 expression and increased mitochondrial fragmentation in FHR PASMC. Note the loss of SOD2 (green) in FHR as evidenced by the reduced intensity and peak height.
B) Confocal immunofluorescence images of 3 control patients (who died from non lung-related conditions, upper 3 rows) and 3 patients who died of PAH (lower rows). Note the medial hypertrophy and plexiform lesions in the PAH patients. SOD2 expression (red) is decreased in the media and adventitia of small PAs and in the plexiform lesions. α-smooth muscle actin (green) is increased in PAH.
C) mean±SEM of red fluorescence intensity showing decreased SOD2 expression in the media of small PAs. * p<0.05.
SOD2 knockdown recapitulates a “PAH phenotype” in normal PASMC
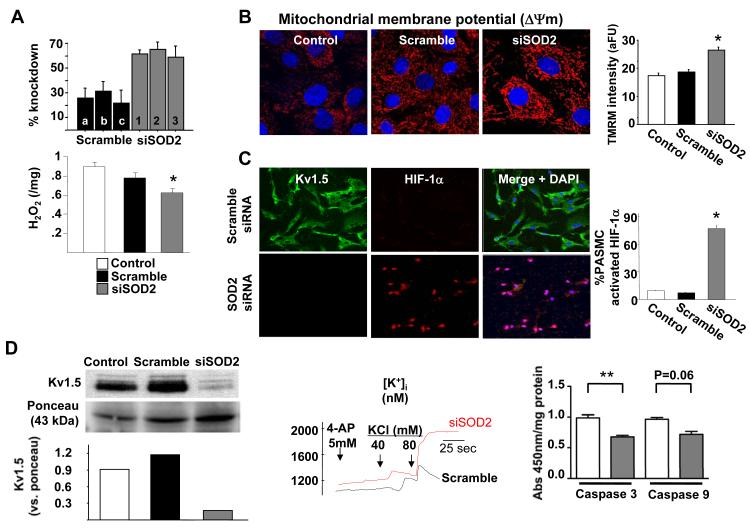
To assess whether downregulation of mitochondrial SOD2 was sufficient to contribute to the initiation of PAH, we used SOD2 siRNA to reduce SOD2 levels in normal Sprague-Dawley PASMC. SOD2 siRNA decreased SOD2 expression (mRNA and protein, Figure 2 and Supplemental Figure 1). siSOD2 also decreased H2O2 production (Figure 2A). SOD2 knockdown reduced the production of superoxide in normal PASMC, as demonstrated by a reduction of L-012 chemiluminescence. The L-012 signal is specifically inhibited by pegylated-SOD but not pegylated-catalase, confirming it is a measure of superoxide (Supplemental Figure 2). The mitochondrial membrane potential (ΔΨm) of SOD2 siRNA-treated cells became hyperpolarized (Figure 2B). We investigated the effects of SOD2 siRNA on two known abnormalities implicated in creating the excess proliferation/apoptosis ratio in FHR PAH: HIF-1α activation (translocation to the nucleus) and Kv1.5 downregulation2. SOD2 siRNA activated HIF-1α and downregulated Kv1.5 channel protein expression (Figure 2C&D) in Sprague-Dawley PASMC. The loss of the Kv1.5 channel had two consequences that are associated with apoptosis resistance and increased cell proliferation7, 15 namely, increased cytosolic K+ (Figure 2D) and Ca2+ (Supplemental Figure 1). This elevation of cytosolic K+ was associated with decreased caspase activity in PASMCs (Figure 2D)7. Therefore, reducing SOD2 expression recapitulated many abnormalities seen in FHR PASMC.
Figure 2. Inhibition of SOD2 expression in normal Sprague-Dawley PASMC recapitulates the PAH phenotype.
A) siRNAs reduce SOD2 mRNA and H2O2 production.
B) siSOD2 hyperpolarizes ΔΨm (increased red TMRM intensity).
C) SOD2 siRNA activates HIF-1α (note translocation to the nucleus-red in middle panel) and decreases Kv1.5 expression (decreased green, left panel).
D) Immunoblotting confirmed that siSOD2 decreases Kv1.5 expression (left panel). In line with loss of Kv channels, siSOD2 increased intracellular K+ concentration (middle panel). siSOD2 also reduced caspase activity (right panel).
Hypermethylation of the SOD2 gene in FHR and the therapeutic effects of the methyltransferase inhibitor 5-aza-2′-deoxycytidine
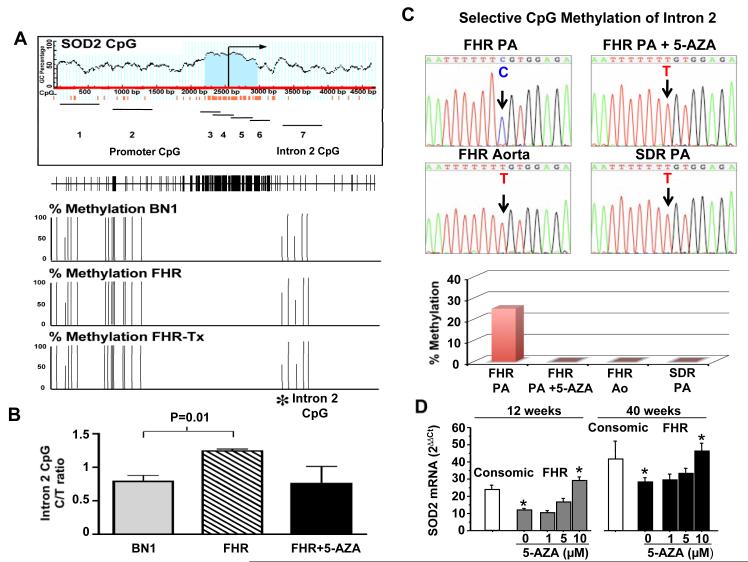
Sequencing of the entire SOD2 gene, beginning 600bp upstream from the transcriptional start site in Sprague Dawley and FHR, demonstrated identical sequences (data not shown). This suggested that decreased SOD2 expression in FHR might reflect epigenetic silencing of gene transcription, as occurs in cancer16, 17. We validated a bisulfite sequencing assay to measure methylation of key regions of the SOD2 gene and examined the effects of demethylation, using the methyltransferase inhibitor 5-aza-2′-deoxycytidine. Genomic DNA isolated from PAs of FHR, consomic FH-BN1 rats, and 5-aza-2′-deoxycytidine-treated FHR was analyzed. Although there are many CpG islands in the SOD2 gene, differential hypermethylation (relative to consomic FH-BN1 DNA) was found only in an enhancer region in intron 2 (Figure 3A-B). To test the effects of 5-aza-2′-deoxycytidine in vivo, we treated FHR and then isolated genomic DNA from PAs for SOD2 gene methylation analysis. 5-aza-2′-deoxycytidine decreased methylation of an intron 2 sequence (Figure 3 A-B). We next cultured PASMC from FHR and SDR and treated them with 5-aza-2′-deoxycytidine. Again, we identified a differentially methylated region within intron 2 (Figure 3C), which was demethylated in response to 5-aza. There was tissue heterogeneity in the differentially methylated sites, with methylation being present in the SOD2 gene isolated from FHR PA but not in FHR aortae. An additional site of differential methylation was found in the FHR’s SOD2 promoter (Supplemental Figure 3). 5-aza-2′-deoxycytidine increased SOD2 mRNA (Figure 3D) and protein (Figure 4A-B) in FHR PASMC in a dose-dependent manner (maximum 80% SOD2 induction at 10μM). 5-aza-2′-deoxycytidine also restored Kv1.5 expression (Figure 4C).
Figure 3. Methylation of SOD2 in FHR PASMC is reversible by 5-aza-2′-deoxycytidine.
A) Schematic of the CG dinucleotide percentage and CpG islands within the SOD2 promoter and first 2 KB after the transcriptional start site. 7 amplicons were surveyed within the SOD2 gene. Their approximate locations are represented by solid, horizontal lines. The positions of individual CpG dinucleotides are shown as vertical tick marks in the panel below the amplicon map. * denotes the differentially methylated CpG dinucleotides in FHR within amplicon 7 compared to BN1 tissue. No methylated CpG pairs were identified in amplicons 3-6.
B) Corresponding methylation percentage of the differentially methylated CpG in intron 2. Results are expressed as a frequency of cytosine methylation in PAs from consomic control BN1 rats (n=2), FHR (n=3) and FHR treated with 5-aza-2′-deoxycytidine (n=3).
C) Representative sequencing traces of genomic DNA from cultured PASMCs. Only methylated cytidines are protected against bisulfite-mediated deamination of cytidine into uridine (which is recognized as thymidine when the PCR product is amplified). As indicated by the arrow, the cytidine in FHR PASMCs was methylated (and therefore remains a cytidine, upper left) and this is reversed by 5-Azacytidine. The site is not methylated in FHR aortic SMC or in SDR PASMCs. The bar graph shows the mean data indicating the reversibility and tissue specificity of this SOD2 methylation in intron 2 in cultured PASMCs.
D) FHR PASMC have lower SOD2 mRNA levels versus consomic PASMC. 5-aza-2′-deoxycytidine (5-AZA) causes a dose-dependent increase in SOD2 expression.
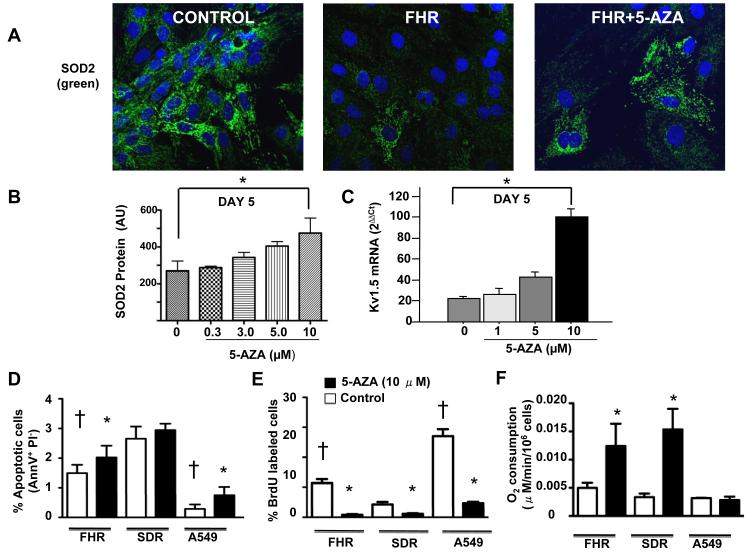
Figure 4. 5-aza-2′-deoxycytidine restores SOD2 expression, reduces proliferation and enhances apoptosis in FHR PASMC.
A-B) Representative and mean data showing that FHR PASMC express less SOD2 than SDR PASMC and this is partially reversible with 5-AZA treatment, n=4 per group. *p<0.05.
C) 5-aza-2′-deoxycytidine-induced, dose-dependent increase in Kv1.5 mRNA in FHR PASMC, *p<0.05.
D-E) 5-aza-2′-deoxycytidine (black bars) increases apoptosis and decreases proliferation in FHR PASMC and lung cancer cells (A549), but not SDR PASMC, n=4-6/group. *p<0.05 between baseline and 5-aza-2′-deoxycytidine, † significant difference from SDR PASMC.
F) 5-aza-2′-deoxycytidine increases O2-consumption in FHR and SDR PASMC, but not A549 cells (n=3-8/group).
SOD2 hypermethylation promotes a proliferative, apoptosis-resistant phenotype
Using flow cytometry we confirmed that under identical conditions FHR PASMC had roughly double the rate of proliferation and half the rate of apoptosis as Sprague-Dawley PASMC (Figure 4D-E). Remarkably, incubating FHR PASMC and lung adenocarcinoma A549 cells with 5-aza-2′-deoxycytidine for 3-days significantly decreased proliferation and caused a modest increase in apoptosis (Figure 4D-E). O2-consumption increased in FHR PASMC in response to 5-aza-2′-deoxycytidine (Figure 4F). These data suggest that methylation-induced SOD2 downregulation creates a low-O2-consumption metabolic state that favors proliferation and suppresses apoptosis.
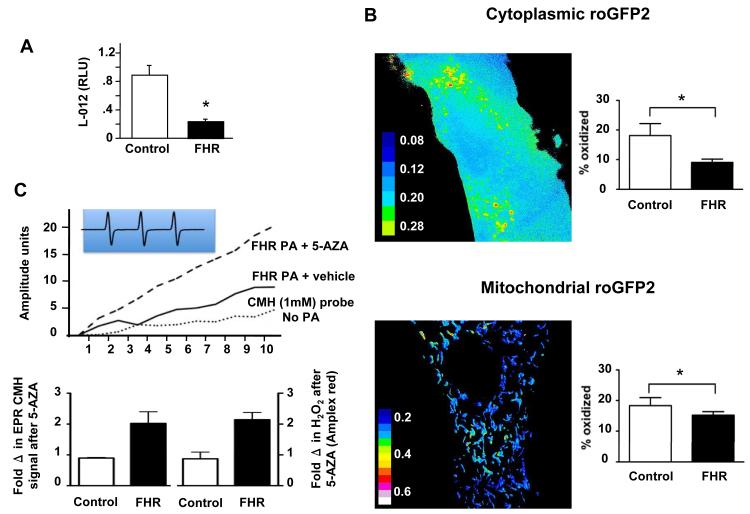
FHR PASMC produce less ROS and are in a reduced state
Baseline L-012 chemiluminescence was lower in FHR versus control PASMC (Figure 5A) and this was mirrored in a reduced redox state in the cytoplasm and mitochondria, as measured with compartment specific roGFPs (Figure 5B and Supplemental Figure 4). Consistent with this, SOD2 siRNA reduced L-012 chemiluminescence production in PASMCs (Supplemental Figure 2A) and 5-aza-2′-deoxycytidine restored ROS production in FHR PAs, as assessed by both chemiluminescence and electron paramagnetic resonance (EPR, Figure 5C). Conversely, 5-aza-2′-deoxycytidine had no effect on H2O2 production in normal PAs. Control experiments with pegylated-SOD and pegylated-catalase demonstrate the specificity of our measurements (Supplemental Figure 2B). Thus, there is a reversible, methylation-induced depression of H2O2 production in FHR.
Figure 5. Lower ROS levels and reduced cellular environment in FHR PASMC.
A) Decreased ROS levels in freshly isolated resistance PAs from FHR versus consomic rats, measured using L-012 chemiluminescence at 37°C.
B) PASMCs were transfected with redox-sensitive GFP constructs targeted to the cytoplasm (upper panel) and mitochondria (lower panel). FHR PASMC had a reduced cytoplasm and mitochondria, relative to Sprague Dawley PASMC. Dithiothreitol and tert-butyl hydroperoxide were used to completely reduce and oxidize the cells, respectively.
C) ROS mean data and representative trace (inset) showing the CMH triplet signal. Peak height (EPR amplitude units) is proportional to ROS levels and is normalized to wet weight. PAs from FHR treated with 5-aza-2′-deoxycytidine (black bars) have increased production of ROS (left) and H2O2 (right) versus untreated FHR. Control rats show no induction of ROS or H2O2, n=5/group.
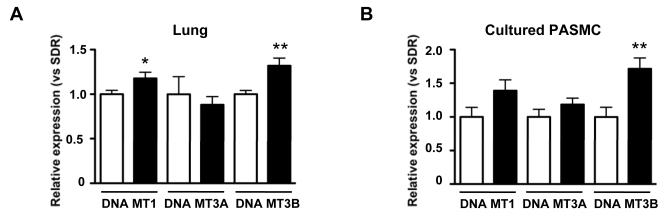
Increased expression of DNA methyltransferases in FHR
Since gene methylation is dependent on the activity of DNA methyltransferases (DNA MT), we measured their expression in FHR and control rats. In lung tissue, both the maintenance DNA methyltransferase (DNA MT1) and the de novo methyltransferase 3B (DNA MT3B) were significantly upregulated versus control (Figure 6A); a similar pattern was found in PASMCs from FHR (Figure 6B).
Figure 6. DNA methyltransferase expression is increased in FHR lung and PASMCs.
A)DNA MT1 and 3B mRNA is increased in FHR versus SDR lungs (n=12 each). *, **p<0.05 and 0.01, respectively. In low passage (3-4) PASMCs (n=8 in each group), FHR had higher DNA MT3B expression and a trend toward increased DNA MT1.
SOD2 augmentation restores FHR PASMC gene expression
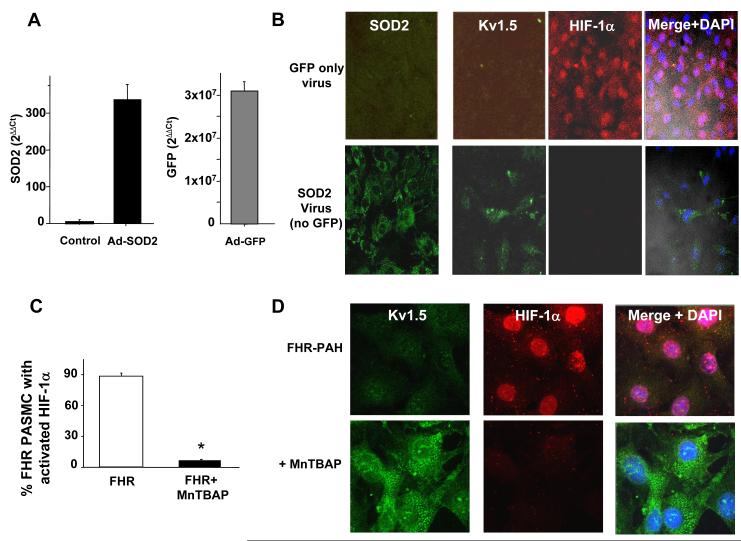
Since 5-aza-2′-deoxycytidine is a non-specific demethylating agent and may affect a multitude of genes, we investigated the therapeutic effects of direct SOD2 augmentation to assess its role in PAH. We used two complementary strategies: 1) direct SOD2 augmentation using adenoviral gene transfer carrying mitochondrially-targeted SOD2 and 2) administration of a membrane permeable SOD analog, MnTBAP.
Adenoviral-SOD2 significantly increased SOD2 expression in FHR PASMC (Figure 7A). This inactivated the spontaneously active HIF-1α seen in FHR and increased Kv1.5 expression (Figure 7B). Likewise, incubation of FHR PASMC with MnTBAP for 48-hours inactivated nuclear HIF-1α (Figure 7C-D) and restored Kv1.5 expression (Figure 7D).
Figure 7. SOD2 supplementation inactivates HIF-1α and increases Kv1.5 expression in FHR PASMC.
A) The SOD2 virus and a control GFP virus are effective, increasing expression of SOD2 and GFP mRNA, respectively, in FHR PASMC.
B) Immunofluorescence using an Alexa647 secondary antibody shows that the SOD2 adenovirus increases human SOD2 expression (green, left lower panel). This restores Kv1.5 expression (green) and inactivates HIF-1α (red nuclei). Nuclei are counterstained blue (DAPI).
C-D) 48-hours incubation with MnTBAP decreases nuclear localization of HIF-1α and restores Kv1.5 expression.
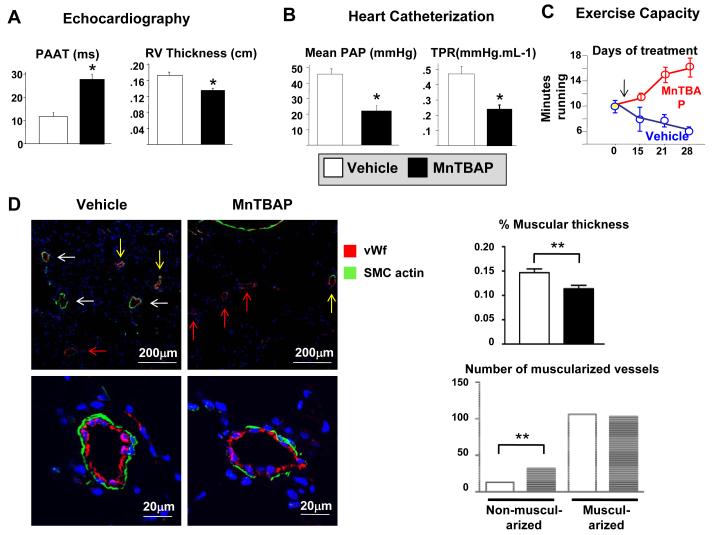
SOD2 augmentation regresses PAH in vivo
To test the therapeutic effects of SOD2 augmentation in vivo, we treated FHR with proven PAH with MnTBAP. MnTBAP was chosen over SOD2 due to the very transient upregulation of transgene expression (~2 weeks) that is often found with adenoviral gene therapy. MnTBAP doubled PAAT (Figure 8A) and caused a concomitant reduction in the mean PA pressure (Figure 8B) and RV free wall thickness (Figure 8A). During the 4-week treatment, exercise capacity deteriorated in vehicle-treated FHR whereas the MnTBAP group showed improvement (Figure 8C). Quantitative histology performed by blinded readers demonstrated that MnTBAP caused a significant reduction in the % medial thickness of precapillary resistance arteries and an increase in the percentage of small (25-50μm) non-muscular PAs (Figure 8D).
Figure 8. MnTBAP regresses PAH in FHR.
A) MnTBAP reduces mean PA pressure measured by Doppler (lengthens PAAT) and decreases right ventricular thickness in FHR treated for 4 weeks, n=5/group. * p<0.05.
B) MnTBAP therapy reduces mean PAP and total pulmonary resistance (TPR).
C) FHR treated with MnTBAP exercise longer on a graded treadmill, n=15/group.
D) Lung sections were stained for von Willebrand factor (red), alpha smooth muscle cell actin (green) and DAPI (blue). Note the fully muscularized (white arrows), partially muscularized (yellow arrows) and non-muscularized blood vessels (red arrows). Lower panel: A representative fully muscularized PA in a vehicle-treated FHR (left) versus MnTBAP (right). The % medial thickness of precapillary resistance PAs was reduced and the number of non-muscularized resistance PAs was increased by MnTBAP. ** p<0.01 versus control.
DISCUSSION
One of the major findings of this study is the identification of SOD2 methylation as a potential new, epigenetic mechanism for PAH in an animal model of spontaneous and heritable PAH. This may be relevant for clinical PAH, since SOD2 is also downregulated in human PAH (Figure 1). We identified hypermethylation of a CpG island in an enhancer region within intron 2 and the promoter of SOD2 as the basis for SOD2 downregulation in FHR. This appears to reflect significantly higher expression of DNA methyltransferase1 and 3B in lungs and of methyltranferase 3B in isolated PASMCs of FHR (Figure 6). This epigenetic down-regulation of SOD2 impairs H2O2-mediated redox signaling, activates HIF-1α and creates a proliferative, apoptosis-resistant state (Figures 4,5,7). Both the mechanism of SOD2 downregulation and its consequences parallel those recently discovered in human cancer16, 17, but to our knowledge, this is the first report of an epigenetic cause of a pulmonary vascular disease. The epigenetic down-regulation of SOD2 was reversible by treatment with the DNA methyltransferase inhibitor 5-aza-2′-deoxycytidine. Furthermore, our study demonstrates that augmentation of SOD2 (by 3 complementary strategies) restores mitochondrial function, inhibits PASMC proliferation and increases cell apoptosis in vitro. In vivo, MnTBAP causes partial regression of established PAH and decreases the muscularization of pulmonary precapillary resistance vessels (Figure 8A-D).
The FHR’s SOD2 deficiency is the consequence of covalent cytosine methylation that occurs in dinucleotide CpGs (Figure 3). Methylation at cytosine’s C5 atom inhibits gene expression by preventing the binding of transcription factors18. Methylation is reversible, heritable and can be tissue-specific19. Indeed, SOD2 methylation occurred in the FHR PA but not in the FHR aorta (Figure 3), thus pointing to tissue specific epigenetic mechanisms. This finding may contribute to understanding the localization of the pathology in human and FHR PAH to the pulmonary circulation. The SOD2 promoter and several of its introns have CpG islands that offer potential methylation sites. Using genomic bisulfite sequencing we directly confirmed that FHR have 2 discrete sites of differential hypermethylation (one in intron 2, the other in the promoter) (Figure 3A-C and Supplemental Figure 3). Although many regions in the SOD2 gene are heavily methylated, these two were the only CpG islands that were both differentially methylated and demethylated by 5-aza-2′-deoxycytidine. The intron 2 site is in the same region of the SOD2 gene that has been identified in transformed human lung fibroblast cell lines20. 5-aza-2′-deoxycytidine covalently binds and irreversibly inhibits DNA methyltransferases21, resulting in reactivation of transcription of previously methylated genes after a requisite cell division. It is unclear why 5-aza-2′-deoxycytidine had no effect on other areas of methylation, but other studies have described similar observations17.
CpG methylation is established and maintained by 3 DNA methyltransferases22. DNA methyltransferase 1 is expressed in proliferating tissues and its activity is coupled to DNA replication, copying methylation patterns from the parental to the daughter strand. Therefore, it is considered primarily a maintenance methyltransferase. In contrast, DNA methyltransferases 3A and 3B are considered “de novo” methyltransferases that can establish new methylation patterns. We identified an upregulation of DNA MT3B in FHR PASMC (Figure 6), which would be an elegant explanation for the observed hypermethylation of the SOD2 gene. Interestingly, DNA MT3B depletion is sufficient to reactivate methylation-silenced genes and decrease proliferation in both human breast adenocarcinoma and A549 cells23.
It is interesting that changes in the activity or expression of DNA methyltransferases can have a relatively specific outcome in terms of site-specific methylation and regulation of specific genes in certain tissues. Current models suggest that the specificity of DNA methyltransferase activity can depend on their expression levels or their interaction with other epigenetic regulators. In the current study, for example, we found that FHR animals with PAH had higher expression levels of DNA MT3B. This DNA methyltransferase has been shown to have distinct CpG methylation activity patterns, which depend on the tissue environment and on the expression of differentially spliced isoforms24. Another level of DNA methylation specificity is achieved by the interaction of DNA methyltransferases with other epigenetic regulators such as histone deacetylases. For example, DNA MT3B contains an ATRX homology domain that interacts with histone deacetylase 125. Interestingly, such an association between increased CpG methylation and decreased histone acetylation has been observed for the SOD2 gene16.
Gene methylation has an established role in promoting pathological cell proliferation in cancer. SOD2, a candidate tumor-suppressor gene8, 9, is silenced in several malignancies16, 17, 26. In multiple myeloma and pancreatic carcinoma the epigenetic silencing of SOD2 is caused by hypermethylation of CpG islands within SOD2’s promoter17, 26, 27. Demethylation of SOD2 in cancer restores SOD2, increases H2O2 and decreases cell proliferation and tumor growth16, 17, 26, consistent with our observations (Figure 3D and 4D-E). The effects of directly overexpressing SOD2 (by 3 complementary means) are concordant with the effects of SOD2 demethylation in cancer28 (Figure 7A-B). Our study demonstrates that reversing gene methylation is beneficial in FHR PASMC in vitro. Treatment with 5-aza-2′-deoxycytidine decreases SOD2 methylation and causes a dose-dependent increase in SOD2 and Kv1.5 expression in FHR PASMC (Figure 4A-C).
Production of H2O2 is a critical link between SOD2 expression and regulation of proliferation (Supplemental Figure 5 provides a schematic representation of the cascade of the proposed transcriptional, metabolic and redox consequences of SOD2 downregulation). Simply knocking down SOD2 expression in a normal PASMC diminishes endogenous H2O2 production and leads to an associated activation of HIF-1α (Figure 2). Moreover, an anti-SOD2 siRNA caused many of the other abnormalities seen in human and FHR PAH, including a decrease in expression of Kv1.5 channels and a rise in cytosolic calcium (Supplemental Figure 1B). Conversely, augmenting SOD2 elevates production of H2O2 in FHR PAs (Figure 5C) and reduces cell proliferation (Figure 4E). Similarly, in prostate cancer, over-expressing SOD2 increases H2O2 and reduces cell proliferation29. Thus, in both PAH and cancer there is an inverse correlation between SOD2 activity and H2O2-mediated cell proliferation29. Catalase, which reduces H2O2 levels, prevents SOD2’s ability to inhibit cancer cell proliferation29. These observations suggest that the effect of SOD2 augmentation in PAH and cancer is mediated by increasing H2O2 production (from a deficient starting level). We support this contention by showing that 5-aza-2′-deoxycytidine only increases H2O2 production in FHR PASMC, having little effect on normal PASMC (Figure 5C).
Our study also identifies the downstream mechanism by which this mitochondrial abnormality promotes cell proliferation-namely, normoxic activation of HIF-1α. HIF-1α activation has previously been identified in human PAH2, 6 and FHR PAH2. The current study supports a key role for H2O2 as the link between SOD2 and cell proliferation, as schematized in Supplemental Figure 5. Although ROS are toxic at high levels, there is a physiologic level of H2O2 production by mitochondria during normal oxidative metabolism12, 30. Physiologic levels of H2O2 serve as redox signaling molecules, involved in oxygen-sensing12. The evidence linking SOD2 levels to ROS in PAH is clear. Simply lowering SOD2 message levels (using siRNA) decreases mitochondrial H2O2 production (Figure 2A); conversely, demethylating the SOD2 gene enhancer in FHR PAs restores SOD2 expression, leading to increased ROS and mitochondrial H2O2 production (Figure 5C). Compartment-specific, redox-sensitive, green fluorescent proteins demonstrated that the net change in redox state in FHR (vs Sprague-Dawley) PASMC is reduction (both in the cytosol and mitochondria), consistent with the observed decreased ROS levels in FHR (Figure 5).
Therapeutic implications
Evidence for the relevance of SOD2 deficiency in PAH comes from the concordant benefit of 3 different strategies that restore SOD2 expression or SOD activity in our study. In FHR PASMC, SOD2 gene therapy, application of the SOD2 mimetic MnTBAP, or 5-aza-2′-deoxycytidine each led to HIF-1α inactivation and restoration of Kv1.5 expression (Figure 7). Although these surrogate endpoints are important, the key therapeutic finding of our study is that MnTBAP treatment regresses PAH in vivo (Figure 8A-D). This hemodynamic benefit is associated with a reduction in right ventricular hypertrophy, improved functional capacity and lung histology. The concordant findings in response to complementary strategies to modulate SOD2 expression reduces the possibility of artifacts related to flaws in any single strategy such as the stress of intraperitoneal MnTBAP injections, the inflammation from the SOD2 adenovirus, and potential confounding effects of demethylation of non-target genes by 5-aza-2′- deoxycytidine.
The benefits of MnTBAP are consistent with those of tempol (another membrane permeable SOD mimetic) which decreases hypoxic pulmonary hypertension in rats31 and recombinant SOD1, which ameliorates persistent pulmonary hypertension in newborn lambs32. Bowers et al. have also noted decreased SOD2 in the PAs in human PAH4; however, their interpretation was that PAH is a condition of increased oxidative stress (in part because other oxidant markers were elevated). Our data indicate that in FHR, H2O2 is subphysiologic due to both a primary effect, methylation-induced reduction of the SOD2 gene expression, and a secondary effect, pyruvate dehydrogenase kinase-mediated inhibition of oxidative metabolism2. Identification of the therapeutic potential for 5-aza-2′-deoxycytidine is particularly relevant because 5-aza-2′-deoxycytidine (Decitabine) is approved for human use in myeloproliferative disorders (where it demethylates p15).
Limitations
There are limitations in using human tissue, notably we are unsure of their smoking status. We acknowledge smoking could change SOD2 levels33, however this is unlikely to be an important confounder as the rodent data are consistent with the human data. We acknowledge that the net effect of loss of SOD2 is controversial (oxidation in SOD2 knockout mice34 versus reduction in our paper). Interestingly the SOD2 haploinsufficient mouse has a doubling of cancer risk34, supporting our central finding that downregulation of this mitochondrial enzyme is a proliferative, anti-apoptotic signal. We suspect the differences in ROS relate to the duration and severity of SOD2 loss (life long and more profound) for the knockout mouse versus acquired and more modest in FHR.
Finally, pyruvate dehydrogenase kinase activation in PAH thwarts mitochondrial respiration, limiting input to the mitochondrial electron transport chain. While in FHR low ROS and a reduced state activate HIF-1α, others find that ROS stabilizes HIF-1α35-37.
Interestingly, Oberley’s group noted in cancer that siSOD2 activates HIF-1α, consistent with our findings. However, they found that siSOD2 increased superoxide levels without changing H2O2 levels37. This again may relate to the magnitude of the SOD2 decrease achieved and cell specific differences in metabolic activity, prolyl hydroxylase activity and the status of the many other antioxidant systems. Our findings are supported by earlier studies showing that short pre-exposure of cells to H2O2 selectively prevents hypoxia-induced accumulation of HIF-1α protein10.
In summary, the recognition of a novel epigenetic mechanism of PAH (methylation-induced attenuation of SOD2 expression) may partially explain the excessive cell proliferation and decreased apoptosis in PAH and could offer new therapeutic targets.
Clinical perspective.
Pulmonary arterial hypertension (PAH) is characterized by remodeling of small precapillary resistance arteries, leading to increased vascular resistance and right ventricular failure. PAH is increasingly seen as a disease in which vascular obstruction is caused not only by vasoconstriction and inflammation, but also by enhanced proliferation and impaired apoptosis of vascular cells. The discovery that most patients with familial PAH have mutations in the bone morphogenetic protein receptor type II gene highlighted a potential genetic basis for PAH. However, most sporadic PAH patients do not have these mutations. Here we report that both fawn hooded rats (a strain with spontaneous PAH) and humans with PAH have a fragmented mitochondrial network and decreased expression of mitochondrial superoxide dismutase 2 (SOD2) in their pulmonary artery smooth muscle cells (PASMC). SOD2 is an important generator of hydrogen peroxide, which at physiological levels is a signaling molecule. In cancer, SOD2 is considered a potential oncogene and its expression is depressed. Suppression of SOD2 in normal PASMC recapitulates the proliferative, PAH phenotype. Interestingly, SOD2 downregulation is not due to gene mutation, rather the SOD2 gene is epigenetically silenced by specific (and reversible) methylation of specific CpG islands in the promoter and intron 2. This epigenetic inhibition of gene transcription is associated with increased DNA methyltransferase expression. SOD supplementation or demethylation strategies reverse the hyperproliferative phenotype of the FHR PASMC and regress experimental PAH in vivo. This is the first demonstration of an epigenetic basis for a heritable vascular disease and has etiologic and therapeutic implications.
Supplementary Material
Acknowledgments
Funding Sources This work is supported by NIH-RO1-HL071115 and 1RC1HL099462-01, the American Heart Association (AHA) and the Roche Foundation for Anemia Research.
Footnotes
Disclosures None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Stephen L. Archer, Section of Cardiology, Department of Medicine, University of Chicago, Chicago, Illinois.
Glenn Marsboom, Section of Cardiology, Department of Medicine, University of Chicago, Chicago, Illinois.
Gene H. Kim, Section of Cardiology, Department of Medicine, University of Chicago, Chicago, Illinois.
Hannah J. Zhang, Section of Cardiology, Department of Medicine, University of Chicago, Chicago, Illinois.
Peter T. Toth, Section of Cardiology, Department of Medicine, University of Chicago, Chicago, Illinois.
Eric C. Svensson, Section of Cardiology, Department of Medicine, University of Chicago, Chicago, Illinois.
Mardi Gomberg-Maitland, Section of Cardiology, Department of Medicine, University of Chicago, Chicago, Illinois.
Jalees Rehman, Section of Cardiology, Department of Medicine, University of Chicago, Chicago, Illinois.
References
- 1.Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1{alpha}-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol. 2008;294:H570–578. doi: 10.1152/ajpheart.01324.2007. [DOI] [PubMed] [Google Scholar]
- 2.Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thébaud B, Bonnet SN, Haromy A, Harry G, Moudgil R, McMurtry MS, Weir E, Archer SL. An abnormal mitochondrial-HIF-1-Kv channel pathway disrupts oxygen-sensing and triggers pulmonary arterial hypertension (PAH) in fawn-hooded rats: similarities to human PAH. Circulation. 2006;113:2630–2641. doi: 10.1161/CIRCULATIONAHA.105.609008. [DOI] [PubMed] [Google Scholar]
- 3.Voelkel NF, Cool C, Lee SD, Wright L, Geraci MW, Tuder RM. Primary pulmonary hypertension between inflammation and cancer. Chest. 1998;114:225S–230S. doi: 10.1378/chest.114.3_supplement.225s. [DOI] [PubMed] [Google Scholar]
- 4.Bowers R, Cool C, Murphy RC, Tuder RM, Hopken MW, Flores SC, Voelkel NF. Oxidative stress in severe pulmonary hypertension. Am J Respir Crit Care Med. 2004;169:764–769. doi: 10.1164/rccm.200301-147OC. [DOI] [PubMed] [Google Scholar]
- 5.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Tuder RM, Chacon M, Alger L, Wang J, Taraseviciene-Stewart L, Kasahara Y, Cool CD, Bishop AE, Geraci M, Semenza GL, Yacoub M, Polak JM, Voelkel NF. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: evidence for a process of disordered angiogenesis. J Pathol. 2001;195:367–374. doi: 10.1002/path.953. [DOI] [PubMed] [Google Scholar]
- 7.Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta L, Bonnet S, Harry G, Hashimoto K, Porter CJ, Andrade MA, Thebaud B, Michelakis ED. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11:37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 8.Li N, Oberley TD, Oberley LW, Zhong W. Overexpression of manganese superoxide dismutase in DU145 human prostate carcinoma cells has multiple effects on cell phenotype. Prostate. 1998;35:221–233. doi: 10.1002/(sici)1097-0045(19980515)35:3<221::aid-pros8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 9.Bravard A, Sabatier L, Hoffschir F, Ricoul M, Luccioni C, Dutrillaux B. SOD2: a new type of tumor-suppressor gene? Int J Cancer. 1992;51:476–480. doi: 10.1002/ijc.2910510323. [DOI] [PubMed] [Google Scholar]
- 10.Huang LE, Arany Z, Livingston DM, Bunn HF. Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its alpha subunit. J Biol Chem. 1996;271:32253–32259. doi: 10.1074/jbc.271.50.32253. [DOI] [PubMed] [Google Scholar]
- 11.Wang GL, Jiang BH, Semenza GL. Effect of altered redox states on expression and DNA-binding activity of hypoxia-inducible factor 1. Biochem Biophys Res Commun. 1995;212:550–556. doi: 10.1006/bbrc.1995.2005. [DOI] [PubMed] [Google Scholar]
- 12.Weir EK, Lopez-Barneo J, Buckler KJ, Archer SL. Acute oxygen-sensing mechanisms. N Engl J Med. 2005;353:2042–2055. doi: 10.1056/NEJMra050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dooley CT, Dore TM, Hanson GT, Jackson WC, Remington SJ, Tsien RY. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J Biol Chem. 2004;279:22284–22293. doi: 10.1074/jbc.M312847200. [DOI] [PubMed] [Google Scholar]
- 14.Martin RC, Liu Q, Wo JM, Ray MB, Li Y. Chemoprevention of carcinogenic progression to esophageal adenocarcinoma by the manganese superoxide dismutase supplementation. Clin Cancer Res. 2007;13:5176–5182. doi: 10.1158/1078-0432.CCR-07-1152. [DOI] [PubMed] [Google Scholar]
- 15.Platoshyn O, Yu Y, Golovina VA, McDaniel SS, Krick S, Li L, Wang JY, Rubin LJ, Yuan JX. Chronic hypoxia decreases K(V) channel expression and function in pulmonary artery myocytes. Am J Physiol Lung Cell Mol Physiol. 2001;280:L801–812. doi: 10.1152/ajplung.2001.280.4.L801. [DOI] [PubMed] [Google Scholar]
- 16.Hitchler MJ, Oberley LW, Domann FE. Epigenetic silencing of SOD2 by histone modifications in human breast cancer cells. Free Radic Biol Med. 2008;45:1573–1580. doi: 10.1016/j.freeradbiomed.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurt EM, Thomas SB, Peng B, Farrar WL. Molecular consequences of SOD2 expression in epigenetically silenced pancreatic carcinoma cell lines. Br J Cancer. 2007;97:1116–1123. doi: 10.1038/sj.bjc.6604000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ngo VM, Laverriere JN, Gourdji D. CpG methylation represses the activity of the rat prolactin promoter in rat GH3 pituitary cell lines. Mol Cell Endocrinol. 1995;108:95–105. doi: 10.1016/0303-7207(94)03462-3. [DOI] [PubMed] [Google Scholar]
- 19.Razin A, Kantor B. DNA methylation in epigenetic control of gene expression. Prog Mol Subcell Biol. 2005;38:151–167. doi: 10.1007/3-540-27310-7_6. [DOI] [PubMed] [Google Scholar]
- 20.Huang Y, He T, Domann FE. Decreased expression of manganese superoxide dismutase in transformed cells is associated with increased cytosine methylation of the SOD2 gene. DNA Cell Biol. 1999;18:643–652. doi: 10.1089/104454999315051. [DOI] [PubMed] [Google Scholar]
- 21.Taylor SM, Jones PA. Mechanism of action of eukaryotic DNA methyltransferase. Use of 5-azacytosine-containing DNA. J Mol Biol. 1982;162:679–692. doi: 10.1016/0022-2836(82)90395-3. [DOI] [PubMed] [Google Scholar]
- 22.Goll MG, Bestor TH. Eukaryotic cytosine methyltransferases. Annu Rev Biochem. 2005;74:481–514. doi: 10.1146/annurev.biochem.74.010904.153721. [DOI] [PubMed] [Google Scholar]
- 23.Beaulieu N, Morin S, Chute IC, Robert MF, Nguyen H, MacLeod AR. An essential role for DNA methyltransferase DNMT3B in cancer cell survival. J Biol Chem. 2002;277:28176–28181. doi: 10.1074/jbc.M204734200. [DOI] [PubMed] [Google Scholar]
- 24.Robertson KD, Uzvolgyi E, Liang G, Talmadge C, Sumegi J, Gonzales FA, Jones PA. The human DNA methyltransferases (DNMTs) 1, 3a and 3b: coordinate mRNA expression in normal tissues and overexpression in tumors. Nucleic Acids Res. 1999;27:2291–2298. doi: 10.1093/nar/27.11.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bachman KE, Rountree MR, Baylin SB. Dnmt3a and Dnmt3b are transcriptional repressors that exhibit unique localization properties to heterochromatin. J Biol Chem. 2001;276:32282–32287. doi: 10.1074/jbc.M104661200. [DOI] [PubMed] [Google Scholar]
- 26.Hurt EM, Thomas SB, Peng B, Farrar WL. Integrated molecular profiling of SOD2 expression in multiple myeloma. Blood. 2007;109:3953–3962. doi: 10.1182/blood-2006-07-035162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodge DR, Xiao W, Peng B, Cherry JC, Munroe DJ, Farrar WL. Enforced expression of superoxide dismutase 2/manganese superoxide dismutase disrupts autocrine interleukin-6 stimulation in human multiple myeloma cells and enhances dexamethasone-induced apoptosis. Cancer Res. 2005;65:6255–6263. doi: 10.1158/0008-5472.CAN-04-4482. [DOI] [PubMed] [Google Scholar]
- 28.Kim A, Zhong W, Oberley TD. Reversible modulation of cell cycle kinetics in NIH/3T3 mouse fibroblasts by inducible overexpression of mitochondrial manganese superoxide dismutase. Antioxid Redox Signal. 2004;6:489–500. doi: 10.1089/152308604773934251. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez AM, Carrico PM, Mazurkiewicz JE, Melendez JA. Mitochondrial or cytosolic catalase reverses the MnSOD-dependent inhibition of proliferation by enhancing respiratory chain activity, net ATP production, and decreasing the steady state levels of H(2)O(2) Free Radic Biol Med. 2000;29:801–813. doi: 10.1016/s0891-5849(00)00362-2. [DOI] [PubMed] [Google Scholar]
- 30.Schroder E, Eaton P. Hydrogen peroxide as an endogenous mediator and exogenous tool in cardiovascular research: issues and considerations. Curr Opin Pharmacol. 2008;8:153–159. doi: 10.1016/j.coph.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 31.Elmedal B, de Dam MY, Mulvany MJ, Simonsen U. The superoxide dismutase mimetic, tempol, blunts right ventricular hypertrophy in chronic hypoxic rats. Br J Pharmacol. 2004;141:105–113. doi: 10.1038/sj.bjp.0705580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinhorn RH, Albert G, Swartz DD, Russell JA, Levine CR, Davis JM. Recombinant human superoxide dismutase enhances the effect of inhaled nitric oxide in persistent pulmonary hypertension. Am J Respir Crit Care Med. 2001;164:834–839. doi: 10.1164/ajrccm.164.5.2010104. [DOI] [PubMed] [Google Scholar]
- 33.Harju T, Kaarteenaho-Wiik R, Sirvio R, Paakko P, Crapo JD, Oury TD, Soini Y, Kinnula VL. Manganese superoxide dismutase is increased in the airways of smokers’ lungs. Eur Respir J. 2004;24:765–771. doi: 10.1183/09031936.04.00121203. [DOI] [PubMed] [Google Scholar]
- 34.Van Remmen H, Ikeno Y, Hamilton M, Pahlavani M, Wolf N, Thorpe SR, Alderson NL, Baynes JW, Epstein CJ, Huang TT, Nelson J, Strong R, Richardson A. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics. 2003;16:29–37. doi: 10.1152/physiolgenomics.00122.2003. [DOI] [PubMed] [Google Scholar]
- 35.BelAiba RS, Djordjevic T, Bonello S, Flugel D, Hess J, Kietzmann T, Gorlach A. Redox-sensitive regulation of the HIF pathway under non-hypoxic conditions in pulmonary artery smooth muscle cells. Biol Chem. 2004;385:249–257. doi: 10.1515/BC.2004.019. [DOI] [PubMed] [Google Scholar]
- 36.Bell EL, Klimova TA, Eisenbart J, Moraes CT, Murphy MP, Budinger GR, Chandel NS. The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production. J Cell Biol. 2007;177:1029–1036. doi: 10.1083/jcb.200609074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaewpila S, Venkataraman S, Buettner GR, Oberley LW. Manganese superoxide dismutase modulates hypoxia-inducible factor-1 alpha induction via superoxide. Cancer Res. 2008;68:2781–2788. doi: 10.1158/0008-5472.CAN-07-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.