Abstract
Mice harboring a null mutation in Abca4/Abcr serve as a model of autosomal recessive Stargardt disease. Consistent with the human retinal disorder, deficiency in Abcr is associated with substantial accumulations of lipofuscin pigments in retinal pigment epithelial (RPE) cells. To observe for photoreceptor cell degeneration in these mutant mice, outer nuclear layer (ONL) thickness was measured at 200 μm intervals superior and inferior to the optic nerve head. ONL width in Abcr−/− mouse was reduced at 8–9 month and 11 and 13 months relative to Abcr+/+ mice; thinning was more pronounced centrally and in superior retina. The numbers of photoreceptor nuclei spanning the width of the outer nuclear layer were also reduced. No evidence of age-related ONL thinning was observed in Abcr+/+ mice at these ages. We conclude that albino Abcr−/− mice exhibit progressive photoreceptor cell loss that is detectable at 8 months of age and that has worsened by 11 and 13 months of age. The measurement of ONL thickness is an established approach to assessing photoreceptor cell integrity and can be used in preclinical studies using Abcr−/− mice.
61.1 Introduction
Mutations in ABCA4 (ABCR), the gene encoding the photoreceptor-specific ATP-binding cassette transporter (Sun et al. 1999), are responsible for some types of inherited retinal degeneration including an autosomal recessive form of retinitis pigmentosa, recessive cone-rod dystrophy and recessive Stargardt disease (Klevering et al. 2004; Maugeri et al. 2000; Shroyer et al. 2001). All of these inherited blinding disorders are characterized by excessive accumulations of autofluorescent lipofuscin in retinal pigment epithelial (RPE) cells. This disease feature is replicated in the Abcr null mutant mouse (Weng et al. 1999) wherein levels of the lipofuscin fluorophores A2E and isoA2E are increased several fold (Kim et al. 2004, 2007; Mata et al. 2001; Weng et al. 1999). Even greater increases in another lipofuscin pigment all-trans-retinal dimer-phosphatidylethanolamine (atRAL dimer-PE) are observed (Kim et al. 2007). Characterization of the Abcr−/− mouse retina also revealed delayed dark adaptation, increased levels of all-trans-retinal and elevated phosphatidylethanolamine (Weng et al. 1999). Although at 6 months of age, the numbers of photoreceptor nuclei were found not to be diminished (Mata et al. 2001), it was recently reported that in 11 month old Abcr−/− mice fed both control and vitamin A supplemented diet, the numbers of rows of nuclei across the outer nuclear layer was reduced as compared to wild-type mice (Radu et al. 2008).
Several therapeutic strategies aimed at alleviating vision loss in recessive Stargardt disease have been tested in Abcr−/− mice. These approaches include vector-based gene therapies (Kong et al. 2008) and the administration of compounds that limit the visual cycle including isotretinoin (Radu et al. 2003), an inhibitor of 11-cis-retinol dehydrogenase; the retinoid analog fenretinide that lowers serum vitamin A (Radu et al. 2005); and compounds that target RPE65 (Maeda et al. 2008; Maiti et al. 2006). In these pre-clinical studies quantitation of A2E served as the therapeutic outcome measure.
Although HPLC quantitation of the lipofuscin pigment A2E serves as an objective measure of therapeutic efficacy, additional endpoint measures are desirable. Since the measurement of outer nuclear layer thickness is a widely accepted approach to assessing photoreceptor cell integrity (Lavail et al. 1987; Michon et al. 1991), we have undertaken to compare outer nuclear layer (ONL) thickness in age-matched albino Abcr−/− and Abcr+/+ mice, homozygous for the Leu-450 allele of Rpe65.
61.2 Methods
61.2.1 Animals and Rearing
Albino Abca4/Abcr null mutant mice homozygous for Rpe65-Leu450, were generated and genotyped for the Abcr null mutation and Rpe65-Leu450Met variant by PCR-amplification of tail DNA as previously reported (Kim et al. 2004). For Rpe65, digestion of the 545-bp product with MwoI restriction enzyme (New England Biolabs), yielded 180- and 365-bp fragments if the sequence corresponded to Leu-450; Met-450 was associated with the undigested 545-bp band; and heterozygous mice exhibited all 3 bands. Abcr−/− and Abcr+/+ mice were raised under 12-h on-off cyclic lighting with in-cage illuminance of 30–80 lux. Mice were anaesthetized and perfused with 4% paraformaldehyde in phosphate buffered saline. Following enucleation, eyes were immersed in 4% paraformaldehyde for 24 h at 4°C. The proposed research involving animals has been approved by the Institutional Animal Care and Use Committee (IACUC).
61.2.2 Measurement of Outer Nuclear Layer Thickness
Sagittal 6-mm paraffin serial sections of murine retina were prepared and stained with hematoxylin and eosin. Microscopic images were acquired and analyzed using a digital imaging system (Leica Microsystems; Leica Application suite; Welzlar, Germany). For measurement of ONL thickness, two to three sections through the optic nerve head of the left eyes were imaged with a 10 × objective. ONL thickness was measured at 200 μm intervals superior and inferior to the edge of the optic nerve head along the vertical meridian; ONL width in pixels was converted to microns (1 pixel: 0.92 μm) and data from the three sections were averaged. For groups of Abcr−/− and Abcr+/+ mice at each age, mean ONL thickness at each position along the vertical meridian was plotted as a function of eccentricity from the optic nerve head (Mittag et al. 1999; Tanito et al. 2005). Values were compared by unpaired t-test or one-way analysis of variance (ANOVA) as appropriate, and significance was assessed at the 0.05 level (GraphPad Software Inc, La Jolla CA).
61.2.3 Counting Photoreceptor Nuclei
The numbers of nuclei extending across the width of the ONL were determined in superior hemiretina at a fixed distance of 600 μm from the edge of the optic disc. Counting was performed using a digital image obtained from one section per eye photographed with a 63 × objective. Three lines were drawn (5 μm apart) at this position and nuclei traversed by the line were counted by 2 individuals, one of whom was masked to mouse age and genotype. Nuclei counts obtained by the 2 individuals along the three lines were averaged to give a value for each eye. Values were compared by unpaired t-test.
61.3 Results
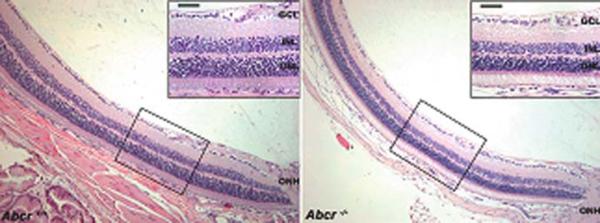
We probed for evidence of photoreceptor cell degeneration in Abcr−/− mice at ages 5 months, 8–9 months, 11 and 13 months using standard morphological methods based on measurement of ONL thickness. Shown in Fig. 61.1 are representative images of hematoxylin and eosin stained superior central retinas obtained with a 10 × objective; images captured at higher magnification (40 × objective) are in the insets. A difference in ONL width between Abcr−/− and Abcr+/+ retina was visible at 8–9 months of age (Fig. 61.1).
Fig. 61.1.
Representative light micrographs of Abcr+/+ and Abcr−/− mouse retinas. Images of inferior hemisphere along the vertical meridian; age 9 months. Insets, higher magnification images obtained in the regions indicated. ONH, optic nerve head; GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Magnification bar, 50 μm
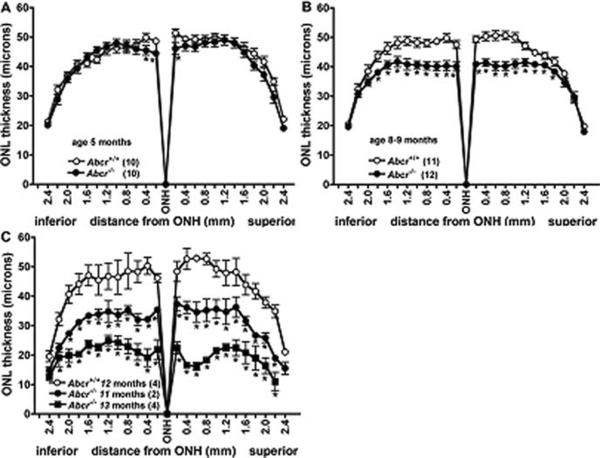
ONL thicknesses were plotted as a function of distance in 200 μm intervals superior and inferior to the optic nerve head in the vertical plane. Examination of ONL measurements in Abcr+/+ at 5 months, 8–9 months (mean age 8.2 months) and 12 months of age revealed that these measurements did not vary as a function of these ages (one-way ANOVA, p > 0.05) (Fig. 61.2). Conversely, in Abcr−/− mice at 8–9 months (mean age 8.5 months) a decrease in ONL thickness was observed, the thinning being most noticeable in central retina. For example, comparison of Abcr−/− and Abcr+/+ mice at 8–9 months of age revealed a 15–20% reduction in ONL thickness, 0.2–1.0 mm superior and inferior to ONH (p < 0.05) (Fig. 61.2). In Abcr−/− mice at 11 months of age, the difference in ONL thickness was further accentuated, there being a 23–36% decrease at eccentricities of 0.2–1.0 mm in Abcr−/− relative to Abcr+/+ mice (p < 0.05) (Fig. 61.2). At 8–9 months of age, the degenerative changes were slightly more distinct in superior retina as compared to inferior retina, the reduction in the inferior hemiretina ranging from 15 to 19%, while the decrease in superior hemiretina was 17–20%. In Abcr−/− mice aged 13 months, the thinning of ONL in superior retina was clearly more pronounced, decreases in the 0.2–1.0 mm zones being 54–69% superiorly and 48–62% inferiorly when compared to Abcr+/+ mice (age 12 months). Statistically significant differences (p < 0.05) between Abcr+/+ and Abcr−/− mice in terms of ONL thickness were observed at 5 months of age only at 0.2 and 0.4 mm from the ONH inferiorly and 0.2 mm superiorly (Fig. 61.2).
Fig. 61.2.
Quantification of outer nuclear layer (ONL) thickness in Abcr+/+ and Abcr−/− mice at age 5 month (a), 8–9 months (b) and 11–13 months (c). Measurements are plotted as a function of distance from the optic nerve head (ONH) in the inferior and superior hemispheres. Mean ± SEM; numbers of mice presented in parentheses
Measurements of ONL thickness agreed with the counts of nuclei spanning the width of the ONL. The numbers of nuclei at 5 months of age in Abcr−/− mice were not significantly different than in Abcr+/+ mice (Abcr+/+: 9.8 ± 0.24; Abcr−/−: 9.6 ± 0.33, mean ± SEM; p > 0.05). Conversely at 8–9 months, the mean number of nuclei in Abcr−/− mice was reduced by 22% relative to Abcr+/+ (Abcr+/+: 9.5 ± 0.25; Abcr−/−: 7.4 ± 0.39, mean ± SEM; p < 0.05).
61.4 Discussion
The Abcr−/− mouse is notable for exhibiting an excessive accumulation of the bis-retinoid pigments that constitute the lipofuscin of RPE cells (Kim et al. 2004; 2007; Weng et al. 1999). By morphometric analysis of ONL thickness combined with counting of photoreceptor cell nuclei spanning the ONL, we have demonstrated that albino Abcr−/− mice display photoreceptor cell loss that is clearly detectable at 8 months of age and that has worsened by 12 and 13 months of age. Thinning of the ONL was more marked in the superior hemisphere of retina. The amassing of RPE lipofuscin to pronounced levels in Abcr−/− mice precedes the loss of photoreceptor cells; for instance by 3 months of age, A2E levels in the mutant mice are approximately 5-fold greater than in Abcr+/+ mice (Kim et al. 2007). Indeed it is potentially significant that by 8 months of age, A2E levels appear to reach a plateau.
As compared to some other mouse models of retinal degeneration, the loss of photoreceptors in the Abcr−/− mice occurred with later onset. For instance, mice expressing the P23H substitution in rhodopsin, a mutation prevalent in human autosomal dominant retinitis pigmentosa, exhibit substantial reduction in ONL thickness, even at 2 months of age (Naash et al. 1993). Mice carrying a naturally occurring autosomal recessive mutation in Rpe65 (Rpe65rd12) develop a retinal degeneration that is considered to be slowly progressing, yet even then, ONL width is reduced by 30–40% at age 6–7 months of age (Pang et al. 2005; Redmond et al. 1998).
In the present study ONL thicknesses in Abcr+/+ mice were consistent with previous reports (Kurth et al. 2007); moreover, we did not observe an age-related thinning of ONL in Abcr+/+ mice examined between 5 and 12 months. Consistent with this, most studies of wild-type mice have reported age-related photoreceptor cell loss only after 1 year of age. For example, ONL thickness measurements were reported to be the same at 2, 4, 6 and 12 months of age in BALB/cJ, C57BL/6 and C57BL/6-C2 J mice (Bravo-Nuevo et al. 2004; Li et al. 2001), while another study described a 40% decline in rows of photoreceptor nuclei between 2 and 17 months of age (BALB/c mice) (Gresh et al. 2003). Similarly, screening of several in-bred laboratory strains of mice, including BALB/cJ, BALB/cByJ, A/J, NZW/LacJ and 129P3/J, revealed normal retinal morphology at 10–12 months of age but noticeable ONL thinning by 22–24 months (Chang 2008). On the other hand, Danciger et al. presented ONL thickness data that reflected a decline of approximately 6 μm between 6 and 12 months of age in BALB/c mice (Danciger et al. 2003).
In conclusion, we suggest that two measures of therapeutic efficacy are available for preclinical studies of recessive Stargardt disease utilizing the Abcr−/− mouse: HPLC quantitation of RPE lipofuscin fluorophores such as A2E and ONL thickness measurement. Both approaches are also translatable to non-invasive endpoint measures in human clinical trials – analysis of fundus autofluorescence in human subjects serves as a measure of RPE lipofuscin while segmentation of the outer retinal complex [ORC: thickness of ONL, inner segments (IS) and outer segments (OS)] in OCT images of the human eye is akin to ONL thickness measurements in mice.
Acknowledgments
This work was supported by National Institutes of Health Grant EY12951 (to JRS), a gift from Dr. Gertrude Neumark Rothschild and a grant from Research to Prevent Blindness to the Department of Ophthalmology. JRS is the recipient of a Research to Prevent Blindness Senior Investigator Award.
References
- Bravo-Nuevo A, Walsh N, Stone J. Photoreceptor degeneration and loss of retinal function in the C57BL/6-C2J mouse. Invest Ophthalmol Vis Sci. 2004;45:2005–2012. doi: 10.1167/iovs.03-0842. [DOI] [PubMed] [Google Scholar]
- Chang B. Age-related eye disease. In: Chalupa LM, Williams RW, editors. Eye, retina and visual system of the mouse. The MIT Press; Cambridge, MA: 2008. pp. 581–590. [Google Scholar]
- Danciger M, Lyon J, Worrill D, et al. A strong and highly significant QTL on chromosome 6 that protects the mouse from age-related retinal degeneration. Invest Ophthalmol Vis Sci. 2003;44(6):2442–2449. doi: 10.1167/iovs.02-1252. [DOI] [PubMed] [Google Scholar]
- Gresh J, Goletz PW, Crouch RK, et al. Structure-function analysis of rods and cones in juvenile, adult, and aged C57BL/6 and Balb/c mice. Vis Neurosci. 2003;20:211–220. doi: 10.1017/s0952523803202108. [DOI] [PubMed] [Google Scholar]
- Kim SR, Fishkin N, Kong J, et al. The Rpe65 Leu450Met variant is associated with reduced levels of the RPE lipofuscin fluorophores A2E and iso-A2E. Proc Natl Acad Sci U S A. 2004;101(32):11668–11672. doi: 10.1073/pnas.0403499101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SR, Jang YP, Jockusch S, et al. The all-trans-retinal dimer series of lipofuscin pigments in retinal pigment epithelial cells in a recessive Stargardt disease model. Proc Natl Acad Sci U S A. 2007;104:19273–19278. doi: 10.1073/pnas.0708714104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevering BJ, Maugeri A, Wagner A, et al. Three families displayinng the combination of Stargardt's disease with cone-rod dystrophy or retinitis pigmentosa. Ophthalmology. 2004;111:546–553. doi: 10.1016/j.ophtha.2003.06.010. [DOI] [PubMed] [Google Scholar]
- Kong J, Kim SR, Binley K, et al. Correction of the disease phenotype in the mouse model of Stargardt disease by lentiviral gene therapy. Gene Ther. 2008;15:1311–1320. doi: 10.1038/gt.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth I, Thompson DA, Rüther K, et al. Targeted disruption of the murine retinal dehydrogenase gene Rdh12 does not limit visual cycle function. Mol Cell Biol. 2007;27:1370–1379. doi: 10.1128/MCB.01486-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail MM, Gorrin GM, Repaci MA, et al. Genetic regulation of light damage to photoreceptors. Invest Ophthalmol Vis Sci. 1987;28:1043–1048. [PubMed] [Google Scholar]
- Li C, Cheng M, Yang H, et al. Age-related changes in the mouse outer retina. Optom Vis Sci. 2001;78:425–430. doi: 10.1097/00006324-200106000-00015. [DOI] [PubMed] [Google Scholar]
- Maeda A, Maeda T, Golczak M, et al. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J Biol Chem. 2008;283:26684–26693. doi: 10.1074/jbc.M804505200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti P, Kong J, Kim SR, et al. Small molecule RPE65 antagonists limit the visual cycle and prevent lipofuscin formation. Biochem. 2006;45:852–860. doi: 10.1021/bi0518545. [DOI] [PubMed] [Google Scholar]
- Mata NL, Tzekov RT, Liu X, et al. Delayed dark adaptation and lipofuscin accumulation in Abcr+/− mice: implications for involvement of ABCR in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2001;42:1685–1690. [PubMed] [Google Scholar]
- Maugeri A, Klevering BJ, Rohrschneider K, et al. Mutations in the ABCA4 (ABCR) gene are the major cause of autosomal recessive cone-rod dystrophy. Am J Hum Genet. 2000;67(4):960–966. doi: 10.1086/303079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michon JJ, Li ZL, Shioura N, et al. A comparative study of methods of photoreceptor morphometry. Invest Ophthalmol Vis Sci. 1991;32:280–284. [PubMed] [Google Scholar]
- Mittag TW, Bayer AU, LaVail MM. Light-induced retinal damage in mice carrying a mutated SOD I gene. Exp Eye Res. 1999;69:677–683. doi: 10.1006/exer.1999.0748. [DOI] [PubMed] [Google Scholar]
- Naash MI, Hollyfield JG, Al-Ubaidi MR, et al. Simulation of human autosomal dominant retinitis pigmentosa in transgenic mice expressing a mutated murine opsin gene. Proc Natl Acad Sci U S A. 1993;90:5499–5503. doi: 10.1073/pnas.90.12.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Chang B, Hawes NL, et al. Retinal degeneration 12 (rd12): a new, spontaneously arising mouse model for human Leber congenital amaurosis (LCA) Mol Vis. 2005;11:152–162. [PubMed] [Google Scholar]
- Radu RA, Han Y, Bui TV, et al. Reductions in serum vitamin A arrest accumulation of toxic retinal fluorophores: a potential therapy for treatment of lipofuscin-based retinal diseases. Invest Ophthalmol Vis Sci. 2005;46:4393–4401. doi: 10.1167/iovs.05-0820. [DOI] [PubMed] [Google Scholar]
- Radu RA, Mata NL, Nusinowitz S, et al. Treatment with isotretinoin inhibits lipofuscin and A2E accumulation in a mouse model of recessive Stargardt's macular degeneration. Proc Natl Acad Sci U S A. 2003;100(8):4742–4747. doi: 10.1073/pnas.0737855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radu RA, Yuan Q, Hu J, et al. Accelerated accumulation of lipofuscin pigments in the RPE of a mouse model for ABCA4-mediated retinal dystrophies following vitamin A supplementation. Invest Ophthalmol Vis Sci. 2008;49:3821–3829. doi: 10.1167/iovs.07-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond TM, Yu S, Lee E, et al. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20:344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- Shroyer NF, Lewis RA, Yatsenko AN, et al. Null missense ABCR (ABCA4) mutations in a family with Stargardt disease and retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2001;42:2757–2761. [PubMed] [Google Scholar]
- Sun H, Molday RS, Nathans J. Retinal stimulates ATP hydrolysis by purified and reconstituted ABCR, the photoreceptor-specific ATP-binding cassette transporter responsible for Stargardt disease. J Biol Chem. 1999;274(12):8269–8281. doi: 10.1074/jbc.274.12.8269. [DOI] [PubMed] [Google Scholar]
- Tanito M, Elliot MH, Kotake Y, et al. Protein modifications by 4-hydroxynonenal and 4-hydroxyhexenal in light-exposed rat retina. Invest Ophthalmol Vis Sci. 2005;46:3859–3868. doi: 10.1167/iovs.05-0672. [DOI] [PubMed] [Google Scholar]
- Weng J, Mata NL, Azarian SM, et al. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt's disease from the phenotype in Abcr knockout mice. Cell. 1999;98(1):13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]