Abstract
The hippocampus is crucial for the consolidation of new declarative long-term memories. Genetic and behavioral experimentation have revealed that several protein kinases are critical for the formation of hippocampus-dependent long-term memories. Cyclic-AMP dependent protein kinase (PKA) is a serine–threonine kinase that has been strongly implicated in the expression of specific forms of hippocampus-dependent memory. We review evidence that PKA is required for hippocampus-dependent memory in mammals, and we highlight some of the proteins that have been implicated as targets of PKA. Future directions and open questions regarding the role of PKA in memory storage are also described.
Keywords: memory, learning, cyclic AMP-dependent protein kinase, hippocampus, synaptic plasticity, long-term potentiation
Introduction
Protein kinases modulate a plethora of important processes, including synaptic plasticity, learning, and memory. Multiple chemical neurotransmitters, hormones, and other signaling substances use cyclic adenosine 3′,5′monophosphate (cAMP) as an intracellular second messenger. The principal target for cAMP in mammalian cells is cAMP- dependent protein kinase (PKA), which is ubiquitously expressed and mediates intracellular signal transduction. Pioneering work by Earl Sutherland identified cAMP as the first intracellular second messenger (Sutherland and Rall, 1957; Sutherland et al., 1965). Subsequently, Edwin Krebs, Paul Greengard, and their colleagues purified PKA from rabbit skeletal muscle (Walsh et al., 1968; Reimann et al., 1971), and they showed that PKA activity was stimulated by cAMP (Miyamoto et al., 1968, 1969; Walsh et al., 1968; Beavo et al., 1974). Other advances, made possible by genetic, molecular, and cell biological techniques, have shed light on the molecular characteristics, dynamics, and functional plurality of the PKA holoenzyme (reviewed by McKnight et al., 1988; Beebe, 1994; Skalhegg and Tasken, 2000). It is now well established that PKA regulates many biological processes through its phosphorylation of proteins. Also, phosphatases such as protein phosphatase 1 and the calcium-regulated phosphatase, calcineurin, can dephosphorylate proteins that had been phosphorylated by PKA, thus allowing PKA signaling events to be reversible (Fig. 1).
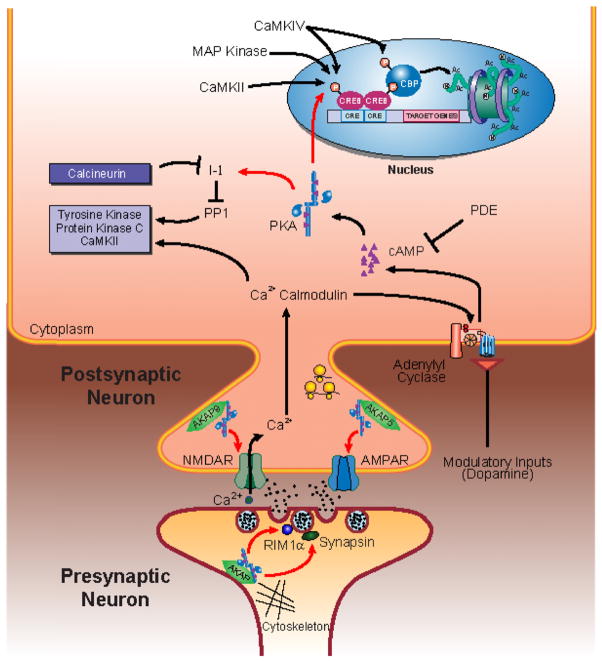
Fig. 1.
The cAMP/PKA signaling pathway critically regulates molecular components underlying long-term potentiation and long-term memory. In the postsynaptic neuron, PKA targets include NMDA and AMPA receptors, inhibitor-1 (I-1) and CREB. In the presynaptic terminal, PKA targets include RIM1α and synapsin. (See Color Plate 6.1 in color plate section.)
A fundamental process that is modulated by PKA is synaptic transmission, which can be modified by the electrical activity of a neuron —a process termed “activity-dependent synaptic plasticity” (reviewed by Nguyen and Woo, 2003). Because synaptic plasticity involves long-lasting modifications of intercellular signaling in the nervous system, it plays significant roles in regulating learning and memory (Castellucci et al., 1970; Kupfermann et al., 1970; McKernan and Shinnick-Gallagher, 1997; Abraham et al., 2002; Whitlock et al., 2006). Not surprisingly, certain types of long-lasting synaptic plasticity, such as long-term potentiation (LTP) and long-term depression (LTD), are believed to underlie some forms of learning and memory in the mammalian brain (Bliss and Lomo, 1973; Dudek and Bear, 1992; Martin et al., 2000; Abraham et al., 2002; Whitlock et al., 2006).
Cyclic AMP/PKA signaling has been shown to be pivotal for specific types of long-term synaptic plasticity and for long-term memory (Fig. 1), as demonstrated in the landmark studies of learning and memory in Aplysia and Drosophila (reviewed by Burrell and Sahley, 2001). In particular, electrophysiological and behavioral studies of the marine snail, Aplysia californica, have revealed a requirement for cAMP/PKA signaling in the establishment of short- and long-lasting forms of synaptic plasticity, learning, and memory (Kandel, 2001). These studies demonstrated an important role for PKA in mediating short-term synaptic facilitation (by reversible phosphorylation of ion channels), and long-term facilitation and long-term memory for behavioral sensitization (by modulation of gene expression and protein synthesis) (Kandel, 2001). Studies in Drosophila have also been instrumental in defining the role of PKA in learning and memory (reviewed by McGuire et al., 2005; Davis, 1996). The learning mutant, dunce, was the first learning mutant isolated in Drosophila, and dunce encodes a cAMP phosphodiesterase. Similarly, mutations in adenylyl cyclase (rutabaga) and mutations in PKA itself impair learning and memory in Drosophila. Overall, these landmark experiments have laid much of the conceptual foundations for subsequent research on the roles of PKA in learning and memory in the mammalian brain. In this article, the roles of PKA in hippocampus-dependent memory are reviewed. We focus only on the mammalian hippocampus, because of its pivotal roles in the consolidation of spatial and non-spatial long-term memories. We discuss studies that have shown a requirement for PKA signaling in the hippocampus. We then consider some of the downstream targets of PKA, emphasizing studies that have identified requirements for PKA-mediated phosphorylation and signaling in hippocampal LTP, a form of synaptic plasticity that has been linked to memory formation (Abel et al., 1997; Gruart et al., 2006; Whitlock et al., 2006).
cAMP-dependent protein kinase (PKA)
The mammalian PKA family consists of four regulatory (R) subunits (RIα, RIβ, RIIα, RIIβ) and three catalytic (C) subunits (Cα, Cβ, Cγ). Each subunit is encoded by a unique gene (McKnight et al., 1988; Doskeland et al., 1993) and they are all expressed in the mammalian brain (Cadd and McKnight, 1989). Two major isozymes of PKA, termed type I (with RIα and RIβ dimers) and type II (with RIIα and RIIβ dimers), have been characterized and were initially identified based on their patterns of chromatographic elution (Tasken et al., 1993; Francis and Corbin, 1999; Skalhegg and Tasken, 2000). In the absence of cAMP, PKA is an inactive tetrameric holoenzyme composed of two R subunits bound to two C subunits. Each regulatory subunit contains two tandem cAMP binding sites, a high-affinity site and a low-affinity site (Taylor et al., 1990). Sequential and cooperative binding of cAMP to these two sites releases monomeric C subunits (Taylor et al., 1990; Gibbs et al., 1992). These dissociated C subunits can then phosphorylate serine and threonine residues on numerous proteins. In essence, the regulatory subunits inhibit the phosphotransferase activities of the catalytic subunits.
Properties of memory
Learning, the change in behavior as a result of experience, and memory, the retention of changes in behavior that result from learning, have been of great interest to neurobiologists. The psychological study of memory began over 100 years ago with the experiments of Herman Ebbinghaus; since then, researchers have sought to define the principles underlying memory storage. This work has defined three major properties of memory storage (Milner et al., 1998). First, the study of patients like H. M. revealed that there are multiple memory systems that function in distinct but overlapping ways. Thus, declarative or explicit memory, the conscious memory of facts and events, is mediated by the hippocampal memory system, whereas non-declarative or implicit memory, involving unconscious learning of emotional tasks or skills and habits, is mediated by other brain systems such as the amygdala and striatum. Secondly, memory consists of specific temporal phases, including short-term and long-term memory. As we will see in our discussion here of the cAMP/PKA signaling pathway, these phases of memory can be mediated by distinct molecular pathways. Thirdly, memory consists of specific stages. Memory is acquired during training, consolidated in the period following training into a more stable form, and then retrieved when needed. Following retrieval, memory can be modified by processes of reconsolidation and extinction. In this review, we will focus on the role of the cAMP/PKA signaling pathway in hippocampus-dependent forms of memory in rodents.
The role of the cAMP/PKA system in spatial memory
In rodents, the quintessential hippocampus-dependent behavioral task is spatial learning and memory in the hidden platform version of the Morris water maze. In this task, animals learn to use the distal cues located in the room to find the location of a submerged platform during repeated trials. Performance is measured by an increase in latency to find the platform during training trials and memory is tested in a probe (or transfer) test in which the platform is removed from the pool and animals swim freely. During this probe test, the spatial preference of the animals is measured by a tracking device and spatial learning is reflected in a spatial bias to this search pattern: well-trained animals spend most of their time swimming in the vicinity of the platform location. This form of spatial learning depends on the dorsal hippocampus and it is NMDA receptor-dependent (Morris et al., 1982, 1986; Tsien et al., 1996). Importantly, the water maze can be configured in many different ways: with a visible platform that is shifted from trial to trial to test for procedural memory, or with a hidden platform that is shifted from day to day to test for working memory. As typically configured with a positionally fixed hidden platform, the water maze tests spatial reference memory. In addition to the water maze, spatial memory can also be tested in the radial arm maze and in the Y maze.
What is the role of the cAMP/PKA signaling pathway in spatial memory? This issue has been difficult to address genetically because of redundancy in the genes encoding various components of the cAMP/PKA signaling pathway, particularly PKA itself. In addition, the large number of training trials in the water maze makes region-specific pharmacological manipulation difficult. Initial work in the mammalian hippocampus defined a role for cAMP and PKA in synaptic plasticity (reviewed in Nguyen and Woo, 2003). The first demonstration of a role for cAMP in spatial memory came in 1995 when Daniel Storm and colleagues examined the effects of mutations in the gene encoding type I adenylyl cyclase on behavior (Wu et al., 1995). They found that mutant mice lacking type I adenylyl cyclase had reductions in the levels of calcium-stimulated cAMP synthesis and exhibited spatial memory deficits in the hidden platform version of the water maze (Wu et al., 1995). Studies of similar conventional null mutations in the genes encoding specific regulatory and catalytic subunits of PKA were difficult to interpret because of compensatory changes in the expression of genes encoding other PKA subunits in these mutant mice (Brandon et al., 1997). Also, the behavioral phenotypes observed in mutant mice is sensitive to genetic background (Howe et al., 2002). To define the role of PKA in learning and memory in rodents, Abel et al. (1997) created R(AB) transgenic mice expressing a dominant negative form of the regulatory subunit of PKA. By using a transgenic approach, PKA can be inhibited in specific cell types at particular times, thereby reducing potential compensatory effects. Furthermore, the R(AB) transgene will interfere broadly with type I PKA within the cell types in which it is expressed. In the initial R(AB) transgenics, the CaMKIIα promoter restricted expression to postnatal excitatory neurons within the forebrain. These transgenic mice have provided the clearest demonstration that PKA activity is critical for spatial memory, as these mice exhibited normal initial learning in the hidden platform version of the water maze, but showed deficits in memory in retrieval tests. Biochemical studies of PKA activation following training have revealed that spatial learning activates PKA in the hippocampus. Interestingly, such activation is observed early in learning at a time when PKA inhibition does not impair performance, suggesting that PKA activation during training sets into motion a biochemical cascade that leads to memory storage (Vazquez et al., 2000; Mizuno et al., 2002). Such a cascade may likely involve the activation of ERK (extracellular signal-regulated protein kinases) and the transcription factor, CREB (cAMP response element binding protein).
Few studies have probed the effects of pharmacological inhibitors of PKA on spatial learning, perhaps because of the assumption that PKA would need to be inhibited during each training trial. Surprisingly, a single infusion of H-89, an inhibitor of PKA, into area CA1 of the dorsal hippocampus after the last trial on the third day of training significantly impaired spatial memory (Sharifzadeh et al., 2005). Clearly, additional pharmacological studies are needed to define the role that PKA might play earlier in acquisition or during retrieval, reconsolidation, and extinction of spatial memory. As always with pharmacological studies, it is important to note that the effects of inhibitors are rarely specific (Davies et al., 2000).
The role of the cAMP/PKA system in contextual conditioning
Experiments with spatial memory clearly establish the role of the cAMP/PKA pathway in hippocampus-dependent memory. However, because of the repeated training trials necessary for spatial learning, these tasks do not allow researchers to precisely define the role of this signaling pathway in short- and long-term memory. Since 1890, when William James made the distinction between short-term and long-term memory (James, 1890), researchers have sought to determine the relationship between these processes. Using single-trial learning tasks such as contextual fear conditioning and step-down inhibitory avoidance, researchers can define the mechanisms underlying short-term and long-term memory in the mammalian brain. In contextual fear conditioning, animals are exposed to a novel context and receive a mild footshock. A single 2- to 3-min training trial is sufficient for rodents to exhibit fear when re-exposed to the conditioning context. Such a fear response is measured most frequently by immobility (“freezing”). Contextual fear conditioning is sensitive to lesions of the amygdala and hippocampus (reviewed by Maren, 2001). Importantly, cued fear conditioning, in which animals are exposed to a tone and a footshock, is sensitive to lesions of the amygdala but not to hippocampal lesions, providing a good behavioral control for experiments aimed at addressing the impact of manipulations on hippocampal function.
The first evidence that PKA played a role in contextual fear conditioning came from studies of R(AB) transgenic mice (Abel et al., 1997; Bourtchouladze et al., 1998). These mutant mice exhibited selective deficits in long-term memory for contextual fear conditioning. Short-term memory for contextual fear as well as cued fear conditioning were intact. More recent R(AB) transgenic lines have used the tetracycline system for conditional regulation in adulthood, and these studies have confirmed that genetic inhibition of neuronal PKA in adulthood selectively impairs contextual long-term memory (Isiegas et al., 2006). Mice lacking the calcium-responsive forms of adenylyl cyclase also exhibit selective long-term memory deficits in contextual conditioning and passive avoidance, but the time course of these deficits appears to be distinct from that observed in the PKA mutant mice, perhaps because of differences in experimental procedures or genetic backgrounds of the different mutant mice (Wong et al., 1999). Although most research has focused on aversive conditioning, recent studies using novel object recognition have also revealed an important role for cAMP signaling in long-term memory in this non-aversive task (Pineda et al., 2004; Wang et al., 2004). Interestingly, studies in invertebrates (Drosophila, Aplysia) have found that the cAMP/PKA pathway is involved in short-term and long-term memory. In contrast, the majority of the studies in vertebrate systems have found that the cAMP/PKA signaling pathway plays a selective role in long-term memory. The reasons for this distinction are unclear.
Intra-ventricular or intra-hippocampal injection of PKA inhibitors has been shown to impair long-term contextual memory (Bourtchouladze et al., 1998; Wallenstein et al., 2002; Ahi et al., 2004). In experiments using step-down inhibitory avoidance, Izquierdo and colleagues have also found that inhibitors of PKA administered into hippocampal area CA1 selectively impair long-term memory (Bernabeu et al., 1997; Vianna et al., 2000; Quevedo et al., 2004). In rodents, inhibitors of protein synthesis impair long-term memory, similar to inhibitors of PKA, suggesting that PKA plays a role in activating the biochemical mechanisms that engage protein synthesis and transcription.
Much research has focused on the idea that PKA targets transcriptional machinery (Fig. 1). One target of PKA of great interest has been CREB, and CREB phosphorylation is increased after fear conditioning, as is expression of a CRE-driven reporter gene (Impey et al., 1998; Stanciu et al., 2001; Athos et al., 2002; Mizuno et al., 2002). It is important to note in this context that PKA is one of several kinases that can phosphorylate CREB on serine 133, and identifying which kinase is most critical under specific behavioral circumstances remains a challenge (Athos et al., 2002; Sindreu et al., 2007). The analysis of CREB mutant mice, however, has not been as clearly in support of a selective role for CREB in long-term memory as one might have expected, perhaps because of compensatory changes in other CRE-binding proteins in CREB mutant mice (Hummler et al., 1994) or because of effects of genetic background (Bourtchouladze et al., 1994; Gass et al., 1998; Bucan and Abel, 2002; Graves et al., 2002; Balschun et al., 2003). Indeed, the data appear to be more consistent with the idea that a CRE-binding protein related to CREB is involved in long-term memory storage, because the clearest phenotypes have been observed in genetically modified mice expressing dominant-negative forms of CREB (Kida et al., 2002; Pittenger et al., 2002), which effectively interfere with many proteins binding to CRE sites, and in CBP mutant mice, which also interfere with the action of a number of transcription factors (Oike et al., 1999; Bourtchouladze et al., 2003; Alarcon et al., 2004; Korzus et al., 2004; Wood et al., 2005, 2006).
The study of the molecular mechanisms underlying memory storage has highlighted important questions about the nature of memory. First, what is the relationship between long-term and short-term memory? Is short-term memory a process on the way towards long-term memory or are these two forms of memory mediated by independent, parallel processes? Most treatments that impair short-term memory also impair long-term memory, suggesting that in many cases they are serial processes. However, under certain circumstances, treatments can lead to impairments in short-term memory without impairing long-term memory, implying that these forms of memory can be mediated by distinct, parallel mechanisms (Emptage and Carew, 1993; Izquierdo et al., 1998; Sherff and Carew, 2004). Secondly, is memory consolidation a single process set into motion by training or does it have distinct phases? Here the data from studies of the role of PKA in long-term memory storage suggest that there are at least two time periods during which PKA and protein synthesis are required during memory consolidation (Bernabeu et al., 1997; Bourtchouladze et al., 1998). Finally, what is the behavioral role of cAMP/PKA signaling in the hippocampus and how does this compare to the role of this signaling pathway in other brain regions?
It is interesting to note that genetically modified mice in which the PKA pathway has been manipulated exhibited selective deficits in contextual fear conditioning, with intact cued fear conditioning (Abel et al., 1997; Isiegas et al., 2006). This suggests that the cAMP/PKA signaling pathway has distinct roles in the hippocampus and the amygdala, because direct pharmacological manipulation of PKA in the amygdala impairs cued fear conditioning (Schafe and LeDoux, 2000). In addition, it may be that the hippocampus forms a representation of the context during training whereas the amygdala may be the site of the association of the conditioned and unconditioned stimuli (CS and US) (Maren, 2001; Keeley et al., 2006). If this is the case, then there may be fundamental biochemical differences in the ways in which circuits in the hippocampus and amygdala are activated by training. Future experiments will be required to investigate these issues.
The cAMP/PKA pathway as a target of cognition-enhancing drugs
Our discussion has focused on the impact of impairments n i cAMP/PKA signaling, but researchers have also investigated the behavioral effects of increased activity n i the cAMP/PKA pathway. Pharmacological experiments have revealed a time window of 3–6 h after training when treatment with drugs that directly or indirectly activate PKA can enhance memory storage (Bernabeu et al., 1997). Blockade of the degradation of cAMP by treatment with inhibitors of phosphodiesterases such as the PDE4 inhibitor, rolipram, enhances memory (Barad et al., 1998). Interestingly, these PDE inhibitors can reverse the memory impairments found n i aged mice (Bach et al., 1999) and in mouse models of mental retardation (Bourtchouladze et al., 2003) and Alzheimer’s Disease (Vitolo et al., 2002; Gong et al., 2004). Although the benefits of pharmacological blockade of PDE for memory storage have been known for some time, the use of this approach in human patients is still n i its nascent stages (Rose et al., 2005).
Studies of genetic manipulations designed to increase levels of cAMP have revealed that it is important to distinguish between manipulations that increase cAMP levels and those that increase cAMP signals. Genetic manipulations that increase cAMP signals (such as overexpression of calcium-responsive adenylyl cyclase) lead to memory enhancements like those observed with PDE inhibitors, whereas genetic manipulations that increase basal cAMP levels lead to memory impairments (Pineda et al., 2004; Wang et al., 2004; Bourtchouladze et al., 2006).
Synaptic tagging
Neurons typically receive inputs from thousands of synaptic contacts. However, L-LTP is input-specific (Andersen et al., 1977; Nguyen et al., 1994). To preserve the input specificity of L-LTP, a mechanism to mark, or “tag”, active synapses has been proposed to allow newly synthesized gene products to be captured and utilized at appropriately activated synapses (Sossin, 1996; Frey and Morris, 1997; Schuman, 1997). Frey and Morris (1997) first provided evidence for the synaptic tag theory in the rat hippocampus. They proposed that proteins synthesized in response to long-term synaptic changes at one set of synapses could be captured and utilized by other synapses to express L-LTP if a synaptic tag is generated by appropriate synaptic activity. In accordance with this idea, they found that transient potentiation resulting from weaker synaptic activation could be prolonged to resemble L-LTP if paired with established protein synthesis-dependent L-LTP at separate synaptic inputs (Frey and Morris, 1997).
Interestingly, low-frequency stimulation (LFS) can impair L-LTP subsequently induced at the previously activated synapses (homosynaptic inhibition), as well as at other synapses converging on the same postsynaptic cells (heterosynaptic inhibition) (Young and Nguyen, 2005). Both homosynaptic and heterosynaptic inhibition of L-LTP by prior LFS required protein phosphatase activity (Young et al., 2006), and LFS impaired signaling through the cAMP/PKA pathway (Young et al., 2006). More importantly, pharmacological or genetic inhibition of PKA impaired synaptic capture, and pharmacological activation of cAMP/PKA signaling was sufficient to generate a synaptic tag that enabled persistent synaptic facilitation (Young et al., 2006). Thus, PKA has a key role in synaptic tagging that may be a novel control point in the consolidation of L-LTP, and perhaps, of long-term memory. PKA-mediated signaling can be constrained by prior episodes of synaptic activity to regulate future L-LTP expression and the spatiotemporal integration of multiple synaptic events. Because there is evidence that a behavioral analog of synaptic tagging and capture may occur during spatial exploration of a novel environment (Moncada and Viola, 2007), it will be of interest to determine, by in vivo inhibition of PKA, whether PKA also has a role in producing this modification of behavioral learning and memory.
Downstream substrates for PKA in synaptic plasticity and memory storage
The cAMP/PKA signaling cascade has garnered much attention because of its critical requirement for both long-term memory and for specific forms of long-term synaptic plasticity that are believed to underlie memory storage. The precise molecular mechanisms underlying these processes have not been fully elucidated, but many proteins that are important mediators of synaptic plasticity are regulated by cAMP/PKA signaling (Fig. 1; reviewed by Nguyen and Woo, 2003). Here, we discuss several key proteins that are targets of PKA and that have been implicated in mediating the induction and expression of hippocampal LTP, an activity-dependent enhancement of synaptic strength that is believed to be a cellular mechanism for information storage in the mammalian brain (Bliss and Collingridge, 1993; Martin et al., 2000; Gruart et al., 2006; Whitlock et al., 2006). These proteins include NMDA (N-methyl-D-aspartate) and AMPA (alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate) glutamatergic receptors, RIM1α, inhibitor-1 (I-1, an inhibitor of protein phosphatase-1), and the transcription factor, CREB (Fig. 1). We highlight these substrates to underscore the notion that PKA signaling is important for the expression of hippocampus-dependent forms of memory because it can modify specific signal transduction molecules that are critical for hippocampal LTP, a type of synaptic plasticity that has been strongly correlated with memory storage.
NMDA receptors (NMDARs)
In rat (Collingridge et al., 1983) and mouse (Tsien et al., 1996; Woo et al., 2003) hippocampal area CA1, LTP induction requires activation of NMDARs. Pharmacological block of brain NMDARs impairs spatial learning and attenuates hippocampal LTP in rats (Morris et al., 1986). Genetic knockout of NMDARs in area CA1 per se impairs spatial learning in the Morris water maze and blocks induction of LTP (Tsien et al., 1996). At the molecular level, PKA is coupled directly to NMDARs through an A-kinase anchoring protein (AKAP), yotiao, which permits modulation of NMDAR channel activity by PKA (Westphal et al., 1999). Recent studies by Suzanne Zukin and her colleagues have shown that pharmacological blockers of PKA reduce both the calcium permeability of NMDARs and NMDAR-mediated calcium signals in active dendritic spines of rodent hippocampal neurons (Skeberdis et al., 2006). The same blockers of PKA attenuated LTP at Schaeffer collateral-CA1 synapses (Skeberdis et al., 2006), thus linking PKA-dependent synaptic plasticity to calcium signaling in spines and to calcium permeability of NMDARs. Interestingly, this regulation of NMDAR currents by PKA was more prominent in neurons cultured from immature animals, suggesting developmental regulation of PKA modulation of these currents (Skeberdis et al., 2006). Thus, PKA phosphorylates the NMDAR or another associated protein, thereby altering the size of the NMDA channel pore to modify calcium permeability. This mechanism would regulate the amount of calcium influx into hippocampal neurons, which is a critical determinant of the type of plasticity (LTP or LTD) that is expressed. PKA regulation of calcium permeability can thus serve as a critical “front line” gate for controlling the strength of downstream intracellular signaling leading to induction and long-term expression of LTP. It is unclear whether this modulation of NMDAR calcium influx by PKA per se is critical for hippocampus-dependent memory, but given the established links between NMDARs, PKA, and hippocampal memory (Tsien et al., 1996; Abel et al., 1997), this idea is a testable one that will require more exact mutations of the NMDAR channel complex to selectively modify NMDAR calcium permeability in the intact animal.
AMPA receptors (AMPARs)
AMPA receptors (AMPARs) are targets of PKA (and other kinases), and PKA-mediated phosphorylation of these receptors has been implicated in the expression of hippocampal LTP. These receptors are composed of four subunits (GluR1–GluR4), which combine in different ratios to form functional receptors (Hollmann and Heinemann, 1994; Dingledine et al., 1999). Alteration of subunit compositions of AMPARs is a key mechanism for regulating synaptic strength (Liu and Cull-Candy, 2000) through control of trafficking of these receptors (Shi et al., 2001). Furthermore, reversible phosphorylation of serine residues in specific subunits is critically linked to the expression of distinct forms of synaptic plasticity. Serine residues 831 and 845, located in the intracellular C-terminal domain of GluR1, are phosphorylated by CaMKII (Barria et al., 1997; Mammen et al., 1997) and PKA (Roche et al., 1996), respectively.
The first demonstrations that PKA can regulate AMPAR currents in hippocampal neurons were accomplished by intracellular or extracellular application of modifiers of PKA activity (Greengard et al., 1991; Wang et al., 1991). AMPA/kainate receptor whole-cell currents in dissociated hippocampal neurons were potentiated by intracellular application of catalytic subunits of PKA (Wang et al., 1991), and this same treatment increased the opening frequency and open times of these channels (Greengard et al., 1991), thereby providing a molecular basis for the enhancement of whole-cell EPSC amplitudes seen following bath applications of PKA activators (Wang et al., 1991; Duffy and Nguyen, 2003).
It is noteworthy that direct infusion of membrane-impermeant PKI (a specific peptide inhibitor of PKA) into CA1 pyramidal neurons of mouse hippocampal slices blocks maintenance of LTP, and that infusion of catalytic subunits of PKA into the same CA1 neurons is sufficient to elicit long-lasting synaptic potentiation of AMPAR-mediated excitatory postsynaptic currents (Duffy and Nguyen, 2003). Also, spatial “enrichment” of mice facilitates both PKA-dependent potentiation of AMPAR-mediated synaptic responses in area CA1 and hippocampus-dependent contextual fear memory (Duffy et al., 2001), suggesting that PKA-dependent synaptic plasticity is linked to improved performance on a hippocampal memory task (cf. Abel et al., 1997).
Lee et al. (2000) have shown that induction of homosynaptic LTD in area CA1 of naïve hippocampal slices elicited dephosphorylation of serine-845 (the PKA site) of GluR1, whereas induction of LTD in previously potentiated slices dephosphorylated serine-831 (the CaMKII site). Conversely, if LTP was induced at previously depressed synapses, serine-845 was phosphorylated, whereas LTP induction at naïve synapses elicited serine-831 phosphorylation (Lee et al., 2000). Interestingly, hippocampal learning (inhibitory avoidance performance) is associated with LTP-like increases in synaptic strength in the rat hippocampus and with GluR1 phosphorylation patterns similar to those seen after tetanus-induced LTP (Whitlock et al., 2006). These findings are consistent with the general concept that protein phosphorylation favors LTP, whereas dephosphorylation favors LTD. The results also demonstrate that different phosphorylation sites on a single receptor subunit are responsible for opposite polarities of synaptic plasticity.
AMPA receptor trafficking also plays an important role in hippocampal synaptic plasticity (Song and Huganir, 2002; Derkach et al., 2007), experience-dependent synaptic strengthening in cortical circuits (Clem and Barth, 2006), and associative learning (Rumpel et al., 2005). Recycling and sorting of AMPARs are activity-dependent, and they can be regulated by the phosphorylation state of the receptor (Carroll et al., 1999; Shi et al., 1999; Hayashi et al., 2000; Lu et al., 2001). Indeed, phosphorylation and dephosphorylation of AMPARs have been implicated in hippocampal LTD (Kameyama et al., 1998; Lee et al., 1998) and in LTP (reviewed by Soderling and Derkach, 2000), which in turn, involve the removal (Carroll et al., 1999; Man et al., 2000) or addition (Shi et al., 1999; Hayashi et al., 2000) of synaptic AMPARs, respectively. By expressing a recombinant construct of a specific subunit tagged with green fluorescent protein, Malinow and colleagues have defined some of the characteristics of AMPAR insertion (Shi et al., 1999, 2001; Hayashi et al., 2000), including a requirement for PKA-dependent phosphorylation of AMPAR subunits during synaptic plasticity (Esteban et al., 2003). The latter study showed that PKA controls the synaptic delivery of GluR4 subunits in rat hippocampal neurons, by relieving a retentive process that inhibits subunit incorporation at synapses. Interestingly, the effects of PKA are not restricted to GluR4: incorporation of GluR1, which requires CaMKII, was also blocked by inhibition of PKA (Esteban et al., 2003). Phosphorylation of serine-845 by PKA promotes AMPAR insertion and reduces AMPAR endocytosis (Man et al., 2007). More importantly, GluR1 is incorporated into synapses in a PKA-dependent manner during LTP, and stable LTP required PKA-mediated phosphorylation of serine-845 in the GluR1 subunit (Esteban et al., 2003). It should be noted, however, that ser-845 is phosphorylated under basal conditions (Lee et al., 2000); this is consistent with the observation that acute PKA activity is not necessary for induction of early stages of LTP (Huang and Kandel, 1994; but see Otmakhova et al., 2000; Yasuda et al., 2003).
In addition to AMPAR phosphorylation, AMPAR synthesis can increase several hours after L-LTP induction, and this synthesis, along with late LTP (L-LTP), were both attenuated by inhibitors of either PKA or transcription (Nayak et al., 1998). Thus, increased AMPA receptor synthesis is another mechanism by which PKA may cause L-LTP, which is known to be PKA-dependent. Overall, AMPAR incorporation at hippocampal synapses, resulting from increased synthesis and insertion, requires PKA, and it may explain the PKA-dependence of some types of LTP.
RIM1α and presynaptic LTP
The mossy fiber (MF) pathway, which connects granule cells of the dentate gyrus to pyramidal neurons of area CA3, displays a form of LTP (MF-LTP) that is expressed presynaptically and is independent of NMDA receptor activation (for a review, see Nguyen and Woo, 2003). Activation of PKA is required for MF-LTP (Huang et al., 1994; Weisskopf et al., 1994). One presynaptic substrate for PKA during MF-LTP is RIM1α, a presynaptic active zone protein that binds to Rab3A, a synaptic vesicle protein also implicated in MF-LTP (Castillo et al., 2002). RIM1α knockout mice display poor MF-LTP (Castillo et al., 2002), and impaired spatial learning on the Morris water maze (Powell et al., 2004). The presynaptic localization of RIM1α positions it for a role in vesicular docking and fusion, processes that are logically vital for presynaptic expression of MF-LTP. Thus, MF-LTP is one form of synaptic plasticity that requires a functional interface between synaptic vesicles and active zones, mediated through binding between Rab3A and RIM1α.
Interestingly, LTP in other brain regions also display a requirement for RIM1α and, by inference, involvement of presynaptic PKA activation. In the cerebellum, proof that PKA phosphorylation of RIM1α can elicit LTP was obtained by Lonart et al. (2003). LTP in RIM1α mutant mice was rescued by presynaptic expression of RIM1α, whereas introduction of mutant RIM1α lacking the PKA phosphorylation site impaired LTP in wildtype neurons (Lonart et al., 2003). Also, in the Schaffer collateral pathway of CA1, genetic knockout of RIM1α impaired L-LTP but not E-LTP, and this impairment of L-LTP may result from defective synaptic capture of gene products (Huang et al., 2005). Thus, PKA-mediated phosphorylation of RIM1α may impact numerous processes during synaptic plasticity and possibly at multiple sites. In a broader framework, a challenge for future research is to elucidate how the pre- and postsynaptic contributions of PKA are regulated to orchestrate the multiple molecular events that underlie PKA-dependent synaptic plasticity in distinct brain structures.
CREB and CRE-mediated gene expression
Hippocampal L-LTP, but not E-LTP (early LTP), requires transcription (Nguyen et al., 1994). Activation of PKA may cause L-LTP by regulating gene expression. PKA can modify transcription by phosphorylating several different transcription factors, one of which is CREB. CREB modulates transcription of genes containing cAMP response elements (CRE) in their promoters (Montminy, 1997; Lonze and Ginty, 2002). Following stimulation that leads to an increase in cytosolic cAMP, C subunits of PKA translocate into the nucleus to phosphorylate serine-133 on CREB (Bacskai et al., 1993). This phosphorylation can initiate transcription of CRE-associated genes (Yamamoto et al., 1988; Gonzalez and Montminy, 1989).
Studies in Aplysia (Dash et al., 1990; Bartsch et al., 1995; Martin et al., 1997) and Drosophila (Tully, 1991; Yin et al., 1994) demonstrated that members of the CREB family of transcription factors are necessary for long-lasting forms of synaptic plasticity and for long-term memory. In mice, it is unclear whether CREB activation is necessary for L-LTP. Bourtchouladze et al. (1994) found that knockout of the alpha and delta isoforms of CREB impaired LTP induced by just one 100-Hz train. Balschun et al. (2003) found normal LTP induced by three trains of 100-Hz stimulation in slices from CREB mutants, and Pittenger et al. (2002) similarly found intact multi-train LTP in mice expressing a dominant negative inhibitor of CREB. Thus, the requirement for CREB in L-LTP in the mammalian hippocampus may involve regulatory mechanisms that are more complex than previously hypothesized from studies on invertebrates. Genetic backgrounds of CREB mutant mice may also play key roles in determining the synaptic phenotypes of these animals.
An important question is whether PKA-mediated activation of CREB is critical for L-LTP. Direct evidence that PKA is critical for CREB-mediated changes in gene expression that may be linked to L-LTP comes from a study by Matsushita et al. (2001), in which specific blockade of nuclear PKA activity, by targeted delivery of PKI (a specific PKA inhibitor) to the nucleus, resulted in deficient CA1 L-LTP with a corresponding reduction in CREB phosphorylation (Matsushita et al., 2001). Thus, L-LTP in area CA1 of mouse hippocampal slices involves, but may not require, CREB-mediated transcription that requires nuclear PKA activation. Furthermore, whether CREB itself, or a related CRE-binding protein, is the effector for PKA-dependent L-LTP remains to be determined as the antibodies that bind to phosphorylated CREB also recognize phosphorylated forms of closely related proteins.
Inhibitor-1 and regulation of protein phosphatase-1 (PP-1)
Protein phosphatases can interact with the cAMP signaling pathway. Activation of dopaminergic D1 receptors, which are positively coupled to cAMP production, leads to phophorylation of DARPP-32 (dopamine and cAMP-regulated phosphoprotein, 32 kDa), an isoform of I-1 that is an endogenous inhibitor of PP-1 (Huang and Glinsmann, 1976; Hemmings et al., 1984; Greengard et al., 1999). I-1 and PP-1 have been implicated in E-LTP by studies suggesting that activation of the cAMP/PKA pathway may permit expression of E-LTP by suppressing PP-1’s inhibition of E-LTP (Blitzer et al., 1995, 1998). PKA is thought to phosphorylate I-1, thereby enabling I-1 to inactivate PP-1; this would then allow E-LTP to be expressed (Blitzer et al., 1995, 1998). Indeed, stimulation that induced LTP triggered cAMP-dependent phosphorylation of I-1 and decreased PP-1 activity (Blitzer et al., 1998). Conversely, dephosphorylation of I-1 by calcineurin is thought to enable expression of LTD under conditions when calcium binding to calmodulin can activate calcineurin, which is known to be required for LTD (Mulkey et al., 1994). Calcineurin thus appears to act as an inhibitory constraint on LTP, because genetic inhibition of calcineurin enhances expression of E-LTP (Malleret et al., 2001).
The gating model of PKA/PP-1 regulation of E-LTP was also extended to L-LTP. R(AB) transgenic mice display reduced hippocampal PKA activity and deficient L-LTP in area CA1 (Abel et al., 1997; Woo et al., 2000). L-LTP in hippocampal slices from these mice was rescued by acute application of PP-1 inhibitors (Woo et al., 2002), indicating that PP-1 acts downstream of PKA to impair L-LTP. Similarly, pharmacological inhibition of PP-1 in R(AB) mutant slices also rescued forskolin-induced synaptic facilitation, which was defective in mutants (Woo et al., 2002). Interestingly, pharmacological inhibition of PP-1 had no effect on E-LTP induced by a single 100-Hz train in mutant slices (Woo et al., 2002). Thus, suppression of PP-1 by PKA selectively regulates L-LTP in mice. These collective data show that PP-1 acts as an inhibitory constraint on L-LTP, and that genetic reduction of PKA activity impairs L-LTP by enhancing this inhibitory constraint.
Some evidence indicates that I-1 is not required for hippocampal spatial learning and memory (Allen et al., 2000). Genetic knockout of I-1 did not impair performance on the Morris water maze, but it did attenuate LTP at lateral perforant path synapses (but not at Schaeffer collateral-CA1 synapses) (Allen et al., 2000). Thus, some caution is required in attributing a critical role for I-1 in specific types of hippocampal learning and memory, and not all hippocampal synapses may rely on I-1-mediated regulation of LTP.
The role of PKA in shaping the essence of memory
Our review has highlighted multiple roles for PKA in hippocampal memory. However, much remains unexplored. For example, what are the specific roles of PKA in the distinct phases of memory processing? These phases include acquisition, consolidation, reconsolidation, and retrieval of memories, and their mechanistic definitions are essential goals that must be achieved before researchers can fully grasp the essence of memory in the mammalian brain. Reversible pharmacological inactivation of hippocampal synaptic activity, by blockade of AMPAR-mediated transmission, has revealed that synaptic activity is required for encoding, consolidation, and retrieval of spatial memories in rats (Riedel et al., 1999). Reversible, genetic reduction of hippocampal PKA activity might definitively shed light on the roles of PKA in these important temporal phases of memory processing (see Isiegas et al., 2006, for evidence of PKA’s inhibitory role in extinction), but more refinement is needed to achieve increasingly rapid temporal and spatial control of transgene expression to definitively resolve the contributions of PKA to the phases of memory processing.
We also need to explore the functional role of specific forms of PKA. Broadly speaking, PKA is present as RI or RII containing tetramers, referred to as type 1 or type II PKA respectively. These forms of PKA have different affinities for cAMP and they are localized to specific subcellular locations by AKAPs (reviewed in Bauman et al., 2004). Identifying isoform specific functions of PKA and the function of specific PKA-containing AKAP complexes is a challenge, but selective pharmacological tools are becoming available to examine these questions (e.g., Gold et al., 2006). Appropriate genetic approaches, such as the expression of specific inhibitors or the conditional mutation of appropriate genes, may also provide effective resolutions to these questions. Most of the current genetic experiments use broad-based inhibitors of either PKA activity or PKA anchoring. Identifying the specific PKA complex involved in memory storage will be critical for identifying the relevant PKA targets among the many that have been found to date.
PKA may selectively control specific aspects of memory processing. If so, this might lead to novel strategies, involving pharmacological, environmental, or genetic manipulations of PKA signaling, for alleviating memory disorders that arise from deficits of one or more of these temporal stages of memory processing. However, such strategies must be tempered by the fact that PKA activation in several brain structures, such as the prefrontal cortex and nucleus accumbens, can impair, rather than improve, cognition (Arnsten et al., 2005).
Central for further advancing our understanding of the roles of PKA in memory is the need for more research on PKA’s roles in regulating synaptic plasticity in multiple brain structures. The history of this field is rich with examples of how the elucidation of mechanisms of synaptic plasticity can enhance our understanding of the cellular and molecular bases of learning and memory. Understanding the essence of memory requires a clear grasp of the integrative nature of memory processing, which involves coordinated communication between many distinct brain structures and subregions. Thus, the role of PKA in extrahippocampal synaptic physiology and cognition is ripe for future investigation. There is evidence that PKA is important for synaptic plasticity in brain regions outside of the hippocampus, such as prefrontal cortex (Jay et al., 1998), amygdala (Huang and Kandel, 1998), neostriatum (Colwell and Levine, 1995), thalamus (Castro-Alamancos and Calcagnotto, 1999), visual cortex (Hensch et al., 1998), and cerebellum (Salin et al., 1996; Chen and Regehr, 1997; Chavis et al., 1998; Linden and Ahn, 1999). Another important goal will be to distinguish between PKA’s role as a mediator, versus its role as a modulator, of memory function. PKA may act as a mediator by directly controlling memory functions, whereas a modulatory role for PKA will be evident if PKA modifies the efficacies of other signaling pathways and neurotransmitter systems to alter memory dynamics.
Most, if not all, studies on hippocampal memory and synaptic plasticity are correlative because it is very difficult to establish causality between a molecular event, such as PKA activation, and a more complex process, such as synaptic potentiation or memory. At the molecular level, a critical requirement that needs to be satisfied before a definitive causal relationship between PKA activation and memory is established is direct visualization of PKA activation during memory processing. Apart from in vitro cellular imaging of PKA activity (Zhang et al., 2001; Gervasi et al., 2007), it will also be critical to refine techniques for in vivo imaging of kinase expression or activities. Such methods may be used to assess the causal relationships between PKA activity and specific behaviors, such as spatial and contextual learning. Some progress has been achieved in this domain, particularly with the emergence of two-photon imaging of neural activity in freely moving animals (Helmchen et al., 2001), but further fine-tuning is necessary to accommodate the stringent requirements for stable and specific monitoring of signal transduction in neural circuits in vivo.
Another technical approach that may yield important insights on the roles of PKA in behavior is in vivo recording of neural network activity patterns. Simultaneous recording of electrical activity in two or more interconnected brain regions in awake, freely moving animals is an important step towards elucidating the roles of identified signaling molecules in complex brain functions such as perception, learning, and memory. The relative paucity of data on multi-regional network activity patterns in genetically modified mice may now be remedied by the application of multi-electrode methods that can record oscillatory firing patterns in awake rodents, including mice (Buzsáki et al., 2003). It will be vital to apply these approaches to genetically modified PKA mutant mice (Abel et al., 1997) to directly assess the roles of PKA in regulating multi-region brain activity. Overall, the integrated application of methods from molecular and systems neuroscience will reveal novel roles for intracellular signaling in general, and for PKA signaling in particular, in learning and memory.
Acknowledgments
Our research was supported by the Canadian Institutes of Health Research (PVN), the Alberta Heritage Foundation for Medical Research (PVN), the National Institutes of Health (TA), and the Human Frontiers Science Program (TA). PVN thanks the University of Pennsylvania Provost’s Distinguished International Scholars Program for supporting a sabbatical visit during which this article was written. We thank Ted Huang (University of Pennsylvania) for his help with constructing the figure. We thank S. Belleville, V. Castellucci, M. Dumas, J. C. Lacaille, and W. Sossin for making the “Essence of Memory” meeting in Montreal both memorable and fruitful. We apologize to colleagues whose research we could not discuss because of space limitations.
References
- Abel T, Nguyen PV, Barad M, Deuel TAS, Kandel ER, Bourtchuladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- Abraham WC, Logan B, Greenwood JM, Dragunow M. Induction and experience-dependent consolidation of stable long-term potentiation lasting months in the hippocampus. J Neurosci. 2002;22:9626–9634. doi: 10.1523/JNEUROSCI.22-21-09626.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahi J, Radulovic J, Spiess J. The role of hippocampal signaling cascades in consolidation of fear memory. Behav Brain Res. 2004;149:17–31. doi: 10.1016/s0166-4328(03)00207-9. [DOI] [PubMed] [Google Scholar]
- Alarcon JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A. Chromatin acetylation, memory, and LTP are impaired in CBP(+/−) Mice: a model for the cognitive deficit in Rubinstein–Taybi syndrome and its amelioration. Neuron. 2004;42:947–959. doi: 10.1016/j.neuron.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Allen PB, Hvalby O, Jensen V, Errington ML, Ramsay M, Chaudry FA, Bliss TVP, Storm-Mathisen J, Morris RGM, Andersen P, Greengard P. Protein phosphatase-1 regulation in the induction of LTP: heterogeneous molecular mechanisms. J Neurosci. 2000;20:3537–3543. doi: 10.1523/JNEUROSCI.20-10-03537.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P, Sundberg SH, Sveen O, Wigstrom H. Specific long-lasting potentiation of synaptic transmission in hippocampal slices. Nature. 1977;266:736–737. doi: 10.1038/266736a0. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Ramos BP, Birnbaum SG, Taylor JR. Protein kinase A as a therapeutic target for memory disorders: rationale and challenges. Trends Neurosci. 2005;11:121–128. doi: 10.1016/j.molmed.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Athos J, Impey S, Pineda VV, Chen X, Storm DR. Hippocampal CRE-mediated gene expression is required for contextual memory formation. Nat Neurosci. 2002;5:1119–1120. doi: 10.1038/nn951. [DOI] [PubMed] [Google Scholar]
- Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci USA. 1999;96:5280–5285. doi: 10.1073/pnas.96.9.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacskai BJ, Hochner B, Mahaut-Smith M, Adams SR, Kaang BK, Kandel ER, Tsien RY. Spatially resolved dynamics of cAMP and protein kinase A subunits in Aplysia sensory neurons. Science. 1993;260:222–226. doi: 10.1126/science.7682336. [DOI] [PubMed] [Google Scholar]
- Balschun D, Wolfer DP, Gass P, Mantamadiotis T, Welzl H, Schutz G, Frey JU, Lipp HP. Does cAMP response element-binding protein have a pivotal role in hippocampal synaptic plasticity and hippocampus-dependent memory? J Neurosci. 2003;23:6304–6314. doi: 10.1523/JNEUROSCI.23-15-06304.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barad M, Bourtchouladze R, Winder DG, Golan H, Kandel E. Rolipram, a type IV-specific phosphodiesterase inhibitor, facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc Natl Acad Sci USA. 1998;95:15020–15025. doi: 10.1073/pnas.95.25.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Derkach V, Soderling T. Identification of the Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in the alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate-type glutamate receptor. J Biol Chem. 1997;272:32727–32730. doi: 10.1074/jbc.272.52.32727. [DOI] [PubMed] [Google Scholar]
- Bartsch D, Ghirardi M, Skehel PA, Karl KA, Herder SP, Chen M, Bailey CH, Kandel ER. Aplysia CREB2 represses long-term facilitation: relief of repression converts transient facilitation into long-term functional and structural change. Cell. 1995;83:979–992. doi: 10.1016/0092-8674(95)90213-9. [DOI] [PubMed] [Google Scholar]
- Bauman AL, Goehring AS, Scott JD. Orchestration of synaptic plasticity through AKAP signaling complexes. Neuropharmacology. 2004;46:299–310. doi: 10.1016/j.neuropharm.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Beavo JA, Bechtel PJ, Krebs EG. Activation of protein kinase by physiological concentrations of cyclic AMP. Proc Natl Acad Sci USA. 1974;71:3580–3583. doi: 10.1073/pnas.71.9.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe SJ. The cAMP-dependent protein kinases and cAMP signal transduction. Semin Cancer Biol. 1994;5:285–294. [PubMed] [Google Scholar]
- Bernabeu R, Bevilaqua L, Ardenghi P, Bromberg E, Schmitz P, Bianchin M, Izquierdo I, Medina JH. Involvement of hippocampal cAMP/cAMP-dependent protein kinase signaling pathways in a late memory consolidation phase of aversively motivated learning in rats. Proc Natl Acad Sci USA. 1997;94:7041–7046. doi: 10.1073/pnas.94.13.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitzer R, Wong T, Nouranifar R, Iyengar R, Landau E. Postsynaptic cAMP pathway gates early LTP in hippocampal CA1 region. Neuron. 1995;15:1403–1414. doi: 10.1016/0896-6273(95)90018-7. [DOI] [PubMed] [Google Scholar]
- Blitzer RD, Connor JH, Brown GP, Wong T, Shenolikar S, Iyengar R, Landau EM. Gating of CaMKII by cAMP-regulated protein phosphatase activity during LTP. Science. 1998;280:1940–1942. doi: 10.1126/science.280.5371.1940. [DOI] [PubMed] [Google Scholar]
- Bourtchouladze R, Abel T, Berman N, Gordon R, Lapidus K, Kandel ER. Different training procedures for contextual memory in mice can recruit either one or two critical periods for memory consolidation that require protein synthesis and PKA. Learn Mem. 1998;5:365–374. [PMC free article] [PubMed] [Google Scholar]
- Bourtchouladze R, Frenguelli B, Blendy J, Cioffi D, Schütz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Bourtchouladze R, Lidge R, Catapano R, Stanley J, Gossweiler S, Romashko D, Scott R, Tully T. A mouse model of Rubinstein–Taybi syndrome: defective long-term memory is ameliorated by inhibitors of phosphodiesterase 4. Proc Natl Acad Sci USA. 2003;100:10518–10522. doi: 10.1073/pnas.1834280100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourtchouladze R, Patterson SL, Kelly MP, Kreibich A, Kandel ER, Abel T. Chronically increased Gs alpha signaling disrupts associative and spatial learning. Learn Mem. 2006;13:745–752. doi: 10.1101/lm.354106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon EP, Idzerda RL, McKnight GS. PKA isoforms, neural pathways, and behavior: making the connection. Curr Opin Neurobiol. 1997;7:397–403. doi: 10.1016/s0959-4388(97)80069-4. [DOI] [PubMed] [Google Scholar]
- Bucan M, Abel T. The mouse: genetics meets behaviour. Nat Rev Genet. 2002;3:114–123. doi: 10.1038/nrg728. [DOI] [PubMed] [Google Scholar]
- Burrell BD, Sahley CL. Learning in simple systems. Curr Opin Neurobiol. 2001;11:757–764. doi: 10.1016/s0959-4388(01)00281-1. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Buhl DL, Harris KD, Csicsvari J, Czeh B, Morozov A. Hippocampal network patterns of activity in the mouse. Neuroscience. 2003;116:201–211. doi: 10.1016/s0306-4522(02)00669-3. [DOI] [PubMed] [Google Scholar]
- Cadd G, McKnight GS. Distinct patterns of cAMP-dependent protein kinase gene expression in mouse brain. Neuron. 1989;3:71–79. doi: 10.1016/0896-6273(89)90116-5. [DOI] [PubMed] [Google Scholar]
- Carroll RC, Lissin DV, von Zastrow M, Nicoll RA, Malenka RC. Rapid redistribution of glutamate receptors contributes to long-term depression in hippocampal cultures. Nat Neurosci. 1999;2:454–460. doi: 10.1038/8123. [DOI] [PubMed] [Google Scholar]
- Castellucci V, Pinsker H, Kupfermann I, Kandel ER. Neuronal mechanisms of habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science. 1970;167:1745–1748. doi: 10.1126/science.167.3926.1745. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Schoch S, Schmitz F, Sudhof TC, Malenka RC. RIM1alpha is required for presynaptic long-term potentiation. Nature. 2002;415:327–330. doi: 10.1038/415327a. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Calcagnotto ME. Pre-synaptic long-term potentiation in corticothalamic synapses. J Neurosci. 1999;19:9090–9097. doi: 10.1523/JNEUROSCI.19-20-09090.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavis P, Mollard P, Bockaert J, Manzoni O. Visualization of cyclic AMP-regulated presynaptic activity at cerebellar granule cells. Neuron. 1998;20:773–781. doi: 10.1016/s0896-6273(00)81015-6. [DOI] [PubMed] [Google Scholar]
- Chen C, Regehr WG. Mechanism of cAMP-mediated enhancement at a cerebellar synapse. J Neurosci. 1997;17:8687–8694. doi: 10.1523/JNEUROSCI.17-22-08687.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem RL, Barth A. Pathway-specific trafficking of native AMPARs by in vivo experience. Neuron. 2006;49:663–670. doi: 10.1016/j.neuron.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Kehl SJ, McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS, Levine MS. Excitatory synaptic transmission in neostriatal neurons: regulation by cyclic AMP-dependent mechanisms. J Neurosci. 1995;15:1704–1713. doi: 10.1523/JNEUROSCI.15-03-01704.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Hochner B, Kandel ER. Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature. 1990;345:718–721. doi: 10.1038/345718a0. [DOI] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL. Physiology and biochemistry of Drosophila learning mutants. Physiol Rev. 1996;76:299–317. doi: 10.1152/physrev.1996.76.2.299. [DOI] [PubMed] [Google Scholar]
- Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8:101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Doskeland SO, Maronde E, Gjertsen BT. The genetic subtypes of cAMP-dependent protein kinase —functionally different or redundant? Biochim. Biophys Acta. 1993;1178:249–258. doi: 10.1016/0167-4889(93)90201-y. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci USA. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy SN, Craddock KJ, Abel T, Nguyen PV. Environmental enrichment modifies the PKA-dependence of hippocampal LTP and improves hippocampus-dependent memory. Learn Mem. 2001;8:26–34. doi: 10.1101/lm.36301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy SN, Nguyen PV. Postsynaptic application of a peptide inhibitor of cAMP-dependent protein kinase blocks expression of long-lasting synaptic potentiation in hippocampal neurons. J Neurosci. 2003;23:1142–1150. doi: 10.1523/JNEUROSCI.23-04-01142.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emptage NJ, Carew TJ. Long-term synaptic facilitation in the absence of short-term facilitation in Aplysia neurons. Science. 1993;262:253–256. doi: 10.1126/science.8211146. [DOI] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6:136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- Francis SH, Corbin JD. Cyclic nucleotide-dependent protein kinases: intracellular receptors for cAMP and cGMP action. Crit Rev Clin Lab Sci. 1999;36:275–328. doi: 10.1080/10408369991239213. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RG. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- Gass P, Wolfer DP, Balschun D, Rudolph D, Frey U, Lipp H, Schutz G. Deficits in memory tasks of mice with CREB mutations depend on gene dosage. Learn Mem. 1998;5:274–288. [PMC free article] [PubMed] [Google Scholar]
- Gervasi N, Hepp R, Tricoire L, Zhang J, Lambolez B, Paupardin-Tritsch D, Vincent P. Dynamics of protein kinase A signaling at the membrane, in the cytosol, and in the nucleus of neurons in mouse brain slices. J Neurosci. 2007;27:2744–2750. doi: 10.1523/JNEUROSCI.5352-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs CS, Knighton DR, Sowadski JM, Taylor SS, Zoller MJ. Systematic mutational analysis of cAMP-dependent protein kinase identifies unregulated catalytic subunits and defines regions important for the recognition of the regulatory subunit. J Biol Chem. 1992;267:4806–4814. [PubMed] [Google Scholar]
- Gold MG, Lygren B, Dokurno P, Hoshi N, McConnachie G, Tasken K, Carlson CR, Scott JD, Barford D. Molecular basis of AKAP specificity for PKA regulatory subunits. Mol Cell. 2006;24:383–395. doi: 10.1016/j.molcel.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Gong B, Vitolo OV, Trinchese F, Liu S, Shelanski M, Arancio O. Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model after rolipram treatment. J Clin Invest. 2004;114:1624–1634. doi: 10.1172/JCI22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Graves L, Dalvi A, Lucki I, Blendy JA, Abel T. Behavioral analysis of CREB alphadelta mutation on a B6/129 F1 hybrid background. Hippocampus. 2002;12:18–26. doi: 10.1002/hipo.10003. [DOI] [PubMed] [Google Scholar]
- Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- Greengard P, Jen J, Nairn A, Stevens CF. Enhancement of the glutamate response by cAMP-dependent protein kinase in hippocampal neurons. Science. 1991;253:1135–1138. doi: 10.1126/science.1716001. [DOI] [PubMed] [Google Scholar]
- Gruart A, Munoz MD, Delgado-Garcia JM. Involvement of the CA3–CA1 synapse in the acquisition of associative learning in behaving mice. J Neurosci. 2006;26:1077–1087. doi: 10.1523/JNEUROSCI.2834-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Helmchen F, Fee MS, Tank DW, Denk W. A miniature head-mounted two-photon microscope. high-resolution brain imaging in freely moving animals. Neuron. 2001;31:903–912. doi: 10.1016/s0896-6273(01)00421-4. [DOI] [PubMed] [Google Scholar]
- Hemmings HC, Jr, Nairn AC, Greengard P. DARPP-32, a dopamine- and adenosine 3′:5′-monopho-sphate-regulated neuronal phosphoprotein. II Comparison of the kinetics of phosphorylation of DARPP-32 and phosphatase inhibitor 1. J Biol Chem. 1984;259:14491–14497. [PubMed] [Google Scholar]
- Hensch TK, Gordon JA, Brandon EP, McKnight GS, Idzerda RL, Stryker MP. Comparison of plasticity in vivo and in vitro in the developing visual cortex of normal and protein kinase A RIbeta-deficient mice. J Neurosci. 1998;18:2108–2117. doi: 10.1523/JNEUROSCI.18-06-02108.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Howe DG, Wiley JC, McKnight GS. Molecular and behavioral effects of a null mutation in all PKA C beta isoforms. Mol Cell Neurosci. 2002;20:515–524. doi: 10.1006/mcne.2002.1119. [DOI] [PubMed] [Google Scholar]
- Huang FL, Glinsmann WH. Separation and characterization of two phosphorylase phosphatase inhibitors from rabbit skeletal muscle. Eur J Biochem. 1976;70:419–426. doi: 10.1111/j.1432-1033.1976.tb11032.x. [DOI] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. Recruitment of long-lasting and protein kinase A-dependent long-term potentiation in the CA1 region of hippocampus requires repeated tetanization. Learn Mem. 1994;1:74–82. [PubMed] [Google Scholar]
- Huang YY, Kandel ER. Postsynaptic induction and PKA-dependent expression of LTP in the lateral amygdala. Neuron. 1998;21:169–178. doi: 10.1016/s0896-6273(00)80524-3. [DOI] [PubMed] [Google Scholar]
- Huang YY, Li XC, Kandel ER. cAMP contributes to mossy fiber LTP by initiating both a covalently mediated early phase and macromolecular synthesis-dependent late phase. Cell. 1994;79:69–79. doi: 10.1016/0092-8674(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Huang YY, Zakharenko SS, Schoch S, Kaeser PS, Janz R, Sudhof TC, Siegelbaum SA, Kandel ER. Genetic evidence for a protein-kinase-A-mediated presynaptic component in NMDA-receptor-dependent forms of long-term synaptic potentiation. Proc Natl Acad Sci USA. 2005;102:9365–9370. doi: 10.1073/pnas.0503777102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummler E, Cole T, Blendy J, Ganss R, Aguzzi A, Schmid W, Beermann F, Schulz G. Targeted mutation of the CREB gene: compensation within the CREB/ATF family of transcription factors. Proc Natl Acad Sci USA. 1994;91:5647–5651. doi: 10.1073/pnas.91.12.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impey S, Smith DM, Obrietan K, Donahue R, Wade C, Storm DR. Stimulation of cAMP response element (CRE)-mediated transcription during contextual learning. Nat Neurosci. 1998;1:595–601. doi: 10.1038/2830. [DOI] [PubMed] [Google Scholar]
- Isiegas C, Park A, Kandel ER, Abel T, Lattal KM. Transgenic inhibition of neuronal protein kinase A activity facilitates fear extinction. J Neurosci. 2006;26:12700–12707. doi: 10.1523/JNEUROSCI.2743-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo I, Barros DM, Mello e Souza T, de Souza MM, Izquierdo LA, Medina JH. Mechanisms for memory types differ. Nature. 1998;393:635–636. doi: 10.1038/31371. [DOI] [PubMed] [Google Scholar]
- James W. The Principles of Psychology. Holt; New York: 1890. [Google Scholar]
- Jay TM, Gurden H, Yamaguchi T. Rapid increase in PKA activity during long-term potentiation in the hippocampal afferent fibre system to the prefrontal cortex in vivo. Eur J Neurosci. 1998;10:3302–3306. doi: 10.1046/j.1460-9568.1998.00389.x. [DOI] [PubMed] [Google Scholar]
- Kameyama K, Lee HK, Bear MF, Huganir RL. Involvement of a postsynaptic PKA substrate in expression of homosynaptic LTD. Neuron. 1998;21:1163–1175. doi: 10.1016/s0896-6273(00)80633-9. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: A dialogue between genes and synapses [Nobel Lecture] Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Keeley MB, Wood MA, Isiegas C, Stein J, Hellman K, Hannenhalli S, Abel T. Differential transcriptional response to nonassociative and associative components of classical fear conditioning in the amygdala and hippocampus. Learn Mem. 2006;13:135–142. doi: 10.1101/lm.86906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida S, Josselyn SA, de Ortiz SP, Kogan JH, Chevere I, Masushige S, Silva AJ. CREB required for the stability of new and reactivated fear memories. Nat Neurosci. 2002;5:348–355. doi: 10.1038/nn819. [DOI] [PubMed] [Google Scholar]
- Korzus E, Rosenfeld MG, Mayford M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron. 2004;42:961–972. doi: 10.1016/j.neuron.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfermann I, Castellucci V, Pinsker H, Kandel E. Neuronal correlates of habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science. 1970;167:1743–1745. doi: 10.1126/science.167.3926.1743. [DOI] [PubMed] [Google Scholar]
- Lee H, Barbarosie M, Kameyama K, Huganir RL, Bear MF. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405:955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- Lee H, Kameyama K, Huganir RL, Bear MF. NMDA induces long-term synaptic depression and dephosphorylation of the GluR1 subunit of AMPA receptors in hippocampus. Neuron. 1998;21:1151–1162. doi: 10.1016/s0896-6273(00)80632-7. [DOI] [PubMed] [Google Scholar]
- Linden DJ, Ahn S. Activation of presynaptic cAMP-dependent protein kinase is required for induction of cerebellar long-term potentiation. J Neurosci. 1999;19:10221–10227. doi: 10.1523/JNEUROSCI.19-23-10221.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SG, Cull-Candy GG. Synaptic activity at calcium-permeable AMPA receptors induces a switch in receptor subtype. Nature. 2000;405:454–458. doi: 10.1038/35013064. [DOI] [PubMed] [Google Scholar]
- Lonart G, Schoch S, Kaeser PS, Larkin CJ, Sudhof TC, Linden DJ. Phosphorylation of RIM1alpha by PKA triggers presynaptic long-term potentiation at cerebellar parallel fiber synapses. Cell. 2003;115:49–60. doi: 10.1016/s0092-8674(03)00727-x. [DOI] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Lu W, Man H, Ju W, Trimble WS, MacDonald JF, Wang YT. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron. 2001;29:243–254. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- Malleret G, Haditsch U, Genoux D, Jones MW, Bliss TV, Vanhoose AM, Weitlauf C, Kandel ER, Winder DG, Mansuy IM. Inducible and reversible enhancement of learning, memory, and long-term potentiation by genetic inhibition of calcineurin. Cell. 2001;104:675–686. doi: 10.1016/s0092-8674(01)00264-1. [DOI] [PubMed] [Google Scholar]
- Mammen AL, Kameyama K, Roche KW, Huganir RL. Phosphorylation of the alpha-amino-3-hydroxy-5-methylisoxazole4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J Biol Chem. 1997;272:32528–32533. doi: 10.1074/jbc.272.51.32528. [DOI] [PubMed] [Google Scholar]
- Man HY, Lin JW, Ju WH, Ahmadian G, Liu L, Becker LE, Sheng M, Wang YT. Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization. Neuron. 2000;25:649–662. doi: 10.1016/s0896-6273(00)81067-3. [DOI] [PubMed] [Google Scholar]
- Man HY, Sekine-Aizawa Y, Huganir RL. Regulation of AMPA receptor trafficking through PKA phosphorylation of the GluR1 subunit. Proc Natl Acad Sci USA. 2007;104:3579–3584. doi: 10.1073/pnas.0611698104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Martin KC, Casadio A, Zhu H, EY, Rose JC, Chen M, Bailey CH, Kandel ER. Synapse-specific long-term facilitation of Aplysia sensory to motor synapses: a function for local protein synthesis in memory storage. Cell. 1997;91:927–938. doi: 10.1016/s0092-8674(00)80484-5. [DOI] [PubMed] [Google Scholar]
- Martin S, Grimwood P, Morris RGM. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Tomizawa K, Moriwaki A, Li ST, Terada H, Matsui H. A high-efficiency protein transduction system demonstrating the role of PKA in long-lasting LTP. J Neurosci. 2001;21:6000–6007. doi: 10.1523/JNEUROSCI.21-16-06000.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire SE, Deshazer M, Davis RL. Thirty years of olfactory learning and memory research in Drosophila melanogaster. Prog Neurobiol. 2005;76:328–347. doi: 10.1016/j.pneurobio.2005.09.003. [DOI] [PubMed] [Google Scholar]
- McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390:607–611. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- McKnight GS, Clegg CH, Uhler MD, Chrivia JC, Cadd GG, Correll LA, Otten AD. Analysis of the cAMP-dependent protein kinase system using molecular genetic approaches. Rec Prog Horm Res. 1988;44:307–335. doi: 10.1016/b978-0-12-571144-9.50014-4. [DOI] [PubMed] [Google Scholar]
- Milner B, Squire LR, Kandel ER. Cognitive neuroscience and the study of memory. Neuron. 1998;20:445–468. doi: 10.1016/s0896-6273(00)80987-3. [DOI] [PubMed] [Google Scholar]
- Miyamoto E, Kuo JF, Greengard P. Adenosine 3′,5′-monophosphate-dependent protein kinase from brain. Science. 1968;165:63–65. [PubMed] [Google Scholar]
- Miyamoto E, Kuo JF, Greengard P. Cyclic nucleotide-dependent protein kinases. 3 Purification and properties of adenosine 3′,5′-monophosphate-dependent protein kinase from bovine brain. J Biol Chem. 1969;244:6395–6402. [PubMed] [Google Scholar]
- Mizuno M, Yamada K, Maekawa N, Saito K, Seishima M, Nabeshima T. CREB phosphorylation as a molecular marker of memory processing in the hippocampus for spatial learning. Behav Brain Res. 2002;133:135–141. doi: 10.1016/s0166-4328(01)00470-3. [DOI] [PubMed] [Google Scholar]
- Moncada D, Viola H. Induction of long-term memory by exposure to novelty requires protein synthesis: evidence for a behavioral tagging. J Neurosci. 2007;27:7476–7481. doi: 10.1523/JNEUROSCI.1083-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montminy M. Transcriptional regulation by cyclic AMP. Annu Rev Biochem. 1997;66:807–822. doi: 10.1146/annurev.biochem.66.1.807. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Garrud P, Rawlins JNP, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Endo S, Shenolikar S, Malenka RC. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994;369:486–488. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- Nayak A, Zastrow DJ, Lickteig R, Zahniser NR, Browning MD. Maintenance of late-phase LTP is accompanied by PKA-dependent increase in AMPA receptor synthesis. Nature. 1998;394:680–683. doi: 10.1038/29305. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Abel T, Kandel ER. Requirement of a critical period of transcription for induction of a late phase of LTP. Science. 1994;265:1104–1107. doi: 10.1126/science.8066450. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Woo NH. Regulation of hippocampal synaptic plasticity by cyclic AMP-dependent protein kinases. Prog Neurobiol. 2003;71:401–437. doi: 10.1016/j.pneurobio.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Oike Y, Hata A, Mamiya T, Kaname T, Noda Y, Suzuki M, Yasue H, Nabeshima T, Araki K, Yamamura K. Truncated CBP protein leads to classical Rubinstein–Taybi syndrome phenotypes in mice: implications for a dominant-negative mechanism. Hum Mol Genet. 1999;8:387–396. doi: 10.1093/hmg/8.3.387. [DOI] [PubMed] [Google Scholar]
- Otmakhova NA, Otmakhov N, Mortenson L, Lisman JE. Inhibition of the cAMP pathway decreases early LTP at CA1 hippocampal synapses. J Neurosci. 2000;20:4446–4451. doi: 10.1523/JNEUROSCI.20-12-04446.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda VV, Athos JI, Wang H, Celver J, Ippolito D, Boulay G, Birnbaumer L, Storm DR. Removal of G(ialpha1) constraints on adenylyl cyclase in the hippocampus enhances LTP and impairs memory formation. Neuron. 2004;41:153–163. doi: 10.1016/s0896-6273(03)00813-4. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Huang YY, Paletzki RF, Bourtchouladze R, Scanlin H, Vronskaya S, Kandel ER. Reversible inhibition of CREB/ATF transcription factors in region CA1 of the dorsal hippocampus disrupts hippocampus-dependent spatial memory. Neuron. 2002;34:447–462. doi: 10.1016/s0896-6273(02)00684-0. [DOI] [PubMed] [Google Scholar]
- Powell CM, Schoch S, Monteggia L, Barrot M, Matos MF, Feldmann N, Sudhof TC, Nestler EJ. The presynaptic active zone protein RIM1alpha is critical for normal learning and memory. Neuron. 2004;42:143–153. doi: 10.1016/s0896-6273(04)00146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo J, Vianna MR, Martins MR, Barichello T, Medina JH, Roesler R, Izquierdo I. Protein synthesis, PKA, and MAP kinase are differentially involved in short- and long-term memory in rats. Behav Brain Res. 2004;154:339–343. doi: 10.1016/j.bbr.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Reimann EM, Walsh DA, Krebs EG. Purification and properties of rabbit skeletal muscle adenosine 3′,5′-monophosphate-dependent protein kinases. J Biol Chem. 1971;246:1986–1995. [PubMed] [Google Scholar]
- Riedel G, Micheau J, Lam AG, Roloff E, Martin SJ, Bridge H, Hoz L, Poeschel B, McCulloch J, Morris RG. Reversible neural inactivation reveals hippocampal participation in several memory processes. Nat Neurosci. 1999;2:898–905. doi: 10.1038/13202. [DOI] [PubMed] [Google Scholar]
- Roche KW, O’Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Rose GM, Hopper A, De Vivo M, Tehim A. Phosphodiesterase inhibitors for cognitive enhancement. Curr Pharm Des. 2005;11:3329–3334. doi: 10.2174/138161205774370799. [DOI] [PubMed] [Google Scholar]
- Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- Salin PA, Malenka RC, Nicoll RA. Cyclic AMP mediates a presynaptic form of LTP at cerebellar parallel fiber synapses. Neuron. 1996;16:797–803. doi: 10.1016/s0896-6273(00)80099-9. [DOI] [PubMed] [Google Scholar]
- Schafe GE, LeDoux JE. Memory consolidation of auditory Pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci. 2000;20:RC96. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuman EM. Synapse specificity and long-term information storage. Neuron. 1997;18:339–342. doi: 10.1016/s0896-6273(00)81234-9. [DOI] [PubMed] [Google Scholar]
- Sharifzadeh M, Sharifzadeh K, Naghdi N, Ghahremani MH, Roghani A. Posttraining intrahippocampal infusion of a protein kinase AII inhibitor impairs spatial memory retention in rats. J Neurosci Res. 2005;79:392–400. doi: 10.1002/jnr.20358. [DOI] [PubMed] [Google Scholar]
- Sherff CM, Carew TJ. Parallel somatic and synaptic processing in the induction of intermediate-term and long-term synaptic facilitation in Aplysia. Proc Natl Acad Sci USA. 2004;101:7463–7468. doi: 10.1073/pnas.0402163101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- Sindreu CB, Scheiner ZS, Storm DR. Ca2+ -stimulated adenylyl cyclases regulate ERK-dependent activation of MSK1 during fear conditioning. Neuron. 2007;53:79–89. doi: 10.1016/j.neuron.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalhegg BS, Tasken K. Specificity in the cAMP/PKA signaling pathway. Differential expression, regulation, and subcellular localization of subunits of PKA. Front Biosci. 2000;5:D678–D693. doi: 10.2741/skalhegg. [DOI] [PubMed] [Google Scholar]
- Skeberdis VA, Chevaleyre V, Lau CG, Goldberg JH, Pettit DL, Suadicani SO, Lin Y, Bennett MV, Yuste R, Castillo PE, Zukin RS. Protein kinase A regulates calcium permeability of NMDA receptors. Nat Neurosci. 2006;9:501–510. doi: 10.1038/nn1664. [DOI] [PubMed] [Google Scholar]
- Soderling TR, Derkach VA. Postsynaptic protein phosphorylation and LTP. Trends Neurosci. 2000;23:75–80. doi: 10.1016/s0166-2236(99)01490-3. [DOI] [PubMed] [Google Scholar]
- Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002;25:578–588. doi: 10.1016/s0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- Sossin WS. Mechanisms for the generation of synapse specificity in long-term memory: the implications of a requirement for transcription. Trends Neurosci. 1996;19:215–218. doi: 10.1016/0166-2236(96)20016-5. [DOI] [PubMed] [Google Scholar]
- Stanciu M, Radulovic J, Spiess J. Phosphorylated cAMP response element binding protein in the mouse brain after fear conditioning: relationship to Fos production. Brain Res Mol Brain Res. 2001;94:15–24. doi: 10.1016/s0169-328x(01)00174-7. [DOI] [PubMed] [Google Scholar]
- Sutherland EW, Oye I, Butcher RW. The action of epinephrine and the role of the adenyl cyclase system in hormone action. Rec Prog Horm Res. 1965;21:623–646. [PubMed] [Google Scholar]
- Sutherland EW, Rall TW. The properties of an adenine ribonucleotide produced with cellular particles, ATP, Mg, and epinephrine or glucagons. J Am Chem Soc. 1957;79:3608. [Google Scholar]
- Tasken K, Skalhegg BS, Solberg R, Andersson KB, Taylor SS, Lea T, Blomhoff HK, Jahnsen T, Hansson V. Novel isozymes of cAMP-dependent protein kinase exist in human cells due to formation of RI alpha–RI beta heterodimeric complexes. J Biol Chem. 1993;268:21276–21283. [PubMed] [Google Scholar]
- Taylor SS, Buechler JA, Yonemoto W. cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu Rev Biochem. 1990;59:971–1005. doi: 10.1146/annurev.bi.59.070190.004543. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- Tully T. Physiology of mutations affecting learning and memory in Drosophila — the missing link between gene product and behavior. Trends Neurosci. 1991;14:163–164. doi: 10.1016/0166-2236(91)90096-d. [DOI] [PubMed] [Google Scholar]
- Vazquez SI, Vazquez A, Pena de Ortiz S. Different hippocampal activity profiles for PKA and PKC in spatial discrimination learning. Behav Neurosci. 2000;114:1109–1118. doi: 10.1037//0735-7044.114.6.1109. [DOI] [PubMed] [Google Scholar]
- Vianna MR, Izquierdo LA, Barros DM, Ardenghi P, Pereira P, Rodrigues C, Moletta B, Medina JH, Izquierdo I. Differential role of hippocampal cAMP-dependent protein kinase in short- and long-term memory. Neurochem Res. 2000;25:621–626. doi: 10.1023/a:1007502918282. [DOI] [PubMed] [Google Scholar]
- Vitolo OV, Sant’Angelo A, Costanzo V, Battaglia F, Arancio O, Shelanski M. Amyloid beta-peptide inhibition of the PKA/CREB pathway and long-term potentiation: reversibility by drugs that enhance cAMP signaling. Proc Natl Acad Sci USA. 2002;99:13217–13221. doi: 10.1073/pnas.172504199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenstein GV, Vago DR, Walberer AM. Time-dependent involvement of PKA/PKC in contextual memory consolidation. Behav Brain Res. 2002;133:159–164. doi: 10.1016/s0166-4328(01)00476-4. [DOI] [PubMed] [Google Scholar]
- Walsh DA, Perkins JP, Krebs EG. An adenosine 3′,5′-monophosphate-dependant protein kinase from rabbit skeletal muscle. J Biol Chem. 1968;243:3763–3765. [PubMed] [Google Scholar]
- Wang H, Ferguson GD, Pineda VV, Cundiff PE, Storm DR. Overexpression of type-1 adenylyl cyclase in mouse forebrain enhances recognition memory and LTP. Nat Neurosci. 2004;7:635–642. doi: 10.1038/nn1248. [DOI] [PubMed] [Google Scholar]
- Wang LY, Salter MW, MacDonald JF. Regulation of kainate receptors by cAMP-dependent protein kinase and phosphatases. Science. 1991;253:1132–1135. doi: 10.1126/science.1653455. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Castillo PE, Zalutsky RA, Nicoll RA. Mediation of hippocampal mossy fiber long-term potentiation by cyclic AMP. Science. 1994;265:1878–1882. doi: 10.1126/science.7916482. [DOI] [PubMed] [Google Scholar]
- Westphal RS, Tavalin SJ, Lin JW, Alto NM, Fraser ID, Langeberg LK, Sheng M, Scott JD. Regulation of NMDA receptors by an associated phosphatase-kinase signaling complex. Science. 1999;285:93–96. doi: 10.1126/science.285.5424.93. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Wong ST, Athos J, Figueroa XA, Pineda VV, Schaefer ML, Chavkin CC, Muglia LJ, Storm DR. Calcium-stimulated adenylyl cyclase activity is critical for hippocampus-dependent long-term memory and late phase LTP. Neuron. 1999;23:787–798. doi: 10.1016/s0896-6273(01)80036-2. [DOI] [PubMed] [Google Scholar]
- Woo NH, Abel T, Nguyen PV. Genetic and pharmacological demonstration of a role for cyclic AMP-dependent protein kinase-mediated suppression of protein phosphatases in gating the expression of late LTP. Eur J Neurosci. 2002;16:1871–1876. doi: 10.1046/j.1460-9568.2002.02260.x. [DOI] [PubMed] [Google Scholar]
- Woo NH, Duffy SN, Abel T, Nguyen PV. Genetic and pharmacological demonstration of differential recruitment of cAMP-dependent protein kinases by synaptic activity. J Neurophysiol. 2000;84:2739–2745. doi: 10.1152/jn.2000.84.6.2739. [DOI] [PubMed] [Google Scholar]
- Woo NH, Duffy SN, Abel T, Nguyen PV. Temporal spacing of synaptic stimulation critically modulates the dependence of LTP on cyclic AMP-dependent protein kinase. Hippocampus. 2003;13:293–300. doi: 10.1002/hipo.10086. [DOI] [PubMed] [Google Scholar]
- Wood MA, Attner MA, Oliveira AM, Brindle PK, Abel T. A transcription factor-binding domain of the coactivator CBP is essential for long-term memory and the expression of specific target genes. Learn Mem. 2006;13:609–617. doi: 10.1101/lm.213906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood MA, Kaplan MP, Park A, Blanchard EJ, Oliveira AM, Lombardi TL, Abel T. Transgenic mice expressing a truncated form of CREB-binding protein (CBP) exhibit deficits in hippocampal synaptic plasticity and memory storage. Learn Mem. 2005;12:111–119. doi: 10.1101/lm.86605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ZL, Thomas SA, Villacres EC, Xia Z, Simmons ML, Chavkin C, Palmiter RD, Storm DR. Altered behavior and long-term potentiation in type 1 adenylyl cyclase mutant mice. Proc Natl Acad Sci USA. 1995;92:220–224. doi: 10.1073/pnas.92.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto KK, Gonzalez GA, Biggs WH, III, Montminy MR. Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature. 1988;334:494–498. doi: 10.1038/334494a0. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Barth AL, Stellwagen D, Malenka RC. A developmental switch in the signaling cascades for LTP induction. Nat Neurosci. 2003;6:15–16. doi: 10.1038/nn985. [DOI] [PubMed] [Google Scholar]
- Yin JCP, Wallach JS, Del Vecchio M, Wilder EL, Zhou H, Quinn WG, Tully T. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell. 1994;79:49–58. doi: 10.1016/0092-8674(94)90399-9. [DOI] [PubMed] [Google Scholar]
- Young JZ, Isiegas C, Abel T, Nguyen PV. Metaplasticity of late-LTP: a critical role for PKA in synaptic tagging. Eur J Neurosci. 2006;23:1784–1794. doi: 10.1111/j.1460-9568.2006.04707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JZ, Nguyen PV. Homosynaptic and heterosynaptic inhibition of synaptic tagging and capture of LTP by prior synaptic activity. J Neurosci. 2005;25:7221–7231. doi: 10.1523/JNEUROSCI.0909-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ma Y, Taylor S, Tsien RY. Genetically encoded reporters of PKA activity reveal impact of substrate tethering. Proc Natl Acad Sci USA. 2001;98:14997–15002. doi: 10.1073/pnas.211566798. [DOI] [PMC free article] [PubMed] [Google Scholar]